Abstract
We investigated the effect of PKA treatment (1 U/ml) on the mechanical properties of isolated human cardiac myofibrils. PKA treatment was associated with significant incorporation of radiolabeled phosphate into several sarcomeric proteins including troponin I and myosin binding protein C and was also associated with a right shift in the tension-pCa relation (ΔpCa50 = 0.2 ± 0.1). PKA treatment also caused right shifts in the pCa dependence of the rate of tension development, tension redevelopment, and the linear and exponential phases of myofibril relaxation. However, there was no change in the same measures of crossbridge turnover when expressed as a function of tension. We conclude that the changes in crossbridge kinetics as a function of calcium concentration reflect a reduced tension due to a lower calcium sensitivity and that the relationship between crossbridge kinetics and tension was unchanged, indicating no direct effect of PKA treatment on crossbridge cycling.
Keywords: muscle contraction, phosphatase, relaxation, heart
the cardiac β-receptor, acting through G proteins, activates adenylate cyclase, increasing the level of intracellular cAMP, which, in turn, activates cAMP-dependent protein kinase (PKA). PKA phosphorylates many intracellular targets including calcium channels, ryanodine receptors, and myofilament proteins, most notably troponin I (TnI; Refs. 26, 30). The phosphorylation of TnI in cardiac muscle decreases the calcium sensitivity of myocytes (31). PKA treatment of demembranated or “skinned” cardiac muscle decreases the sensitivity of the contractile proteins to calcium, most likely due to the phosphorylation of cardiac TnI at serine residues in the N-terminal region of the protein (21).
Several studies have also suggested that PKA treatment alters crossbridge kinetics. For example, Kentish et al. (20) made transgenic mice where slow skeletal troponin I (ssTnI) replaced cardiac troponin I (cTnI). cTnI has N-terminal serines that ssTNI lacks, and these are thought to be important in regulating calcium sensitivity. In this study, flash photolysis of a calcium chelator produced a faster relaxation in PKA-treated cTnI mouse trabeculae than was seen in PKA-treated ssTnI mouse trabeculae. This suggested that PKA phosphorylation of the N-terminal serines could affect crossbridge kinetics (20).
The minimum of the Fourier transform of the changes in tension in response to a sequence of swept or randomized frequency length changes is termed fmin. Under steady-state conditions, fmin is used as an indicator of crossbridge turnover rate (19). In intact rat papillary muscle, the inhibition of cyclic AMP-dependent phosphatase produced a increase in fmin, suggesting that crossbridge cycling rate increased (35). Saeki et al. (29) studied length transients in response to step unloading of rat cardiac trabeculae and concluded that adrenaline increased crossbridge cycling. Adrenaline has also been found to increase ATPase activity in slices of myocardial wall (38). All of these findings are supportive of changes in crossbridge cycling.
However, contrary findings also exist; there was no difference in relaxation rate in response to flash photolysis of a calcium chelator between PKA-treated and control guinea pig trabeculae (18), measurements of shortening velocity in rat skinned cardiomyocytes showed no effect of PKA treatment (13), isoproterenol treatment produced no change in unloaded shortening velocity in intact rat cardiac trabeculae (10), nor did PKA treatment alter maximum shortening velocity (17) or the tension cost in skinned rat cardiac trabeculae (9).
Some studies (14) have found more complex responses; assessment of actomyosin interaction by use of the in vitro motility assay showed changes in velocity with PKA treatment only at submaximal levels of activation. The ATPase activity of a slurry of bovine cardiac myofibrils was less sensitive to calcium after the addition of PKA (26). Consequently, while changes in calcium sensitivity due to PKA treatment are well accepted, the effect of PKA on crossbridge kinetics and cycling rate remains controversial.
In this study, we used single isolated human cardiac myofibrils to assess the effect of PKA on crossbridge kinetics. The single myofibril technique offers the ability to examine the kinetics of both tension development and relaxation following step changes in ambient calcium that are complete within a few milliseconds (25). Alterations in crossbridge kinetics are reflected in changes in the rate of tension development or the rate of relaxation. This allows the direct effect of PKA on crossbridge kinetics to be examined.
We divided samples of human myofibrils into two aliquots, treated one aliquot with PKA and the other with an equal amount of buffer, and examined the mechanical response of single myofibrils to changes in the concentration of calcium in the bathing solution. While we did not measure baseline phosphorylation of sarcomeric proteins and so cannot exclude the possibility that some PKA targets sites were already fully phosphorylated, PKA treatment of myofibrils caused 32P incorporation into several sarcomeric proteins, indicating phosphorylation by active PKA. We found a decrease in calcium sensitivity consistent with previous studies of the effects of PKA. We also found a shift in the dependence of measures of crossbridge cycling rate on calcium but no change in their dependence on tension. This suggests that apparent PKA-induced changes in crossbridge cycling rate are secondary to reductions in tension at a given calcium and that there was no direct effect of PKA treatment on crossbridge cycling rate.
METHODS
Collection of human samples.
All human cardiac tissue samples were from nonfailing donor hearts deemed unsuitable for transplantation for noncardiac reasons. All donors were women, aged between 16–64 with normal ejection fractions (range: 52 to 60%). Sample collection was approved by the Temple University Institutional Review Board.
All hearts underwent cardioplegia in situ. Small segments (≈1–2 g) were cut from the hearts and frozen by immersion in liquid N2 for later study. The frozen segments were stored in liquid N2 until used for mechanical and biochemical studies.
For each experiment, a small sample (≈10 mg) was cut from the frozen segment using a liquid N2 cooled scalpel. The sample was then placed in a glass vial containing ice-cold rigor solution (see below for composition) to which was added Triton X-100 at a final concentration of 1%. The sample was homogenized at 9,000 rpm for 10 s. The resultant slurry was centrifuged at 2,000 rpm for 15 min, and the pellet was resuspended in rigor solution without Triton X-100. This procedure was repeated twice with a final resuspension in relaxing solution (contains ATP; see below). This process removes the majority of cytosolic and nonmyofilament material from the sample, and single myofibrils and small bundles of myofibrils can be visualized under the microscope.
The resultant myofibril suspension was divided into four 300-μl aliquots; two for mechanical studies and two for radioactive phosphate 32P incorporation studies.
PKA treatment.
The two aliquots for mechanical studies were resuspended in standard relaxing solution; one aliquot was treated with PKA by adding 10 μl of a 1 U/ml solution of cAMP-dependent protein kinase catalytic subunit (Promega V5161) in 350 mM KH2PO4 buffer . The other aliquot received 10 μl of 350 mM KH2PO4 buffer. The samples were gently rocked for 1 h at 20°C. They were then twice centrifuged and resuspended in 1 ml rigor to remove ATP and PKA. The resulting myofibril suspensions were stored at 4°C and used within 3 days from preparation.
The aliquots of myofibrils for measurement of 32P incorporation were resuspended in a modified relaxing solution containing 0.5 mM nonradioactive ATP. Twenty-five microcuries of 32P-labeled ATP were added to each aliquot. The final concentration of ATP was similar to that in standard relaxing solution used in the mechanical studies.
As in the aliquots for mechanical studies, PKA (1 U/ml) was added to one aliquot of each pair, an equivalent amount of buffer was added to the other, and they were then incubated for 1 h at 20°C. Following PKA treatment, the myofibrils were centrifuged as for the mechanical studies (described above) and resuspended in Laemmli buffer for gel electrophoresis.
Experimental solutions.
The solutions used for the mechanical experiments had the following compositions in (mM): rigor solution: 50 Tris, 100 KCl, 2 MgCl2, 1 EGTA, 2 DTT, and 0.2 PMSF; 0.01 leupeptin, and 0.005 pepstatin; activating solution: 2 CaCl2, 5.9 MgCl2, 1 EGTA, 51 K-propionate, 26 Na2SO4, 10 MOPS, 6 ATP, and 1 DTT; relaxing solution: 0 CaCl2, 6 MgCl2, 1 EGTA, 54 K-propionate, 27 Na2SO4, 10 MOPS, 5.4 ATP, and 1.0 DTT; and bath solution: 0 CaCl2, 6 MgCl2, 10 EGTA, 54 K-propionate, 17 Na2SO4, 10 MOPS, 5.4 ATP, and 1.0 DTT.
Intermediate pCa concentrations for the mechanical experiments (see below) were made by mixing activating and relaxing solutions to achieve intermediate calcium concentrations. Calcium concentrations were selected for each individual experiment to generate tensions that covered a range from 10% of maximum tension to maximum tension.
Tension measurements.
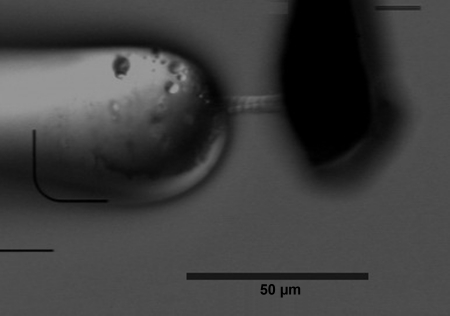
All mechanical experiments were performed at 15°C. Mechanical studies of myofibrillar activation were carried out on an apparatus similar to that of Pogessi and colleagues (7, 8, 23). Briefly, the myofibrils were delivered as a concentrated aliquot (50 μl) into a large volume (2.5 ml) of bath solution in a temperature controlled, gold-plated, brass bath. The bath had a coverslip window in the base and was mounted on the stage of an inverted, phase contrast microscope (Olympus IX70). The myofibrils were lifted between two glass microtools, a holding probe and force probe, the latter with an L-shaped cantilever design. Both were held by three axis micromanipulators, and the myofibril was attached at the tip of the base of the L-shaped cantilever and at the tip of the larger, stiffer holding probe (Fig. 1).
Fig. 1.
Two human cardiac myofibrils suspended between the force measuring cantilever (right) and the positioning probe (left).
Sarcomere length was calculated as the length of the visible portion of the myofibril (from a calibrated camera image) divided by the number of sarcomeres. Sarcomere length was set at 2.1 μm at the outset of the experiment and checked again at the conclusion of the experiment. If the sarcomere length changed by more than ≈10% during the experiment, the myofibril was discarded. Sarcomere lengths reported are the lengths measured at the end of each experiment.
The force probe had a tip diameter of ∼10 μm that tapered little along a 1-mm length (2). The force probes were calibrated by measuring the deflection of the probe with a nano-machined cantilever of known stiffness (a generous gift from Gerald Pollack; Ref. 11). The stiffness of the force probe used to measure myofibril tension was estimated to be 128 pN/nm, and the typical amount of shortening experienced by the myofibrils at maximum tension was ∼0.5 μm. This represented ∼1% of the average length of myofibrils used in this study. The deflection of the force probe in response to contraction of the myofibril was detected by a split photo-diode placed at the camera port of the inverted microscope. The deflection was linearly related to tension over the range of deflections observed, and the probe had a resonant frequency in air of ∼2,000 Hz as measured by plucking the cantilever and finding the dominant frequency of the resultant oscillation.
The holding probe was straight and had a tip diameter of 40 μm and a stiffness such that there was negligible deflection in response to a maximal contraction of the myofibril.
Myofibril kinetics.
Activation was achieved via rapid solution switching that had a delay of 2–3 ms from initiating signal to completion of switching. An activation-relaxation cycle included superfusion of the myofibril with relaxing solution, a switch to activating solution until the contraction stabilized, and then a return to relaxing solution prior to ending flow when relaxation was complete. Each activation-relaxation cycle was performed with an activating solution with a pCa randomly selected from five different concentrations. The tension for each pCa was measured as the stable tension immediately before relaxation of the myofibril.
When the contraction was stable, the myofibril was subjected to a rapid (<2 ms) step shortening followed by a short (100 ms) quiescent period to allow shortening and a subsequent rapid (<2 ms) restretch to its original length. This procedure “breaks” crossbridge attachments within the myofibril, and as they reform, there is an exponential rise in tension that is a function of actomyosin crossbridge turnover. The exponential curve was fit with an equation of the form P = Pmax[1 − exp(−ktr·t)], where P is the tension, Pmax is the asymptotic maximum tension, and the rate constant ktr is an index of crossbridge turnover rate (5).
Relaxation kinetics in isolated myofibrils are characterized by a initial linear decrease in tension followed by a complex fall that can be approximated by a exponential function. The relaxation kinetics were assessed by measuring the slope of the initial linear phase (klin; see Fig. 4) and by fitting a single exponential decay to the early portion of the nonlinear phase of relaxation [P = Pel(−kexp·t)], where P is the tension, Pel is the tension at the end of the linear phase, t is duration, and kexp is a rate constant (25).
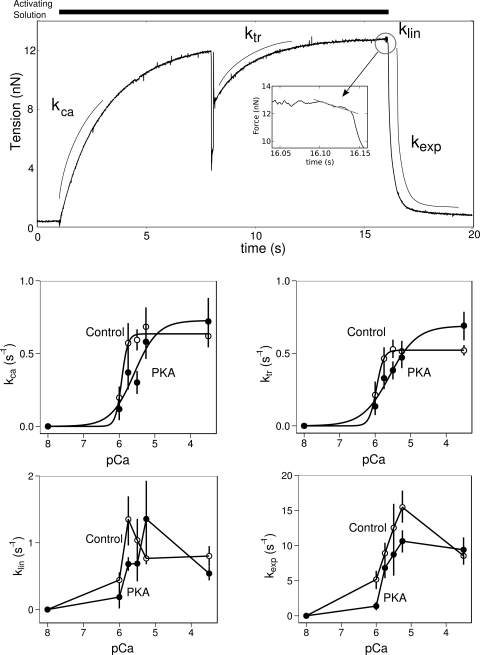
Fig. 4.
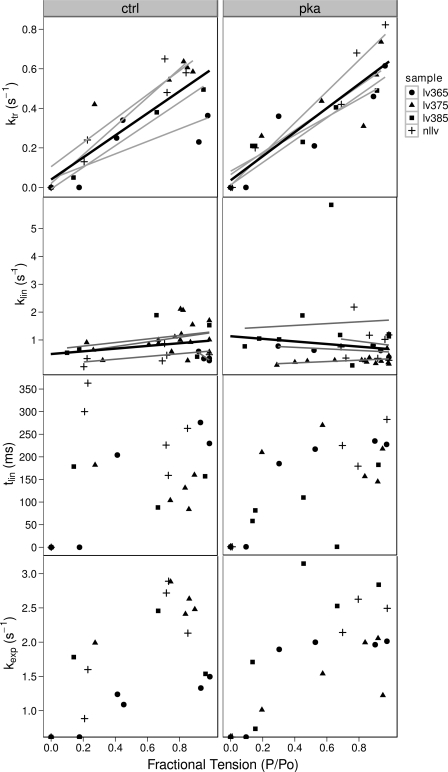
PKA effects on the kinetics of contraction as a function of calcium concentration. In top, middle, and bottom rows, error bars indicate the means ± SE. Top row: a typical force trace from an isolated myofibril with annotations that illustrate the regions of the trace. Inset: enlarged portion of the force trace during the early linear relaxation phase. Middle row left: rate of tension development (kca) is an approximately hyperbolic function of the ambient calcium. Lines represent the result of fitting a Hill curve to the data. PKA treatment results in a right-shifted curve (0.2 ± 0.10 pCa units; P = 0.04; n = 4) relative to control. Middle row right: rate of tension redevelopment (ktr) is a similar hyperbolic function of the ambient calcium concentration and PKA treatment also results in a right-shifted curve. Bottom row left: slope of the initial linear phase (klin) has a complex dependence on the ambient calcium and no simple curve fits the data. Relation shifts to the right in response to PKA treatment. Bottom row right: decay in force after the linear phase (kexp) also has a complex relation to ambient calcium and also appears to right shift in response to PKA treatment.
Tension-pCa relation.
No measurements of the thickness of the myofibrils were made, and consequently, we can make no estimates of myofibril stress (tension/cross-sectional area). Tensions may be compared when expressed as a fraction of the maximum tension recorded during a repeated random sequence of contractions. This is termed the fractional tension or P = Po. The mean fractional tension at pCa 3.5 may be <1 because a repeated maximum contraction was often less than the first. If the final maximum contraction developed <80% of the tension of the first, then the preparation was discarded.
We used the following form of the Hill equation,
where P is the tension developed by the muscle, Po is the maximum tension developed by the muscle, pCa is the log of the calcium concentration in the bath, h is the Hill coefficient, and pCa50 is the log of the calcium concentration where the tension is half of Po. The Hill equation was fit to the steady-state developed tension at each pCa from each experiment (36). The fits were carried out using the R statistical language version 2.9 with the nls and nlme libraries (16).
Assessment of 32P incorporation.
Following incubation with 32P-ATP (see above) and subsequent resuspension in Laemmli buffer, samples were matched for protein concentration and loaded on 12% SDS-PAGE gels that were then run at 150-V constant voltage for 2 h. Gels were fixed in 40% methanol and 10% acetic acid for a minimum of 1 h. Gels were rinsed with water, stained with Coomassie brilliant blue, and dried. Dried gels were placed on film (Kodak Biomax MR-1), and the 32P incorporation was visualized using autoradiography.
Statistical calculations.
All summary statistics, Student's t-tests, ANOVAs, and regressions were performed using the R statistical language version 2.9 (16). A nonlinear mixed effects model was used to describe the effects of PKA treatment on the parameters of the Hill nonlinear fit. Data described in text are given as means ± SE unless otherwise noted. Differences were held to be statistically significant at P < 0.05 (12). Because batches of myofibrils were randomized to be treated with PKA or not, the experimental unit is the myofibril batch; however, the underlying biological variation was due to only four human hearts.
RESULTS
Human cardiac myofibrils.
Data was obtained from 13 myofibrils from 4 patient hearts. The average physical characteristics of the myofibrils used in this experiment, subdivided by treatment, are given in Table 1 . There was no significant difference in the morphology of myofibrils from the control and PKA-treated groups.
Table 1.
Average physical characteristics of control myofibrils and myofibrils treated with PKA
| Parameter | Control | PKA |
|---|---|---|
| Sarcomere length, μm | 2.05 ± 0.02 | 2.1 ± 0.18 |
| Length, μm | 35.99 ± 14.25 | 27.69 ± 16.9 |
| Width, μm | 6.94 ± 3.13 | 4.73 ± 1.92 |
Data are means ± SD; n = 13.
The average width of the myofibril preparations used in this study indicates that they were typically small bundles of three to four myofibrils, assuming a single myofibril diameter of ∼2 μm (15, 32).
32P incorporation.
We examined 32P incorporation into myofilament proteins to confirm the activity of PKA and that phosphorylation of myofilament proteins was in fact occurring. We found significant incorporation of 32P into the several myofilament proteins (Fig. 2 shows results typical of 4 experiments). These results are consistent with PKA actively phosphorylating sarcomeric proteins.
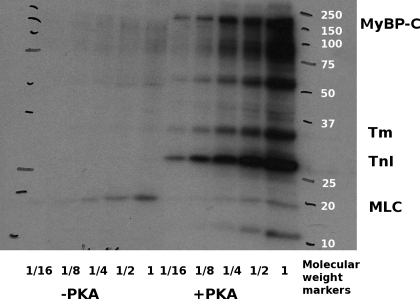
Fig. 2.
Typical autoradiogram of a myofibril preparation incubated with (+PKA) and without (−PKA) 32P-labeled ATP and PKA. Fractions indicate serial 1 in 2 dilutions of the original sample. Increased intensity in the +PKA samples indicates that there is significant incorporation of 32P into the myofilament proteins in the presence of PKA. MyBP-C, myosin binding protein C; Tm, tropomyosin; TnI, troponin I; MLC, myosin light chain.
Tension-pCa measures.
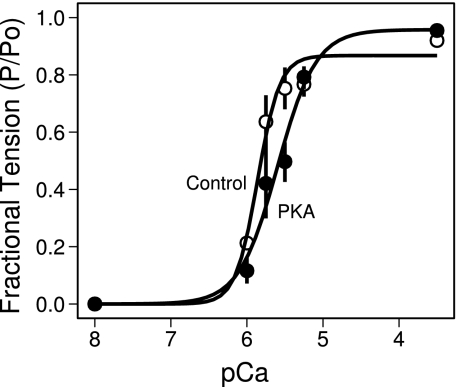
A nonlinear mixed effects model relating tension to pCa via the Hill equation was fit globally to the data from all 13 myofibrils (36). The model allowed all Hill equation parameters (Po, pCa50, and h) to depend on PKA treatment and accounted for the nesting of treatments within samples. Control myofibrils had a pCa50 of 5.83 ± 0.05 and PKA treatment elicited a decrease of 0.20 ± 0.09 (P = 0.04; Fig. 3). The Hill coefficient for control myofibrils was 3.65 ± 0.82, and PKA treatment caused a decrease of 1.5 ± 0.88 (P = 0.08; Fig. 3).
Fig. 3.
Typical data showing the change in the force-pCa relation of isolated human cardiac myofibrils in response to treatment with PKA. Data were normalized to highest force level recorded in a repeated randomized sequence. This resulted in averaged normalized forces being <1 if subsequent contractions at pCa 3.5 developed less tension than the first. Control samples (○) had a pCa50 of 5.83 ± 0.05 (n = 4). PKA treatment (●) shifted the force-pCa relation 0.20 ± 0.09 pCa units (n = 4; P = 0.04) at right. Lines represent best fit of the Hill equation to the underlying data.
Kinetics of contraction.
The effect of PKA treatment on the contraction kinetics of human myofibrils as a function of calcium is illustrated in Fig. 4 and as a function of fractional tension (P/Po) is illustrated in Fig. 5.
Fig. 5.
PKA effects on the kinetics of contraction as a function of normalized force. Because the force is not a true independent variable in these experiments, individual data points and a robust-nonlinear curve fit for each sample (gray lines) and for the overall data set (black line) have been used to describe the data. Top row: kca and ktr are approximately linear functions of the developed tension and there is no significant difference between control and PKA-treated curves. Second row: klin has no dependence on force and PKA treatment has little effect, which would fit with the interpretation of klin as an index of the crossbridge detachment rate constant g. Third row: duration of the linear phase (tlin) has little dependence on the force with or without PKA. This is consistent with klin being an index of apparent detachment rate (gapp). Bottom row: kexp is a rising function of the developed tension but PKA again has little effect.
The rate of tension development (kca) and the rate of tension redevelopment (ktr) are representative of similar processes, and when we plotted ktr against the kca, we found a straight line with a slope not significantly different from 1 (P > 0.05; data not shown), therefore, both constants will be considered together.
Both kca and ktr had a hyperbolic dependence on pCa of the form ktr = Kmax/[1 + 10(pCa−pCa50)h], where Kmax is the asymptotic value of the reate constant, as shown by the fitted curves. The parameter values for the fitted curves are given in Table 2. Treatment with PKA shifted the hyperbolic curve to the right by ∼0.3 pCa units and reduced the cooperativity from ∼4–5 to ∼1 (Fig. 4).
Table 2.
Parameters for hyperbolic curves fitted to average ktr and kca data as function of pCa
| Constant and Treatment | Kmax | pCa50 | Hill |
|---|---|---|---|
| kca | |||
| Control | 0.63 ± 0.04 | 5.93 ± 0.05 | 5.05 ± 2.40 |
| PKA | 0.73 ± 0.07 | 5.57 ± 0.10 | 1.32 ± 0.42 |
| ktr | |||
| Control | 0.52 ± 0.02 | 5.96 ± 0.02 | 4.32 ± 1.22 |
| PKA | 0.69 ± 0.03 | 5.59 ± 0.05 | 1.13 ± 0.17 |
Data are fitted values ± SE. kca; Rate of tension development; ktr; rate of tension redevelopment. Kmax is the asymptotic value of ktr or kca.
The slope of the linear phase of relaxation (klin) had a complicated dependence on pCa. It rose to a peak at pCa 6 in control myofibrils and PKA treatment shifted the peak to the right (Fig. 4). The rate constant (kexp) of the exponential phase of relaxation shows a similar peaked relation, and PKA treatment appeared to reduce the exponential rate constant at all submaximal calcium concentrations. The changes in kexp can be explained by a right shift, where a shifted peak is undetected due to the lack of data points between pCa 5 and pCa 3.5.
In summary, PKA treatment right shifts curves for ktr, kca, and klin vs. pCa, and a right shift also explains the PKA-induced change in the kexp-pCa relation.
However, when the rate constants are plotted as functions of the fractional tension, very different relationships emerge. The rate constants ktr and kca are approximately linearly dependent on the fractional tension, and PKA treatment appears to have little effect on this relation. No significant differences were detected between the slopes of the control and PKA-treated relations (Fig. 5, top). The slope of the control kca-pCa line was 0.60 ± 0.12, and PKA increased the slope by only 0.06 ± 0.16 (P = 0.69). The slope of the control ktr-pCa line was 0.55 ± 0.07, and the change in slope was 0.05 ± 0.09 (P = 0.61) with PKA treatment.
The regressions of the linear phase of relaxation, klin, on the fractional tension had slopes not significantly different from zero in either control or PKA-treated myofibrils. This is consistent with a constant value for klin, independent of the level of tension. The mean klin was 0.74 ± 0.25 s−1 for control myofibrils and 0.84 ± 0.35 s−1 for PKA-treated myofibrils (P = 0.78).
The duration of the linear phase, tlin, did not have a strong dependence on the fractional tension (Fig. 5). The average duration of the linear phase, excluding zero values, was 202 ± 24 ms in the absence of PKA treatment and 203 ± 26 ms with PKA treatment (P = 0.98).
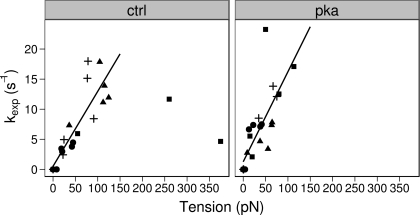
The exponential constant, kexp, also had a weak dependence on the fractional tension (Fig. 5). The exponential phase of relaxation is thought to be due to sequential rapid sarcomere lengthening as the load-bearing capacity of half-sarcomeres decays below the tension borne by the myofibril (25). Since each crossbridge contributes a unitary force and load-bearing capacity to the myofibril tension, we might expect to see a dependence of relaxation on tension rather than on stress (tension/cross-sectional area). We therefore examined the relation between kexp and tension and found that a linear relationship existed for much of the data (Fig. 6). Several data points were identified as possible outliers and were not used in the fitting of the data. The dependence on tension is consistent with load-bearing capacity playing a role in the exponential phase of relaxation. Regardless of the detailed mechanism of exponential relaxation, we could detect no effect of PKA treatment on the kexp-tension relation, suggesting that the exponential phase of relaxation was unaffected by PKA treatment.
Fig. 6.
Effects of PKA on the kinetics of human cardiac myofibril exponential relaxation as a function of absolute force. Each sample is indicated by a symbol as before and the solid lines represent a least squares linear fit. The 2 data points at the far right on the left were designated outliers and were not used to fit the lines to the data. An ANOVA showed there was no significant difference in the intercepts (control: 0.52 ± 1.3 vs. PKA: 1.27 ± 1.76 s−1; P = 0.25) or slopes of the lines (control 0.12 ± 0.02 vs. PKA 0.14±0.03 s−1·mN·mm2; P = 0.42).
In short, PKA treatment produced no significant differences in the relationships between the rate constants and the tension or fractional tension. This suggests that the PKA-induced changes in kinetic parameters as a function of calcium were due to a reduction in tension for a given calcium rather than direct effects on crossbridge turnover.
DISCUSSION
Our data showed PKA-dependent 32P incorporation into sarcomeric proteins that confirmed the activity of PKA and the phosphorylation of a number of sarcomeric proteins (Fig. 2). We observed a right shift in the tension-pCa relationship (Fig. 3) upon treatment with PKA that was consistent with data from previous studies (9, 21). While we found shifts in the dependence of indexes of crossbridge cycling (e.g., ktr) on calcium (Fig. 4), we also found that the relationship between the indexes of crossbridge cycling and the developed tension were unchanged by treatment with PKA (Fig. 5).
The ranges of data values from the present work are comparable to other studies of human myofibrils or myocytes. Our measures of myofibril function were similar to those found by Narolska et al. (22). Our pCa50 for myofibrils was ∼5.8, and they reported ∼5.5 in control myocytes and up to 5.8 in treated myocytes. The differences could be due to solution differences, calibration of calcium buffers, or storage of samples. We found the Hill coefficient to be ∼2, and this was within the range (1.88 to 2.13) they reported for cardiomyocytes. Our treatment of human myofibrils with PKA produced a shift in the fractional tension-pCa relationship of ∼0.2 pCa units. This was somewhat larger than that seen by Narolska et al. (22) in PKA-treated human cardiomyocytes (≈0.09), but other studies have shown shifts of 0.2 pCa units in response to PKA treatment (9).
The amount of change in pCa50 is likely dependent on the preexisting phosphorylation state of the phosphoproteins involved in changing calcium sensitivity. Because kinase treatment phosphorylates many proteins and because quantitative measurement of phosphorylation is difficult (see Ref. 37), we focused on whether a shift in pCa50 was necessarily accompanied by changes in crossbridge cycling rate rather than on assigning effects to specific phosphorylation events.
The rates of tension redevelopment (ktr) at maximal calcium in the present study (peak values ∼0.7 s−1; see Fig. 4) were similar to that seen in human cardiomyocytes by Narolska et al. (22; their range: 0.53 to 0.76 s−1) and to values for human ventricular muscle published by Piroddi et al. (24). Narolska et al. (22) also reported kca values that were not statistically different from the ktr under the same conditions. The relaxation rates seen in the present study were significantly higher than those seen by Narolska et al. in human ventricular muscle. We found linear phase relaxation rates at peak calcium to be ∼0.7–0.8 s−1, while Narolska et al. found rates of 0.2 to 0.3 s−1 and Pirrodi et al. reported linear relaxation rates of 0.1 s−1. Our exponential relaxation rates were significantly higher as well at ∼10 s−1 compared with ∼3 s−1.
Given the relative paucity of data on human cardiac myofibrils, these comparisons would seem to suggest that our myofibrils were very similar to the human cardiac tissue studied by Narolska et al. (22) and Pirrodi (24). Consequently, we feel our preparations can be taken as representative of human cardiac muscle.
PKA activity.
Autoradiograms of one-dimensional PAGE gels showed that in the absence of PKA treatment, there was incorporation of phosphate into myosin light chain-2 (MLC2) but very little into other myofilament proteins (Fig. 2). This suggests the existence of a constitutive MLC2-specific kinase activity. The nature of this kinase activity is as yet unknown. Addition of PKA (1 U/ml) significantly increased the amount of 32P incorporation into myosin binding protein C (MyBPC), tropomyosin, and TnI, as well as several other unidentified sarcomeric proteins, and reduced the incorporation into MLC2. These results confirm that PKA was active in our samples. More importantly, we obtained the expected decrease in the pCa50 that had been reported in prior studies for similar concentrations of PKA (9, 17).
Effects of PKA on kinetics of tension development.
As previously mentioned, several studies (26, 30) have shown a change in calcium sensitivity with PKA treatment and this is generally accepted to be the result of the phosphorylation of TnI at Ser23,24 . In the present study, PKA treatment produced a right shift in the tension-pCa curve consistent with previous work by other investigators (21).
We used the rate of tension redevelopment as an indicator of crossbridge isometric kinetics (4, 5). An analysis by Brenner (5) shows that ktr is proportional to the sum of the apparent attachment ( fapp) and apparent detachment (gapp) rate constants (ktr ∝ fapp + gapp) at a given steady-state tension.
We found an approximately linear relationship between ktr and the amount of fractional tension (Fig. 5). Brenner (4) reported a nonlinear relation between ktr and tension in rabbit psoas. Piroddi et al. (24) also found curvilinear relationships between ktr and fractional tension for both atrial and ventricular myofibrils from human samples but noted that the ventricular relationships showed only modest curvature (24). We cannot exclude a gently curved relation in our data from human ventricles. However, the exact form of the relation is not essential to our finding of a lack of significant difference in the ktr-fractional tension relation between control and PKA-treated tissues.
Models of crossbridge cycling suggest that relaxation should dominated by the apparent detachment rate constant (gapp) and that this rate constant should be independent of the tension (39). In isolated myofibrils, the relevant phase of relaxation dominated by the detachment rate is thought to be the early linear phase (25). We found that the slope of the klin-tension relation is not significantly different from zero (Fig. 5). This is consistent with a constant detachment rate that is independent of the level of developed tension. This finding is also consistent with prior descriptions of the dependence of gapp on tension (4).
The rate constant of the exponential phase of relaxation had a linear dependence on tension. The exponential phase of relaxation is thought to occur as the load-bearing capacity of half-sarcomeres decays below the tension borne by the myofibril and the half-sarcomere rapidly lengthens (25). At present, the basis for the linear relationship between kexp and tension is not clear and requires further study. Nevertheless, we could find no change in this linear relationship with PKA treatment. This is consistent with no effect of PKA on crossbridge turnover.
Does PKA affect crossbridge turnover?
The question of whether PKA treatment affects crossbridge turnover is complicated by fact that the measurement of tension and crossbridge tunrover is carried out by varying the ambient calcium. The measures of crossbridge kinetics as a function of calcium all show an apparent right shift in the underlying relationships (see Fig. 4). This is similar to the shift seen in the tension-pCa relation (see Fig. 3) and suggests an underlying dependence of the shift in the kinetic parameters on the shift in the tension-pCa relation. The right shift is easily understood if the relationship between crossbridge turnover, as indicated by ktr and the number of active crossbridges, the tension, is considered the more fundamental; the right shift in the tension-pCa curve due to PKA treatment results in a lower tension at a given calcium and the lower tension results in a lower net crossbridge turnover rate. The net result of PKA treatment is a right shift in turnover as a function of calcium. However, the underlying relation between crossbridge turnover and tension remains unchanged by PKA treatment (see Fig. 5).
The consideration of the related tension-pCa and tension-ktr relations curves offers a possible explanation for the confusion in previous studies of the effect of PKA on the kinetics of tension development.
A study by Turnbull et al. (35) showed changes in crossbridge turnover as measured by pseudorandom binary noise in twitching intact muscle. In this case, both calcium sensitivity and intracellular calcium transients are likely affected by the administration of noradrenergic agonists. The more rapid rise in intracellular calcium secondary to the noradrenergic stimulation results in a more rapid rise in tension due to an increase in the net attachment rate that is due to a greater activation of the thin filament by the higher calcium. No change in the fundamental attachment rate constant ( fapp) need occur for the net attachment rate to increase, only an increase in the number of available attachment sites. This results in more crossbridges being attached per unit time. Under the nonsteady-state conditions seen in twitching intact cardiac muscle, this could be potentially be responsible for the shift in the minimum dynamic stiffness (fmin) in response to noradrenergic stimulation.
In a study by Kentish et al. (20), the principal measurement of kinetics was of the normalized exponential relaxation following flash photolysis of a calcium chelator (20). The exponential phase of relaxation is determined in large part by intersarcomere dynamics, which is dependent on the decay of the load-bearing capacity of sarcomeres (25). This decay is, in turn, influenced by the calcium sensitivity and the rate of reduction in calcium. A reduction in calcium sensitivity in response to PKA treatment would result in an increased rate of decline in load-bearing capacity for a given rate of calcium decline. This would result in a more rapid rate of relaxation. Kentish et al. (20) reported their relaxation rates as the sum of two exponentials, a fast phase and a slow phase, in wild-type (cTnI) and transgenic (ssTnI for cTnI) animals. PKA treatment produced no change in pCa50 or in the slow phase of relaxation with ssTnI, but it decreased the pCa50 and increased the rate of the slow phase of relaxation in tissues with cardiac TnI. They also found that ssTnI tissues were significantly less sensitive to calcium than the cTnI tissues and yet the slow phase had a similar rate in both cTnI and ssTnI tissues. This latter finding suggested that the relaxation rate was not strictly dependent on shifts in calcium sensitivity and consequently PKA effects were due to changes in crossbridge turnover. It is of note, however, that the fast exponential component of relaxation was markedly different in the cTnI and ssTnI tissues, suggesting that the total rate of relaxation was different when calcium sensitivity was different, consistent with an effect of calcium sensitivity alone.
However, not all such studies can be explained. For example, Strang et al. (34) used skinned rat cardiomyocytes and measured unloaded shortening velocity at maximal calcium using the slack test and found that the unloaded shortening velocity at maximal calcium was increased by PKA treatment. However, in contrast to the study by Strang et al, more recent studies (6) have found that crossbridge kinetics at maximal calcium do not differ significantly with PKA treatment but that submaximal shortening velocities are affected.
In studies of the effects of PKD and PKA phosphorylation of TnI on the mechanics of cardiomyocytes, Bardswell et al. (3) found that the shifts in pCa50 are separable from the changes in ktr. This finding is consistent with our conclusion that PKA-induced pCa shifts of ∼0.2 in human cardiac myofibrils produce little to no direct effect on the kinetics of crossbridge cycling.
Several studies (33, 27, 28, 1) have implicated MyBPC as a potential regulator of crossbridge cycling rate, and indeed there is good evidence to suggest that MyBPC is a target of PKA and can modify stretch activation in cardiac muscle. However, while we found a significant incorporation of 32P into MyBPC, we found no evidence for changes in crossbridge cycling rate to associate with the phosphorylation of MyBPC. We cannot exclude the possibility that changes in ktr may depend on MyBPC in a dose-dependent fashion and that our samples are sufficiently phosphorylated at baseline so as to exclude any further effects of MyBPC phosphorylation. However, separable effects, that is independent changes in calcium sensitivity and crossbridge cycling rate, would also be consistent with our finding that changes in calcium sensitivity secondary to PKA treatment are not accompanied by changes in crossbridge cycling.
While several studies that apparently contradict our findings may be interpreted in terms of changes in calcium sensitivity alone, many prior studies cannot be reconciled with the present work. While these contradictory studies suggest that crossbridge cycling rate is regulated by PKA, we find no evidence in favor of this in isolated human myofibrils.
Conclusions.
Our treatment of human myofibrils with PKA was accompanied by a net increase in the phosphorylation of TnI, tropomyosin, and MyBPC and several other sarcomeric proteins, as measured by 32P incorporation. The treatment with PKA resulted in a right shift in the tension-pCa curve of ∼0.2 pCa units as was expected from prior studies of mammalian cardiac tissue. We similarly observed right shifts in several measures of crossbridge turnover when expressed as functions of calcium. These shifts were due to an underlying, unchanged dependence of crossbridge turnover on tension and a reduction in the tension for a given calcium due to a reduction in calcium sensitivity. While we cannot exclude that changes in crossbridge cycling rate may occur under other conditions, we conclude that PKA treatment sufficient to significantly reduce calcium sensitivity did not have a direct effect on crossbridge turnover.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-62426 and HL-077195.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Ababou A, Rostkova E, Mistry S, Le Masurier C, Gautel M, Pfuhl M. Myosin binding protein C positioned to play a key role in regulation of muscle contraction: structure and interactions of domain C1. J Mol Biol 384: 615–630, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ayittey PN, Walker JS, Rice JJ, de Tombe PP. Glass microneedles for force measurements: a finite-element analysis model. Pflügers Arch 457: 1415–1422, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bardswell SC, Cuello F, Rowland AJ, Sadayappan S, Robbins J, Gautel M, Walker JW, Kentish JC, Avkiran M. Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling. J Biol Chem 285: 5674–5682, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci USA 85: 3265–3629, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brenner B. Muscle Mechanics II: Skinned muscle fibres. In: Current Methods in Muscle Physiology, edited by Sugi H. Oxford, UK: Oxford University Press pp; 33–69 1998 [Google Scholar]
- 6. Chen PP, Patel JR, Rybakova IN, Walker JW, Moss RL. Protein kinase a-induced myofilament desensitization to Ca2+ as a result of phosphorylation of cardiac myosin-binding protein C. J Gen Physiol 136: 615–627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colomo F, Piroddi N, Poggesi C, te Kronnie G, Tesi C. Active and passive forces of isolated myofibrils from cardiac and fast skeletal muscle of the frog. J Physiol 500: 535–548, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Tombe PP, Belus A, Piroddi N, Scellini B, Walker JS, Martin AF, Tesi C, Poggesi C. Myofilament calcium sensitivity does not affect cross-bridge activation-relaxation kinetics. Am J Physiol Regul Integr Comp Physiol 292: R1129–R1136, 2007 [DOI] [PubMed] [Google Scholar]
- 9. de Tombe PP, Stienen GJ. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ Res 76: 734–741, 1995 [DOI] [PubMed] [Google Scholar]
- 10. de Tombe PP, ter Keurs HE. Lack of effect of isoproterenol on unloaded velocity of sarcomere shortening in rat cardiac trabeculae. Circ Res 68: 382–391, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Fauver ME, Dunaway DL, Lilienfeld DH, Craighead HG, Pollack GH. Microfabricated cantilevers for measurement of subcellular and molecular forces. IEEE Trans Biomed Eng 45: 891–898, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Fisher RA. Statistical Methods, Experimental Design and Scientific Inference. Oxford, UK: Oxford University Press, 1990 [Google Scholar]
- 13. Hofmann PA, Lange JH. Effects of phosphorylation of troponin I and C protein on isometric tension and velocity of unloaded shortening in skinned single cardiac myocytes from rats. Circ Res 74: 718–726, 1994 [DOI] [PubMed] [Google Scholar]
- 14. HunlichM , Begin KJ, Gorga JA, Fishbaugher DE, LeWinterMM , VanBuren P. Protein kinase A mediated modulation of acto-myosin kinetics. J Mol Cell Cardiol 38: 119–125, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Huxley AF. Reflections on Muscle. Princeton, NJ: Princeton University Press, 1980 [Google Scholar]
- 16. Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comp Graph Stat 5: 299–314, 1996 [Google Scholar]
- 17. Janssen PM, de Tombe PP. Protein kinase A does not alter unloaded velocity of sarcomere shortening in skinned rat cardiac trabeculae. Am J Physiol Heart Circ Physiol 273: H2415–H2422, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Johns EC, Simnett SJ, Mulligan IP, Ashley CC. Troponin I phosphorylation does not increase the rate of relaxation following laser flash photolysis of diazo-2 in guinea-pig skinned trabeculae. Pflügers Arch 433: 842–844, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Kawai M, Brandt PW. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil 1: 279–303, 1980 [DOI] [PubMed] [Google Scholar]
- 20. Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res 88: 1059–1065, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res 66: 12–21, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Narolska NA, Piroddi N, Belus A, Boontje NM, Scellini B, Deppermann S, Zaremba R, Musters RJ, dos Remedios C, Jaquet K, Foster DB, Murphy AM, van Eyk JE, Tesi C, Poggesi C, van der Velden J, Stienen GJ. Impaired diastolic function after exchange of endogenous troponin I with C-terminal truncated troponin I in human cardiac muscle. Circ Res 99: 1012–1020, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Piroddi N, Belus A, Eiras S, Tesi C, van der Velden J, Poggesi C, Stienen GJ. No direct effect of creatine phosphate on the cross-bridge cycle in cardiac myofibrils. Pflügers Arch 452: 3–6, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Pirrodi N, Belus A, Scellini B, Tesi C, Giunti G, Cerbai E, Mugelli A, Poggesi C. Tension generation and relaxation in single myofibrils from human and ventricular myocardium. Pflügers Arch 454: 63–73, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Poggesi C, Tesi C, Stehle R. Sarcomeric determinants of striated muscle relaxation kinetics. Pflügers Arch 449: 505–517, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Ray KP, England PJ. Phosphorylation of the inhibitory subunit of troponin and its effect on the calcium dependence of cardiac myofibril adenosine triphosphatase. FEBS Lett 70: 11–16, 1976 [DOI] [PubMed] [Google Scholar]
- 27. Razumova MV, Bezold KL, Tu AY, Regnier M, Harris SP. Contribution of the myosin binding protein C motif to functional effects in permeabilized rat trabeculae. J Gen Physiol 132: 575–585, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Razumova MV, Shaffer JF, Tu AY, Flint GV, Regnier M, Harris SP. Effects of the N-terminal domains of myosin binding protein-C in an in vitro motility assay: Evidence for long-lived cross-bridges. J Biol Chem 281: 35846–35854, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Saeki Y, Shiozawa K, Yanagisawa K, Shibata T. Adrenaline increases the rate of cross-bridge cycling in rat cardiac muscle. J Mol Cell Cardiol 22: 453–460, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Solaro RJ, Moir AJ, Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature 262: 615–617, 1976 [DOI] [PubMed] [Google Scholar]
- 31. Solaro RJ, Robertson S, Johnson J, Holroyde M, Potter J. Troponin-I phosphorylation: a unique regulator of the amounts of caclium required to activate cardiac myofibrils. Cold Spring Harbor Conference. Cell Proliferation 8: 901–911, 1981 [Google Scholar]
- 32. Squire JM. The Structural Basis of Muscular Contraction. New York: Plenum, 1981 [Google Scholar]
- 33. Stelzer JE, Patel JR, Walker JW, Moss RL. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase a phosphorylation. Circ Res 101: 503–511, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Strang KT, Sweitzer NK, Greaser ML, Moss RL. Beta-adrenergic receptor stimulation increases unloaded shortening velocity of skinned single ventricular myocytes from rats. Circ Res 74: 542–549, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Turnbull L, Hoh JF, Ludowyke RI, Rossmanith GH. Troponin I phosphorylation enhances crossbridge kinetics during beta-adrenergic stimulation in rat cardiac tissue. J Physiol 542: 911–920, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walker J, Li X, Buttrick P. Analysing force-pCa curves. J Muscle Res Cell Motil 31: 59–69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walker JS, Walker LA, Etter EF, Murphy RA. A dilution immunoassay to measure myosin light chain phosphorylation. Anal Biochem 284: 173–182, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Winegrad S, Weisberg A, Lin LE, McClellan G. Adrenergic regulation of myosin adenosine triphosphatase activity. Circ Res 58: 83–95, 1986 [DOI] [PubMed] [Google Scholar]
- 39. Woledge RC, Curtin NA, Homsher E. Energetic Aspects of Muscle Contraction. London: Academic, 1985 [PubMed] [Google Scholar]