Abstract
The myofibroblastic differentiation of hepatic stellate cells (HSC) is a critical event in liver fibrosis and is part of the final common pathway to cirrhosis in chronic liver disease from all causes. The molecular mechanisms driving HSC differentiation are not fully understood. Because macroscopic tissue stiffening is a feature of fibrotic disease, we hypothesized that mechanical properties of the underlying matrix are a principal determinant of HSC activation. Primary rat HSC were cultured on inert polyacrylamide supports of variable but precisely defined shear modulus (stiffness) coated with different extracellular matrix proteins or poly-l-lysine. HSC differentiation was determined by cell morphology, immunofluorescence staining, and gene expression. HSC became progressively myofibroblastic as substrate stiffness increased on all coating matrices, including Matrigel. The degree rather than speed of HSC activation correlated with substrate stiffness, with cells cultured on supports of intermediate stiffness adopting stable intermediate phenotypes. Quiescent cells on soft supports were able to undergo myofibroblastic differentiation with exposure to stiff supports. Stiffness-dependent differentiation required adhesion to matrix proteins and the generation of mechanical tension. Transforming growth factor-β treatment enhanced differentiation on stiff supports, but was not required. HSC differentiate to myofibroblasts in vitro primarily as a function of the physical rather than the chemical properties of the substrate. HSC require a mechanically stiff substrate, with adhesion to matrix proteins and the generation of mechanical tension, to differentiate. These findings suggest that alterations in liver stiffness are a key factor driving the progression of fibrosis.
Keywords: myofibroblast, liver fibrosis, matrix stiffness, transforming growth factor-β, Matrigel
hepatic stellate cells (HSC) are multifunctional cells making up ∼15% of the cells in the healthy liver (11). Characterized by the presence of vitamin A-filled lipid droplets in their normal, or “quiescent”, state, HSC differentiate in chronic liver disease into fibrogenic, highly proliferative, contractile and migratory myofibroblasts, with associated loss of retinoids and increased expression of α-smooth muscle actin (α-SMA). These myofibroblastic HSC are responsible for much of the abnormal extracellular matrix (ECM) deposition (scar formation) in fibrosis and cirrhosis. Because of their central role in liver fibrosis, HSC are prime targets for pharmacological intervention in many forms of chronic liver disease.
HSC myofibroblastic differentiation can be modeled in vitro by culturing freshly isolated primary cells on glass or uncoated tissue culture plastic. Cells progressively lose their vitamin A droplets and acquire the spread morphology and α-SMA expression typical of myofibroblasts, reaching the fully differentiated state after 7 or more days. The effects of multiple soluble factors have been studied using this in vitro system, and many have been confirmed to be important regulators of HSC behavior in vivo (11). Matrix proteins represent another category of important mediators of HSC myofibroblastic differentiation in vitro, although their function is poorly understood. HSC cultured on a basement membrane-like matrix (Matrigel) remain quiescent, and HSC myofibroblasts placed on Matrigel lose their myofibroblastic characteristics (13, 14, 36). It has proven impossible, however, to identify an individual component of Matrigel that regulates differentiation. HSC cultured on glass or plastic coated with laminin, type IV collagen, or heparin sulfate proteoglycans, the components of Matrigel, differentiate in vitro similarly to cells on uncoated supports (13).
Our laboratory has recently demonstrated that portal fibroblasts, myofibroblast precursors in the liver that also contribute to fibrosis, require a mechanically stiff environment in addition to the soluble factor transforming growth factor-β (TGF-β) for myofibroblastic differentiation (26). Preliminary studies suggest that mechanical stiffness also plays a role in HSC differentiation (26). The importance of mechanical tension to myofibroblast differentiation has been recognized for many years (1, 19, 38). Mechanical tension, such as that generated internally by the cell and resisted by ECM stiffness, is increasingly appreciated as a mediator of a variety of cell behaviors, including growth and viability, motility, adhesion, and differentiation (7, 40); however, mechanical signals, like their chemical counterparts, affect different cellular systems in characteristically different ways (17, 43). Liver stiffness increases significantly as fibrosis develops, and there is evidence that it also increases early after injury as a function, not of fibrosis, but of altered matrix protein cross-linking (16), which increases progressively as fibrosis develops (31). Thus understanding the effect of matrix mechanics on HSC behavior is important to understanding the progression of liver fibrosis.
We hypothesized that mechanical stiffness is required for HSC differentiation, regardless of the underlying matrix or presence of soluble factors. We made use of a mechanically tunable cell culture system in which cells were cultured on polyacrylamide supports of variable but precisely defined shear modulus (G′) and coated with thin layers of various ECM proteins, including Matrigel, that do not alter the overall stiffness of the system (30). In this way, the effects of the mechanical properties of the substrate (the polyacrylamide support) were studied independently of the chemical properties (the ECM protein coating).
MATERIALS AND METHODS
Isolation of rat HSC.
Primary HSC were isolated from the livers of 450- to 900-g male retired breeder Sprague-Dawley rats by sequential in situ pronase and collagenase perfusion, followed by density gradient centrifugation, as previously described (12, 27, 39). HSC were cultured in medium 199 (Invitrogen, Carlsbad, CA) with 10% fetal calf serum (Gemini Bio-Products, Woodland, CA). Purity and viability (determined by desmin staining, UV autofluorescence, and Trypan blue exclusion) were >95%. Cells from a single animal were used for all data points for each individual experiment. All animal work was approved by the University of Pennsylvania Institutional Animal Care and Use Committee and was conducted in accordance with National Institutes of Health guidelines.
Polyacrylamide supports.
Polyacrylamide gels were prepared on glass coverslips by a modification of methods previously described (10, 26, 30). Stiffness (as defined by the G′ and measured with a Rheometrics RFS-II controlled strain rheometer) of the gels was varied by adding bis-acrylamide to a final concentration ranging from 0.01 to 0.3% to a mixture of 7.5% acrylamide (10). Final gel thickness after polymerization was uniform and at least 100 μm. Note that pore size is too small to permit cell invasion. Pore size in these hydrogels depends on total polyacrylamide concentration, not on the density of cross-links; thus, because of the small contribution of bis-acrylamide to the final acrylamide concentration, pore size and solute diffusion are identical in gels of different stiffnesses (2, 43). The polyacrylamide surface was prepared for cross-linking by two cycles of UV light activation of Sulfo-SANPAH (Pierce, Rockford, IL). Thin layers of amine-containing proteins [type I collagen (0.1 mg/ml), plasma fibronectin (0.2 mg/ml) and growth factor-depleted Matrigel (0.5 mg/ml) (BD Biosciences, Bedford, MA)], as well as poly-l-lysine (PLL) (0.1 mg/ml; Sigma, St. Louis, MO) were cross-linked to the gel surface by overnight incubation at 4°C. Cells require matrix to adhere and will not attach to uncoated polyacrylamide; cross-linked matrix proteins at these concentrations provide a uniform coating, but do not alter the underlying G′ (30). There is no change in the density of matrix coatings on gels of different stiffnesses (8, 9, 29); coatings are uniform and indistinguishable on hard and soft gel surfaces (6, 43). Excess matrix was removed with HEPES washes, and gels were incubated in serum-free medium 199 for 2 h at 37°C. Freshly isolated HSC were then seeded at a density of 102 HSC/mm2 gel surface and cultured for the times described in the Fig. 1–6 legends. There was minimal deposition of additional matrix on the gels over the time of culture (Supplemental Fig. S1; the online version of this article contains supplemental data), as determined by staining with antibodies against type I collagen (Accurate Scientific) and fibronectin (BD Biosciences).
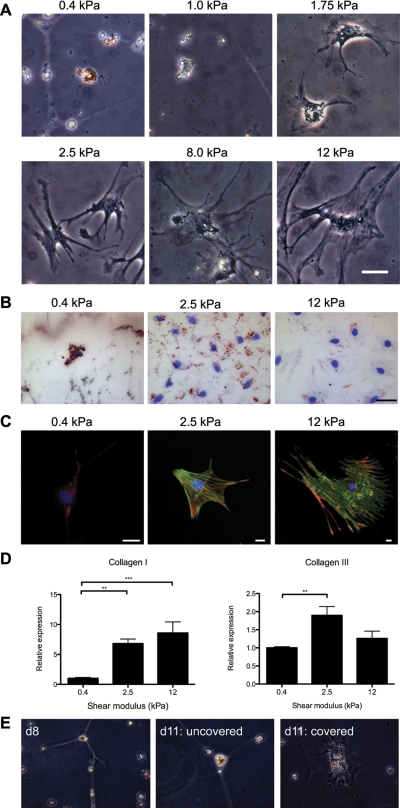
Fig. 1.
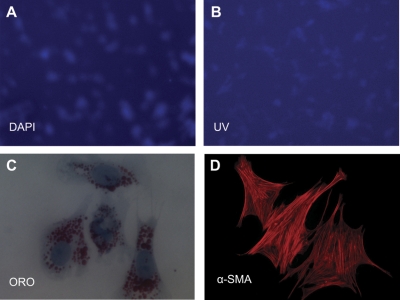
Hepatic stellate cells (HSC) demonstrate increased spreading and α-smooth muscle actin (α-SMA) expression on stiffer substrates. A: freshly isolated rat HSC were cultured for 7 days on type I collagen-coated polyacrylamide gels of varying shear modulus (G′), ranging from 0.4 to 12 kPa. By light microscopy, HSC appear morphologically quiescent on soft supports (0.4–1.0 kPa). These cells also demonstrated UV autofluorescence (data not shown). HSC plated on stiff (8–12 kPa) polyacrylamide supports displayed an activated, myofibroblastic-like phenotype. HSC cultured on intermediate supports (1.75–2.5 kPa) showed intermediate phenotypes. These results are representative of 3 experiments. Bar, 50 μm. B: HSC were cultured as in A and were stained with Oil Red O (ORO) and counterstained with 4,6-diamidino-2-phenylindole (DAPI; blue). Lipid droplets decrease on stiffer gels. Representative cells are from 2 different experiments Bar, 50 μM. C: HSC were cultured as in A and were immunostained with antibodies against the rat stellate cell marker desmin (red) and α-SMA (green); nuclei were stained with DAPI (blue). Desmin expression decreases in cells on stiffer gels, whereas α-SMA expression and cell size increase. α-SMA is organized in stress fibers in cells on 12-kPa gels. Photos are representative of 3 experiments (bars, 10 μm, note different magnifications). D: HSC were cultured as in A, and quantitative RT-PCR (qRT-PCR) for collagen I and collagen III was performed. Results are normalized to expression of 18s rRNA and are averaged from 3–4 independent experiments, each done in triplicate. Values are means ± SE. **P < 0.01. ***P < 0.005. E: primary HSC were cultured for 8 days (d8) on type I collagen-coated polyacrylamide gels of 0.4-kPa stiffness, maintaining a quiescent phenotype (left). Cells cultured under the same conditions for an additional 3 days remained quiescent in appearance (middle), whereas cells on a region of the gel covered with a glass coverslip for the additional 3 days began to spread and lose lipid droplets (right). Representative cells are shown from 2 independent experiments; ×40 magnification.
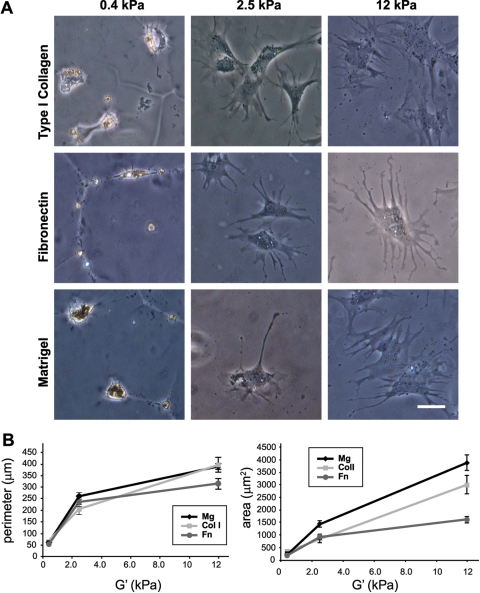
Fig. 2.
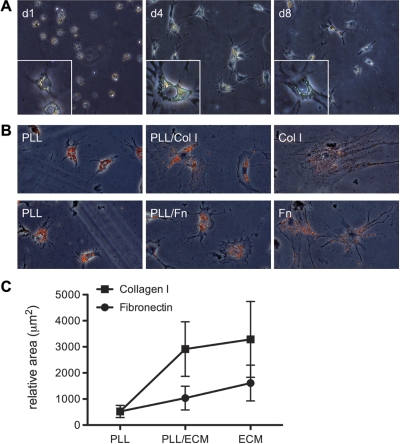
HSC undergo myofibroblastic differentiation on stiff substrates, independent of the matrix coating. A: HSC were cultured for 7 days on polyacrylamide gels of varying stiffness coated with type I collagen, plasma fibronectin, or Matrigel. Regardless of the extracellular matrix (ECM) coating, HSC adopted a myofibroblastic phenotype on rigid (12 kPa) supports, and they remained quiescent on soft (0.4 kPa) supports. Results are representative of at least 3 experiments. Bar, 50 μm. B: cells on the different matrices and supports were traced using Image J software for quantification of perimeter (left) and area (right). Values are means ± SD. Mg, Matrigel; Col I, type I collagen; Fn, plasma fibronectin. A minimum of 40 cells were analyzed for each condition.
Fig. 3.
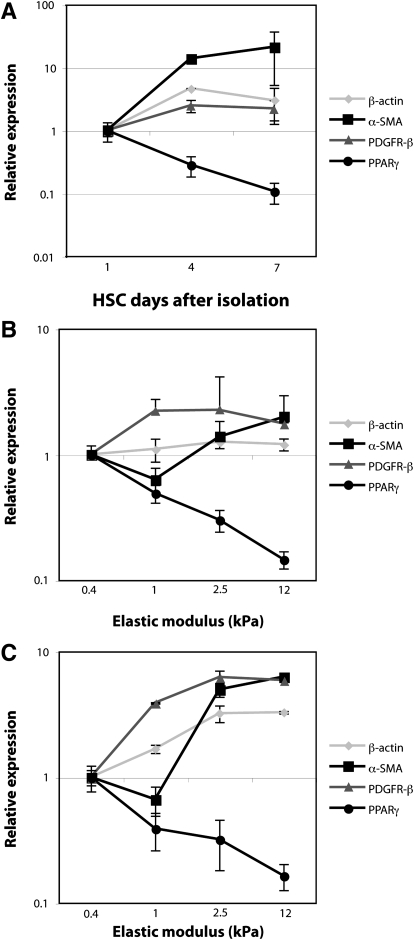
Changes in gene expression on increasingly stiff substrates parallel those occurring on plastic over time. A: primary HSC were cultured on tissue culture plastic and harvested on day 1 (quiescent phenotype), day 4 (intermediate), and day 7 (myofibroblastic). mRNA expression of four genes [β-actin, α-SMA, PDGF receptor-β (PDGF-Rβ), and peroxisome proliferator-activated receptor-γ (PPAR-γ)] was assayed by qRT-PCR and normalized to the 18S rRNA internal control. Changes in gene expression are shown relative to expression levels at day 1. This experiment was performed in duplicate, with 2 independent isolates of HSC (n = 4 replicates total). B and C: primary HSC were cultured for 7 days on polyacrylamide supports of variable stiffness, coated with either 0.1 mg/ml type I collagen (B) or 0.2 mg/ml plasma fibronectin (C). mRNA expression of the same panel of genes was determined by qRT-PCR, with levels at each point expressed relative to those for soft supports (0.4 kPa). With increasing stiffness of polyacrylamide supports, changes in HSC gene expression paralleled those observed with progressive HSC activation. Each experiment (B and C) was performed in duplicate, with 3 independent isolates of cells (n = 6 total). Values are means ± SD.
Fig. 4.
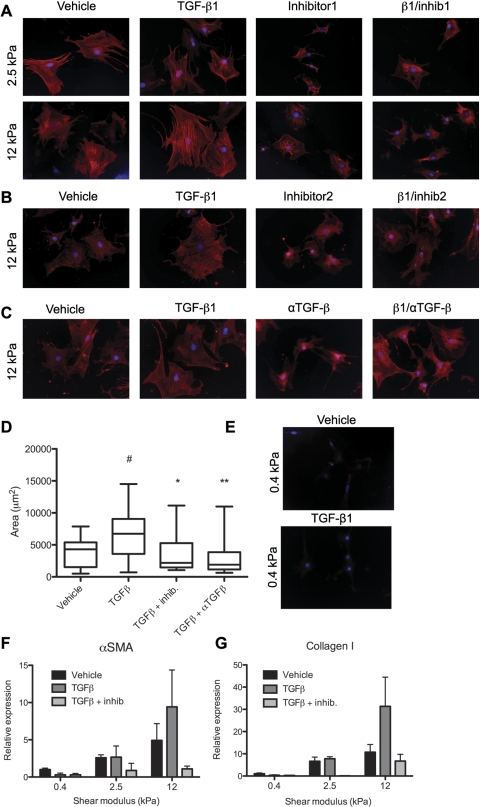
HSC on stiff substrates express α-SMA, even in the absence of transforming growth factor (TGF)-β. Primary rat HSC were cultured on type I collagen-coated polyacrylamide substrates of 2.5 or 12 kPa. A: beginning on the day after isolation, after cells adhered to the gels, they were treated with either vehicle, 100 pM TGF-β1, 5 μM TGF-β kinase inhibitor (NPC-34016; inhibitor 1), or a combination of TGF-β and the kinase inhibitor. After 7 days, cells were fixed and stained with antibody against α-SMA (red). Nuclei are stained with DAPI (blue). ×20 Magnification. B and C: cells were treated as in A, but with a different kinase inhibitor (616451; inhibitor 2) or a pan-TGF-β blocking antibody (αTGF-β). Cells were stained as in A. Results are representative of 2 independent experiments. Controls were treated with vehicle and mouse serum IgG. D: cell area was calculated using National Institutes of Health Image J. Results are representative of 2 independent experiments. Values are means ± SE. #Significantly different from vehicle, P < 0.05. *Significantly different from TGF-β treatment, P < 0.05. **Significantly different from TGF-β treatment, P < 0.01. E: primary rat HSC were cultured on type I collagen-coated polyacrylamide substrates of 0.4-kPa stiffness. Beginning on the day after isolation, after cells adhered to the gels, they were treated with either vehicle or 100 pM TGF-β1. After 7 days, cells were fixed and stained with antibody against α-SMA (red). Nuclei are stained with DAPI (blue). ×20 Magnification. F and G: cells were treated as in B, then analyzed by real-time PCR for expression of α-SMA (F) and type I collagen (G). Values are means ± SE.
Fig. 5.
HSC require matrix protein interactions to undergo myofibroblastic differentiation on stiff supports. A: primary rat HSC were cultured on 12-kPa polyacrylamide supports coated with 0.1 mg/ml poly-l-lysine (PLL) for up to 8 days. Even at day 8, the cells are minimally spread and retain vitamin A droplets (bright field). Photos are representative of cells in 4 experiments. ×10 Magnification. B: primary rat HSC were cultured for 7 days on 0.1 mg/ml PLL alone, on a mixture of 0.05 mg/ml PLL and 0.05 mg/ml type I collagen or plasma fibronectin, or on 0.1 mg/ml collagen or plasma fibronectin alone. ORO staining demonstrates retention of lipid droplets in cells on PLL, but increasing spreading and loss of lipid droplets with increasing amounts of matrix proteins. ×20 Magnification. C: quantification of the cell area in B, where ECM represents either collagen or fibronectin. Cells (15–32 per data point) were traced with Image J to determine area, with data shown as means ± SD. Two-way ANOVA demonstrated statistical significance for PLL and PLL/ECM points compared with PLL alone, with P < 0.0001.
Fig. 6.
HSC require the generation of mechanical tension to undergo myofibroblastic differentiation on stiff supports. Primary rat HSC were cultured on Teflon supports for 7 days. A: nuclei were stained with DAPI (blue). B: vitamin A-containing lipid droplets were visualized by UV autofluorescence. ×20 Magnification. C and D: cells treated as above were gently removed from the Teflon by rinsing and were replated on glass. C: after 6 h, cells stained with ORO demonstrated persistent lipid droplets. ×60 Magnification. D: cells cultured for 5 days on glass (after 7 days on Teflon), then stained with antibodies against α-SMA (red), demonstrated α-SMA in stress fibers. ×20 Magnification. ORO and immunostains are representative of 3 independent experiments.
For sandwich experiments (Fig. 1E), cells were cultured for 8 days, then a small glass coverslip and 35-g weight (sufficient to enable the glass coverslip to adhere to the gel) were placed on top. The weight was removed after 30 s, and the cells cultured for an additional 3 days with the glass covering remaining in place. For culture on polytetrafluoroethylene (Teflon), primary cells were plated on Teflon cell culture inserts (Millipore, Billerica, MA). After 7 days of culture, cells were removed from the insert by gentle rinsing and were replated on glass coverslips for staining or further culture. For TGF-β experiments (see Fig. 4), cells were treated with either 100 pM TGF-β1 (R&D Systems, Minneapolis, MN), 5 μM of the well-characterized TGF-β type I receptor kinase inhibitor NPC-34016 (a kind gift of David Liu, Scios, Freemont, CA) (15), 100 nM of a second TGF-β type I receptor kinase inhibitor (616451; Calbiochem), or a TGF-β1, -β2, -β3 neutralizing antibody (10 μm; R&D Systems).
Immunostaining and Oil Red O staining.
For immunostaining, HSC cultured on polyacrylamide supports were fixed in 1% formaldehyde (for Fig. 1B, 4% paraformaldehyde) for 30 min and permeabilized using 0.1% Triton X-100 for 3 min. Cells were then washed extensively with PBS, and immunofluorescence was performed using desmin (1:1,000, clone DE-U-10, Sigma) and α-SMA (1:1,600, clone 1A4, Sigma) monoclonal antibodies. Before mounting, cells underwent nuclear staining with 4,6-diamidino-2-phenylindole (1:10,000, Molecular Probes, Eugene, OR). In all cases, control cells treated in parallel without primary antibody showed no staining. For Oil Red O staining, cells were fixed in 10% formalin for 15 min, incubated in Oil Red O solution (0.5% in propylene glycol, Poly Scientific, Bay Shore, NY) for 1 h at room temperature, and treated with 85% propylene glycol for 2 min.
Quantitative PCR.
Total RNA was isolated from HSC cultured on either polyacrylamide gels (day 7) or tissue culture plastic (days 1, 4, and 7) (RNeasy miniprep kit, Qiagen, Valencia, CA). For Fig. 3, one-step quantitative RT-PCR (qRT-PCR) was performed. Briefly, RNA template was reverse transcribed into cDNA and quantitatively amplified by PCR using FullVelocity SYBR Green (Stratagene, La Jolla, CA) in an Mx3000P instrument (Stratagene), according to the manufacturer's instructions. For Fig. 4, two-step qRT-PCR was performed, with RNA template first reverse transcribed using SuperScript III (Invitrogen, Carlsbad, CA), then quantitatively amplified by PCR using Fast SYBR Green (Applied Biosystems, Carlsbad, CA) in a StepOnePlus instrument (Applied Biosystems). Individual qRT-PCR reactions were performed in duplicate or triplicate, and analysis was by the ΔΔCt method. Primers were designed online using Primer3 software (http://frodo.wi.mit.edu). For sequences, see Supplemental Table S1. mRNA expression of all measured transcripts was normalized to 18S ribosomal RNA.
Statistical analysis.
All data are expressed as means ± SE and are representative of at least three experiments, unless otherwise specified.
RESULTS
HSC differentiate as stiffness increases.
Primary rat HSC were cultured on polyacrylamide gels of variable stiffness, ranging from 0.4 to 12 kPa, for 7 days (Fig. 1A). This range of stiffnesses was chosen because the stiffness (G′) of normal rat liver is 0.3–0.6 kPa, whereas cirrhotic livers have a G′ ranging from 3 kPa to upwards of 12 kPa (16). Although there is regional variability in the stiffness of different microenvironments in the liver (25), due, in part, to cell interactions with matrix proteins (41), these are nonetheless reasonable approximations of the mechanical forces encountered by an individual cell and are significantly more physiological than tissue culture plastic and glass, which are at least several orders of magnitude stiffer than even cirrhotic liver (24, 29). Cells demonstrated both the morphological changes of myofibroblast differentiation (progressive spreading, loss of lipid droplets, and de novo expression of α-SMA), as well as increased fibrogenesis as the stiffness increased. HSC plated on the most compliant supports (G′ of 0.4–1.0 kPa) for 7 days retained the phenotypic appearance of freshly isolated, quiescent HSC, with a rounded morphology and abundant, UV autofluorescent, lipid droplets (Fig. 1, A and B). Cells on stiffer supports (8–12 kPa), similar to control cells plated on uncoated tissue culture plastic, lost their vitamin A droplets and demonstrated the spread-out morphology characteristic of myofibroblasts, while cells on supports of intermediate stiffness (1.75–2.5 kPa) demonstrated a range of intermediate morphologies. These intermediate phenotypes remained stable even after cells were cultured on gels for more than 2 wk (data not shown). Immunostaining for the myofibroblast marker α-SMA demonstrated increased α-SMA expression and organization in stress fibers in cells on stiffer substrates, consistent with myofibroblastic activation (Fig. 1C). The increase in α-SMA correlated with increased expression of collagen I mRNA, although collagen III expression was highest on intermediate stiffness gels (Fig. 1D). To confirm that cells on the softest supports were viable, quiescent cells initially cultured on soft supports were gently covered with a stiff support (glass coverslip) for 3 additional days. These cells demonstrated increased spreading and loss of lipid droplets compared with uncovered cells (Fig. 1E), indicating both that cells on soft supports were viable and that stiffness sensing was dynamic. The ultimate degree of HSC activation, over at least 2 wk, correlated with the stiffness of the substrate. The G′ of the substrate did not appear to regulate the rate of phenotypic change, and there was no stiffness threshold separating the quiescent and differentiated states. There was no evidence of increased apoptosis in cells on soft compared with stiff supports (Supplemental Fig. S2).
Cells on soft supports were unable to differentiate, regardless of the coating matrix.
The role of the mechanical relative to the chemical properties of the matrix was tested by culturing cells on polyacrylamide supports of variable elastic modulus coated with thin layers of different matrix proteins, including type I collagen, plasma fibronectin, or Matrigel (Fig. 2). Cells on stiff substrates differentiated, regardless of the underlying matrix, while cells on soft substrates retained a quiescent appearance. Notably, cells plated on supports coated with thin layers of Matrigel (diluted to prevent gel formation) demonstrated a range of phenotypes, from quiescent to differentiated, depending on the stiffness of the support. There were modest matrix-dependent differences in mean cell area, suggesting that, while mechanical factors (i.e., substrate stiffness) were the primary determinant of HSC phenotype, the chemical identity of the matrix also played a role (Fig. 2B; see also Fig. 3, B vs. C, and Fig. 5C).
Changes in gene expression on increasingly stiff substrates parallel those observed with myofibroblastic differentiation on plastic.
HSC on 12-kPa substrates had the morphological appearance and α-SMA expression typical of myofibroblasts. mRNA expression patterns of HSC cultured on polyacrylamide supports of increasing stiffness for 7 days (Fig. 3, B and C) paralleled the expression patterns of morphologically similar HSC at various time points during differentiation on plastic (Fig. 3A). Specifically, as HSC differentiated on plastic, expression of β-actin and PDGF receptor-β (PDGF-Rβ) increased modestly, α-SMA increased markedly, and peroxisome proliferator-activated receptor-γ (PPAR-γ) decreased. Cells cultured on polyacrylamide supports demonstrated similar changes in gene expression with increasing gel stiffness, regardless of whether the ECM coating was type I collagen or plasma fibronectin. Expression of β-actin increased modestly from soft to stiff supports, whereas α-SMA and PDGF-Rβ expression increased markedly, and PPAR-γ decreased. The order of magnitude of many of the gene changes (particularly for α-SMA) was higher for cells on plastic than on stiff gels, potentially due to the overwhelming nature of the mechanical stimulus provided by plastic or (given that α-SMA stress fibers are prominent even on gels) to a disconnect between the protein and mRNA levels of α-SMA. Nonetheless, the findings are consistent with the conclusion that cells on supports of increasing, physiological range stiffness undergo increasing degrees of myofibroblastic differentiation.
TGF-β is not sufficient for α-SMA expression.
Our laboratory has previously shown that portal fibroblasts, another myofibroblast precursor population in the liver, require both TGF-β and a mechanically stiff environment for myofibroblastic differentiation (26); when treated with TGF-β inhibitors, portal fibroblasts fail to express any α-SMA, regardless of matrix stiffness. Because HSC have been reported to express α-SMA, even in the absence of TGF-β signaling (18, 27, 39), we treated cells with two different TGF-β kinase inhibitors, as well as a pan-TGF-β blocking antibody, while culturing them on supports of physiological stiffness (Fig. 4, A–C). HSC produce TGF-β in an autocrine fashion (4), so an inhibitor is necessary to assess its effects. Immunostaining for α-SMA showed that, although cells treated with the inhibitors spread significantly less than TGF-β-treated cells, and although TGF-β treatment resulted in increased α-SMA organization into stress fibers (39), a low level of α-SMA expression was evident, even in the absence of TGF-β signaling in cells on intermediate (2.5 kPa) and stiff (12 kPa) supports (Fig. 4, A–D). This is in marked contrast to portal fibroblasts (26). Treatment with exogenous TGF-β was not sufficient to induce α-SMA expression on 0.4-kPa gels (Fig. 4, E and F). Real-time PCR demonstrated that stiffness was required for α-SMA and collagen I expression, regardless of TGF-β exposure, and that culture on stiff supports resulted in some TGF-β-independent expression, although TGF-β significantly enhanced the effects of stiffness (Fig. 4, F and G).
Matrix protein interactions are required for HSC myofibroblastic differentiation on stiff substrates.
Freshly isolated HSC were cultured on polyacrylamide substrates coated with PLL, which permits cell adhesion via electrostatic interactions. Cells on PLL maintained a round morphology and lipid droplets even after 8 days, demonstrating minimal evidence of myofibroblastic differentiation (Fig. 5A). Although there was a small amount of fibronectin deposited on the PLL-coated supports (binding directly to the PLL), likely accounting for the minimal degree of cell spreading, there was a dramatic difference between cells cultured on fibronectin or collagen vs. PLL (Fig. 5B). In experiments in which the total concentration of proteins coating the gels remained constant, but the mix of PLL and ECM protein varied, differentiation increased as a function of the matrix protein concentration (Fig. 5, B and C). Adding PLL to cells on tissue culture plastic or to high concentrations of matrix proteins on polyacrylamide does not inhibit differentiation (data not shown). Thus matrix protein interactions, in addition to adhesion to a stiff substrate, enhance myofibroblastic differentiation.
HSC require the generation of mechanical tension for myofibroblastic differentiation on stiff substrates.
To determine whether mechanical tension was also required for the response to stiff substrates, freshly isolated cells were cultured on Teflon, which has previously been reported to maintain HSC in a quiescent state (5). Teflon is stiff, with an elastic modulus four to five orders of magnitude stiffer than normal or cirrhotic liver (33). Although matrix, in particular fibronectin, is deposited by cells onto Teflon (23), cells are unable to generate mechanical tension due to the nature of the polymer. Cells cultured on Teflon for 7 days remained viable, with UV autofluorescence indicating the persistence of vitamin A droplets (Fig. 6, A and B). When cells were removed from the supports and stained 6 h later, Oil Red O staining demonstrated a persistently quiescent morphology (Fig. 6C); however, when cells were plated on glass after 7 days on Teflon and cultured for an additional 5 days, they demonstrated the ability to undergo myofibroblastic activation, with loss of lipid droplets and expression of α-SMA (Fig. 6D). Thus HSC myofibroblastic differentiation requires a mechanically stiff substrate, adhesion to matrix proteins, and the ability to generate tension.
DISCUSSION
We have used a physiologically relevant cell culture system to demonstrate that HSC myofibroblastic differentiation requires a mechanically stiff environment. This held true even in the presence of TGF-β and regardless of the chemical identity of the coating matrix.
The ability of HSCs to adopt a range of stable phenotypes when cultured under different mechanical conditions implies that cells continuously probe the substrate and that HSC stiffness sensing is an active process, as has been shown for other kinds of cells (7, 21, 30, 40). Our observation that stable quiescent cells (for example, those cultured on soft gels or Teflon) will differentiate when their mechanical environment changes also supports this conclusion. The mechanism for sensing and transmitting mechanical signals has not been identified in HSC, but, based on data in the literature from other systems, almost certainly involves integrins, their interactions with the cytoskeleton, and downstream signaling networks (3, 32). Investigators studying HSC have demonstrated that RGD sequence-mediated interactions, as well as integrin-linked kinase and possibly focal adhesion kinase, are required for HSC differentiation, consistent with the stiffness and matrix dependence we describe (20, 34, 35, 44). Further investigation will be required to determine the role of integrins in HSC mechanotransduction.
HSC differentiated to myofibroblasts on stiff gels, regardless of the matrix coating. This included cells on Matrigel, which has previously been reported to maintain HSC quiescence (13). When used at standard concentrations (diluted <50%), Matrigel forms a gel with a G′ of <0.4 kPa (37). Because this gel is significantly less stiff than tissue culture plastic, plating cells on Matrigel exposes them to a physical, as well as a chemical, environment that differs significantly from standard culture conditions and mimics the mechanical environment of our softest polyacrylamide supports. Thus our data provide a mechanistic explanation for the observation that HSC plated on Matrigel are phenotypically quiescent and may explain why it has proven impossible to isolate the individual chemical components of Matrigel responsible for maintenance of the quiescent phenotype (13). Our findings also suggest that alternate culture systems could be used for the maintenance of HSC quiescence, including soft supports such as polyacrylamide and other hydrogels (22).
In a previous study of liver stiffness and fibrosis in a rodent model, we observed myofibroblast differentiation at whole liver stiffnesses significantly lower than the 8–12 kPa required in the system described here (16). This result can be explained by important differences between polyacrylamide and biologically relevant matrix proteins. Polyacrylamide is an ideal elastic substrate, and its modulus is independent of the strain applied. Many matrix proteins, however, demonstrate strain stiffening, such that the stress vs. strain curve is nonlinear: essentially, these proteins become stiffer as force is applied to them. This is relevant to the liver, as rheometry studies demonstrate that the liver stiffens under increasing strain (unpublished results). Recently published work demonstrates that contractile mesenchymal cells respond to the nonlinear properties of the ECM proteins fibrin and collagen by creating long-distance stiffness gradients (41). This suggests that small changes in HSC phenotype (and contractility) may generate large changes in local stiffness, thereby enhancing the phenotype.
We do not observe an absolute requirement for TGF-β (see Fig. 4). Our data are consistent with work showing that primary HSC from TGF-β1 null mice activate normally when cultured on a stiff substrate, such as tissue culture plastic (18), although TGF-β is clearly required for complete expression of the myofibroblast phenotype (Fig. 4 and Ref. 39). Certain functions of HSC-derived myofibroblasts may be mediated by both chemical signals from TGF-β and physical signals from the matrix, as suggested by the observation that fully activated HSC treated with TGF-β and cultured on tethered rather than floating collagen lattices demonstrate increased expression of fibrillin-1 (28) and by our observation that collagen synthesis is enhanced when cells on a stiff support are treated with TGF-β (Fig. 4G). Physical cues, such as those provided by increased stiffness of the surrounding matrix, may provide the initial stimulus for activation, which is then enhanced by chemical signals, such as TGF-β (via Smad3), resulting in the morphological and functional maturation of activated HSC. The stiffness of the physical environment may also play an important role mediating the release of active TGF-β from its latent form (42).
Our findings have important implications for understanding the development and progression of liver disease. The observation that HSC undergo myofibroblastic differentiation in the presence of a stiff environment (for example, a fibrotic scar) could explain the perpetuation of established fibrosis. Similarly, the finding that modest increases in mechanical stiffness can cause partial phenotypic changes in HSC, combined with recently published data on the strain stiffening of matrix proteins (41), suggests a mechanism for the amplification of the repair process early after injury. An intriguing question is whether localized increases in liver stiffness, which occur early after injury (25), mediate the very first round of HSC differentiation. Our laboratory has observed in an animal model of chronic liver injury that an increase in liver stiffness precedes measurable increases in fibrosis, and that this is mediated, at least in part, by alterations in collagen cross-linking (16). Whether this is a generally applicable mechanism is not yet clear; however, our findings suggest that mechanical factors need to be incorporated into models of HSC differentiation.
GRANTS
This work was supported by an American Gastroenterological Association (AGA)/Miles and Shirley Fiterman Basic Research Award to R. G. Wells, and by National Institutes of Health (NIH) R01 Grants DK058123 (to R. G. Wells) and GM083272 (to P. A. Janmey). E. P. Chan was supported by NIH institutional training Grant T32 DK07066 and by an AGA Fellowship to Faculty Transition Award. A. L. Olsen is supported by grant F30 DK081265.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Jia-Ji Hui and Min Li for HSC isolation; Klaus Kaestner for use of qRT-PCR equipment; and Gary Swain and the UPenn/National Institute of Diabetes and Digestive and Kidney Diseases Center for Molecular Studies in Digestive and Liver Disease (P30 DK50306) Morphology Core for assistance with microscopy.
REFERENCES
- 1. Arora PD, Narani N, McCulloch CA. The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol 154: 871–882, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basu A, Wen Q, Lubensky T, Janmey P, Yodh A. Non-affine displacements in flexible polymer gels. Macromol. 44: 1671–1679, 2011 [Google Scholar]
- 3. Bershadsky A, Kozlov M, Geiger B. Adhesion-mediated mechanosensitivity: a time to experiment, and a time to theorize. Curr Opin Cell Biol 18: 472–481, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Bissell DM, Wang SS, Jarnagin WR, Roll FJ. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest 96: 447–455, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bridle KR, Crawford DH, Ramm GA. Identification and characterization of the hepatic stellate cell transferrin receptor. Am J Pathol 162: 1661–1667, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Byfield FJ, Wen Q, Levental I, Nordstrom K, Arratia PE, Miller RT, Janmey PA. Absence of filamin A prevents cells from responding to stiffness gradients on gels coated with collagen but not fibronectin. Biophys J 96: 5095–5102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139–1143, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance vs. ligand density in cell on gel responses. Biophys J 86: 617–628, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 166: 877–887, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport 13: 2411–2415, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88: 125–172, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem 161: 207–218, 1987 [DOI] [PubMed] [Google Scholar]
- 13. Friedman SL, Roll FJ, Boyles J, Arenson DM, Bissell DM. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem 264: 10756–10762, 1989 [PubMed] [Google Scholar]
- 14. Gaca MD, Zhou X, Issa R, Kiriella K, Iredale JP, Benyon RC. Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix Biol 22: 229–239, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Ge R, Rajeev V, Subramanian G, Reiss KA, Liu D, Higgins L, Joly A, Dugar S, Chakravarty J, Henson M, McEnroe G, Schreiner G, Reiss M. Selective inhibitors of type I receptor kinase block cellular transforming growth factor-beta signaling. Biochem Pharmacol 68: 41–50, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol 293: G1147–G1154, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Georges PC, Janmey PA. Cell type-specific response to growth on soft materials. J Appl Physiol 98: 1547–1553, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol 30: 77–87, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol 159: 1009–1020, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iwamoto H, Sakai H, Kotoh K, Nakamuta M, Nawata H. Soluble Arg-Gly-Asp peptides reduce collagen accumulation in cultured rat hepatic stellate cells. Dig Dis Sci 44: 1038–1045, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng 9: 1–34, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Kloxin AM, Benton JA, Anseth KS. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials 31: 1–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koenig AL, Gambillara V, Grainger DW. Correlating fibronectin adsorption with endothelial cell adhesion and signaling on polymer substrates. J Biomed Mater Res 64A: 20–37, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter 3: 299–306, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Levental I, Levental KR, Klein EA, Assoian R, Miller RT, Wells RG, Janmey PA. A simple indentation device for measuring micrometer-scale tissue stiffness. J Phys Condens Matter 22: 194120, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology 46: 1246–1256, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Liu C, Gaca MD, Swenson ES, Vellucci VF, Reiss M, Wells RG. Smads 2 and 3 are differentially activated by transforming growth factor-beta (TGF-beta) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-beta-independent. J Biol Chem 278: 11721–11728, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Lorena D, Darby IA, Reinhardt DP, Sapin V, Rosenbaum J, Desmouliere A. Fibrillin-1 expression in normal and fibrotic rat liver and in cultured hepatic fibroblastic cells: modulation by mechanical stress and role in cell adhesion. Lab Invest 84: 203–212, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Materials Res 7: 1564–1583, 1992 [Google Scholar]
- 30. Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A 94: 13661–13665, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Popov Y, Sverdlov DY, Sharma AK, Bhaskar KR, Li S, Freitag TL, Lee J, Dieterich W, Melino G, Schuppan D. Tissue transglutaminase does not affect fibrotic matrix stability or regression of liver fibrosis in mice. Gastroenterology 140: 1642–1652, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J Cell Sci 122: 179–186, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rae PJ, Dattelbaum DM. The properties of poly(tetrafluoroethylene) (PTFE) in compression. Polymer 45: 7615–7625, 2004 [Google Scholar]
- 34. Reif S, Lang A, Lindquist JN, Yata Y, Gabele E, Scanga A, Brenner DA, Rippe RA. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J Biol Chem 278: 8083–8090, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Shafiei MS, Rockey DC. The role of integrin-linked kinase in liver wound healing. J Biol Chem 281: 24863–24872, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Sohara N, Znoyko I, Levy MT, Trojanowska M, Reuben A. Reversal of activation of human myofibroblast-like cells by culture on a basement membrane-like substrate. J Hepatol 37: 214–221, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Soofi SS, Last JA, Liliensiek SJ, Nealey PF, Murphy CJ. The elastic modulus of Matrigel as determined by atomic force microscopy. J Struct Biol 167: 216–219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Uemura M, Swenson ES, Gaca MD, Giordano FJ, Reiss M, Wells RG. Smad2 and Smad3 play different roles in rat hepatic stellate cell function and alpha-smooth muscle actin organization. Mol Biol Cell 16: 4214–4224, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology 47: 1394–1400, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Winer JP, Oake S, Janmey PA. Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation. PLoS One 4: e6382, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 179: 1311–1323, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60: 24–34, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Zhang Y, Ikegami T, Honda A, Miyazaki T, Bouscarel B, Rojkind M, Hyodo I, Matsuzaki Y. Involvement of integrin-linked kinase in carbon tetrachloride-induced hepatic fibrosis in rats. Hepatology 44: 612–622, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.