Abstract
The divalent metal transporter (DMT1, Slc11a2) is an important molecule for intestinal iron absorption. In the Belgrade (b/b) rat, the DMT1 G185R mutation markedly decreases intestinal iron absorption. We used b/b rats as a model to examine the genes that could be compensatory for decreased iron absorption. When tissue hypoxia was assayed by detecting pimonidazole HCl adducts, the b/b liver and intestine exhibited more adducts than the +/+ rats, suggesting that hypoxia might signal altered gene expression. Total RNA in the crypt-villus bottom (C-pole) and villus top (V-pole) of +/+, b/b, and iron-fed b/b rats was isolated for gene array analyses. In addition, hepatic hepcidin and intestinal hypoxia-inducible factor-α (Hifα) expression were examined. The results showed that expression of hepatic hepcidin was significantly decreased and intestinal Hif2α was significantly increased in b/b and iron-fed b/b than +/+ rats. In b/b rats, the expression of Tfrc mRNA in the C-pole and of DMT1, Dcytb, FPN1, Heph, Hmox1, and ZIP14 mRNAs in the V-pole were markedly enhanced with increases occurring even in the C-pole. After iron feeding, the increased expression found in b/b rats persisted, except for Heph and ZIP14, which returned to normal levels. Thus in b/b rats depressed liver hepcidin production and activated intestinal Hif2α starting at the C-pole resulted in increasing expression of iron transport genes, including DMT1 G185R, in an attempt to compensate for the anemia in Belgrade rats.
Keywords: Belgrade (b/b) rats, iron absorption, divalent metal transporter and ferroportin
iron is an essential element for all mammals serving to coordinate oxygen binding in hemoglobin and myoglobin and acting as a cofactor in multiple enzymes that are critical for cell growth and division (2). Iron excess has the potential for catalyzing formation of reactive oxygen radicals, causing oxidative damage to DNA, proteins, and lipids (15). Since the body does not possess a mechanism for active excretion of iron, iron homeostasis is regulated through the hepatic hepcidin-intestinal absorptive pathway (16, 21). Intestinal iron absorption requires that iron be transported across three cellular sites: the brush border membrane (BBM), the cytoplasm, and the basolateral membrane (BLM) of the epithelium. The molecules involved in the transport across each site have been largely identified, but the detailed process of how the molecules function at each of these sites remains unclear. The initial step in the absorption of dietary iron depends on the reduction of iron by duodenal cytochrome b reductase (Cybrd1) for transport by the divalent metal transporter (DMT1, Sla11a2) across the BBM (11, 20). A Slc11a2 mutation, G185R in the Belgrade (b/b) rat and mk mice (8, 9) and the G75R mutation in humans (3), results in marked decrease of iron transport with a resulting severe, but not lethal, iron deficiency anemia. Survival may occur because the mutation does not result in complete loss of iron transport (12, 36) or other gene products such as the zinc transporter 14 (ZIP14 or Slc39a14) (21), heme carrier protein (HCP1, Slc46a1), or proton-coupled folate transporter (PCFT) (23, 26) or the lipocalin 2 (Lcn2) receptor Slc22a17 (24p3) offer alternative routes for iron absorption (6, 10).
Whatever the mechanism for survival, the mutation in DMT1 does provide an analytic system to examine the compensatory changes in the duodenal enterocyte as the enterocyte attempts to increase iron transport. By studying the crypt-villus axis, it may be possible to determine where in the axis compensatory changes occur. In this report, we took advantage of a technique that allows isolation of mRNA from the crypt-villus bottom (C-pole) and the villus tops (V-pole) of the crypt-villus axis and compares b/b, wild-type (+/+), and iron-fed b/b rats to examine the potential compensatory gene(s). Compared with +/+ rats, the expression of DMT1 and Cybrd1 mRNAs is increased in b/b rats (4). Since increases of intestinal DMT1 and Cybrd1 are induced by hypoxia-inducible factor-2α [Hif2α also known as endothelial PAS1 (Epas1)] (19, 25), and since hypoxia has also been reported to activate iron regulatory protein 2 (IRP2) (13, 14), we hypothesized that hypoxia in the b/b rat induced expression of iron absorptive and metabolic genes. Since several nuclear factors such as Epas1 and Egln3 are known to be activated by hypoxia in the liver and intestine, we investigated whether the transcriptional factor Epas1 activated target genes at the C- or V-pole of the intestine, resulting in the increased iron absorptive gene expression and hence survival of b/b rats.
MATERIALS AND METHODS
Animal and tissue preparation.
A Belgrade rat colony was maintained in the animal quarter according to Institutional Animal Care and Use Committee guidelines with animals fed a Harlan Teklad 22/5 rodent chow and water ad libitum approved by Louisiana State University Health Sciences Center. At 28 days of age, four wild-type (+/+) and eight b/b rats were shifted to a purified rodent diet (AIN-76A, Dyet no. 100000) with deionized water ad libitum and kept in individual cages for 4 wk. The wild-type (+/+) and b/b rats were identified by genomic typing using four primers: wild-type forward (5′-TTCAGTGGACGGGCTTCGC-3′), wild-type reverse (5′-TGCGATGGTGATGAGGACTCC-3′), mutant forward (5′- TTCTAATCTATGGTTCCCCTGTATGGTA-3′), and mutant reverse (5′-AGCCTGCAACACTTGCCTCATAT-3′). The amplicons produced by primer pairs 1 and 4 generated 423 bp of +/+, b/b, and +/b common band; primer pairs 1 and 2 produced a 196-bp +/+ and +/b band; and the primer pairs 3 and 4 generated a 275-bp band of b/b and +/b. Three days before the experiment, four randomly selected b/b rats were given deionized water containing ferric ammonium citrate (100 μM) ad libitum. The 3 days of iron feeding was thought to significantly replenish iron including enterocytes since there was significant improvement in the anemia present in b/b rats (Fig. 1). The weight of the b/b rats was markedly lower than the +/+ rats and did not change after iron feeding (212 ± 5, 124 ± 1, and 126 ± 2 g for +/+, b/b, and iron-fed b/b, respectively). Rats were killed by injection of an overdose of pentobarbital sodium followed by heart puncture to collected blood for measurements of hemoglobin (HGB), hematocrit (HCT), and mean corpuscular volume (MCV). A 2-cm-long duodenal segment 1 cm from the pylorus was removed and rinsed with ice-cold normal saline, and the distal 1 cm of the removed duodenum was embedded in optimal cutting temperature (OCT) compound (Miles, Elkhart, IN) and longitudinally sectioned to determine the mucosal thickness and for immunofluorescent staining for sucrase-isomaltase (Si). The mucosal thickness and Si immunofluorescent staining allow determination of the sections collected from crypt-villus axis (see Verification of the iron status and confirmation of the position of the C-V sections prior to microarray analysis).
Fig. 1.
Hemoglobin (HGB), hematocrit (HCT), and red blood cell mean corpuscular volume (MCV) in Belgrade (b/b) and iron-fed b/b compared with +/+ rats. Blood was collected by intracardiac puncture to measure HGB, HCT, and MCV in 4 +/+, 4 b/b, and 3 iron-fed b/b rats. Data are means ± SE. *P < 0.05 vs. the previous column.
The proximal 1 cm of the removed duodenum was opened longitudinally and subjected to cryostat sectioning from the luminal surface (villus top) to the crypt bottom (see results). On the basis of Si immunofluorescent staining, the sequential sections form the bottom to the top were separated into six sequential groups and total RNA was obtained for Northern blot analysis and quantitative PCR. The total RNA isolated was then pooled from sections a and b representing the bottom and designated as the C-pole, and from sections e and f representing the villus top and designated as the V-pole. These pools were used for microarray analysis. In addition, for RNA electrophoretic mobility shift assay (EMSA), the duodenum from nine rats consisting wild-type, iron-deficient, and iron-fed iron-deficient rats (3 rats for each group) was similarly sectioned and the lysate from groups from a–f were isolated for the measurement of IRP activities. To examine the specific effect of Hif2α on intestinal transport and iron-related gene, an experiment consisted of three rats each for wild-type, iron-deficient, and iron-refed for 3 days were performed. In this experiment, the iron-deficient +/+ rats were fed an iron-deficient diet (AIN-76A, Dyet no. 115001) (Dyets, Bethlehem, PA) with distilled water ad libitum. The total RNA from the C- and V-poles isolated for gene array as described above.
cRNA preparation and microarray analysis.
cRNA was prepared and used for microarray analysis. Briefly, total RNA from the C- and V-poles of each rat was quantified spectrophotometrically and checked for degradation with an Agilent 2100 Bioanalzyer (Agilent Technologies, Palo Alto, CA). The intact RNA was used for analysis. For cDNA synthesis ∼10 μg total RNA was incubated with a T7-(dT)24 primer by using the Superscript cDNA Synthesis Kit (Invitrogen, Carlsbad, CA). Biotinylated cRNA was transcribed in vitro by using the GeneChip IVT Labeling Kit (Affymetrix, Santa Clara, CA) and purified using the GeneChip Sample Cleanup Module (Affymetrix). Twenty micrograms of the purified cRNA was fragmented, biotin labeled, and hybridized to the Rat Genome 230 2.0 Array (Affymetrix). The arrays were washed, stained with streptavidin-R phycoerythrin (10 μg/ml) (Vector Laboratories, Burlingame, CA), incubated with biotinylated goat anti-streptavidin antibody (Vector Laboratories), stained once again with streptavidin-R phycoerythrin, and then scanned with a GeneChip Scanner 3000. Pixel intensities were measured, expression signals were analyzed, and features were extracted by use of a commercial software package (GeneChip Operating Software 1.2,). Data mining analysis was performed with Data Mining Tool version 3.0 (Affymetrix) algorithms.
Validation of gene expression by real-time RT-PCR.
In addition to the analysis of marker gene Si and Descr4 (defensin 5, a Paneth cell marker) expression by Northern blots, the differential expression of selected mRNAs was verified by real-time RT-PCR. Because of the limited RNA samples, the C- and V-pole RNA samples from microarrays were pooled and first-strand cDNA synthesized from 5 μg total RNA with random primers and the Superscript II RT kit (Invitrogen). For real-time PCR, 1 μl of first-strand cDNA was used as a template and mixed with a SYBR Green Master Mix and primer pairs (Supplemental Table S1; the online version of this article contains supplemental data). PCR amplification was performed with the Bio-Rad iCycler with following cycling parameters: 95°C for 5 min, 45 cycles of 95°C for 30 s, 57°C for 30 s, and then 72°C for 30 s. In parallel, a standard curve was generated with β-actin by using 0.0001, 0.0125, 0.05, 0.20, 0.80, and 1.60 μl of first-strand cDNA.
Immunofluorescent detection of hypoxia.
Nitroimidazole and its derivative pimonidazole (Hypoxyprobe-1) with immunochemical reagents have been used to detect hypoxic cells in different cellular compartments in the liver and the intestine in vivo (1, 28). These compounds enter viable cells, where they convert to form reactive intermediate species. In the presence of normal oxygen levels, the molecule is reoxidized and diffuses out of the cells. Under hypoxic conditions, the molecules interact with thiol groups of intracellular proteins forming protein adducts that can be localized with antibodies. In the present study, we intravenously injected 60 mg/100 g body wt of Hypoxyprobe-1 (Natural Pharmacia International, Burlington, MA) via the tail veins into 3 +/+, 3 b/b, and 3 iron-fed b/b. The rats were euthanized 1 h later, and the liver and intestine were collected, embedded in OCT compound in the same block to avoid thickness and staining variation amount tissues, and cryostat sectioned at 5 μm, and immunofluorescent staining was performed with the rabbit anti-serum to the protein adduct of Hypoxyprobe-1 with subsequent staining with goat anti-rabbit antiserum and staining of the nuclei with diamidine-2-phenylindole (Pierce, Rockford, IL). The sections were then extensively washed and mounted with Vectashield (Vector Laboratories, Burlingame, CA). The slides were examined with a Zeiss Z1 AxioObserver and images recorded with an ApoTome device with the same setting in all tissues.
Northern and Western blot analyses.
Northern blots were performed for further verification of expression of some genes involved in iron metabolism as described (32). 32P-labeled probes were generated by random primer labeling for Si, Descr4, Cybrd1, DMT1, H-ferritin (FtH), heme oxygenase (Hmox1), hephaestin (Heph), ferroportin (FPN1), and β-actin (β-Act). The hybridization signals were detected by a PhosphorImager and quantitated with the ImageQuant software. The protein levels of selected genes were determined by Western blot analysis according to established methods (32). The antibodies for Si, ferritin (Ft), DMT1, FPN1, and Heph were generated in our laboratory (30, 32, 34–36). Antibodies against human Arnt, Hif1α, Epas1, Egln3, Hmox1, and β-Act were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and ZIP14 from Sigma Life Science (St. Louis, MO). These antibodies against the human proteins were reacted with the rat counterparts. The signals were detected by ECL Western blotting system (Amersham, Arlington Heights, IL), normalized with β-Act, and quantitated by transmittance densitometry using volume integration with ImageQuant application software.
Determination of IRP activities by EMSA.
IRP activity was assayed along the crypt-villus axis by using lysates obtained from cryostat section described previously (33). Unlabeled and 32P-labeled RNA containing the iron regulatory element (IRE) was prepared by in vitro transcription of the linearized pGL-66 as described previously (31). The pGL-66 clone was a gift provided by Dr. Elizabeth A. Liebold. Protein-RNA complexes were resolved on a 6.5% nondenaturing PAGE. Radioactive signals were detected by phosphorimage analysis.
Statistical analysis.
For comparison of gene expression in the C-pole or V-pole among wild-type, b/b, and iron-fed b/b rats, the ANOVA test using GraphPad Prism 4 software was performed. For comparisons between bottom and top gene expression in normal and b/b or iron-fed b/b rats, the one-way ANOVA with Benjamin and Hochberg correction was applied by use of GeneShifer (Geospiza).
RESULTS
Verification of the iron status and confirmation of the position of the C-V sections prior to microarray analysis.
The iron status of each rat in each of the three groups was assessed for HGB, HCT, and red blood cell MCV as shown in Fig. 1. The HGB, HCT, and MCV were significantly lower in the b/b rats. In the b/b rats fed iron for 3 days, the HGB and HCT were significantly increased and the MCV reached normal levels.
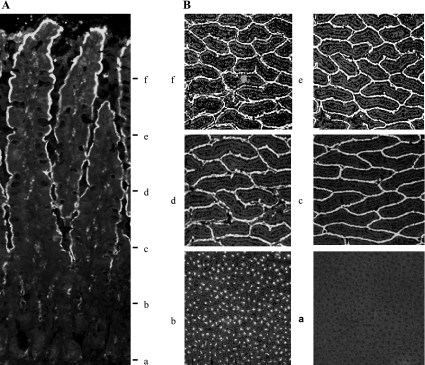
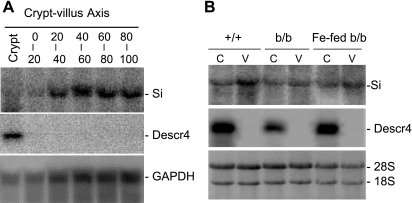
Compared to +/+ rats, the duodenum of the b/b rats had increased girth and thickness and these changes persisted after 3 days of iron repletion (1,158 ± 60, 1,303 ± 84, and 1,268 ± 53 μm for the duodenal thickness of +/+, b/b, and iron-fed b/b rats, n = 4, respectively). On the basis of the location of Si, sequential sections were pooled into levels a, b, c, d, e, and f, representing the crypts and 0–20, 20–40, 40–60, 60–80, and 80–100% levels of villus (Figs. 2 and 3A). In these six groups of sections, i.e., a through f, the Descr4 mRNA was expressed only in a and Si mRNA was expressed in b–f (Fig. 3, A and B). On the basis of the Descr4 and Si localization, the C-pole (groups a and b) and the V-pole (sections e and f) from +/+, b/b, and iron-fed b/b rats were used for microarray analysis.
Fig. 2.
Immunofluorescent staining of sucrase-isomaltase (Si) in the duodenal crypt-villus axis. A: a longitudinal section of the mucosa stained for Si as described in materials and methods showing that Si is absent in the crypts and present in the villus cells. B: the mucosa layer as shown in A was sectioned at right angles to the crypt-villus axis to obtain sections from the villus tips to the crypt bottom as described in materials and methods. The last section representing each level was stained for the presence of Si.
Fig. 3.
Northern blot analysis of Si and Descr4 in sections from duodenal crypt-villus axis. A: RNA was isolated from mucosal sections a–f of +/+ rats and subsequently subjected to electrophoretic separation and Northern blot analysis with Si and Descr4. P32-labeled Si and Descr4 were prepared as described in materials and methods. Figures shown are representative of all 3 rat groups that were sectioned at a similar way. B: RNA was extracted from the pooled mucosal sections a and b, and e and f representing the villus bottoms and crypts (C-pole; C) and villus tops (V-pole; V), respectively and subjected to Northern blot analysis as in A. Data are representative blots of all rats used for the experiment.
Immunofluorescent staining of hypoxic protein adducts.
Since under normal circumstances intestine and rat pericentral regions in liver are hypoxic (1, 19), we hypothesized that the liver and intestine of the anemic b/b rats would be even more hypoxic than those of +/+ rats. To determine the presence of an in vivo hypoxic state, the formation of hypoxic protein adducts in the liver, the organ secretes hepcidin to reduce FPN expression, and the intestine, the organ expresses FPN, after intravenous infusion of Hypoxyprobe 1 was examined. In the liver, +/+ rats showed slight evidence of hypoxia with occasional aggregates of intense fluorescence in the pericentral region (Fig. 4A, arrows). Comparing with b/b rats, more aggregates that are fluorescent were present (Fig. 4B, arrows). The hepatic hypoxic adducts were still present in the iron-fed b/b rats (Fig. 4C). In the duodenum of +/+ rats, weak fluorescence was present (Fig. 4D) comparing with the present of intense fluorescence, particularly in the cytoplasm of the upper villus cells (arrows) in b/b rats (Fig. 4E). With iron feeding, the fluorescent signal decreased but was still more intense than in the +/+ rats with occasional aggregates being present (Fig. 4F, arrowhead).
Fig. 4.
Immunofluorescent staining of Hypoxyprobe-1 adducts in the liver and intestine in +/+, b/b, and iron-fed b/b rats. Rats were injected by the tail vein as described in materials and methods with Hypoxyprobe-1, and the livers (a–c) and the intestines (d–f) were collected 1 h later. The tissues were frozen sectioned and immunofluorescent stained with antibodies against the Hypoxyprobe-1 protein adducts and nuclei stained with diamidine-2-phenylindole as described in materials and methods. Arrows designate the present but weak immunological staining in the liver of +/+ rats (A), with stronger staining in the b/b rats (B) and then subsided in the iron-fed b/b rats (C). Similarly, the duodenum of +/+ rats (D) showed weak fluorescent staining compared with strong staining, particularly in the cytoplasm in the upper villus cells (arrows) and intramucosal macrophages (arrowheads) (E). The positive staining had decreased in the iron-fed b/b rats (F) but was still higher than the +/+ rats. The data were representative of 3 sets of experiments with n = 3 for each group.
Verification of gene expression observed by microarray analysis by RT-PCR and Northern blot.
Of the 31,042 tags represented on the chip, the rat duodenum expressed ∼15,368 genes (accession no. GSE7970). On the basis of the GeneShift software analysis, +/+ rats transcripts for cell cycle and lipid transport-related genes were appropriately expressed in the crypt and villus as reported in the literature (Supplemental Tables S1 and S2). In addition, quantitative RT-PCR of selected genes with primer pairs listed in Supplemental Table S3 confirmed the relative expression of genes in the C- and V-pole as shown by the microarray analyses (Supplemental Table S4 and Supplemental Fig. S1). Furthermore, several genes, namely DMT1, FPN1, Hmox1, and HCP1, that were identified by microarray analyses to be differentially expressed in the C- and V-poles and between the +/+, b/b, and iron-fed b/b were quantitated by Northern blot analysis with the relative amounts of mRNA being in agreement with microarray data (Supplemental Fig. 2 and Supplemental Table S4).
Identification of genes associated with iron absorption in b/b rats.
Since hepatic hepcidin induces intestinal FPN1 internalization and degradation, the livers of +/+, b/b, and iron-fed b/b rats were examined for hepcidin mRNA expression. As expected, the liver expressed significantly lower levels of hepcidin mRNA in b/b compared with +/+ rats with expression increasing in the iron-fed b/b rats (Supplemental Table S6). However, hepatic Hif1α and Epas1 (Hif2α) mRNAs expression were significantly increased in b/b compared with +/+ rats and remained significantly higher in the iron-fed b/b rats (Supplemental Table S6).
In the intestine, the iron status is only one potential regulatory factor affected diverse and extensive changes in gene expression (accession no. GSE7970). We examined a selected set of genes that are known to be associated with iron absorption and iron or metal metabolism and found that these genes were preferentially expressed in either the C- or V-poles of the duodenal mucosa (Table 1). In the set of genes more highly expressed in the C-pole than the V-pole, three major responses were noted to the iron deficiency imposed by the Belgrade mutation: increased expression in the C-pole in both b/b and iron-fed b/b rats (Tfrc, Hba-a1, Hbb, and Lcn2); increased expression in the V-pole only in b/b rats with suppressed expression in both poles in iron-fed rats [metallothionein 1a (Mt1a)] and decreased expression in the C-pole only in b/b rats [v-myc myelocytomatosis viral oncogene 1 (Myc), STEAP family member 3 (Steap3), and Lcn2 receptor (Slc22a17)] (Table 1). Among these genes there were four different responses to iron feeding: a return to normal levels (Myc, Steap3, and Slc22A17), no or little response (Tfrc, Hba and Hbb), suppression (Mt1a), or increased expression (Lcn2).
Table 1.
Predominant C- and V-pole gene expression of related to iron transport
| +/+ |
b/b |
Iron-Fed b/b |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| GenBank | Gene ID | C-pole | V-pole | C-pole | V-pole | C-pole | V-pole | Gene Name | Probe ID |
| Increased expression in the C-pole in both b/b and iron-fed b/b rats | |||||||||
| 1388750_at | Tfrc | 11.60 | 3.26 | 22.66 | 8.46 | 19.61 | 8.90 | transferrin receptor | BF417032 |
| 1370239_at | Hba-a1 | 11.59 | 7.59 | 26.22 | 21.67 | 20.56 | 18.15 | hemoglobin alpha | AI179404 |
| 1367553_x_at | Hbb | 6.95 | 3.42 | 15.6 | 11.38 | 12.33 | 10.11 | hemoglobin beta | NM_033234 |
| 1387011_at | Lcn2 | 0.10 | 0.03 | 0.23 | 0.04 | 0.64 | 0.07 | lipocalin 2 | NM_130741 |
| Increase expression in the V-pole only in b/b rats and suppressed in both poles in iron-fed rats | |||||||||
| 1371237_a_at | Mt1a | 22.95 | 7.54 | 25.49 | 11.51 | 12.13 | 4.32 | metallothionein 1a | AF411318 |
| Decreased expression in the C-pole only in b/b rats | |||||||||
| 1368308_at | Myc | 1.67 | 0.14 | 0.81 | 0.18 | 1.51 | 0.31 | v-myc viral oncogene | NM_012603 |
| 1370374_at | Steap3 | 0.48 | 0.15 | 0.26 | 0.14 | 0.46 | 0.16 | STEAP 3 | AF335281 |
| 1371889_at | Slc22a17 | 0.37 | 0.29 | 0.22 | 0.21 | 0.3 | 0.21 | Lcn2 receptor | AW915448 |
| Increased expression in the C-pole in b/b rats and the increase continued in iron-fed b/b rats | |||||||||
| 1387130_at | Slc40a1 | 4.71 | 32.26 | 14.79 | 41.37 | 9.31 | 37.84 | FPN1 | NM_133315 |
| 1377369_at | Cybrd1 | 1.12 | 28.1 | 14.16 | 49.89 | 5.28 | 44.07 | cytochrome b reductase 1 | BF419070 |
| 1367877_at | Slc11a2 | 6.37 | 25.7 | 29.75 | 42.86 | 19.05 | 39.64 | DMT1 | NM_013173 |
| 1368174_at | Egln3 | 0.77 | 2.96 | 2.18 | 8.95 | 1.25 | 7.41 | PHD3 | NM_019371 |
| 1370080_at | Hmox1 | 0.48 | 0.98 | 1.51 | 4.21 | 0.91 | 3.47 | heme oxygenase 1 | NM_012580 |
| 1369703_at | Epas1 | 0.20 | 0.62 | 0.30 | 0.96 | 0.31 | 1.13 | Epas1 or Hif2a | NM_023090 |
| 1369342_at | Atp7a | 0.08 | 0.33 | 0.3 | 1.69 | 0.15 | 2.11 | ATPase, Cu2+ transport a | NM_052803 |
| 1368149_at | Hif1a | 0.10 | 0.17 | 0.40 | 0.88 | 0.19 | 0.63 | hypoxia-inducible factor 1a | NM_024359 |
| Increased expression in the C-pole in b/b rats and recovered in iron-fed b/b rats | |||||||||
| 1368533_at | Heph | 3.83 | 25.80 | 6.65 | 26.96 | 4.28 | 24.03 | hephaestin | NM_133304 |
| 1373984_at | Slc39a14 | 3.68 | 10.6 | 5.86 | 11.25 | 4.99 | 10.29 | zinc transporter a14 | AA850715 |
| Increased expression in the C-pole of b/b rats but decreased in V-pole of b/b and iron-fed b/b rats | |||||||||
| 1397740_at | Sfxn1 | 2.58 | 6.58 | 3.69 | 4.44 | 2.84 | 3.77 | sideroflexin 1 | AA964229 |
| 1374073_at | Slc46a1 | 0.13 | 4.74 | 0.20 | 1.36 | 0.04 | 0.82 | HCP1/PCFT | BI285135 |
| No change in either C- and V-pole in b/b and iron-fed b/b rats | |||||||||
| 1367565_a_at | Fth1 | 40.85 | 57.46 | 47.09 | 59.32 | 41.02 | 55.73 | ferritin, heavy chain | NM_012848 |
| 1367559_at | Ftl | 11.05 | 15.95 | 11.79 | 19.48 | 11.63 | 16.51 | ferritin, light chain | L01122 |
| 1390874_at | Ireb2 | 1.89 | 11.6 | 2.7 | 10.56 | 2.03 | 9.25 | IRP2 | BE109206 |
Expression of normal (+/+), Belgrade (b/b), and iron-fed b/b rats involved in iron metabolism and transport expressed ≥1.5-fold in the crypt-villus bottom (C-pole) or villus top (V-pole). Data are gene expression ± SE with 4 each for +/+, b/b, and iron-fed b/b rats. Gene expression is categorized only if comparison of +/+ to b/b or to iron-fed b/b had ≥1.5-fold difference in the comparison with P < 0.05.
Similarly, in genes with predominant expression in the V-pole in the +/+ rats several categories of responses were noted: increased expression in the C-pole in b/b rats with the increase continuing in iron-fed b/b rats, increased expression in the C-pole in b/b rats with recovery in iron-fed b/b rats, increased expression in the C-pole of b/b rats, but decreased in the V-pole of b/b and iron-fed b/b rats, and no change in either C- and V-pole in b/b and iron-fed b/b rats (Table 1). Surprisingly, in the first group of genes there was a robust increase of expression at the C-pole, which we label as precocious, since the products of these genes are predominantly active in the villus tops where iron absorption occurs. The genes in this category included Slc40a1, Cybrd1, Slc11a2, Hmox1, Heph, and Slc39a14 (Table 1). Also the response to iron feeding differed between the C-pole and V-pole in this same group of genes with maintenance of expression in the V-pole and decreased expression in the C-pole.
Intriguingly, the transcription factor Epas1 (i.e., Hif2α), as well as Egln3 (i.e., PHD3), expression increased in the b/b rats and remained elevated after iron feeding (Table 1). These increases appeared to be specific for iron deficiency, since the wild-type rats with iron deficiency also increased Epas1 and Egln3, but not Hif1a mRNA expression (Supplemental Table S5). The mitochondrial iron transporter (Sfxn1) (7) and heme/folate transporter HCP1/PCFT (Slc46a1) (23, 26) transcripts were reduced in the V-pole of b/b rats and further decreased in iron-fed b/b rats (Table 1).
Activation of IRPs.
The expression of various iron-related proteins is regulated by IRP activity, and iron deficiency is known to increase IRP activity. To determine whether IRP activity in the b/b rat duodenum was changed as expected, and whether those changes were uniform along the villi, we first measured IRP1 and IRP2 binding activities in the duodenal villi of +/+, b/b, and b/b-fed rats (Fig. 5, A–C). As shown, in all three groups of rats, IRP1 activity increased to be maximal in the midvillus. In the +/+ rats, IRP1 activity was clearly greater than IRP2 activity (Fig. 5A). However, in the b/b and iron-fed b/b rats, IRP2 activity was increased and was particularly active in the villus tops with more activity in the b/b rats than the b/b-fed iron rats (Fig. 5C). Total IRP activity was determined by the addition of β-mercaptoethanol and demonstrated that the total content of IRP1 was about the same, whereas a decrease of IRP2 binding activity in the +/+ villus tops remained (Fig. 5A).
Fig. 5.
Iron regulatory protein (IRP) binding activity in +/+, b/b, and iron-fed b/b rats determined by RNA electromobility shift assay (EMSA). A: EMSA assays to assess IRP binding activity were performed as described in materials and methods from extracts of the mucosal sections a through f from +/+, b/b, and iron-fed b/b rats. β-Mercaptoethanol (β-Me) was added to assay to show that V- and C-pole IRP2 activities remained unchanged by the reducing agent. IRP2 binding activity was identified by the formation of a supershift band after addition of anti-IRP2 antiserum. Shown is a representative EMSA of 3 sets of experiments. B and C: the EMSAs as shown in A were quantitated by scanning as described in materials and methods to determine the relative activities of IRP1 (B) and IRP2 (C). Data are means ± SE of 3 samples per group. B: *P < 0.05 vs. the same column in +/+ and iron-fed b/b rats. C: *P < 0.05 vs. the same column in +/+ rats; **P < 0.05 vs. the same column in b/b rats.
Expression of proteins related to intestinal iron transport.
Because of the potential control by HIF in expression of genes involved in iron transport and metabolism (19, 24), we explored at the protein levels of Hif1α, Epas1 (Hif2α), and the ubiquitously expressed β subunit, namely aryl hydrocarbon nuclear translocator (Arnt) (also known as Hif1 β) (Fig. 6). In addition, the iron-dependent prolyl hydroxylase 3 (Egln3 or PHD3) that hydroxylates Hifα subunits for subsequent ubiquitination by the E3 ubiquitin ligase and degradation via the proteasome pathway was determined (Fig. 6). Arnt protein expression was similar in the C- and V-poles of all three types of rats. The Hif1a was too low to be detectable in both C- and V-poles in all rats with the antibody (not shown), whereas Epas1 was increased in the b/b rats compared with the +/+ rats, with expression in the V-pole being greater than in the C-pole for both types of rats (Fig. 6, A and B). Although in general protein expression paralleled the mRNA expression in the iron-fed rats, Epas1 protein levels in the V-pole were significantly reduced despite the mRNA levels persisting after iron feeding (Fig. 6, A and B compared with Supplemental Table S4). Likewise, Egln3 protein expression paralleled the V-pole > C-pole gradient for Egln3 mRNA in the +/+, b/b, and iron-fed b/b rats, except that the level of protein expression appeared relatively greater in the b/b and iron-fed b/b rats than in the +/+ rats (Fig. 6A). Similar to mRNA, Egln3 protein increased from undetectable in the C-pole to low levels in the V-pole in +/+ rats. In both C- and V- poles, Egln3 was increased to significantly higher levels in b/b and iron-fed b/b rats (Fig. 6, A and B, compared with Supplemental Table S4).
Fig. 6.
Western blot analyses of duodenal protein expression of Arnt, Epas1 (Hif2α), and Egln3 (PHD3). Western blot analysis of Arnt, Epas1 (Hif2α), and Egln3 (PHD3) in extracts of the C-poles and V-poles of +/+, b/b, and iron-fed b/b rats was conducted as described in materials and methods. A: representative of 3 sets of experiments. Samples of the Arnt blot were run on the same gel but presented in a noncontiguous order. B: quantitation of A. *P < 0.05 vs. the same column of +/+ rats.
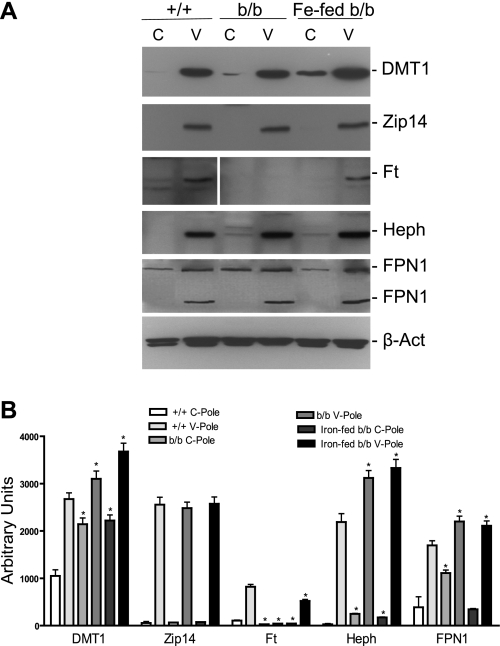
Interestingly, with the increase of the transcription factor Epas1, there was an associated approximately twofold increase of DMT1 in both the C- and V-poles of the b/b and iron-fed b/b rats, and striking gradients in protein expression observed for ZIP14, Heph, and FPN1 (Fig. 7, A and B). Although presumably the changes in FPN1 expression result from changes affected by reduced hepcidin levels (Supplemental Table S6), it is not known whether Heph and ZIP14 protein expression are controlled by HIF activity or other factors. As confirmation of the iron status of the three groups of rats, Ft protein expression in the +/+ rats was greater in the V-pole where iron is absorbed, was nondetectable in the b/b rats, and increased once again in the V-pole of the iron-fed b/b rats (Fig. 7, A and B).
Fig. 7.
Western blot analyses of duodenal protein expression of divalent metal transporter (DMT1), zinc transporter 14 (Zip14), ferritin (Ft), Heph, and FPN1. A: Western blot analysis of DMT1, Zip14, Ft, Heph, and FPN1 in extracts of the C-poles and V-pole of +/+, b/b, and iron-fed b/b rats was conducted as described in materials and methods. Samples in the Ft blot were run on the same gel but presented in a noncontiguous order. A representative of 3 sets of experiments is shown. B: quantitation of DMT1, Zip14, Ft, Heph, and FPN1 as shown in A. *P < 0.05 vs. the same column of +/+ rats, n = 3.
DISCUSSION
The studies presented utilized the Belgrade rat as a model of iron deficiency to examine the changes in expression of gene(s) involved in iron transport and iron metabolism in response to decreased iron absorption. The Belgrade rat has a G184R mutation in DMT1 that decreases intestinal iron absorption, and hence it was of interest to determine by gene array analysis whether there were alterations in gene expression that appeared responsible for compensatory iron absorption. We have previously shown that in the Belgrade rat iron feeding can elicit an increase in intestinal ferritin, a decrease in DMT1, and a decrease in IRP activity (36). Clearly, iron feeding was able to rapidly reverse the hematological indications of iron deficiency with partial correction of the anemia and correction of the erythrocyte MCV. These results suggest either that the mutation does not completely block iron transport or that compensatory uptake pathways are present.
In the b/b rats, most of the genes whose protein products are responsible for intestinal iron transport were upregulated in the C- and V-poles, and after iron feeding for 3 days they continued to be upregulated. Two genes, Heph and ZIP14 (Slc39a14), had a different pattern of expression with an increase in mRNA in the C-pole and no change seen in the V-pole where iron absorption takes place (Table 2). In addition, Western blot analyses showed that the ZIP14 protein did not increase in b/b rats, suggesting that it is unlikely a candidate responsible for compensatory iron absorption. Heph protein expression increased in the V-pole and was accompanied by an increase of FPN1, suggesting that the two proteins, which are known to interact for transport of iron across the basolateral membrane (35), could play a role in increased iron absorption. The mutated DMT1 G184R, as well as Cybrd1, expression were significantly increased in the b/b and iron-fed b/b rats. Since the mutated DMT1 has the ability to respond to iron feeding by internalization (36), the DMT1 in the Belgrade rat might not have a complete block to transport of luminal iron into the enterocyte. This hypothesis is consistent with the results of the backcross of the G185R mutation (Slc11a2mk/mk) onto Slc11a2−/− mice (generated by Slc11a2flox/flox mice with mice carrying a villin-cre transgene), which produced healthier progeny with increased liver iron stores than the DMT1 knockout mice (12). The possibility that the heme transporter HCP1 (Slc46a1) could play a compensatory role in the present study seems unlikely, since HCP1 mRNA was reduced in b/b V-pole and decreased further in the iron-fed b/b rat V-pole. In addition, the rats were fed a purified rodent diet (AIN-76A, Dyet no. 100000) containing no heme. However, we did observe that the mRNA for Lcn2, a putative iron transporter in other tissues, was increased in the C-pole of the b/b rats and increased even more in the iron-fed b/b rats. However, neither the expression nor functionality of Lcn2 protein in the duodenum has been explored. In addition, the Lcn2 receptor Slc22a17 (24p3) (6, 10) was barely detectable in the C-pole and was without significant differences between the +/+, b/b, and iron-fed b/b rats. Hence, it is not clear that Lcn2 could be a compensatory mechanism for iron uptake.
The data presented shows that in the duodenum, the Epas1 transcription factor was increased significantly in b/b and iron-fed b/b compared with +/+ rats, suggesting that the b/b intestine is under hypoxic stress. This hypothesis was verified by demonstrating increased amounts of hypoxic protein adducts generated by b/b with a reduction of adducts in the iron-fed rats (Fig. 4). The increase of Epas1 in the b/b and in iron-fed b/b rats would result in activation of various intestinal iron transport genes such as Tfrc and DMT1 that contain hypoxia response elements (19, 25, 27). Furthermore, the Epas1 mRNA itself contains a functional IRE at the 5′ end of the untranslated region (24), the binding of IRPs to the IRE may stabilize Epas1 mRNA for the increase of Epas1 translation and subsequently increase the translation of DMT1 and Tfrc. Further study will be needed to verify the posttranscriptional control of Epas1 mRNA.
The IRP binding activity that we demonstrated was clearly functional, since both H- and L-Ft proteins were not detectable in the b/b rats when the activities were high. Interestingly, ferritin did increase in the villus tops of iron-fed b/b rats, indicating that luminal iron uptake occurs with the lack of response in the C-pole, confirming that iron absorption occurs in the villus tops. Thus, in the b/b rats, increased hypoxia from the anemia could lead to an increase in HIF2α for transcription and IRP activation for posttranscriptional stabilization of DMT1, which in turn would drive intestinal iron uptake.
The hypoxic changes seen in the duodenum were also seen in the livers of the b/b and iron-fed b/b rats with more adducts detected after intravenous administration of pimonidazole HCl than seen with +/+ rats. Concomitantly, there were increases of Hif1α and Epas1 mRNAs and lower levels of hepcidin mRNAs in the b/b and iron-fed b/b than in the +/+ rats (Supplemental Table S6). The effect of reduced hepcidin expression is consistent with the increase of intestinal FPN1 protein expression observed in the present study: Hepcidin binds to FPN1, inducing FPN1 internalization, phosphorylation, and degradation (5, 22), whereas reduced hepcidin causes the accumulation of intestinal FPN1.
The hypoxic response is mediated by the heterodimeric transcriptional factor HIF, which consists of an α-subunit Hif1α, Hif2α, or Hif3α and the-β subunit Hif1β (Arnt) subunit. The Arnt subunit expression was constant under various iron conditions; however, the HIFα protein levels are controlled by prolyl hydroxylate protein domain (PHDs or Eglns) (18), which lead to HIFα polyubiquitination by the von Hippel-Lindau protein (pVHL) and subsequent degradation. In the present study, Egln3 transcripts and proteins increased in parallel with Hif2α, indicating that Hif2α is tightly regulated without interruption by DMT1 G185R mutation. Thus the increases of nuclear factor Epas1, with subsequent activation of intestinal iron-absorptive genes such as DMT1, Cybrd1, FPN1, and Heph, appear to be the pathway to support iron absorption of b/b rats. This pathway to increase DMT1 and Cybrd1 has recently been reported (25), but to increase FPN1 and Heph expression remains to be determined.
The elevations intestinal Hga-1a and HGB mRNA expression are noted in the present study. The intestinal Hga-1a and HGB were not derived from red blood cell contamination, because glycophorin, a specific marker for reticulocytes, expression was minimal in both of the C- and V-poles. In human idiopathic pulmonary fibrosis and in rat retinal ganglion cells, the hemoglobins were generated to facilitate cellular oxygenation (17, 29). Whether the intestinal Hga-1a and HGB mRNAs translate to protein and function as oxygen carrier remains to be determined.
GRANTS
This study is supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Research Grants DK065101 (K. Y. Yeh) and DK43785 (J. Glass) and by the Feist-Weiller Cancer Center, Louisiana University Health Sciences Center, Shreveport, LA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Arteel GE, Thurman RG, Raleigh JA. Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur J Biochem 253: 743–750, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Beard JL, Hendricks MK, Perez EM, Murray-Kolb LE, Berg A, Vernon-Feagans L, Irlam J, Isaacs W, Sive A, Tomlinson M. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr 135: 267–272, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Blanco E, Kannengiesser C, Grandchamp B, Tasso M, Beaumont C. Not all DMT1 mutations lead to iron overload. Blood Cells Mol Dis 43: 199–201, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Collins JF, Franck CA, Kowdley KV, Ghishan FK. Identification of differentially expressed genes in response to dietary iron deprivation in rat duodenum. Am J Physiol Gastrointest Liver Physiol 288: G964–G971, 2005 [DOI] [PubMed] [Google Scholar]
- 5. De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell 18: 2569–2578, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 123: 1293–1305, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Fleming MD, Campagna DR, Haslett JN, Trenor CC, 3rd, Andrews NC. A mutation in a mitochondrial transmembrane protein is responsible for the pleiotropic hematological and skeletal phenotype of flexed-tail (f/f) mice. Genes Dev 15: 652–657, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA 95: 1148–1153, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fleming MD, Trenor CC, 3rd, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet 16: 383–386, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432: 917–921, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC, Hediger MA. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett 509: 309–316, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Gunshin H, Fujiwara Y, Custodio AO, Direnzo C, Robine S, Andrews NC. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest 115: 1258–1266, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanson ES, Foot LM, Leibold EA. Hypoxia post-translationally activates iron-regulatory protein 2. J Biol Chem 274: 5047–5052, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Hanson ES, Rawlins ML, Leibold EA. Oxygen and iron regulation of iron regulatory protein 2. J Biol Chem 278: 40337–40342, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell 117: 285–297, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of mammalian iron metabolism. Cell 142: 24–38, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Ishikawa N, Ohlmeier S, Salmenkivi K, Myllarniemi M, Rahman I, Mazur W, Kinnula VL. Hemoglobin alpha and beta are ubiquitous in the human lung, decline in idiopathic pulmonary fibrosis but not in COPD. Respir Res 11: 123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem 74: 115–128, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest 119: 1159–1166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, Mudaly M, Richardson C, Barlow D, Bomford A, Peters TJ, Raja KB, Shirali S, Hediger MA, Farzaneh F, Simpson RJ. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 291: 1755–1759, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr 28: 197–213, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127: 917–928, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Sanchez M, Galy B, Muckenthaler MU, Hentze MW. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nat Struct Mol Biol 14: 420–426, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab 9: 152–164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Identification of an intestinal heme transporter. Cell 122: 789–801, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Tacchini L, Bianchi L, Bernelli-Zazzera A, Cairo G. Transferrin receptor induction by hypoxia. HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J Biol Chem 274: 24142–24146, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med 85: 1295–1300, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Tezel G, Yang X, Luo C, Cai J, Kain AD, Powell DW, Kuehn MH, Pierce WM. Hemoglobin expression and regulation in glaucoma: insights into retinal ganglion cell oxygenation. Invest Ophthalmol Vis Sci 51: 907–919, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yeh KY, Alvarez-Hernandez X, Glass J, Yeh M. Rat intestinal and hepatic ferritin subunit expression during development and after dietary iron feeding. Am J Physiol Gastrointest Liver Physiol 270: G498–G505, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Yeh KY, Yeh M, Glass J. Glucocorticoids and dietary iron regulate postnatal intestinal heavy and light ferritin expression in rats. Am J Physiol Gastrointest Liver Physiol 278: G217–G226, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Yeh KY, Yeh M, Glass J. Hepcidin regulation of ferroportin 1 expression in the liver and intestine of the rat. Am J Physiol Gastrointest Liver Physiol 286: G385–G394, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Yeh KY, Yeh M, Glass J, Granger DN. Rapid activation of NF-kappaB and AP-1 and target gene expression in postischemic rat intestine. Gastroenterology 118: 525–534, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Yeh KY, Yeh M, Holt PR. Differential effects of thyroxine and cortisone on jejunal sucrase expression in suckling rats. Am J Physiol Gastrointest Liver Physiol 256: G604–G612, 1989 [DOI] [PubMed] [Google Scholar]
- 35. Yeh KY, Yeh M, Mims L, Glass J. Iron feeding induces ferroportin 1 and hephaestin migration and interaction in rat duodenal epithelium. Am J Physiol Gastrointest Liver Physiol 296: G55–G65, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yeh KY, Yeh M, Watkins JA, Rodriguez-Paris J, Glass J. Dietary iron induces rapid changes in rat intestinal divalent metal transporter expression. Am J Physiol Gastrointest Liver Physiol 279: G1070–G1079, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.