Abstract
Dietary fiber intake links to decreased risk of colorectal cancers. The underlying mechanisms remain unclear. Recently, we found that butyrate, a short-chain fatty acid produced in gut by bacterial fermentation of dietary fiber, enhances TGF-β signaling in rat intestinal epithelial cells (RIE-1). Furthermore, TGF-β represses inhibitors of differentiation (Ids), leading to apoptosis. We hypothesized that dietary fiber enhances TGF-β's growth inhibitory effects on gut epithelium via inhibition of Id2. In this study, Balb/c and DBA/2N mice were fed with a regular rodent chow or supplemented with a dietary fiber (20% pectin) and Smad3 level in gut epithelium was measured. In vitro, RIE-1 cells were treated with butyrate and TGF-β1, and cell functions were evaluated. Furthermore, the role of Ids in butyrate- and TGF-β-induced growth inhibition was investigated. We found that pectin feeding increased Smad3 protein levels in the jejunum (1.47 ± 0.26-fold, P = 0.045, in Balb/c mice; 1.49 ± 0.19-fold, P = 0.016, in DBA/2N mice), and phospho-Smad3 levels (1.92 ± 0.27-fold, P = 0.009, in Balb/c mice; 1.83 ± 0.28-fold, P = 0.022, in DBA/2N mice). Butyrate or TGF-β alone inhibited cell growth and induced cell cycle arrest. The combined treatment of butyrate and TGF-β synergistically induced cell cycle arrest and apoptosis in RIE-1 cells and repressed Id2 and Id3 levels. Furthermore, knockdown of Id2 gene expression by use of small interfering RNA caused cell cycle arrest and apoptosis. We conclude that dietary fiber pectin enhanced Smad3 expression and activation in the gut. Butyrate and TGF-β induced cell cycle arrest and apoptosis, which may be mediated by repression of Id2. Our results implicate a novel mechanism of dietary fiber in reducing the risk of colorectal cancer development.
Keywords: butyrate, cell cycle arrest, apoptosis, inhibitor of differentiation
in the colonic crypt, tissue homeostasis is maintained by a balance between an increase in cell number due to mitosis at the base of the crypt and a decrease in cell number by apoptosis as cells migrate toward the luminal surface (5). The regulation of colonic cell number is influenced by endogenous growth factors. For instance, transforming growth factor (TGF)-β is expressed in the gut epithelium and serves as an important negative regulator of enterocyte and colonocyte proliferation (2, 22). TGF-β signals via its binding to a cell surface receptor complex, which subsequently phosphorylates the intracellular mediators Smad2 and Smad3. Phosphorylated Smad2 or Smad3 form a heteromeric complex with Smad4. This complex translocates into the nucleus and regulates the transcription of target genes (19, 50). One family of target genes regulated by TGF-β includes the inhibitors of differentiation (Ids). Ids are a family of helix-loop-helix (HLH) proteins, which lack the basic DNA-binding domain that is the characteristic of other basic HLH (bHLH) superfamily members. Ids function as negative regulators of bHLH transcription factors by binding and sequestering them, thereby blocking the binding of bHLH proteins to DNA (32). Four mammalian Ids (Id1, Id2, Id3, and Id4) have been identified and found expressed in undifferentiated and proliferating cells (17). By binding to bHLH proteins, Id proteins regulate a variety of cellular processes including cellular growth, senescence, differentiation, apoptosis, angiogenesis, and neoplastic transformation. TGF-β has been shown to inhibit Id1, Id2, and Id3 expression in several cell lines (21, 25), and we have shown that TGF-β repressed Id2 through activation of Smad3 leading to apoptosis induction (8).
TGF-β serves as an important tumor suppressor by inhibiting cellular proliferation and inducing apoptosis. However, during colon carcinogenesis, most colon cancer cells become resistant to the tumor suppressor activities of TGF-β owing to the alteration of various components of the TGF-β signaling pathway. For example, TGF-β type I receptor (44), type II receptor (29), Smad2 (16, 35), and Smad4 (26, 40, 42) have been shown to be either mutated or downregulated in human colorectal cancers.
In addition to endogenous growth factors like TGF-β, dietary factors have been implicated as important players involved in the growth control of colonocytes. Epidemiological studies suggest that a diet low in fat and high in fiber could be protective against colorectal cancer (4, 20). Other studies have demonstrated that diets high in fat and low in fiber, typical of those found in industrialized countries, increase the risk of developing colorectal cancers (36). Dietary fiber is fermented by bacteria in the colonic lumen to generate short-chain fatty acids. Among the many bacterial metabolites, butyrate represents the most biologically relevant metabolite in the colon (10) and is a preferred substrate for colonocytes (30, 41, 43). Short-chain fatty acids, including butyrate, link to decreased incidence of colorectal cancer and inflammatory bowel disease (27, 37, 46). As a naturally potent inhibitor of histone deacetylases (HDACs) (24), butyrate induces expression of specific genes that elicit extensive cellular morphological and metabolic changes (1). Additionally, butyrate exerts various biological effects on cultured mammalian cells, such as inhibition of cell proliferation and induction of differentiation and apoptosis (9, 12–13). Furthermore, HDAC inhibitors have been investigated in clinical trials as potential treatments for human malignancies (15). Therefore, butyrate has been proposed as a dietary tumor suppressor in the gut (15, 28).
Although butyrate and TGF-β both exert growth control over the gut epithelium, it is not known whether butyrate and TGF-β function are interrelated. We began to test the hypothesis that dietary fiber enhances the tumor suppressor properties of TGF-β in the gut in this study.
MATERIALS AND METHODS
Animal feeding and tissue preparation.
Four-week-old male Balb/c and DBA/2N mice, weighing 18–20 g, were purchased from Harlan (Indianapolis, IN). The mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility. All of the following studies were approved by the Institutional Animal Care and Use Committee of The University of Texas Medical Branch. At 5 wk of age, groups of Balb/c and DBA/2N mice were transferred to wire-bottom cages to minimize coprophagy and consumption of bedding (49). The mice were assigned to one of two diets: a control diet (Prolab RMH2500) and an experimental diet (Prolab RMH2500 supplemented with 20% pectin prepared by Bio-Serv, Frenchtown, NJ). The body weight was recorded once a week. After 4 wk of feeding, the mice were euthanized and the jejunum and the colon were harvested. The mucosal tissues were collected by opening the gut lumen and scraping the mucosal surface with a glass slide. The tissue was then used for the preparation of protein lysates.
Immunoprecipitation and Western blotting.
Mouse mucosal tissue and rat intestinal epithelial cells (RIE-1) cells were lysed using 1× cell lysis buffer (Cell Signaling Technology). Protein concentrations of the lysates were quantified by using a protein assay dye (Bio-Rad Laboratories). Immunoprecipitation and Western blotting were performed as previously described (6, 8, 31). Phospho-Smad3 (pSmad3) was detected by immunoprecipitation with anti-Smad3 antibody (Invitrogen) followed by Western blotting using anti-pSmad3 antibody provided by Dr. Edward B. Leof (Mayo Clinic, Rochester, MN). Smad3 and Ids were detected by Western blotting. Anti-Id antibodies were purchased from Santa Cruz Biotechnology. Anti-β-actin and anti-GAPDH antibodies were purchased from Sigma. Horseradish peroxidase-conjugated goat anti-rabbit antibody was purchased from Bio-Rad Laboratories.
Reagents.
Sodium butyrate (Sigma) was dissolved in PBS. TGF-β1 (R&D Systems) was diluted in a vehicle solution (0.1% BSA, 4 mM HCl). Reagents were stored at −20°C in small aliquots.
Cell culture.
The RIE-1 cell line (a gift from Dr. Kenneth D. Brown, Cambridge Research Station, Babraham, Cambridge, UK) has morphological and biological characteristics of intestinal crypt cells (3, 34). This cell line does not have transformed phenotypes in culture, contains intact TGF-β signaling pathway, and responds to TGF-β-induced growth inhibition and apoptosis (6, 8, 23). It has been used by many laboratories including ours as a model of normal gut epithelial cells. RIE-1 cells were maintained as monolayer cultures in DMEM (Mediatech) supplemented with 5% dialyzed fetal bovine serum (dFBS, Invitrogen), and grown at 37°C in a humidified incubator at 5% CO2. The cells were grown to subconfluence and split every 3–4 days.
Cell growth.
Cells were seeded on 12-well plates in 5% dFBS medium for 24 h. The next day, cells were treated with sodium butyrate or TGF-β1 for up to 7 days. Cells were then trypsinized and counted daily via a model Zf Coulter counter (Coulter Electronics).
Cell cycle analysis.
Cells were treated with sodium butyrate or TGF-β1 for 24 h and then pulsed with 5-bromo-2′-deoxyuridine (BrdU) for the last 45 min and harvested for flow cytometric analysis by using a BrdU flow kit (BD Biosciences) according to the manufacturer's instruction as previously described (7).
Apoptosis assays.
DNA fragmentation was quantified by cell death detection ELISA assay (Roche Molecular Biochemicals) according to the manufacturer's instructions as previously described (6, 8). Apoptotic cells were stained with annexin V-FITC and propidium iodide by using a BD ApoAlert Annexin V-FITC Apoptosis kit (BD Bioscience). Cells were analyzed by flow cytometry as previously described. The cells staining positively with annexin V only were identified as apoptotic cells (7).
Real-time quantitative RT-PCR.
Real-time quantitative PCR was performed as previously described (6). Briefly, total RNA was extracted from cells using a RNAqueous Kit (Ambion, TX) and used for detection of specific mRNA expression by real-time quantitative RT-PCR. First, cDNA was synthesized by using a Retroscript cDNA synthesis kit (Ambion). Next, PCR was performed with a Prism 7700 Sequence Detector (Perkin Elmer/Applied Biosystems Division) by use of the TaqMan PCR core reagent kit and the appropriate forward and reverse primers and probes as described previously (Applied Biosystems).
Id gene silencing.
Rat Id1 small interfering RNA (siRNA) (l-080165-01), Id2 siRNA (l-080063-00), Id3 siRNA (l-080095-01, Dharmacon), and the transfection reagent DharmaFECT 3 (Dharmacon) were used according to the manufacturer's instructions as previously described (6, 8). Scrambled siRNA (d-001206-13, Dharmacon) was used as a nonspecific siRNA control.
Statistical analysis.
Data were expressed as means ± SE. P < 0.05 was considered significant. Differences among groups of in vivo studies were analyzed by Student's t-test. Differences among groups of in vitro studies were analyzed by ANOVA with Tukey-Kramer multiple comparisons test; the in vitro experiments were repeated at least twice, and similar results were obtained.
RESULTS
Pectin feeding increases Smad3 expression and activation in mouse intestine.
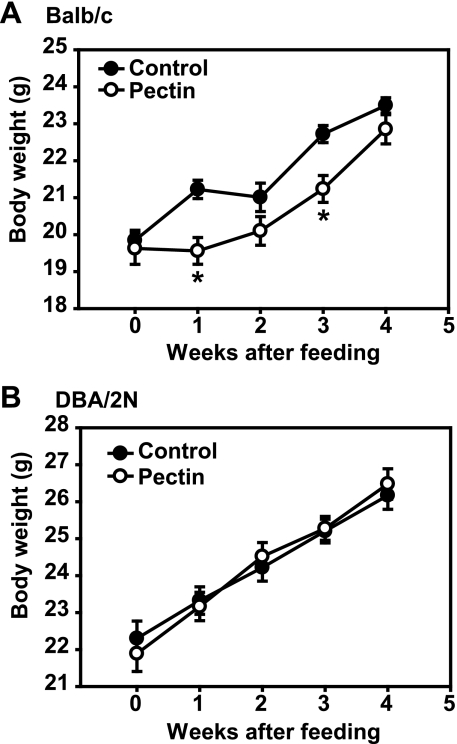
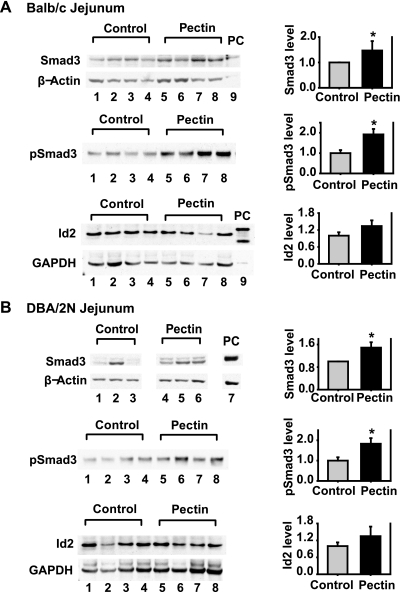
To assess the effects of dietary fiber pectin on the TGF-β signaling pathway in the gut, the mice were fed with an experimental diet (supplemented with 20% pectin) or a control diet consisting of normal rodent chaw. Groups of Balb/c mice fed with dietary fiber pectin tolerated the diet well. Their weight gain was less than the mice fed with control diet at weeks 1 and 3, and was similar to the mice fed with control diet at weeks 2 and 4 (Fig. 1A). Groups of DBA/2N mice fed with the experimental diet tolerated the diet well; their weight gain was similar compared with the mice fed with the control diet (Fig. 1B). The mucosal tissue samples from the mouse jejunum and colon were harvested at the week 4 after feeding and analyzed for the protein levels and phosphorylation/activation of Smad3, a key intracellular mediator of TGF-β signaling pathway. Animals fed with the 20% pectin diet had significant increases in Smad3 protein levels in the jejunum tissue in both Balb/c mice (1.47 ± 0.26-fold, n = 16, P = 0.045) and DBA/2N mice (1.49 ± 0.19-fold, n = 14, P = 0.016, Fig. 2) compared with that of the control mice. On the other hand, Smad3 protein levels in the colon tissue of the experimental mice revealed no significant changes compared with that of the control mice (data not shown). Furthermore, the pectin feeding mice had significant increases in pSmad3 in the jejunum tissue in both Balb/c mice (1.92 ± 0.27-fold, n = 8, P = 0.009) and DBA/2N mice (1.83 ± 0.28-fold, n = 8, P = 0.022) compared with that of the control mice. However, the pectin feeding mice had no significant changes in protein levels of Id2, a known target of TGF-β/Smad3 signaling, in the jejunum tissue in both Balb/c mice and DBA/2N mice. Together, our data demonstrate that dietary fiber pectin feeding increased Smad3 protein expression and activation in the mouse intestine.
Fig. 1.
Effects of fiber feeding on body weight. All mice were weighed prior to the study and then weekly thereafter. The control mice were assigned to a control diet (Prolab RMH2500) and the experimental mice were assigned to an experimental diet (Prolab RMH2500 supplemented with 20% pectin prepared by Bio-Serv, Frenchtown, NJ). The body weight was recorded and plotted as means ± SE. A: Balb/c mice (n = 16). B: DBA/2N mice (n = 16). *P < 0.05 compared with the control mice.
Fig. 2.
Effects of fiber feeding on Smad3 protein expression and phosphorylation and inhibitor of differentiation (Id)2 protein expression in the mouse intestine. At week 4 after the feeding, the Balb/c and DBA/2N mice were euthanized and the jejuna were harvested. Mucosal tissue was collected and protein lysates were prepared. Smad3 and Id2 protein levels were detected by Western blotting using specific antibodies. β-Actin or GAPDH served as a protein loading control. Phospho-Smad3 (pSmad3) was detected by immunoprecipitation using an anti-Smad3 antibody followed by Western blotting using an anti-pSmad3 antibody. The same amount of protein (250 μg) for each sample was applied to IP. The protein levels were quantified and expressed as means ± SE of Smad3/β-actin (n = 16/group), pSmad3 level (n = 8/group), and Id2/GAPDH (n = 8/group) normalized to control. The representative images of Western blotting and quantifications of Smad3, pSmad3, and Id2 are shown. A: Balb/c jejunum. B: DBA/2N jejunum. *P < 0.05 compared with the control mice. PC1, positive control of the endogenous Smad3 protein prepared from rat intestinal epithelial (RIE-1) cells; PC2, positive control of Id2 protein prepared from the Cos-1 cells transiently transfected with flag-Id2.
Butyrate enhances the growth inhibitory effect of TGF-β.
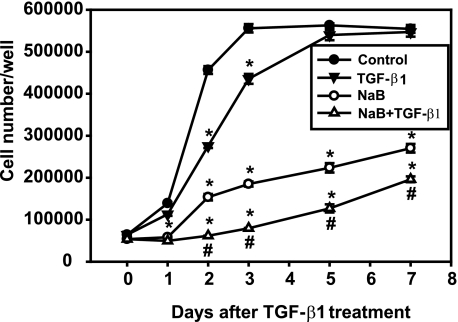
To investigate the mechanisms of action of dietary fiber on gut epithelial cells, we chose butyrate, a biologically relevant short-chain fatty acid among the bacterial metabolites generated from dietary fiber in the gut. We also assessed the effects of butyrate when combined with a known tumor suppressor, TGF-β, on RIE-1 cell growth in vitro. Results from time course experiments showed that TGF-β alone inhibited cell growth on days 1, 2, and 3 by 18.6, 39.6, and 21.7%, respectively, compared with vehicle control. Butyrate alone inhibited cell growth at days 1, 2, 3, 5, and 7 by 58.2, 66.3, 66.7, 60.3, and 51.3%, respectively. The combination of butyrate pretreatment followed by TGF-β treatment induced greater growth inhibition than either alone at days 1, 2, 3, 5, and 7 by 64.4, 86.5, 85.7, 77.4, and 64.6%, respectively (Fig. 3). Similar effects were observed when the cells were cotreated with butyrate and TGF-β (data not shown).
Fig. 3.
Butyrate enhances the growth inhibitory effects of TGF-β. RIE-1 cells were treated with vehicle control, sodium butyrate (NaB, 5 mM), or TGF-β1 (40 pM) or pretreated with NaB (5 mM) followed by TGF-β1 treatment (40 pM). Cells were counted daily via a hemocytometer and expressed as means ± SE. *P < 0.05 compared with the control. #P < 0.05 compared with NaB or TGF-β1 alone.
Butyrate and TGF-β synergistically induce cell cycle arrest and apoptosis.
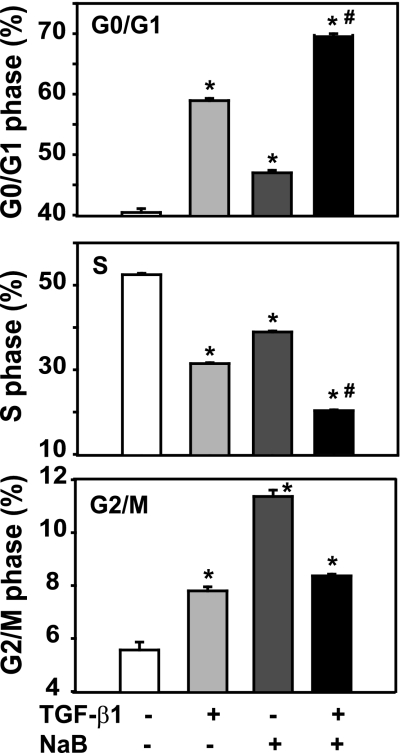
The inhibition of cell growth reflected by a decrease in cell number may result from several biological mechanisms, including cell cycle arrest, apoptosis induction, and/or differentiation. To further study the mechanisms by which butyrate and TGF-β induce growth inhibition in the gut epithelial cells, the RIE-1 cells were pretreated with butyrate followed by TGF-β treatment, and cell cycle progression was analyzed by examining DNA content via flow cytometry. As shown in Fig. 4, TGF-β treatment increased the fraction of cells in G0/G1 phase by 18.5% compared with control and decreased the fraction of cells in S phase by 21.0%. Butyrate treatment slightly increased the fraction of cells in G0/G1 phase by 6.6% and in G2/M phase by 5.8%, with a concomitant decrease in S phase by 13.6%. The combined treatment of TGF-β and butyrate synergistically increased the cells in G0/G1 phase by 29.3% with a concomitant decrease in S phase by 32.2%. However, the combined treatment of TGF-β and butyrate slightly increased the number of cells in G2/M phase by 2.8%. Taken together, our results demonstrate that 1) TGF-β induced cell cycle arrest at G0/G1; 2) butyrate induced cell cycle arrest mainly at G2/M; and 3) butyrate further enhanced the effect of TGF-β-induced G0/G1 cell cycle arrest.
Fig. 4.
Butyrate enhances TGF-β-induced cell cycle arrest. The RIE-1 cells were treated with vehicle control, NaB (5 mM), or TGF-β1 (40 pM) or pretreated with NaB (5 mM) followed by TGF-β1 treatment (40 pM) for 24 h. The cells were pulsed with 5-bromo-2′-deoxyuridine (BrdU) during the last 45 min and harvested for flow cytometric analysis; 10,000 cells were analyzed from each well (n = 3 wells) and expressed as percentage of cells in different phases. *P < 0.05 compared with control. #P < 0.05 compared with NaB or TGF-β1 alone.
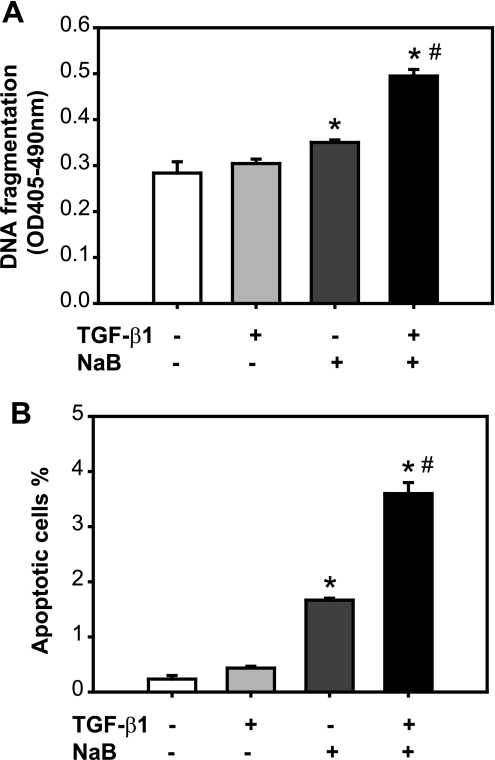
To examine the effect of butyrate and TGF-β on apoptosis induction in RIE-1 cells, the RIE-1 cells were pretreated with butyrate followed by TGF-β treatment. The experiment was carried out using cells maintained in normal growth medium supplemented with 5% serum. Apoptosis was evaluated by DNA fragmentation (Fig. 5A) and annexin V staining (Fig. 5B). TGF-β alone did not induce apoptosis in RIE-1 cells, which is consistent with our previous finding that RIE-1 cells are resistant to TGF-β's effect on apoptosis induction in 5% serum, because of a relatively low ratio of Smad3/Akt (11). Butyrate alone induced apoptosis, consistent with the previous study (18). The combined treatment of butyrate and TGF-β induced apoptosis greater than butyrate treatment alone. This finding suggests that pretreatment with butyrate may sensitize RIE-1 cells to TGF-β's effect on apoptosis induction.
Fig. 5.
Butyrate and TGF-β synergistically induce apoptosis. RIE-1 cells were treated with vehicle control, NaB (5 mM), or TGF-β1 (40 pM) or pretreated with NaB (5 mM) followed by TGF-β1 treatment (40 pM) for 24 h. A: apoptosis was quantified via a cell death detection ELISA assay and expressed as means ± SE. B: apoptotic cells were stained with annexin V-FITC and propidium iodide. Cells were analyzed by flow cytometry. Cells staining with annexin V only were identified as apoptotic cells and expressed as means ± SE. *P < 0.05 compared with the control group. #P < 0.05 compared with TGF-β1 or NaB alone.
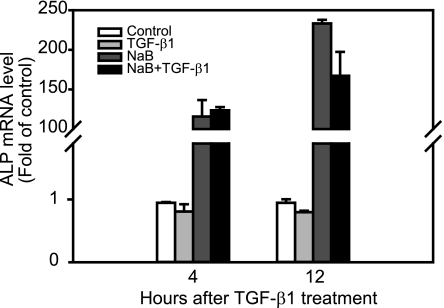
Butyrate is known to induce the differentiation of the gut epithelial cells (9, 38). Here, we determined whether differentiation accounts for one of the mechanisms by which butyrate enhances TGF-β-induced growth inhibitory effects. The RIE-1 cells were treated with butyrate alone or TGF-β alone, or pretreated with butyrate for 12 h, then treated with TGF-β for the indicated time points. The cells were harvested at 4 and 12 h after TGF-β treatment. Alkaline phosphatase (ALP) mRNA expression, a marker of the gut epithelial cell differentiation, was evaluated by real-time quantitative PCR. As shown in Fig. 6, butyrate induced remarkable ALP expression, whereas TGF-β had no effect on ALP expression. The combined treatment of butyrate and TGF-β had similar effects on ALP expression as the butyrate treatment alone. Taken together, our data suggest that butyrate enhances TGF-β growth inhibitory effects through cell cycle arrest and apoptosis induction in RIE-1 cells.
Fig. 6.
Butyrate induces alkaline phosphatase (ALP) expression. RIE-1 cells were treated with vehicle control, NaB (5 mM), or TGF-β1 (40 pM) or pretreated with NaB (5 mM) followed by TGF-β1 treatment (40 pM). Cells were then harvested at 4 and 12 h after TGF-β1 treatment. Total RNA was extracted and converted to cDNA. Real-time PCR was applied to measure ALP gene expression and normalized to 18S mRNA level. ALP mRNA levels were represented as fold of control.
TGF-β and butyrate cooperatively repress Id2 and Id3.
TGF-β has been reported to inhibit Id1, Id2, and Id3 expression in several cell lines and in our previous study (8). Butyrate and TGF-β share similar biological activities and may work through similar signaling pathways. Therefore, we sought to determine whether butyrate regulates Id expression. The RIE-1 cells were treated with TGF-β alone or butyrate alone. As expected, at both mRNA and protein levels, TGF-β repressed Id1, Id2, and Id3 expression, which is consistent with our previous findings (8). Butyrate also repressed Id1, Id2, and Id3 expression except that butyrate did not repress Id1 mRNA expression. The pretreatment of butyrate enhanced TGF-β's repression of Id2 and Id3 expression (Fig. 7).
Fig. 7.
Butyrate enhances TGF-β repression of Id2 and Id3 expression. The RIE-1 cells were treated the same way as in Fig. 6. A: total RNA was extracted and converted to cDNA. Real-time PCR was applied to measure Id1, Id2, and Id3 gene expression and normalized to 18S mRNA level. Id1, Id2, and Id3 mRNA levels were represented as fold of control. B: protein lysates were prepared for Western blotting analysis. Id proteins were detected by using the specific antibodies. β-Actin was a protein loading control. The relative ratio of Id to β-actin was normalized to control and represented as fold of control.
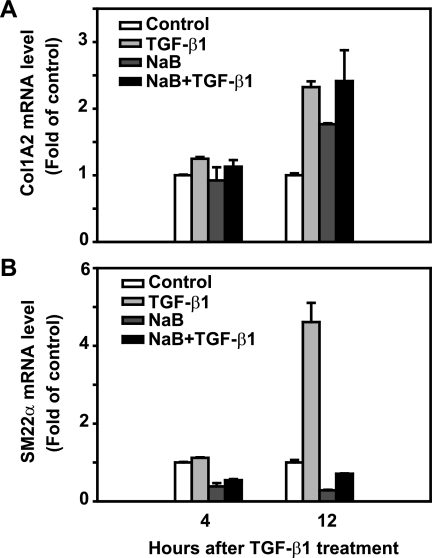
We next examined the effect of butyrate on other known TGF-β target gene expression, such as Col1A2 and SM22α. Col1A2 is one of the major components of the extracellular matrix (47), whereas SM22α is a marker of myofibroblast differentiation (33). As expected, treatment with TGF-β alone for 12 h caused a 2.3-fold induction of Col1A2 mRNA expression compared with control; butyrate treatment induced Col1A2 mRNA expression to a similar magnitude as TGF-β treatment; the combined treatment of butyrate and TGF-β induced Col1A2 expression in a similar level as either treatment alone. Whereas treatment with TGF-β alone induced SM22α mRNA expression 4.6-fold at 12 h compared with control, butyrate alone decreased basal levels of SM22α expression. Interestingly, pretreatment of butyrate blocked TGF-β-induced SM22α expression (Fig. 8). These results suggest that butyrate does not reproduce or enhance every effect of TGF-β, but it specifically affects a subset of TGF-β target genes. These findings are consistent with our previous findings (31).
Fig. 8.
NaB differentially regulates TGF-β-induced Col1A2 and SM22α mRNA expression. The RIE-1 cells were treated the same way as in Fig. 6. Real-time PCR was applied to measure Col1A2 and SM22α gene expression and normalized to 18S mRNA level. Col1A2 (A) and SM22α (B) mRNA levels were represented as fold of control.
Knockdown of Id2 induces cell cycle arrest and apoptosis in RIE-1 cells.
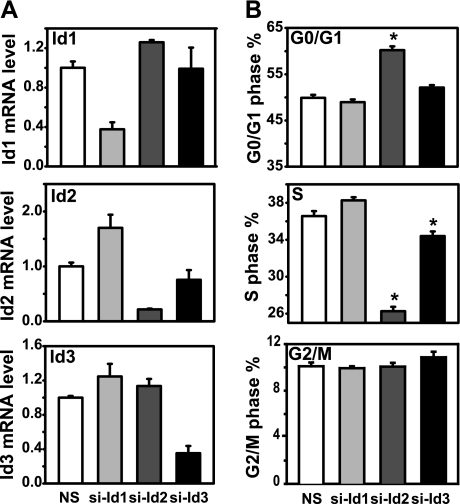
Butyrate and TGF-β synergistically induced cell cycle arrest and apoptosis in gut epithelial cells, which may be mediated through repression of Id2 and Id3 gene expression. This hypothesis predicts that inhibition of Id expression should be sufficient to induce cell cycle arrest and apoptosis. To test this prediction, we used siRNAs specific to rat Id1, Id2, and Id3 to knock down respective Id gene expression. The specificity and efficiency of Id siRNAs were monitored by real-time PCR (Figs. 9A and 10A). Our results showed that Id siRNAs specifically knocked down Id1, Id2, or Id3 gene expression, respectively, compared with nonspecific siRNA control. Id2 siRNA induced G0/G1 arrest and decreased S phase. Id3 siRNA resulted in a marginal decrease of cells in S phase. Id1 siRNA did not alter cell cycle progression, compared with nonspecific siRNA control (Fig. 9B). Furthermore, Id1 and Id2 siRNA transfection caused apoptosis in the RIE-1 cells, whereas Id3 siRNA transfection did not induce apoptosis (Fig. 10B). These observations suggest that downregulation of Id2 expression by butyrate and TGF-β is sufficient to induce cell cycle arrest and apoptosis in RIE cells.
Fig. 9.
Knockdown of Id2 gene expression induces cycle arrest. The RIE-1 cells were transfected with rat Id1, Id2, and Id3 small interfering (si)RNA (50 nmol/l) by using DharmaFECT3 in DMEM supplemented with 5% dialyzed fetal bovine serum (dFBS) for 24 h. Scrambled siRNA was used as nonspecific control (NS). The cells were pulsed with BrdU during the last 45 min and harvested for flow cytometric analysis. A: total RNA was prepared and converted to cDNA for real-time quantitative PCR analysis of Id1, Id2, and Id3 mRNA expression. Id mRNA levels were represented as fold of control. B: cells in different phases of the cell cycle were expressed as means ± SE. *P < 0.05 compared with NS control group.
Fig. 10.
Knockdown of Id2 gene expression induces apoptosis. The RIE-1 cells were transfected with rat Id1, Id2, and Id3 siRNA (50 nmol/l) by using DharmaFECT3 in DMEM supplemented with 5% dFBS for 24 h. Scrambled siRNA was used as nonspecific control. A: total RNA was prepared and converted to cDNA for real-time quantitative PCR analysis of Id1, Id2, and Id3 mRNA expression. Id mRNA levels were represented as fold of control. B: apoptotic cells were stained with annexin V-FITC and propidium iodide. Cells were analyzed by flow cytometry and the cells stained only with annexin V were identified as apoptotic cells. Results are expressed as means ± SE. *P < 0.05 compared with NS control group.
DISCUSSION
Our data show that dietary fiber pectin feeding induced Smad3 protein expression and activation in the mouse intestine mucosal tissue. These in vivo data support the hypothesis that dietary fiber enhances TGF-β/Smad3 signaling in the gut. To study the underlying mechanisms, we chose butyrate, a fermentation product of dietary fiber in the gut, to further examine its effects on cell growth and apoptosis in the presence of the tumor suppressor TGF-β in the RIE-1 cells. We found that at the cellular level butyrate enhanced TGF-β's inhibitory effects on cell growth through induction of cell cycle arrest and apoptosis, not differentiation. At the molecular level, butyrate enhanced TGF-β's effects on Id2 and Id3 repression. Knockdown of Id2 caused cell cycle arrest and apoptosis. Our data suggest that butyrate enhances the tumor suppressor effects of TGF-β at least partly through Id2 repression.
Dietary fiber fermentation by the colonic bacterial flora produces short-chain fatty acids (SCFAs), mainly acetate, propionate, and butyrate, at high concentration (mM) in nearly constant molecular ratio of 1, 0.3, and 0.25, respectively (14). In vitro, butyrate significantly inhibits colon epithelial cell proliferation but increases differentiation and apoptosis, whereas other SCFAs, acetate and propionate, do not have these effects (10). Among these SCFAs, butyrate is considered as the major energy substrate for colonocytes, and it seems to protect against colonic carcinogenesis (48), probably related to its ability to inhibit HDAC activity and thus to impair cellular growth (45). HDAC inhibitors induce the expression of specific genes that are responsible for growth arrest, differentiation, and apoptosis. For example, we have previously reported that butyrate enhances TGF-β signaling by induction of Smad3 gene expression (31). Trichostatin A, another HDAC inhibitor, has been shown to induce cell cycle blockade and differentiation of ovarian cancer cells. This is associated with a decrease in Id1 and no change in Id2 expression (39). In our study, we showed that butyrate repressed Id2 and Id3 mRNA and protein expression and repressed Id1 protein expression without affecting Id1 mRNA expression. One possible explanation for these findings is that different HDAC inhibitors may repress different Id gene expression by specific inhibition of certain groups of HDACs.
Thus far studies on the tumor-suppressive function of butyrate have focused mostly on its intracellular action as a HDAC inhibitor. Most recently, butyrate has been also shown to elicit effects in colon cells extracellularly by serving as a ligand for GPR109A. GPR109A is a G-coupled receptor and a tumor suppressor in colon (41). This suggests that the ability of butyrate to prevent cancer and inflammation in the colon may also be mediated extracellularly via the receptor without entering cells (41).
Together, our studies have revealed an interaction between butyrate, a natural product of dietary fiber, and TGF-β, a major tumor suppressor in the gut, which may explain the beneficial effects of dietary fiber in decreasing the risk of colon cancers. As a general growth inhibition mechanism, TGF-β has been shown to repress Id gene expression (21, 25). To further study the underlying molecular mechanisms of the interaction between butyrate and TGF-β, we found that butyrate and TGF-β cooperatively repressed Id2 and Id3 gene expression. Knockdown of Id2 had a similar effect on cell cycle arrest and apoptosis induction in RIE-1 cells exposed to butyrate and TGF-β. Therefore, downregulation of Id2 is likely a key molecular integrator mediating the combined antiproliferative effects of butyrate and TGF-β in the gut epithelium.
However, in vivo, the high-pectin diet did not decrease protein levels of Id2, a known downstream target of TGF-β/Smad3 signaling compared with control. As reported, Ids are expressed in undifferentiated and proliferating cells. In the case of the gut, such cells localize in the base of the crypt in the villus. Therefore, the effects of the dietary fiber pectin feeding on Id2 expression may be diluted owing to use of the mucosal tissue samples that were collected from whole villus including the crypt and the tip.
Our data suggest that Id2 repression may mediate dietary fiber- and TGF-β-induced cell growth arrest and apoptosis but do not specifically address the role of Id2 in dietary fiber- and TGF-β-induced cell growth arrest and apoptosis. Previously, we have shown that TGF-β represses Id2 expression. Knockdown of Id2 gene expression induces apoptosis in RIE-1 cells, whereas overexpression of Id2 attenuates TGF-β-induced apoptosis. Thus Id2 has been identified as an important downstream target of TGF-β-induced apoptosis in intestinal epithelial cells (8). In the present study, we show that butyrate enhanced TGF-β's effect on Id2 repression, suggesting that repression of Id2 is most likely a mechanism that mediates dietary fiber- and TGF-β-induced cell growth arrest and apoptosis.
Although butyrate potentiated TGF-β-induced Id2 and Id3 repression, this effect was not observed in all TGF-β target gene expression. For example, butyrate did not enhance TGF-β-induced Id1 repression, a close family member of Id2 and Id3. Additionally, butyrate did not enhance TGF-β-induced Col1A2 expression but blocked TGF-β-induced SM22α expression. These results once again suggest that butyrate does not have a broad effect on TGF-β signaling and function but may selectively target a subset of TGF-β signaling and biological activities, specifically those related to its tumor suppressor function.
In summary, our results demonstrate that dietary fiber induced Smad3 expression and activation in the mouse gut mucosal tissue. These results confirm our previous in vitro finding that Smad3 expression was increased in the gut epithelial cells by butyrate (31). Our study suggests a possible interaction between the dietary fiber product butyrate and the TGF-β tumor suppressor signaling pathway. Furthermore, butyrate enhanced TGF-β-induced growth inhibition effects through cell cycle arrest and apoptosis induction, which was partially mediated through the repression of Id2. The potential interaction of dietary fiber/butyrate with the TGF-β signaling pathway is illustrated in Fig. 11. Thus our study reveals a novel molecular mechanism that may account for the beneficial effects of dietary fiber in decreasing the risk of colon cancers.
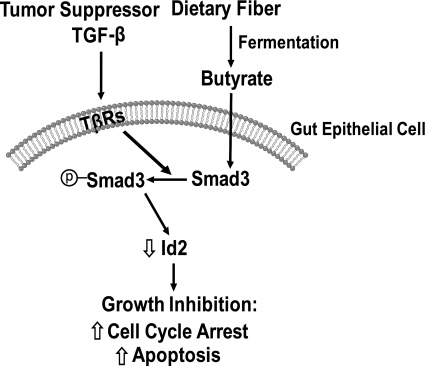
Fig. 11.
Schematic illustration of the potential interaction of dietary fiber with the TGF-β signaling pathway. Upon binding to its receptors (TβRs) on the gut epithelial cells, TGF-β activates (or phosphorylates) Smad3 and inhibits Id2 expression. Butyrate, derived from dietary fiber in the gut, acts on the gut epithelial cells to increase Smad3 expression and subsequently to inhibit Id2 expression, thereby enhancing TGF-β-induced growth inhibition through cell cycle arrest and apoptosis induction.
GRANTS
This study was supported by Public Health Service grants R01 DK060105 (T. C. Ko) and P01 DK035608 (C. M. Townsend and T. C. Ko).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank M. A. Tyler in University of Texas Health Science Center-Houston and A. A. Luo at University of Pennsylvania for critical reading and editing of the manuscript, E. Figueroa and S. Schuenke in Department of Surgery, University of Texas Medical Branch for manuscript preparation, and Dr. H. Guo for real-time quantitative PCR assay at Real-Time PCR Core Facility, Sealy Center for Cancer Cell Biology, University of Texas Medical Branch.
REFERENCES
- 1. Augeron C, Laboisse CL. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res 44: 3961–3969, 1984 [PubMed] [Google Scholar]
- 2. Barnard JA, Beauchamp RD, Coffey RJ, Moses HL. Regulation of intestinal epithelial cell growth by transforming growth factor type beta. Proc Natl Acad Sci USA 86: 1578–1582, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blay J, Brown KD. Characterization of an epithelioid cell line derived from rat small intestine: demonstration of cytokeratin filaments. Cell Biol Int Rep 8: 551–560, 1984 [DOI] [PubMed] [Google Scholar]
- 4. Burkitt DP. Epidemiology of cancer of the colon and rectum. Cancer 28: 3–13, 1971 [DOI] [PubMed] [Google Scholar]
- 5. Cairnie AB, Lamerton LF, Steel GG. Cell proliferation studies in the intestinal epithelium of the rat. I. Determination of the kinetic parameters. Exp Cell Res 39: 528–538, 1965 [DOI] [PubMed] [Google Scholar]
- 6. Cao Y, Chen L, Zhang W, Liu Y, Papaconstantinou HT, Bush CR, Townsend CM, Jr, Thompson EA, Ko TC. Identification of apoptotic genes mediating TGF-beta/Smad3-induced cell death in intestinal epithelial cells using a genomic approach. Am J Physiol Gastrointest Liver Physiol 292: G28–G38, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Cao Y, Deng C, Townsend CM, Jr, Ko TC. TGF-beta inhibits Akt-induced transformation in intestinal epithelial cells. Surgery 140: 322–329, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Cao Y, Liu X, Zhang W, Deng X, Zhang H, Liu Y, Chen L, Thompson EA, Townsend CM, Jr, Ko TC. TGF-beta repression of Id2 induces apoptosis in gut epithelial cells. Oncogene 28: 1089–1098, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung YS, Song IS, Erickson RH, Sleisenger MH, Kim YS. Effect of growth and sodium butyrate on brush border membrane-associated hydrolases in human colorectal cancer cell lines. Cancer Res 45: 2976–2982, 1985 [PubMed] [Google Scholar]
- 10. Comalada M, Bailon E, de Haro O, Lara-Villoslada F, Xaus J, Zarzuelo A, Galvez J. The effects of short-chain fatty acids on colon epithelial proliferation and survival depend on the cellular phenotype. J Cancer Res Clin Oncol 132: 487–497, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Conery AR, Cao Y, Thompson EA, Townsend CM, Jr, Ko TC, Luo K. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-beta induced apoptosis. Nat Cell Biol 6: 366–372, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Coradini D, Biffi A, Costa A, Pellizzaro C, Pirronello E, Di Fronzo G. Effect of sodium butyrate on human breast cancer cell lines. Cell Prolif 30: 149–159, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coradini D, Pellizzaro C, Marimpietri D, Abolafio G, Daidone MG. Sodium butyrate modulates cell cycle-related proteins in HT29 human colonic adenocarcinoma cells. Cell Prolif 33: 139–146, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D'Argenio G, Mazzacca G. Short-chain fatty acid in the human colon. Relation to inflammatory bowel diseases and colon cancer. Adv Exp Med Biol 472: 149–158, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol 45: 495–528, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Eppert K, Scherer SW, Ozcelik H, Pirone R, Hoodless P, Kim H, Tsui LC, Bapat B, Gallinger S, Andrulis IL, Thomsen GH, Wrana JL, Attisano L. MADR2 maps to 18q21 and encodes a TGFbeta-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell 86: 543–552, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Hasskarl J, Munger K. Id proteins—tumor markers or oncogenes? Cancer Biol Ther 1: 91–96, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Heerdt BG, Houston MA, Augenlicht LH. Short-chain fatty acid-initiated cell cycle arrest and apoptosis of colonic epithelial cells is linked to mitochondrial function. Cell Growth Differ 8: 523–532, 1997 [PubMed] [Google Scholar]
- 19. Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature 390: 465–471, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Howe GR, Benito E, Castelleto R, Cornee J, Esteve J, Gallagher RP, Iscovich JM, Deng-ao J, Kaaks R, Kune GA, Kune S, L'Abbé KA, Lee HP, Lee M, Miller AB, Peters RK, Potter JD, Riboli E, Slattery ML, Trichopoulos D, Tuyns A, Tzonou A, Whittemore AS, Wu-Williams AH, Shu Z. Dietary intake of fiber and decreased risk of cancers of the colon and rectum: evidence from the combined analysis of 13 case-control studies. J Natl Cancer Inst 84: 1887–1896, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Kang Y, Chen CR, Massague J. A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol Cell 11: 915–926, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Ko TC, Beauchamp RD, Townsend CM, Jr, Thompson EA, Thompson JC. Transforming growth factor-beta inhibits rat intestinal cell growth by regulating cell cycle specific gene expression.Am J Surg 167: 14–19; discussion 19–20, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Ko TC, Yu W, Sakai T, Sheng H, Shao J, Beauchamp RD, Thompson EA. TGF-beta1 effects on proliferation of rat intestinal epithelial cells are due to inhibition of cyclin D1 expression. Oncogene 16: 3445–3454, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem 42: 65–82, 1982 [DOI] [PubMed] [Google Scholar]
- 25. Ling MT, Wang X, Tsao SW, Wong YC. Down-regulation of Id-1 expression is associated with TGF beta 1-induced growth arrest in prostate epithelial cells. Biochim Biophys Acta 1570: 145–152, 2002 [DOI] [PubMed] [Google Scholar]
- 26. MacGrogan D, Pegram M, Slamon D, Bookstein R. Comparative mutational analysis of DPC4 (Smad4) in prostatic and colorectal carcinomas. Oncogene 15: 1111–1114, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Manning TS, Gibson GR. Microbial-gut interactions in health and disease. Prebiotics. Best Pract Res Clin Gastroenterol 18: 287–298, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 1: 194–202, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Matsushita M, Matsuzaki K, Date M, Watanabe T, Shibano K, Nakagawa T, Yanagitani S, Amoh Y, Takemoto H, Ogata N, Yamamoto C, Kubota Y, Seki T, Inokuchi H, Nishizawa M, Takada H, Sawamura T, Okamura A, Inoue K. Down-regulation of TGF-beta receptors in human colorectal cancer: implications for cancer development. Br J Cancer 80: 194–205, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mortensen PB, Clausen MR. Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol Suppl 216: 132–148, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Nguyen KA, Cao Y, Chen JR, Townsend CM, Jr, Ko TC. Dietary fiber enhances a tumor suppressor signaling pathway in the gut. Ann Surg 243: 619–625; discussion 625–617, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Norton JD. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci 113: 3897–3905, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Qiu P, Feng XH, Li L. Interaction of Smad3 and SRF-associated complex mediates TGF-beta1 signals to regulate SM22 transcription during myofibroblast differentiation. J Mol Cell Cardiol 35: 1407–1420, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 80: 248–265, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riggins GJ, Thiagalingam S, Rozenblum E, Weinstein CL, Kern SE, Hamilton SR, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mad-related genes in the human. Nat Genet 13: 347–349, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Sandler RS, Lyles CM, Peipins LA, McAuliffe CA, Woosley JT, Kupper LL. Diet and risk of colorectal adenomas: macronutrients, cholesterol, and fiber. J Natl Cancer Inst 85: 884–891, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Scheppach W, Weiler F. The butyrate story: old wine in new bottles? Curr Opin Clin Nutr Metab Care 7: 563–567, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Siavoshian S, Segain JP, Kornprobst M, Bonnet C, Cherbut C, Galmiche JP, Blottiere HM. Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: induction of cyclin D3 and p21 expression. Gut 46: 507–514, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Strait KA, Dabbas B, Hammond EH, Warnick CT, Iistrup SJ, Ford CD. Cell cycle blockade and differentiation of ovarian cancer cells by the histone deacetylase inhibitor trichostatin A are associated with changes in p21, Rb, and Id proteins. Mol Cancer Ther 1: 1181–1190, 2002 [PubMed] [Google Scholar]
- 40. Takagi Y, Kohmura H, Futamura M, Kida H, Tanemura H, Shimokawa K, Saji S. Somatic alterations of the DPC4 gene in human colorectal cancers in vivo. Gastroenterology 111: 1369–1372, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, Prasad PD, Ganapathy V. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res 69: 2826–2832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thiagalingam S, Lengauer C, Leach FS, Schutte M, Hahn SA, Overhauser J, Willson JK, Markowitz S, Hamilton SR, Kern SE, Kinzler KW, Vogelstein B. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet 13: 343–346, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81: 1031–1064, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Wang J, Han W, Zborowska E, Liang J, Wang X, Willson JK, Sun L, Brattain MG. Reduced expression of transforming growth factor beta type I receptor contributes to the malignancy of human colon carcinoma cells. J Biol Chem 271: 17366–17371, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Whitlock JP, Jr, Augustine R, Schulman H. Calcium-dependent phosphorylation of histone H3 in butyrate-treated HeLa cells. Nature 287: 74–76, 1980 [DOI] [PubMed] [Google Scholar]
- 46. Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 40: 235–243, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Yang KL, Chang WT, Hung KC, Li EI, Chuang CC. Inhibition of transforming growth factor-beta-induced liver fibrosis by a retinoic acid derivative via the suppression of Col 1A2 promoter activity. Biochem Biophys Res Commun 373: 219–223, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Young GP, McIntyre A, Albert V, Folino M, Muir JG, Gibson PR. Wheat bran suppresses potato starch—potentiated colorectal tumorigenesis at the aberrant crypt stage in a rat model. Gastroenterology 110: 508–514, 1996 [DOI] [PubMed] [Google Scholar]
- 49. Yu CF, Whiteley L, Carryl O, Basson MD. Differential dietary effects on colonic and small bowel neoplasia in C57BL/6J Apc Min/+ mice. Dig Dis Sci 46: 1367–1380, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Zhang Y, Derynck R. Regulation of Smad signalling by protein associations and signalling crosstalk. Trends Cell Biol 9: 274–279, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.