Abstract
The mechanisms by which gastroesophageal reflux disease esophagitis develops are controversial. Although many support the notion that caustic injury leads to reflux esophagitis, others have proposed that reflux esophagitis is caused by esophageal epithelial cytokine-mediated inflammation. We previously demonstrated that Toll-like receptor 3 (TLR3) is highly expressed and functional in the nontransformed human esophageal epithelial cell line EPC2-hTERT. In addition to activation by viral double-stranded RNA, TLR3 can be activated by endogenous mRNA released by necrotic cells. In the present study, we investigated the role of esophageal epithelial TLR3 to sense danger signals released by necrotic esophageal epithelial cells in vitro. Following induction of freeze-thaw necrosis, necrotic EPC2-hTERT cell supernatants (NCS) were used to stimulate EPC2-hTERT monolayers, leading to NF-κB-dependent induction of IL-8 mRNA expression. Responses to self-derived NCS were not observed in transformed gastrointestinal epithelial cell lines, including TE-1 and Caco-2 cells, suggesting that the ability to sense endogenous danger signals is unique to nontransformed esophageal epithelial cells. To determine the immunostimulatory role of epithelial RNA, EPC2-hTERT cells were stimulated with self-derived mRNA, which significantly induced IL-8 mRNA expression. Finally, suppression of TLR3 signaling in a DN-TLR3 cell line, hTERT-ΔTIR-TLR3, led to reduced NCS-induced IL-8 induction by both NCS and mRNA stimulation. Our results demonstrate that human esophageal epithelial cells can sense endogenous danger signals, in part through TLR3 signaling. This supports the concept that epithelial injury plays an inciting role in the pathogenesis of reflux-induced esophagitis, providing important insights into the mechanisms by which epithelial injury leads to inflammation.
Keywords: DAMPs, esophagitis, TLR3
gastroesophageal reflux disease (GERD) is extremely common in Westernized countries, with a prevalence of up to 42% in the United States alone (10). Chronic GERD esophagitis is a known risk factor for the development of Barrett's esophagus, a premalignant condition associated with an increased risk for the development of esophageal adenocarcinoma. Thus a clear understanding of the mechanisms by which GERD leads to esophageal inflammation not only is critical to improving the current treatment of this common disease but is also highly relevant for the prevention of esophageal cancer.
Morphological and electrophysiological events that occur during acid exposure to the esophageal epithelium have been previously described by others (33, 39–43, 51, 52, 54). Using in vivo acid-perfusion models, Orlando et al. (43) reported that the rabbit esophageal epithelium undergoes increased passive epithelial permeability, loss of osmolar regulation, and cellular edema, culminating in epithelial cellular necrosis and inflammation. These results suggested that injury to the esophageal epithelium may be a prerequisite for GERD-induced esophageal inflammation.
In contrast to the notion that caustic epithelial injury causes GERD esophagitis, it was recently proposed that GERD esophagitis may be the result of immune-mediated epithelial injury. Using a rat model of reflux esophagitis, Souza et al. (49) reported that the development of epithelial IL-8 immunoreactivity occurred early in the course of gastroesophageal reflux. Furthermore, the epithelial infiltration of lymphocytes and neutrophils was observed prior to the histological evidence of mucosal erosions, suggesting that epithelial damage was not required for the development of esophagitis in this model system.
Common to both the “caustic injury” and “immune-mediated injury” hypotheses is the clinical observation that GERD esophagitis is characterized by the retention of proinflammatory cells within the esophageal epithelium. IL-8 has been linked to the pathogenesis of GERD (19–20, 22–23) and is presumed to play an important role in the chemoattraction of leukocytes to the esophageal epithelium. However, the mechanisms by which esophageal epithelial IL-8 expression is induced remains incompletely understood.
The “danger hypothesis” first suggested that recognition of tissue damage is crucial for the activation of the host innate immune system (36) and proposed that cell injury can lead to inflammation in the absence of pathogenic stimulation. Cell necrosis is widely recognized as a proinflammatory process, mediated by the release of danger-associated molecular patterns (DAMPs) from dying cells (2, 6, 12, 34, 44). In contrast to apoptotic cells whose intracellular contents remain hidden from immune surveillance (29, 31, 46), the hallmark of cell necrosis is the loss of plasma membrane integrity and the release of DAMPs, which activate the host innate immune system in an effort to repair tissue damage.
We sought to identify a potential mechanism by which esophageal epithelial damage might lead to the expression of proinflammatory cytokines relevant to GERD. We recently characterized the expression profile of primary and immortalized nontransformed human esophageal epithelial cells, and found that Toll-like receptor (TLR) 3 was the most highly expressed and functional TLR in response to stimulation by the double-stranded RNA (dsRNA) analog polyinosinic:polycytidylic acid [poly(I:C)]. In addition to its role in sensing dsRNA derived from viral replication, TLR3 signaling has been previously implicated in the sensing of cell death in animal model systems (8, 25). To date, the role of TLR3 signaling in the context of esophageal epithelial injury has not been investigated. In the present study, we explore a novel hypothesis that esophageal epithelial cell damage and necrosis may incite the innate immune responses by human esophageal epithelial cells through TLR3 signaling, leading to the inducible expression of IL-8.
MATERIALS AND METHODS
Cell culture.
EPC2-hTERT, TE-1, and Caco-2 cells were grown in monolayers at 37°C in a humidified 5% CO2 incubator. EPC2-hTERT cells were maintained in keratinocyte serum-free medium (KSFM) containing 0.9 mM calcium supplemented with epidermal growth factor (1 ng/ml), bovine pituitary extract (50 μg/ml), penicillin (100 units/ml), and streptomycin (100 μg/ml) (Invitrogen, Carlsbad, CA). Caco-2 and TE-1 cells were cultured in DMEM supplemented with fetal bovine serum, penicillin (100 units/ml), and streptomycin (100 μg/ml). Bay11-7082 (10 μM) (Enzo Life Sciences, Plymouth Meeting, PA) or DMSO vehicle control (Sigma) were used in NF-κB inhibition studies.
Induction of necrosis and NCS stimulation.
Confluent monolayers of EPC2-hTERT, TE-1, and Caco-2 cells were harvested by trypsinization, and 1 × 106 cells were resuspended in 3 ml of appropriate maintenance growth medium. Cell suspensions were subjected to five cycles of freeze-thawing, alternating between liquid nitrogen and 37°C. Necrotic cell supernatants (NCS) were separated from necrotic cell pellets (NCP) by centrifugation at 1,000 rpm at 4°C. NCP were washed in PBS, centrifuged, and resuspended in 3 ml of fresh cell culture medium. For control experiments, complete KSFM was subjected to five cycles of freeze-thawing.
For stimulation experiments, EPC2-hTERT cells were seeded at a concentration of 1 × 105 in KSFM in 12-well plates. The following day, cells were washed twice with PBS and stimulated with 333 μl of NCS (derived from ∼1.1 × 105 necrotic cells), NCP, or medium for various time points, at 37°C. This specific volume of NCS was used to stimulate EPC2-hTERT cells with NCS using a 1:1 ratio of live to necrotic cells.
Quantification of RNA in NCS samples and controls was performed by using a Qubit Fluorometer (Invitrogen) according to manufacturer's recommendations.
Induction of acid-induced necrosis.
Confluent monolayers of EPC2-hTERT cells (1 × 106 cells) were incubated in acidified HBSS at pH 2 for 24 h at 37°C. Cells were harvested by scraping, and cell suspensions were centrifuged at 1,000 rpm at 4°C. Acidified necrotic cell supernatants (acid-NCS) were collected and used immediately to stimulate live EPC2-hTERT cells for 3 h at 37°C. For control experiments, live EPC2-hTERT cells were stimulated with fresh HBSS and acidified (pH 2) HBSS. RNA was quantified in acid-NCS samples following neutralization to pH 7.4 by use of a Qubit Fluorometer.
RNA isolation and stimulation.
RNA was isolated from EPC2-hTERT cells by using an RNeasy kit (Qiagen, Valencia, CA) according to manufacturer's recommendations. For RNA stimulation experiments, purified RNA was diluted to 10 μg/ml in complete cell culture medium and used immediately for stimulation experiments.
Quantitative RT-PCR.
RNA samples (0.5 μg/sample) were reverse transcribed by use of a high-capacity cDNA reverse transcriptase kit (Applied Biosystems, Foster City, CA). Preformulated TaqMan Gene Expression Assays were purchased from Applied Biosystems for IL-8 (Hs00174103_m1) and GAPDH (4352934E). Quantitative RT-PCR was performed by using the Taqman Fast Universal PCR Master Mix kit (Applied Biosystems), and reactions were performed in triplicate by using 96-well optical plates on a StepOnePlus Real-Time PCR System (Applied Biosystems). GAPDH was used as an endogenous control to normalize the samples using the ΔΔCT method of relative quantitation, where CT is the threshold cycle.
Cytokine assay.
IL-8 secretion was quantified by enzyme-linked immunosorbent assay (BD OptEIA human IL-8 ELISA kit, BD Biosciences, San Jose, CA) according to manufacturer's instructions. IL-8 levels in each sample were calculated on the basis of a standard curve generated by human recombinant IL-8 provided with the kit. Results were expressed as means + SD in picograms per milliliter.
Western blot.
EPC2-hTERT, pBabe-puro, and pBabe-puro-ΔTIR-TLR3 cells were washed with ice-cold PBS and lysed with RIPA buffer (1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 0.9% NaCl, 25 mM Tris, 1 mM EDTA) containing a protease inhibitor cocktail (Sigma). Cells were scraped and incubated on ice for 1 h. Lysates were cleared by centrifugation for 10 min at 4°C, and supernatants were stored at −80°C until later use. Protein concentrations were determined via a BCA protein assay (Pierce Biotechnology, Rockford, IL). Proteins were separated by electrophoresis by using NuPage 4–12% Bis-Tris gels (Invitrogen) and transferred to nitrocellulose membranes. Membranes were blocked with 2.5% nonfat dry milk and 2.5% bovine serum albumin overnight at 4°C. Membranes were incubated with primary antibody for 1 h at room temperature, washed in Tris-buffered saline Tween-20 (TBST), incubated with secondary antibody for 1 h at room temperature, and washed again in TBST. The signal was developed by using an ECL Western blotting detection kit (GE Healthcare) according to manufacturer's recommendations. Rabbit anti-human IκBα (Santa Cruz Biotechnology, Santa Cruz, CA) was used at a concentration of 1:1,000, and mouse anti-actin (Cell Signaling Technology) was used at a concentration of 1:2,000. Secondary antibodies included horseradish peroxidase-conjugated anti-rabbit (1:2,000) and anti-mouse (1:5,000) (GE Healthcare).
Retroviral vectors and generation of DN-TLR3 cell lines.
A cDNA encoding a dominant negative (DN) form of TLR3 was amplified by polymerase chain reaction with the pZero-hTLR3tirless plasmid (Invivogen) as a template. This DN-TLR3 gene was subcloned into the retroviral vector pBabe-puro at its BamH1 and Sal1 sites, resulting in the pBabe-puro-ΔTIR-TLR3 plasmid. The sequence of the inserted construct was verified by DNA sequencing.
Replication-incompetent retroviruses were produced by transfection of empty pBabe puro vector and pBabe-puro-ΔTIR-TLR3 into Phoenix-Ampho packaging cell lines by using Lipofectamine 2000 according to manufacturer's recommendations. Infected medium was harvested at 48 and 72 h after transfection, filtered, and stored at −80°C until further use; 0.5–1.5 × 105 EPC2-hTERT cells were seeded in six-well tissue culture plates 24 h prior to viral infection. Cells were infected with 0.4 ml of virus-containing medium in complete KSFM, in the presence of 4 μg/ml hexadimethrine bromide (Sigma). Plates were centrifuged at 1,800 rpm at room temperature for 45 min, and medium was replaced with fresh KSFM. At 36 h after infection, 0.5 g/ml of puromycin (Sigma) was added for 5 days to select for cells expressing pBabe-puro genes.
Immunofluorescence.
pBabe-puro-ΔTIR-TLR3 and pBabe-puro cells were seeded in glass chamber slides. The following day, cells were washed with PBS and fixed in 10% neutral buffered formalin for 5 min. For unpermeabilized conditions, cells were blocked with 5% donkey serum, then incubated in mouse anti-TLR3 MAb (1:50) (Imgenex, San Diego, CA) overnight at 4°C. For permeabilized conditions, cells were incubated in 0.2% Triton X-100 for 10 min at room temperature, followed by blocking and primary antibody as above. After washing with PBS, Cy3-conjugated donkey anti-mouse secondary antibody was applied for 2 h at room temperature (1:800). Cells were washed in PBS and stained with 4,6′-diamidino-2-phenylindole (Invitrogen) according to manufacturer's recommendations.
Images were taken using a Plan-Apochromat ×63/1.4 oil objective on an inverted Zeiss Axiovert 200M microscope equipped with Zeiss LSM510 META NLO laser scanning confocal system. For the red and the blue channel, the 543-nm laser line from a HeNe laser and 740-nm wavelength from Coherent Chameleon ultrafast tunable two-photon laser were used, respectively.
Statistical analysis.
The two-tailed Student's t-test was used. A P value of less than 0.05 was considered to be statistically significant.
RESULTS
Stimulation of EPC2-hTERT cells with necrotic cell supernatants leads to the induction of IL-8.
Cell necrosis can be induced by extreme variances from physiological conditions, resulting in the loss of plasma membrane integrity and the release of intracellular contents into the extracellular milieu. Repeated freeze-thawing is a well-established method to induce necrosis in vitro and has been used by others to induce acute necrotic death of healthy cells, leading to the release of endogenous immunostimulatory molecules (14, 45). We investigated whether substances released from necrotically killed human esophageal epithelial cells could activate the esophageal epithelial expression of proinflammatory genes by quantifying the expression of IL-8, a proinflammatory cytokine that has previously been shown to be upregulated in human and animal models of GERD (20, 21, 49).
The immortalized nontransformed human esophageal epithelial cell line EPC2-hTERT was used to establish an in vitro model of human esophageal epithelial cell necrosis. EPC2-hTERT cells overcome replicative senescence and are immortalized by constitutively active telomerase without genetic or epigenetic abnormalities in the p53 and pRb pathways, making this immortalized cell line a useful in vitro model to study normal esophageal epithelial cell physiology (3). In addition, we have previously shown that both EPC2-hTERT cells and the parental primary EPC2 cell lines have similar TLR expression profiles and both cell lines act as innate immune effector cells in response to stimulation with TLR ligands (32).
EPC2-hTERT cells were subjected to five cycles of freeze-thaw necrosis. Freeze-thawed cell suspensions were centrifuged to separate NCS from NCP. The 17- and 19-kDa fragments of caspase 3 were not detected in Western blots using cell lysates from freeze-thawed EPC2-hTERT cells, confirming that freeze-thaw cycles did not induce apoptosis (data not shown).
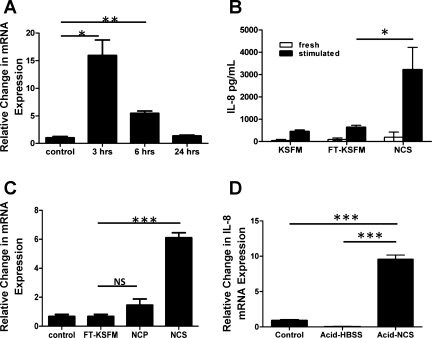
To determine whether immunostimulatory factors were released by necrotic esophageal epithelial cells, live EPC2-hTERT monolayers were stimulated with NCS at 37°C and harvested at various time points after stimulation. Exposure to NCS led to significant increases in IL-8 mRNA expression (Fig. 1A), which peaked at the 3-h time point after stimulation.
Fig. 1.
Necrotic cell supernatant (NCS) stimulation of EPC2-hTERT cells leads to the inducible expression of IL-8. EPC2-hTERT cells were suspended in fresh keratinocyte serum-free medium (KSFM), subjected to 5 cycles of freeze-thaw necrosis, then centrifuged and separated into NCS and necrotic cell pellets (NCP). Fresh medium (control) and freeze-thawed medium (FT-KSFM) were used as controls. A: time course of IL-8 mRNA expression by live EPC2-hTERT monolayers following stimulation with NCS for various time points. B: quantification of IL-8 protein in medium (KSFM), freeze-thawed KSFM (FT-KSFM), and NCS before (fresh) and after (stimulated) being used to stimulate EPC2-hTERT cells for 3 h. C: expression of IL-8 mRNA by EPC2-hTERT cells following stimulation with fresh medium (control), FT-KSFM, NCP, and NCS at the 3-h time point. D: IL-8 mRNA expression in EPC2-hTERT cells following stimulation with neutral HBSS (control), acidified HBSS (acid-HBSS), and NCS made from acid-killed EPC2-hTERT cells (acid-NCS). Results are representative of 3 separate experiments. *P < 0.05, **P < 0.005, ***P < 0.001; NS, not significant.
NCS-induced IL-8 protein secretion by live EPC2-hTERT cells was confirmed by ELISA (Fig. 1B). To verify that IL-8 protein was being produced de novo by live stimulated cell monolayers, IL-8 was also quantified in fresh medium (KSFM), freshly prepared freeze-thawed medium (FT-KSFM), and freshly prepared NCS prior to stimulation of EPC2-hTERT cells.
We next sought to determine whether the substances released by necrotic cells were responsible for the inflammatory response. EPC2-hTERT monolayers were stimulated with NCP for 3 h. Freeze-thawed KSFM (FT-KSFM) was used as a control. Compared with NCS, there was a modest increase in IL-8 expression in NCP-stimulated cells. Neither fresh KSFM nor freeze-thawed KSFM induced an inflammatory response (Fig. 1C). Together, this indicated that substances released by necrotically killed EPC2-hTERT cells, but not the necrotic cells themselves, were primarily responsible for IL-8 induction in this human esophageal epithelial cell line.
Finally, to establish the potential relevance of this model system to physiological events in GERD, we determined whether esophageal epithelial IL-8 could be induced by exposure to supernatants from EPC2-hTERT cells killed by exposure to low pH. Animal models of esophageal acid perfusion have shown that pH ≤2 leads to esophageal epithelial cell necrosis (43, 53). Following prolonged exposure of EPC2-hTERT cells to acidified HBSS (pH 2), acid-killed cell supernatants (acid-NCS) were used to stimulate live monolayers of EPC2-hTERT cells for 3 h at 37°C. Compared with controls stimulated with neutral pH (control) and acid-HBSS, stimulation of esophageal epithelial cells with acid-NCS significantly induced the mRNA expression of IL-8 (Fig. 1D).
Activation of NF-κB signaling by NCS stimulation.
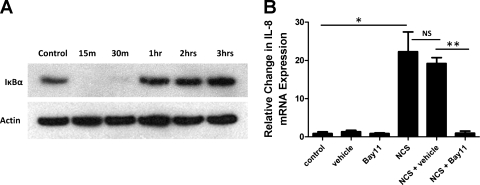
The transcriptional regulation of IL-8 is known to involve interactions between several transcription factors, requiring an NF-κB element and either AP-1 or C/EBP elements (57). We have previously shown that NF-κB signaling pathways are activated by TLR signaling in EPC2-hTERT cells (32). To determine whether endogenous danger signals might induce NF-κB activation, we quantified the expression of IκBα using cell lysates from NCS-stimulated EPC2-hTERT cells. IκBα is a cytoplasmic inhibitor of NF-κB that, when bound to the NF-κB heterodimer, prevents its nuclear translocation (4). Following immune stimulation, IκBα is degraded, allowing the nuclear translocation and activation of NF-κB. IκBα is then resynthesized under the regulatory control of NF-κB, thus providing autoregulation of this signaling pathway (4, 16, 50). As shown in Fig. 2A, NCS stimulation of EPC2-hTERT cells led to the degradation of IκBα within 30 min after exposure. Consistent with known NF-κB-autoregulatory pathways, this was followed by the resynthesis of IκBα at the 1-h time point.
Fig. 2.
NCS-induced activation of NF-κB signaling in EPC2-hTERT cells. A: Western blot analysis of the cytoplasmic NF-κB inhibitor IκBα, using proteins isolated from EPC2-hTERT cells following NCS stimulation for various time points. B: IL-8 mRNA expression by EPC2-hTERT cells following NCS stimulation in the presence or absence of the NF-κB inhibitor, Bay11-7082 (Bay11), compared with vehicle control (DMSO). Results are representative of 2 separate experiments. *P < 0.05, **P < 0.005.
To determine the role of NF-κB in the NCS-mediated induction of IL-8, EPC2-hTERT cells were stimulated with NCS in the presence or the absence of Bay11-7082. Bay11-7082 irreversibly inhibits NF-κB nuclear translocation and activation by selectively inhibiting the phosphorylation and degradation of IκBα (17). Following pretreatment with Bay11-7082, there was a significant inhibition in NCS-mediated activation of IL-8 (Fig. 2B).
Primary nontransformed human esophageal cells are unique in their ability to respond to self-derived NCS.
Others have previously shown that circulating immune effector cells can be activated by DAMPs released by epithelial or endothelial cells in vitro (14, 30). Our observation that esophageal epithelial DAMPs of self-origin can activate innate immune responses has not been previously described in gastrointestinal (GI) epithelial cells.
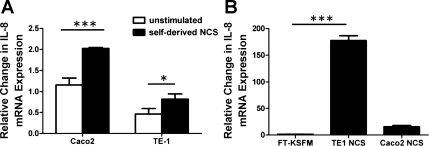
To determine whether the ability to respond to self-derived DAMPs was unique to nontransformed human esophageal epithelial cells, freeze-thaw necrosis was induced in two GI epithelial cell lines: the esophageal squamous cell carcinoma cell line TE-1 and the Caco-2 colon cancer cell line. TE-1 and Caco-2 cells were stimulated with self-derived NCS, and IL-8 was used as a readout of the inflammatory response. Self-derived NCS induced a modest change in IL-8 expression in both cell lines (Fig. 3A). To investigate whether this abrogated response to self-derived NCS could be due to a lack of immunostimulatory substances in the NCS, TE-1, and Caco-2-derived NCS were used to stimulate EPC2-hTERT monolayers. Surprisingly, both TE-1 and Caco-2-derived NCS strongly induced the mRNA expression of IL-8 by EPC2-hTERT cells (Fig. 3B). Together, this suggested that transformed GI epithelial cells may have altered immune responses to self-derived endogenous danger signals.
Fig. 3.
Reduced responses to self-derived NCS by transformed gastrointestinal epithelial cell lines. Esophageal TE-1 squamous cell carcinoma cells and Caco-2 colon cancer cells were subjected to 5 cycles of freeze-thaw necrosis. A: IL-8 expression by live TE-1 and Caco-2 monolayers following stimulation with self-derived NCS for 3 h. TE-1- and Caco-2-derived NCS was generated in DMEM cell culture medium. B: IL-8 expression by EPC2-hTERT monolayers following stimulation with NCS derived from TE-1 and Caco-2 cells. TE-1 and Caco-2 NCS was generated in KSFM. Freeze-thawed KSFM was used as a control. Results are representative of 3 separate experiments. *P < 0.05, ***P < 0.001.
Stimulation of EPC2-hTERT cells with mRNA induces the expression of IL-8.
The potential number of molecules that can be released by a necrotic cell is immense; however, only a small number of substances have been identified thus far as bona fide DAMPs. Among these DAMPs, host-derived RNA was recently identified as an endogenous ligand for TLR3 in several animal models of cell necrosis (8, 25). To determine whether RNA was released by esophageal epithelial cells killed by freeze-thaw necrosis, we quantified RNA concentrations in NCS samples and determined that RNA was present in concentrations ranging from 2.68 to 4.74 μg/ml (data not shown). RNA was also detectable in lower concentrations in NCS from acid-killed EPC2-hTERT cells (0.3 μg/ml) (data not shown). On the basis of our previous study showing that TLR3 is the most abundantly expressed and functional of the human esophageal epithelial TLRs, we hypothesized that endogenously derived RNA might contribute to the proinflammatory properties of NCS.
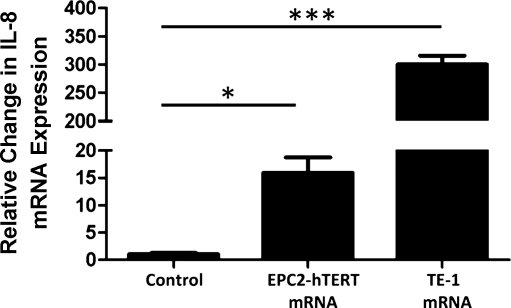
To determine the immunostimulatory ability of mRNA, EPC2-hTERT cells were stimulated with self-derived purified RNA (10 μg/ml) for 2 h at 37°C. Stimulation of EPC2-hTERT cells with endogenous RNA led to a significant increase in IL-8 expression (Fig. 4).
Fig. 4.
Induction of IL-8 expression by stimulation with purified epithelial mRNA. mRNA was isolated from EPC2-hTERT and TE-1 cells and was used to stimulate live EPC2-hTERT cells (10 μg/ml). Results are representative of 3 separate experiments. *P < 0.05, ***P < 0.001.
On the basis of our unexpected observation that stimulation of EPC2-hTERT cells with TE-1-derived NCS resulted in a robust IL-8 response (Fig. 2B), we next stimulated EPC2-hTERT cells with purified TE-1-derived mRNA (10 μg/ml). Surprisingly, stimulation of EPC2-hTERT cells with TE-1-derived mRNA led to a significant induction in IL-8 (Fig. 4), in proportion to the enhanced immunostimulatory effect of TE-1-derived NCS on our cell line.
Inhibition of TLR3 signaling suppresses the innate immune responses to danger signals in human esophageal epithelial cells.
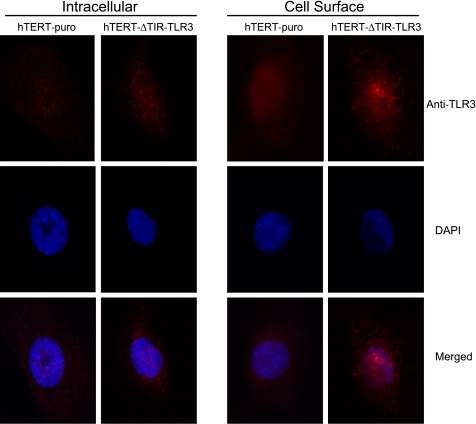
To determine the role of TLR3 in the immune response to NCS stimulation, we generated a DN-TLR3-expressing EPC2-hTERT cell line (hTERT-ΔTIR-TLR3) in which the overexpressed TLR3 transgene lacks the Toll/IL-R (TIR) domain required for intracellular signal transduction (38, 58). We characterized the cellular localization of TLR3 expression in this DN-TLR3 cell line compared with control using immunofluorescence and confocal microscopy. Although most cell types express TLR3 on intracellular endosomal membranes, cell surface expression of TLR3 has also been reported in airway epithelial cells and fibroblasts (18, 35, 47). TLR3 was localized to both the intracellular compartment and to the cell surface in both the control and DN-TLR3 cell line. Although overexpression of the ΔTIR-TLR3 transgene did not significantly alter intracellular TLR3 expression, hTERT-ΔTIR-TLR3 cells demonstrated enhanced cell surface TLR3 expression compared with the control hTERT-puro cell line. Interestingly, the two cell lines exhibited different patterns of cell surface TLR3 expression; whereas surface expression of TLR3 by hTERT-puro cells was diffuse, surface TLR3 expression by hTERT-ΔTIR-TLR3 cells was distributed in a distinct speckled pattern (Fig. 5).
Fig. 5.
Expression of Toll-like receptor (TLR) 3 by hTERT-puro and dominant-negative TLR3 expressing hTERT-ΔTIR-TLR3 cells. Immunofluorescence was performed to determine the localization of TLR3 by both cell lines. For intracellular staining, cells were fixed and permeabilized, followed by incubation with anti-TLR3 MAb. For cell surface staining, cells were fixed and incubated with anti-TLR3 MAb. DAPI, 4,6-diamidino-2-phenylindole; ×63 magnification.
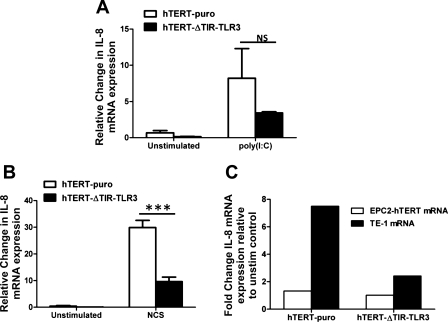
To confirm the functional downregulation of TLR3 signaling in this cell line, hTERT-ΔTIR-TLR3 cell lines were first stimulated with the classic synthetic TLR3 ligand, polyinosinic:polycytidylic acid [poly(I:C)]. Although not statistically significant, diminished IL-8 responses were observed in hTERT-ΔTIR-TLR3 cells compared with cells expressing the empty vector (hTERT-puro) (Fig. 6A).
Fig. 6.
Suppression of NCS and mRNA-induced IL-8 induction in dominant-negative TLR3 expressing human esophageal epithelial cells. A: IL-8 mRNA expression in hTERT-puro and hTERT-ΔTIR-TLR3 cells following poly(I:C)-stimulation (1 μg/ml for 3 h). B: effect of NCS stimulation on IL-8 expression in hTERT-puro and hTERT-ΔTIR-TLR3 cells (3 h). C: change in IL-8 mRNA induction by hTERT-puro and hTERT-ΔTIR-TLR3 cells stimulated with EPC2-hTERT mRNA (open bars) compared with TE-1 mRNA (solid bars). Results represent fold induction in IL-8 compared with unstimulated hTERT-puro and hTERT-ΔTIR-TLR3 cells (10 μg/ml for 2 h). All results are representative of 3 separate experiments. ***P < 0.001.
Both cell lines were then stimulated with NCS derived from wild-type EPC2-hTERT cells to determine the role of TLR3 signaling in the response to endogenous DAMP stimulation. Compared with hTERT-puro cells, there was a significant reduction in NCS-mediated IL-8 induction in hTERT-ΔTIR-TLR3 cells (Fig. 6B). When hTERT-puro and hTERT-ΔTIR-TLR3 cells were stimulated with purified RNA from EPC2-hTERT cells, minimal increases in IL-8 expression were observed in hTERT-puro cells (Fig. 6C). Because TE-1 derived RNA was shown to be a more potent stimulant of IL-8 in EPC-hTERT cells (Fig. 4), we determined its effect on IL-8 expression in both cell lines. TE-1-derived mRNA induced IL-8 expression in hTERT-puro cells, an effect that was suppressed in the hTERT-ΔTIR-TLR3 cell line (Fig. 6C). Together, these results suggested that mRNA-TLR3 interactions may play a role in the innate immune response of human esophageal epithelial cells to danger signals released by dying cells.
DISCUSSION
To date, the contribution of TLR signaling to the pathogenesis of esophagitis has never been explored. When one considers the role of TLRs in pathogen recognition, this knowledge gap is explained by the notion that the esophagus, in contrast to the distal GI tract, is thought to have intermittent contact with low concentrations of potential pathogens. However, when considered in the context of epithelial injury and cell necrosis, a potential role for esophageal TLRs in sensing cell damage and injury becomes highly relevant to this common human disease.
TLR3 has been previously implicated as a sensor of cell damage and death in other model systems (7, 14, 31). In mouse models, TLR3 signaling has been shown to be important for the development of normal inflammatory responses to skin injury (27). In a mouse model of ischemic gut injury, macrophages from TLR3−/− mice were unable to sense RNA released by necrotic neutrophils (8). These and other studies show that TLR3 is an endogenous sensor of tissue necrosis and damage, in the absence of viral activation.
Our study demonstrates that esophageal epithelial cell death is a potent stimulant of IL-8 expression and may therefore support the concept that injury to esophageal epithelial cells is required for the development of GERD esophagitis. Human biopsy studies have shown that IL-8 immunoreactivity of human esophageal biopsies is localized to cells near the stratum basalis in human subjects with GERD (59). Rabbit models of esophageal acid perfusion have also shown that luminal esophageal acid exposure leads to cellular necrosis, which is most pronounced in the metabolically active middle layer of the esophageal epithelium (stratum spinosum), sparing the superficial stratum corneum and the basal proliferating compartment, the stratum basalis (43). Together with our findings, this may suggest a model of GERD in which luminal injury to the esophageal epithelium leads to cell damage and cell death in the stratum spinosum, followed by enhanced chemokine gene expression by adjacent basal cells of the esophageal epithelium. In support of this model, we now show that EPC2-hTERT cells, phenotypically similar to cells of the stratum basalis (3), have the ability to sense cellular stress and injury, functioning as innate immune effector cells in the initiation of esophageal inflammation.
The concept that GERD esophagitis is the result of caustic injury was recently challenged by others. Using a rat model of GERD, Souza et al. (49) showed that esophageal erosions did not develop until 4 wk following esophagoduodenostomy. Furthermore, esophageal epithelial lymphocytic infiltration and IL-8 immunoreactivity preceded the histological development of esophageal erosions. On the basis of these findings, they suggested that GER induces innate esophageal epithelial immune responses that lead to subsequent esophageal injury and inflammation. However, pronounced esophageal epithelial cytoplasmic IL-8 immunoreactivity was not observed until after the development of surface erosions in this model. Notably, edema of suprabasal cells was also observed following the development of surface erosions, similar to Orlando et al.'s (43) previously described models of caustic esophageal injury. Thus, although the early inflammatory events in Souza's model may support an injury-independent model of innate immune responses, their results do not exclude the possibility that acid-induced cell damage may also play a role in the pathogenesis of chronic GERD.
Both TE-1 esophageal squamous cell carcinoma cells and the Caco-2 colon cancer cell line mounted only modest IL-8 responses to self-derived NCS stimulation, demonstrating that the ability to respond to self-derived DAMPs is not a global finding in GI epithelial cells. These results might suggest that TE-1 and Caco-2 cells have reduced ability to detect endogenous danger signals, compared with nontransformed GI epithelial cells. Unexpectedly, we also found that substances released by necrotic TE-1 and Caco-2 cells are highly immunostimulatory to the nontransformed esophageal epithelial cell line used in our studies and that this effect may be attributable to their endogenous RNA. Although further investigation into these signaling mechanisms is beyond the scope of the present study, we speculate that these results could be applicable to cancer models in which dying tumor cells release immunostimulatory signals to normal cells in their local environment.
In contrast to the significant suppression of IL-8 induction in NCS stimulated hTERT-ΔTIR-TLR3 cells, we observed a downward trend in IL-8 induction by poly(I:C) in hTERT-ΔTIR-TLR3 cells that did not reach statistical significance. This is similar to our previous report in which suppression of TLR3 expression through RNA silencing did not completely abrogate the IL-8 response to poly(I:C) stimulation (32). The dsRNA analog poly(I:C) has been well characterized as a potent and prototypical agonist of TLR3. Similar to dsRNA encountered in the context of viral infection, poly(I:C) stimulation activates several dsRNA signaling pathways including RIG-1 and MDA5 (11, 24). Thus the existence of redundant dsRNA signaling pathways may explain the partial suppression of both poly(I:C) and NCS-induced IL-8 expression in this dominant-negative TLR3 cell line.
In contrast to innate immune responses to poly(I:C) and NCS, EPC2-hTERT-derived mRNA did not significantly increase IL-8 expression by hTERT-puro cells (Fig. 6D). Our observation that mRNA from other esophageal cell lines induced variable IL-8 responses in EPC2-hTERT cells (Fig. 4) led us to determine whether this cell line would respond to a more potent mRNA stimulus. Consistent with its enhanced immunostimulatory effect on EPC2-hTERT cells, TE-1-derived mRNA induced IL-8 expression by hTERT-puro cells. The role of TLR3 signaling in detecting mRNA was confirmed by the blunted effect of TE-1 mRNA in the hTERT-ΔTIR-TLR3 cell line.
In this study, we have focused on endogenous RNA-induced TLR3 signaling as a potential mechanism by which EPC2-hTERT cells sense cell injury and necrosis, on the basis of our previous report that TLR3 is both highly expressed and functional in human esophageal epithelial cell lines. Surprisingly, hTERT-ΔTIR-TLR3 cells demonstrated enhanced expression of dominant negative TLR3 on the cell surface, compared with intracellular TLR3. This expression pattern may be consistent with differences between patterns of IL-8 induction in response to poly(I:C) and NCS stimulation. Whereas the expression of IL-8 peaks at 24 h following poly(I:C) stimulation (32), NCS-induced IL-8 expression peaks at 3 h after stimulation. Thus cell surface TLR3 signaling might participate in host innate responses to cell damage, which require a more rapid response compared with the more insidious host responses to viral infection as simulated by the dsRNA analog poly(I:C).
The lack of ssRNA-sensing TLR expression (TLR7 and TLR8) and DNA-sensing TLR9 expression by our cell line (32) likely excludes the involvement of other endogenously derived nucleic acids in this innate immune response. However, the incomplete suppression of IL-8 by NCS-stimulated hTERT-ΔTIR-TLR3 cells clearly indicates that other DAMPs and DAMP receptors play a significant role in this innate immune response. Like TLR3, TLR2 and TLR4 have also been characterized as bona fide DAMP receptors (9, 28, 56). Although our cell line does not express TLR4 (32), an investigation of the role of endogenously derived TLR2 ligands including heat shock proteins (Hsp70, Hsp90) and HMGB1 as DAMPs is needed.
In addition to TLRs, other DAMP receptors have been previously identified, and include RAGE (receptor for advanced glycation end-products) (15, 55), integrins (26), and scavenger receptors (37). The growing list of identified DAMPs includes HMGB1 (1, 46), uric acid/MSU (48), S100 proteins (13), components of the extracellular matrix (12), and heat shock proteins (5). Investigations of these and other esophageal epithelial DAMP-receptor interactions will be important in our understanding of the mechanisms of GERD-induced esophageal inflammation.
In summary, we now propose a mechanism by which human esophageal epithelial cells can detect endogenous danger signals released by necrotic cells, leading to the activation of innate immune signaling. Our findings support the premise that necrotic cell death is a proinflammatory stimulus and may have potential implications for the study of common inflammatory disorders affecting the esophagus.
GRANTS
This work was supported by grants from the National Institutes of Health (K08 DK066206 to M.-L. Wang, and R01DK087789 to M.-L. Wang).
DISCLOSURES
This research was conducted with support from the Investigator Sponsored Study Program of AstraZeneca.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Hiroshi Nakagawa for providing the pBabe-puro vector and Phoenix-Ampho cell lines, as well as technical advice for the generation of dominant-negative cell lines.
REFERENCES
- 1. Abraham E, Arcaroli J, Carmody A, Wang H, Tracey K. Cutting edge: HMG-1 as a mediator of acute lung inflammation. J Immunol 165: 2950–2954, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Aimanianda V, Haensler J, Lacroix-Desmazes S, Kaveri SV, Bayry J. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol Sci 30: 287–295, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Andl CD, Mizushima T, Nakagawa H, Oyama K, Harada H, Chruma K, Herlyn M, Rustgi AK. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem 278: 1824–1830, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Arenzana-Seisdedos F, Thompson J, Rodriguez MS, Bachelerie F. Inducible nuclear expression of newly synthesized I kappa B alpha negatively regulates DNA binding and transcriptional activities of NF-kappa B. Mol Cell Biol 15: 2689–2696, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int Immunol 12: 1539–1546, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81: 1–5, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Brentano F, Schorr O, Gay RE, Gay S, Kyburz D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum 52: 2656–2665, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med 205: 2609–2621, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng N, He R, Tian J, Ye PP, Ye RD. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J Immunol 181: 22–26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delaney BC. Review article: prevalence and epidemiology of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 20: 2–4, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Flur K, Allam R, Zecher D, Kulkarni OP, Lichtnekert J, Schwarz M, Beutler B, Vielhauer V, Anders HJ. Viral RNA induces type I interferon-dependent cytokine release and cell death in mesangial cells via melanoma-differentiation-associated gene-5: implications for viral infection-associated glomerulonephritis. Am J Pathol 175: 2014–2022, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foell D, Wittkowski H, Roth J. Mechanisms of disease: a ‘DAMP’ view of inflammatory arthritis. Nat Clin Pract Rheumatol 3: 382–390, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 81: 28–37, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med 5: 1249–1255, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP, Hess J, Angel P. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med 205: 275–285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16: 225–260, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Goffi F, Boroni F, Benarese M, Sarnico I, Benetti A, Spano PF, Pizzi M. The inhibitor of IkappaBalpha phosphorylation BAY 11–7082 prevents NMDA neurotoxicity in mouse hippocampal slices. Neurosci Lett 377: 147–151, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Groskreutz DJ, Monick MM, Powers LS, Yarovinsky TO, Look DC, Hunninghake GW. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol 176: 1733–1740, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Isomoto H, Nishi Y, Kanazawa Y, Shikuwa S, Mizuta Y, Inoue K, Kohno S. Immune and inflammatory responses in GERD and lansoprazole. J Clin Biochem Nutr 41: 84–91, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Isomoto H, Saenko VA, Kanazawa Y, Nishi Y, Ohtsuru A, Inoue K, Akazawa Y, Takeshima F, Omagari K, Miyazaki M, Mizuta Y, Murata I, Yamashita S, Kohno S. Enhanced expression of interleukin-8 and activation of nuclear factor kappa-B in endoscopy-negative gastroesophageal reflux disease [see comment]. Am J Gastroenterol 99: 589–597, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Isomoto H, Wang A, Mizuta Y, Akazawa Y, Ohba K, Omagari K, Miyazaki M, Murase K, Hayashi T, Inoue K, Murata I, Kohno S. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am J Gastroenterol 98: 551–556, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Isomoto H, Wang A, Mizuta Y, Akazawa Y, Ohba K, Omagari K, Miyazaki M, Murase K, Hayashi T, Inoue K, Murata I, Kohno S. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am J Gastroenterol 98: 551–556, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Isomoto H, Wang A, Nishi Y, Matsumoto A, Shikuwa S, Mizuta Y, Inoue K, Kohno S. Interleukin 8 and 1beta and RANTES levels in esophageal mucosa predict recurrence of endoscopy-negative gastroesophageal reflux disease. Hepatogastroenterology 55: 482–485, 2008 [PubMed] [Google Scholar]
- 24. Kalali BN, Köllisch G, Mages J, Müller T, Bauer S, Wagner H, Ring J, Lang R, Mempel M, Ollert M. Double-stranded RNA induces an antiviral defense status in epidermal keratinocytes through TLR3-, PKR-, and MDA5/RIG-I-mediated differential signaling. J Immunol 181: 2694–2704, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem 279: 12542–12550, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol 8: 279–289, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, Cogen AL, Wu ZR, Hooper LV, Schmidt RR, von Aulock S, Radek KA, Huang CM, Ryan AF, Gallo RL. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med 15: 1377–1382, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leemans JC, Butter LM, Pulskens WP, Teske GJ, Claessen N, van der Poll T, Florquin S. The role of Toll-like receptor 2 in inflammation and fibrosis during progressive renal injury. PLoS One 4: e5704, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leist M, Nicotera P. Cell Death: Apoptosis versus Necrosis. Primer on Cerebrovascular Diseases. San Diego: Academic, 1997, p. 101–104 [Google Scholar]
- 30. Li HS, Verginis P, Carayanniotis G. Maturation of dendritic cells by necrotic thyrocytes facilitates induction of experimental autoimmune thyroiditis. Clin Exp Immunol 144: 467–474, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li M, Carpio DF, Zheng Y, Bruzzo P, Singh V, Ouaaz F, Medzhitov RM, Beg AA. An essential role of the NF-B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol 166: 7128–7135, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Lim DM, Narasimhan S, Michaylira CZ, Wang ML. TLR3-mediated NF-κB signaling in human esophageal epithelial cells. Am J Physiol Gastrointest Liver Physiol 297: G1172–G1180, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Long JD, Marten E, Tobey NA, Orlando RC. Luminal hypertonicity and the susceptibility of rabbit esophagus to acid injury. Dis Esophagus 11: 94–100, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Maher JJ. DAMPs ramp up drug toxicity. J Clin Invest 119: 246–249, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem Biophys Res Commun 293: 1364–1369, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 12: 991–1045, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Mevorach D. The role of death-associated molecular patterns in the pathogenesis of systemic lupus erythematosus. Rheum Dis Clin North Am 30: 487–504, 2004 [DOI] [PubMed] [Google Scholar]
- 38. O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7: 353–364, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Orlando RC. Esophageal epithelial defenses against acid injury. Am J Gastroenterol 89, Suppl 8: S48–S52, 1994 [PubMed] [Google Scholar]
- 40. Orlando RC. Mechanisms of reflux-induced epithelial injuries in the esophagus. Am J Med 108, Suppl 4a: 104S–108S, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Orlando RC. The pathogenesis of gastroesophageal reflux disease: the relationship between epithelial defense, dysmotility, and acid exposure. Am J Gastroenterol 92, Suppl 4: 3S–5S, 1997 [PubMed] [Google Scholar]
- 42.Orlando RC. Reflux esophagitis. In: Textbook of Gastroenterology, edited by Yamada T, Alpers DH, Owyang C, Powell DW, Silverstein FE. Philadelphia, PA: Lippincott, 1991, p. 191–197 [Google Scholar]
- 43. Orlando RC, Powell DW, Carney CN. Pathophysiology of acute acid injury in rabbit esophageal epithelium. J Clin Invest 68: 286–293, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm 2010: 2010. doi:10.1155/2010/672395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 191: 423–433, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Seya T, Matsumoto M, Ebihara T, Oshiumi H. Functional evolution of the TICAM-1 pathway for extrinsic RNA sensing. Immunol Rev 227: 44–53, 2009 [DOI] [PubMed] [Google Scholar]
- 48. Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425: 516–521, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Souza RF, Huo X, Mittal V, Schuler CM, Carmack SW, Zhang HY, Zhang X, Yu C, Hormi-Carver K, Genta RM, Spechler SJ. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 137: 1776–1784, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Sun SC, Ganchi PA, Ballard DW, Greene WC. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science 259: 1912–1915, 1993 [DOI] [PubMed] [Google Scholar]
- 51. Tobey NA, Cragoe EJ, Jr, Orlando RC. HCl-induced cell edema in rabbit esophageal epithelium: a bumetanide-sensitive process. Gastroenterology 109: 414–421, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Tobey NA, Koves G, Orlando RC. HCl-induced cell edema in primary cultured rabbit esophageal epithelium. Gastroenterology 112: 847–854, 1997 [DOI] [PubMed] [Google Scholar]
- 53. Tobey NA, Orlando RC. Mechanisms of acid injury to rabbit esophageal epithelium. Role of basolateral cell membrane acidification. Gastroenterology 101: 1220–1228, 1991 [DOI] [PubMed] [Google Scholar]
- 54. Tobey NA, Reddy SP, Keku TO, Cragoe EJ, Jr, Orlando RC. Mechanisms of HCl-induced lowering of intracellular pH in rabbit esophageal epithelial cells. Gastroenterology 105: 1035–1044, 1993 [DOI] [PubMed] [Google Scholar]
- 55. Turovskaya O, Foell D, Sinha P, Vogl T, Newlin R, Nayak J, Nguyen M, Olsson A, Nawroth PP, Bierhaus A, Varki N, Kronenberg M, Freeze HH, Srikrishna G. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis 29: 2035–2043, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wheeler DS, Chase MA, Senft AP, Poynter SE, Wong HR, Page K. Extracellular Hsp72, an endogenous DAMP, is released by virally infected airway epithelial cells and activates neutrophils via Toll-like receptor (TLR)-4. Respir Res 10: 31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu GD, Lai EJ, Huang N, Wen X. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J Biol Chem 272: 2396–2403, 1997 [PubMed] [Google Scholar]
- 58. Yamamoto M, Akira S. TIR domain-containing adaptors regulate TLR signaling pathways. Adv Exp Med Biol 560: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Yoshida N, Uchiyama K, Kuroda M, Sakuma K, Kokura S, Ichikawa H, Naito Y, Takemura T, Yoshikawa T, Okanoue T. IL-8 expression in the esophageal mucosa of patients with gastroesophageal reflux disease. Scand J Gastroenterol 39: 816–822, 2004 [DOI] [PubMed] [Google Scholar]