Abstract
The water-soluble vitamin B2 (riboflavin, RF) is an essential micronutrient for normal cell function and survival. Recent studies have identified a role for the human riboflavin transporter-2 (hRFT2) in normal intestinal RF absorption. However, little is known about the cell biology of this transporter and specifically about the molecular determinant(s) that dictate its cell surface expression in human intestinal epithelial cells. Here we show that the full-length hRFT2 protein fused to green fluorescent protein (GFP) (GFP-hRFT2) is expressed exclusively at the apical membrane domain of Caco-2 cells. COOH-terminal sequence was essential in dictating cell surface expression with a specific role for conserved cysteine residues (C463 and C467). Mutation of C463 and C467 ablated RF uptake, explained by retention of the constructs within the endoplasmic reticulum. Modeling analysis suggested a potential disulfide bridge between C463 and C386. Consistent with this prediction, mutating the C386 site in the context of the full-length transporter resulted in intracellular retention, whereas mutation of another conserved cysteine (C326A) was without effect on hRFT2 targeting. Intracellular trafficking of hRFT2 was also examined and appeared to involve distinct vesicular structures, the motility of vesicles critically dependent on an intact microtubule network. These results demonstrate a potential role for specific cysteine residues in the cell surface expression of the hRFT2 in human intestinal epithelial cells.
Keywords: epithelia, transport, apical, vitamin B2
the micronutrient riboflavin (RF) is essential for normal cellular functions, growth, and development. In its coenzyme forms (RF-5-phosphate and flavin adenosine dinucleotide), the vitamin plays a key metabolic role in the transfer of electrons in biological oxidation-reduction reactions involving carbohydrate, lipid, and amino acid metabolism, as well as the conversion of pyridoxine and folate into their active forms (6). Recent studies have also demonstrated a role for RF in the oxidative folding of proteins within the endoplasmic reticulum (ER) (36). RF deficiency leads to serious clinical abnormalities that include degenerative changes in the nervous system, anemia, growth retardation, skin lesions, and an increased susceptibility to carcinogens (6, 13, 21). Deficiency of RF occurs in chronic alcoholics, in patients with diabetes mellitus/or inflammatory bowel disease, and those receiving chemotherapy, as well as elderly subjects (2, 8, 9, 16, 22). In contrast to the negative effects of RF deficiency, RF supplementation/optimization appears to have the potential of protecting vital tissues from ischemia-induced oxidative injury (1) and is effective in the treatment of patients with RF-responsive multiple acyl-CoA dehydrogenation defect, Brown-Vialetto-Van Laere and Fazio Londe syndrome (BVVL and FL syndrome) (3, 17, 18). Clinical mutations identified in human RF transporter-2 (hRFT2) manifest as neurological disorders (BVVL and FL syndrome) (14), and the hRFT2 gene has also been linked to esophageal squamous cell carcinoma and gastric cardiac adenocarcinoma (37).
Humans and other mammals cannot synthesize RF and thus must obtain the vitamin from exogenous sources via intestinal absorption. The RF transport process in polarized intestinal epithelia involves the movement of the vitamin molecule across both the brush-border membrane (BBM) and the basolateral membrane (BLM) domains. These processes were characterized using purified BBM and BLM vesicles isolated from the intestine of human donors and animal models and are shown to involve specific carrier-mediated mechanisms at each of these membrane domains (23, 24, 26, 27). Furthermore, the intestinal RF uptake process was found to be sensitive to the inhibitory effect of membrane-impermeant -SH group modifiers (25).
Molecular identity of the mammalian RF transport systems has recently begun to emerge following cloning of three RF transporters from human tissues: hRFT-1, -2, and -3 (39–41). All three hRFTs are expressed in the human intestine, with hRFT2 expression being markedly higher than the others (40). This abundant expression of hRFT2 in intestine together with the similarity of RF transport kinetics mediated by hRFT2 to those of the RF uptake process of intestinal epithelial cells imply a significant physiological role for this transporter in intestinal RF absorption. The hRFT2 is a 469-amino acid protein, which is predicted to have 11 transmembrane domains (TMDs) and an extracellularly oriented COOH terminus (39). Because the intestine plays a critical role in the regulation of overall normal body RF homeostasis, understanding the cell biology of hRFT2 in absorptive cells is important. Our aim in this study was to address this issue with specific focus on identifying molecular determinants that dictate membrane targeting of the hRFT2 protein and to identify the elements important for its intracellular trafficking. This was performed using confocal microscopy to monitor the targeting and intracellular trafficking of full-length, truncated, and mutated hRFT2 constructs fused with green fluorescent protein (GFP) in conjunction with 3H-RF uptake measurements. Our results showed that the hRFT2 protein is exclusively expressed at the apical membrane domain of living Caco-2 cells. By truncation and mutation analysis, we identified an important role for the COOH-terminal tail (and its cysteine residues) in cell surface expression of hRFT2 in intestinal epithelial cells.
MATERIALS AND METHODS
Materials.
[3H]-RF (specific activity 12.3 Ci/mmol) was from Moravek Biochemicals (Brea, CA). GFP-C3, GFP-N3, and DsRed-ER fluorescent protein constructs were from Clontech (Palo Alto, CA). HuTu-80 and Caco-2 cells were from ATCC (Manassas, VA). pFLAG-cytomegalovirus vector and anti-rabbit (rhodamine) conjugate (secondary antibodies) were from Sigma (St. Louis, MO). DNA oligonucleotide primers were synthesized by Sigma Genosys (Woodlands, TX) (Tables 1 and 2). Geneticin (G418) was from Invitrogen (Carlsbad, CA). Cytoskeletal disrupting agents were from Calbiochem (La Jolla, CA).
Table 1.
Combination of primers used to prepare the full-length and COOH-terminal tail truncated constructs of hRFT-2 by PCR
| Construct | Forward and Reverse Primers (5′-3′) | Positions, bp | Fragment, bp |
|---|---|---|---|
| hRFT2[1–469]-GFP | CCGCTCGAGATGGCCTTCCTGATGCAC; | 1–1407 | 1407 |
| CGGGATCCGGCTGGACAGTGCAGATTGCA | |||
| pFLAG-hRFT2[1–469] | GCGTCGACATGGCCTTCCTGATGCAC; | 1–1407 | 1407 |
| CGGGATCCCTAGGCTGGACAGTGCAGATTGCA | |||
| GFP-hRFT2[1–469] | CCGCTCGAGATGGCCTTCCTGATGCAC; | 1–1407 | 1407 |
| CGGGATCCGGCTGGACAGTGCAGATTGCA | |||
| GFP-hRFT2[1–450] | CCGCTCGAGATGGCCTTCCTGATGCAC; | 1–1350 | 1350 |
| CGGGATCCCAGAGGGAACATGAGCAG | |||
| GFP-hRFT2[1–424] | CCGCTCGAGATGGCCTTCCTGATGCAC; | 1–1272 | 1272 |
| CGGGATCCGAGGTCGCGCAGGACCAC | |||
| GFP-hRFT2[451–469] | CCGCTCGAGATGGTCAACGTGCTGCGGCTC; | 1351–1407 | 57 |
| CGGGATCCGGCTGGACAGTGCAGATTGCA |
Primer sequence and combination of primers used to generate each construct are shown. Restriction sites XhoI (boldface text)/SalI (boldface italic text) and BamHI (underlined text) were added to the human riboflavin transporter-2 (hRFT2) primers to allow subsequent subcloning into the green fluorescent protein (GFP)-C3, GFP-N3, and pFLAG vectors.
Table 2.
Overlapping primers encompassing the specified mutation sites for hRFT-2
| Construct | Forward and Reverse Primers (5′-3′) |
|---|---|
| GFP-hRFT2[C326A] | GTGCAGACCTACTCCGCCCTGTCCTATGGGCCA; |
| TGGCCCATAGGACAGGGCGGAGTAGGTCTGCAC | |
| GFP-hRFT2[C386A] | GCGGTGATGAGCCCCGCCCCCCTCTTGCAGGGC; |
| GCCCTGCAAGAGGGGGGCGGGGCTCATCACCGC | |
| GFP-hRFT2[R455A] | CTGGTCAACGTGCTGGCGCTCTTCTCGTCCGCG; |
| CGCGGACGAGAAGAGCGCCAGCACGTTGACCAG | |
| GFP-hRFT2[C463A] | TCGTCCGCGGACTTCGCCAATCTGCACTGTCCA; |
| TGGACAGTGCAGATTGGCGAAGTCCGCGGAGGA | |
| GFP-hRFT2[C467A] | TTCTGCAATCTGCACGCCCCAGCC; |
| GGCTGGGGCGTGCAGATTGCAGAA | |
| Real-Time PCR primers | |
| hRFT2 | CCTTTCCGAAGTGCCCATC; AGAAGGTGGTGAGGTAGTAGG |
| β-Actin | AGCCAGACCGTCTCCTTGTA; TAGAGAGGGCCCACCACAC |
The mutated sequence is shown as underlined text.
Generation of hRFT2 full-length, truncated, and mutated constructs.
The full-length GFP-hRFT2, hRFT2-GFP, and pFLAG-hRFT2 and truncated constructs were generated by PCR amplification using the hRFT2-specific primer combinations listed in Table 1 and conditions as described before (32). The PCR products and the GFP and pFLAG vectors were digested with XhoI/Sal I and BamHI, and products were gel separated and ligated to generate in-frame fusion constructs with the GFP (GFP-C3/N3)/pFLAG fused to the NH2/COOH terminus of each construct. The Quick Change site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used to introduce insertions or deletions of nucleotides into the open reading frame of hRFT2. Overlapping primers containing the mutated nucleotides to the specified mutation sites (Table 2) and full-length GFP-hRFT2 fused plasmid were used as a template for PCR-based site-directed mutagenesis as previously described (28). The nucleotide sequences of all constructs were verified by sequencing (Laragen, Los Angeles, CA).
Cell culture, transient and stable transfection.
Human duodenally derived intestinal epithelial HuTu-80 cells and human-derived intestinal adenocarcinoma Caco-2 cells were maintained in MEM (ATCC). All media were supplemented with 10–20% FBS, glutamine (29 g/l), sodium bicarbonate (2.2 g/l), penicillin (100,000 U/l), and streptomycin (10 mg/l). For transient transfection, cells were grown on sterile glass-bottomed Petri dishes (MartTek Laboratories, Newton, MA) for imaging studies or 12-well plates for uptake analysis and transfected at 95% confluency with 2–4 μg of plasmid DNA using Lipofectamine 2000 (Invitrogen). Transfected cells were visualized using confocal microscopy after 24–48 h. For stable expression, HuTu-80 cells transiently expressing GFP-hRFT2 and GFP were generated by G418 selection (0.8 mg/ml) for 4–8 wk.
Preparation of Xenopus oocytes and nuclear microinjection.
Female adult Xenopus laevis frogs were anesthetized by immersion in 0.1% aqueous solution of 3-aminobenzoic acid ethyl ester (MS-222) for 15 min, and, after death by decapitation, whole ovaries were removed. The procedures used in this study were approved by University of Minnesota Animal Use Committee. The epithelial layers of stage VI oocytes were removed and treated with collagenase (0.5 mg/ml for 30 min) in dissociation solution (in mM: 82.5 NaCl, 2.5 KCl, 10 Na2HPO4, and 5 HEPES, pH 7.8) to ensure complete defolliculation. For expression studies, ∼2 ng of GFP-hRFT2 plasmid cDNA in 5 nl of intracellular solution (in mM: 140 KCl, 10 HEPES, 3 MgCl2, 1 EGTA, and 0.5 CaCl2, pH 7.4) was injected with a Drummond microinjector into the nucleus of each oocyte. Injected oocytes were separated and maintained in Barth's solution with repeated changes of solution at least every 12 h as described before (30, 31).
Immunofluorescence.
Transiently or stably GFP-hRFT2-expressing Caco-2 or HuTu-80 cells were fixed for 10 min in 4% paraformaldehyde solution (Electron Microscopy Sciences, Washington, PA). Cells were permeabilized with 0.2% Triton X-100 and were blocked in 1% BSA for 30 min at room temperature as described before (32). Cells were then incubated with hRFT2 polyclonal antibodies raised in rabbits against the peptide (206-RPREENDLGPAGTVD-280) (Thermo Fisher Scientific, Huntsville, AL) in PBS for 2 h at room temperature and were probed with anti-rabbit rhodamine-conjugated secondary antibodies (Sigma) in PBS (1 h). To visualize the immunofluorescence, cells were mounted by using Fluoromount reagent (Southern Biotechnology, Birmingham, AL) and were imaged by using confocal microscopy.
Uptake assay.
[3H]-RF uptake assays were performed either transiently or stably expressing full-length/truncated/mutated hRFT2 constructs on confluent HuTu-80 or Caco-2 cell lines at 37°C using Krebs-Ringer buffer (pH 7.4) following established procedures (28). After incubation period, the reaction was terminated by the addition of 2 ml of ice-cold Krebs-Ringer buffer followed by immediate aspiration. Cells were then digested with 1 ml of 1 N NaOH, neutralized with 10 N HCl, and then measured for radioactive content using Beckman Coulter LS6500 multipurpose scintillation counter (Fullerton, CA). Protein contents were estimated on parallel wells using a protein assay kit (Bio-Rad, Hercules, CA).
Real-time PCR.
Five micrograms of total RNA were isolated from stable GFP-hRFT2-expressing HuTu-80 cells and control cells as described before (33) using Trizol reagent (Invitrogen). cDNA was synthesized from RNA using reverse transcriptase kit (Invitrogen), and real-time PCR was performed utilizing hRFT2 or β-actin primers (Table 2).
Live cell confocal imaging.
Fluorophores were excited using the 488-nm/543-nm line from an argon/HeNe ion laser, and emitted fluorescence was monitored with a 515 ± 30-nm short-pass filter (GFP) or a 570 ± 50-nm long-pass filter (red fluorescent protein). The motion of individual vesicles was analyzed using frame-to-frame tracking software (Metamorph; Universal Imaging, Downingtown, PA). Videos are provided as supplemental material (Supplemental Movies SM1–3; supplemental material for this article is available online at the American Journal of Physiology Gastrointestinal and Liver Physiology website) (19).
Statistical analysis.
Uptake data presented in this article are the result of at least three separate experiments and are expressed as means ± SE in femtomoles per milligram of protein per 3 min. Differences between the means of samples were tested for significance level at P < 0.05 using Student's t-test. All imaging studies were performed on at least three separate occasions using different cellular preparations.
RESULTS
hRFT2 localization in polarized epithelial cells.
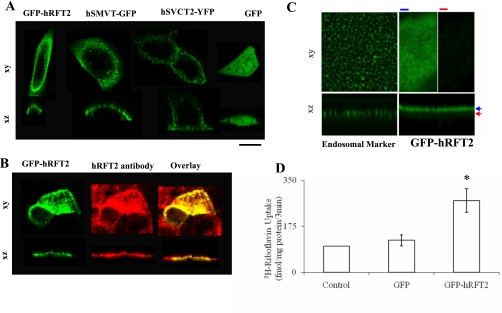
To study cellular expression of hRFT2, we examined the localization of GFP-hRFT2 when expressed in polarized intestinal epithelial Caco-2 cells and in Xenopus oocytes. In live Caco-2 cells imaged by confocal microscopy, the distribution of GFP-hRFT2 mimicked known apical targeted protein (hSMVT-GFP) (28) and was clearly different from basolateral markers (hSVCT2-GFP) (4) or GFP alone (Fig. 1A). The apical expression was also confirmed by immunostaining with specific hRFT2 polyclonal antibodies. Immunofluorescence analysis of Caco-2 cells expressing GFP-hRFT2 revealed strong colocalization between GFP signal and hRFT2 signal at the apical membrane domain (Fig. 1B). Heterologous expression in Xenopus oocytes resulted in cell surface localization of GFP-hRFT2 at the animal pole (data not shown), with fluorescence expression evident in confocal sections peripheral to staining of cortical structures (Fig. 1C). Finally, functionality of the GFP-hRFT2 construct was confirmed by 3H-RF uptake assays in Caco-2 cells transiently expressing different constructs, which showed a significant (2.5-fold; P < 0.05) increase compared with GFP-expressing cells (Fig. 1D). This induction was similar to that caused by transient expression in Caco-2 cells of untagged hRFT2 (i.e., using pFLAG-hRFT2) (517 ± 117 and 1,585 ± 275 fmol/mg protein per 3 min in cells transfected with pFLAG vector and FLAG-hRFT2, respectively). These data demonstrate that full-length NH2-tagged hRFT2 constructs are functionally active at the apical cell surface of polarized intestinal epithelial cells.
Fig. 1.
Distribution of green fluorescent protein-human riboflavin transporter-2 (GFP-hRFT2) in human-derived intestinal Caco-2 cells and Xenopus oocytes. A: lateral (xy) and axial (xz) confocal images of Caco-2 cells expressing GFP-hRFT2, hSMVT-GFP, hSVCT2-YFP, and GFP alone. Imaging was performed 24–48 h after transfection. Scale bar = 10 μm. B: immunofluorescence of Caco-2 cells expressing GFP-hRFT2 showing expression at the apical membrane domain. Specific anti-hRFT2 polyclonal antibodies and anti-rabbit TRITC-conjugated secondary antibodies were used as described in materials and methods. C: expression of GFP-hRFT2 in Xenopus oocytes. GFP-hRFT2 (right) was distributed at the cell surface as evidenced by homogenous peripheral distribution in lateral (xy images, top) and axial (xz scans, bottom) compared with the punctuate distribution of an endosomal marker (left). The xy images for GFP-hRFT2 were taken at the indicated depths (blue, red markers) on the image. D: uptake of 3H-RF by Caco-2 cells transfected with GFP or GFP-hRFT2. Cells were transfected with 4 μg of GFP or GFP-hRFT2, and uptake of 3H-RF (25 nM) was examined after 48 h of transfection in Krebs-Ringer buffer. Data are means ± SE of at least 3 separate determinations. *P < 0.01.
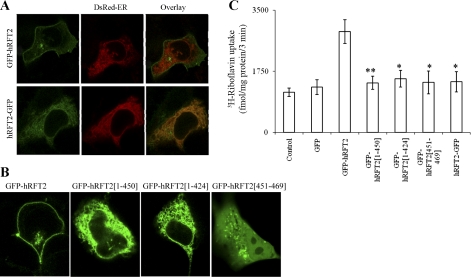
The COOH-terminal tail of hRFT2 is essential for cell surface expression.
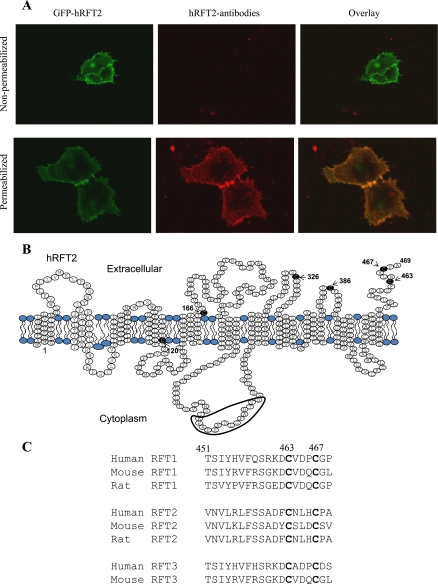
The full-length hRFT2 polypeptide is comprised of 469 amino acids and is predicted to have a very short NH2 terminus, 11 TMDs, and a COOH terminus of 19 amino acids [Fig. 2 (39)]. The result of experiments performed as part of these studies using permeabilized HuTu-80 cells stably expressing GFP-hRFT2 has confirmed this topology prediction. In the latter experiments, we used specific anti-hRFT2 antibodies raised against sequence within the loop between TM6 and TM7 and performed immunostaining using GFP-hRFT2 stably expressing HuTu-80 cells, and confocal images showed no colocalization between GFP-hRFT2 and hRFT2 staining in nonpermeabilized cells (Fig. 2A, top) but strong colocalization in Triton X-100-permeabilized cells (Fig. 2B, bottom). These results suggest that the intracellular loop between the sixth and seventh TMD is located cytosolically and is inaccessible to extracellularly applied antibody without permeabilization. To determine region(s) within the hRFT2 polypeptide important for cell surface expression and functionality, we focused on examining the role of the COOH-terminal because 1) findings with other apically targeted transporters have reported an important role for this region in the targeting events (5, 15, 20, 28, 29, 34), and 2) COOH-terminal tagging of GFP to the hRFT2 transporter (i.e., hRFT2-GFP) prevented cell surface delivery of the fusion construct (Fig. 3A). Thus we examined the effect of truncating the entire COOH-terminal tail (amino acids 451–469) on cell surface expression of the protein. The results showed that complete ablation of the COOH-terminal tail of hRFT2 (GFP-hRFT2[1–450]) prevented cell surface expression; this construct was retained intracellularly within the endoplasmic reticulum in both human intestinal epithelial HuTu-80 (Fig. 3B) and Caco-2 cells (data not shown). Furthermore, uptake of 3H-RF by cells expressing the GFP-hRFT2[1–450] construct was similar to that of control (nontransfected or GFP vector alone transfected) cells (Fig. 3C). Further deletion into the backbone of the hRFT2 polypeptide (GFP-hRFT2[1–424]) also resulted in retention within the ER (Fig. 3B). Reciprocally, expression of the COOH-terminal region of hRFT2 alone (GFP-hRFT2[451–469]) resulted in fluorescence distribution to the entire cytoplasmic volume with some accumulation in vesicular structure (Fig. 3B). No induction in 3H-RF uptake was observed with both these truncation constructs (i.e., GFP-hRFT2[1–424], and GFP-hRFT2[451–469]) compared with controls (Fig. 3C). Therefore, these data suggest that the COOH-terminal domain of hRFT2 is required for cell surface expression.
Fig. 2.
Topology of hRFT2. A: lateral (xy) confocal images of GFP-hRFT2-stably-expressing HuTu-80 cells (green) or antibody staining (red), and their overlay (right). GFP-hRFT2-stably-expressing cells were fixed in 4% paraformaldehyde and either nonpermeabilized with Triton X-100 (top) or permeabilized (bottom). B: predicted 11 transmembrane domains of hRFT2 showing the location of conserved cysteine residues and the antigenic peptide. C: COOH-terminal 19 amino acid sequences for RFT-1, -2, and -3 in human, mouse, and rat showing the conserved COOH-terminal cysteine residues.
Fig. 3.
hRFT2 COOH-terminal determinants and cell surface expression. A: cotransfection of GFP-hRFT2 or hRFT2-GFP with DsRed-ER and their overlay in HuTu-80 cells. Notice the intracellular retention of the construct in the endoplasmic reticulum (ER). B: distribution of hRFT2 COOH-terminal truncation constructs in transiently expressing HuTu-80 cells. C: uptake of 3H-RF (25 nM) in control and transfected HuTu-80 cells. Data are means ± SE of at least 3 separate determinations. **P < 0.01, *P < 0.05.
Role of COOH-terminal cysteines in hRFT2 localization and function.
To help delimit motifs/residues important for hRFT2 expression at the cell surface within the COOH-terminal region (451–469), we performed a sequence alignment of residues in this region of different RFT isoforms from available human and rodent sequences (Fig. 2). Four of the 19 residues within this region are identical across all RFT sequences, with two of these four residues being cysteine moieties (positions 463 and 467). We focused on the role of these (and other; see below) cysteines because previous studies from our laboratory have shown significant inhibition in RF uptake by intestinal epithelial cells treated with the membrane-impermeable p-chloromercuric benzene sulfonate (25); also, a role for cysteine residues in membrane targeting of other proteins to cell surface has been documented (35, 38). The COOH-terminal cysteine residues represent two of six cysteine residues that are conserved across all species and isoforms of the RFT proteins (C120, C166, C326, C386, C463, and C467 using human hRFT2 numbering). Two of these six cysteine residues are closely associated with predicted TMD (C120, TMD4; C166, TMD5) and were not considered further, whereas the remaining four cysteines were predicted to be localized within extracellular regions of the hRFT2 polypeptide (Fig. 2B) and therefore potentially available for disulfide bond formation given the topology of the hRFT2 protein (Fig. 2B).
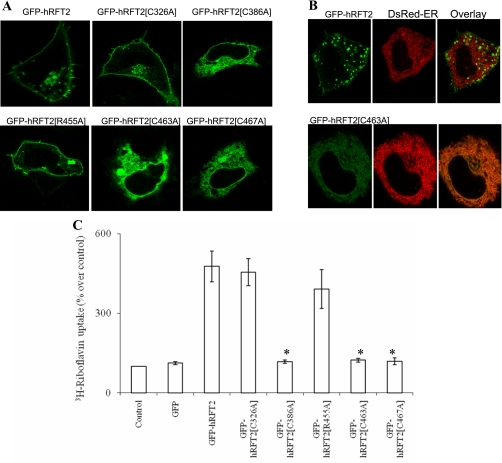
Mutational replacement of cysteine residues within the COOH-terminal tail of the full-length protein (C463 and C467; GFP-hRFT2[C463A] and GFP-hRFT2[C467A]) both resulted in intracellular retention of the fusion construct as shown in Fig. 4A. Cotransfection of an ER marker (DsRed-ER) demonstrated strong overlap (for example, GFP-hRFT2[C463A], Fig. 4B). This intracellular targeting profile was supported by uptake studies demonstrating complete inhibition of induced 3H-RF uptake by both point mutations (P < 0.01) compared with the wild-type hRFT2 (Fig. 4C). In contrast, point mutation of at least one other residue at random in the COOH terminus (R455A) did not impact cell surface expression (Fig. 4A) or 3H-RF accumulation (Fig. 4C).
Fig. 4.
Effect of cysteine residues on hRFT2 targeting and function. A: distribution of GFP-hRFT2 mutant constructs in transiently expressing HuTu-80 cells in lateral (xy) confocal images. B: cotransfection of GFP-hRFT2 (top) or GFP-hRFT2[C463A] (bottom) along with DsRed-ER (middle), shown as overlaid image (right). C: uptake of 3H-RF in control and transfected HuTu-80 cells. Data are means ± SE of at least 3 separate determinations. *P < 0.01.
Next, we examined whether the extracellular cysteines residues in hRFT2 could potentially participate in the formation of disulfide pairs. Assessment of disulfide connectivity using a predictive algorithm [DiANNA 1.1, (10)] identified C386–C463 as a potential disulfide bond from full-length, as well as partial, hRFT2 input sequences. The C386-C463 pairing received the highest predicted scoring of disulfide bonding probability and reflected the only combination compatible with proposed hRFT2 topology (Fig. 2B). Nonetheless, we proceeded to perform mutational analysis of C386 along with other conserved (C326) extracellular cysteine residues within the hRFT2 sequence. Mutation of C326, predicted to lie within the fourth extracellular loop, had little effect on the cell surface expression of the transporter as the GFP-hRFT2[C326A] construct targeted to the cell surface (Fig. 4A) and resulted in equivalent 3H-RF accumulation as observed with wild-type protein (Fig. 4C). In contrast, mutating C386 resulted in intracellular retention (GFP-hRFT2[C386A]) and impaired 3H-RF uptake (Fig. 4, A and C). These experimental data support the in silico prediction regarding the importance of C386 of the hRFT2 polypeptide (Fig. 2B). The data also underscore the importance of the dual cysteine residues in the COOH terminus of the hRFT2 polypeptide, which we have shown to occur in a region essential for cell surface targeting (Fig. 3). In summary, single point mutation of any of these three cysteine residues (C386, C463, or C467, but not C326) in the context of the full-length polypeptide abrogated cell surface expression of hRFT2. Furthermore, functional data (Fig. 4C) extend a bioinformatic prediction of disulfide-bridge on the basis of conformations within the hRFT2 COOH-terminal tail to suggest that the conformation of this region is critical in permitting cell surface expression of this RF transporter in intestinal cells.
Intracellular trafficking of hRFT2.
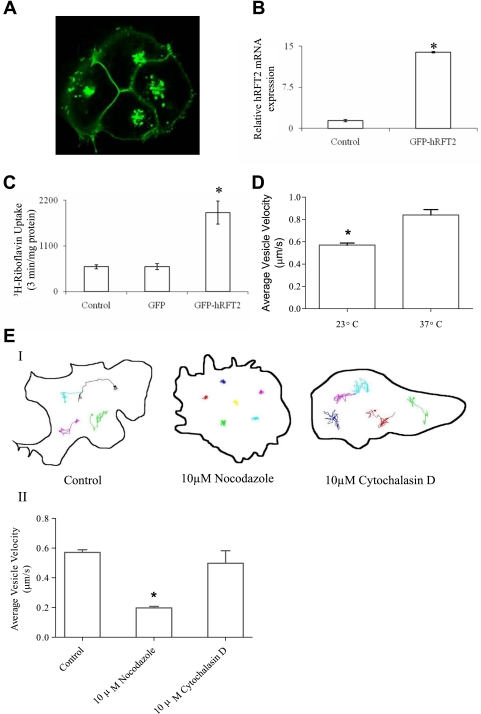
The intracellular trafficking and the specific cytoskeletal components directing intracellular trafficking of hRFT2 to the surface also remain uncharacterized. Stable expression of GFP-hRFT2 resulted in expression of the transporter at the cell surface, as well as in a variety of intracellular punctate structures (Fig. 5A). Quantification of hRFT2 mRNA by real-time RT-PCR revealed an ∼12-fold increase in hRFT2 mRNA expression subsequent to G418 selection (Fig. 5B), with an associated approximately threefold increase in 3H-RF uptake (Fig. 5C). To investigate the dynamics of individual trafficking vesicles and the role of cytoskeletal components in intracellular trafficking of hRFT2 to the cell surface, we used live cell confocal imaging to track the motility of individual structures in the vesicular population. This population was diverse, encompassing various sizes and shapes (for example, spherical and tubular). Some structures exhibited a large range of motion, whereas the majority remained relatively stationary during an imaging period of ∼1 min. Motile vesicles displayed multidirectional movements, often retracing their own paths, with associated changes in velocity and direction (Supplemental Movie S1). To examine the effect of temperature on hRFT2-containing trafficking vesicles in GFP-hRFT2-expressing stable HuTu-80 cells, we measured the velocities of the vesicle movement over a period of time at 23°C and 37°C. Results showed that the velocity of vesicular movements increased in a temperature-dependent manner. The mean population vesicle velocity at 23°C was 0.57 ± 0.02 μm/s compared with 0.84 ± 0.08 μm/s at 37°C (Fig. 5D). We also examined the potential role(s) of cytoskeletal systems in delivering hRFT2 to the cell surface. In this study, we used a pharmacological approach to disrupt cytoskeletal architecture in a stable cell line (GFP-hRFT2-expressing HuTu-80 cells, which are well suited for rapid, live cell confocal imaging). Image stacks were collected before drug addition and following incubation (10 min) in either the microtubule disrupting drug nocodazole (10 μM) or the actin-disrupting agent cytochalasin D (10 μM). To provide an unbiased snapshot of the heterogeneous vesicle population, vesicles were selected at random and their dynamics tracked. After incubation with nocodazole, mean population vesicle velocity was reduced (from 0.57 ± 0.02 μm/s to 0. 19 ± 0.02 μm/s, Fig. 5E and Supplemental Movie S2). In contrast, incubation with cytochalasin D did not affect vesicle movement (0.57 ± 0.02 μm/s vs. 0.50 ± 0.01 μm/s after drug addition, cells; Fig. 5E and Supplemental Movie S3).
Fig. 5.
Effect of cytoskeletal drug treatment on hRFT2 trafficking in GFP-hRFT2-stably-expressing HuTu-80 cells. A: distribution of GFP-hRFT2-trafficking vesicles in stable HuTu-80 cell lines. B: quantification of real-time RT-PCR hRFT2 mRNA expression in the stable HuTu-80 cell lines. C: uptake of 3H-RF in control and GFP-hRFT2-stably-expressing HuTu-80 cells. D: average velocity of vesicles was observed in GFP-hRFT2-stably-expressing HuTu-80 cells maintained at 23°C and 37°C. Data are from >40 vesicles. E: representative vesicle tracks (I) or average vesicle velocity (II) in GFP-hRFT2-stably-expressing HuTu-80 control cells and cells treated with 10 μM nocodazole or 10 μM cytochalasin D for 10 min. Data are means ± SE of at least 3 separate determinations. *P < 0.01.
DISCUSSION
RFT2 is a recently identified RFT that plays an important role in intestinal RF transport and is adaptively upregulated during RF deficiency [(11), and unpublished observation]. In this study we used live cell confocal imaging and 3H-RF uptake approaches to investigate cell biological and physiological aspects of hRFT2 function in human intestinal epithelial cells. Where is the full-length hRFT2 localized in polarized intestinal epithelial cells? What regions/sequences are important for hRFT2 cell surface expression and function? How does hRFT2 traffic to the cell surface?
Our results show the direct evidence of GFP-hRFT2 apical membrane domain localization in human-derived Caco-2 cells (Fig. 1). The apical localization of hRFT2 in intestinal epithelial Caco-2 cells is consistent with carrier-mediated RF uptake observed across the apical BBM of native human intestine (23).
Many nutrient transporters contain targeting motifs/signals embedded within their sequence, including within the COOH terminus of the polypeptide (5, 15, 20, 28, 29, 34). Because of that and because a COOH-terminal GFP fusion of the full-length hRFT2 transporter (hRFT2-GFP) prevented cell surface expression and function of the transporter (Fig. 3, A and C), we focused on this region in our search for such motif(s)/signal(s). Our results showed that complete deletion of the COOH-terminal sequence of hRFT2 (GFP-hRFT2[1–450]) or further deletion into the backbone (GFP-hRFT2[1–424]) leads to retention of the protein within the ER (Fig. 3B) with consequent impaired functionality (Fig. 3C). These findings suggest that the COOH-terminal tail of hRFT2 plays an important role in directing the cell surface expression of the protein. On the basis of COOH-terminal amino acid sequence alignment for different RFT isoforms across human and rodent sequences [Fig. 2C, (39, 40)] we found four of the 19 residues within this region to be conserved. Two of these four conserved residues were found to be cysteines (C463xxxC467). We focused on these cysteine residues because previous studies from our laboratory have shown that membrane-impermeant modifiers of -SH groups lead to marked inhibition in RF uptake by intestinal epithelial cells (25). Furthermore, cysteine residues of other membrane transporters were shown to be important for the function and targeting of these proteins to the plasma membrane (35, 38). Upon mutating C463 and C467, intracellular retention of the hRFT2 protein occurred, which led to impairment in RF uptake. This is clearly suggesting an important role for C463 and C467 in membrane expression of hRFT2. To determine whether these cysteine residues form disulfide bonds with other cysteine in the hRFT2 polypeptide, we subjected the protein to modeling analysis. The modeling predicted a potential disulfide bridge between C463 and C386. This prediction was supported by the finding that mutating C386 of the hRFT2 polypeptide leads to retention of the protein in the ER and impairment in RF uptake. Similar findings on a role for disulfide bridge in membrane expression and functionality of other membrane transporters have been recently reported (7, 12).
Recent genetic analysis of patients afflicted with BVVL and FL syndrome resolved a spectrum of mutations in the hRFT2 (c20orf54) gene. In light of our data highlighting the important role for the COOH-terminal sequence in hRFT2 functionality, it was interesting to note from the clinical literature that patients exhibiting premature truncations (e.g., E71X, Y213X) or a COOH-terminal missense mutation (F457L) died in infancy, whereas patients with point mutations elsewhere in the hRFT2 protein displayed better prognoses following the BVVL and FL diagnosis [R132W, alive; F224L, died after 14 yr (3, 14)]. These results broadly support our structure/function analyses of hRFT2 although precise definition of the cell biological consequences of these specific mutations awaits future definition.
In summary, our data demonstrate for the first time the apical localization of hRFT2 in polarized intestinal epithelial cells, a role for cysteine residues in regulating cell surface expression, and that cell surface delivery depends on intact microtubules.
GRANTS
This work was supported by grants from the Department of Veterans Affairs and the National Institutes of Health (DK84094 to V. Subramanian, GM088790 to J. Marchant, and AA18071 and DK58057 to H. Said).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
REFERENCES
- 1. Betz AL, Ren XD, Ennis SR, Hultquist DE. Riboflavin reduces edema in focal cerebral ischemia. Acta Neurochir Suppl (Wien) 60: 314–317, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Bonjour JP. Vitamins and alcoholism. V Riboflavin, VI Niacin, VII Pantothenic acid, and VIII Biotin. Int J Vitam Nutr Res 50: 425–440, 1980 [PubMed] [Google Scholar]
- 3. Bosch AM, Abeling NG, Ijlst L, Knoester H, van der Pol WL, Stroomer AE, Wanders RJ, Visser G, Wijburg FA, Duran M, Waterham HR. Brown-Vialetto-Van Laere and Fazio Londe syndrome is associated with a riboflavin transporter defect mimicking mild MADD: a new inborn error of metabolism with potential treatment. J Inherit Metab Dis 34: 159–164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyer JC, Campbell CE, Sigurdson WJ, Kuo SM. Polarized localization of vitamin C transporters, SVCT1 and SVCT2, in epithelial cells. Biochem Biophys Res Commun 334: 150–156, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Chuang JZ, Sung CH. The cytoplasmic tail of rhodopsin acts as a novel apical sorting signal in polarized MDCK cells. J Cell Biol 142: 1245–1256, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooperman JM, Lopez R. Riboflavin. In: Handbook of Vitamins: Nutritional, Biochemical and Clinical Aspects, edited by Machlin LJ. New York: Dekker, 1984, p. 299–327 [Google Scholar]
- 7. Dorn M, Weiwad M, Markwardt F, Laug L, Rudolph R, Brandsch M, Bosse-Doenecke E. Identification of a disulfide bridge essential for transport function of the human proton-coupled amino acid transporter hPAT1. J Biol Chem 284: 22123–22132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dreizen S, McCredie KB, Keating MJ, Andersson BS. Nutritional deficiencies in patients receiving cancer chemotherapy. Postgrad Med 87: 163–167, 170, 1990 [DOI] [PubMed] [Google Scholar]
- 9. Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol 84: 744–748, 1989 [PubMed] [Google Scholar]
- 10. Ferre F, Clote P. DiANNA: a web server for disulfide connectivity prediction. Nucleic Acids Res 33: W230–232, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujimura M, Yamamoto S, Murata T, Yasujima T, Inoue K, Ohta KY, Yuasa H. Functional characteristics of the human ortholog of riboflavin transporter 2 and riboflavin-responsive expression of its rat ortholog in the small intestine indicate its involvement in riboflavin absorption. J Nutr 140: 1722–1727, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Fukuda Y, Aguilar-Bryan L, Vaxillaire M, Dechaume A, Wang Y, Dean M, Moitra K, Bryan J, Schuetz JD. Conserved intramolecular disulfide bond is critical to trafficking and fate of ATP-binding cassette (ABC) transporters ABCB6 and sulfonylurea receptor 1 (SUR1)/ABCC8. J Biol Chem 286: 8481–8492, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glodsmith GA. Riboflavin deficiency. In: Riboflavin, edited by Rivlin R. New York: Plenum, 1975, p. 221–224 [Google Scholar]
- 14. Green P, Wiseman M, Crow YJ, Houlden H, Riphagen S, Lin JP, Raymond FL, Childs AM, Sheridan E, Edwards S, Josifova DJ. Brown-Vialetto-Van Laere syndrome, a ponto-bulbar palsy with deafness, is caused by mutations in c20orf54. Am J Hum Genet 86: 485–489, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klapper M, Daniel H, Doring F. Cytosolic COOH terminus of the peptide transporter PEPT2 is involved in apical membrane localization of the protein. Am J Physiol Cell Physiol 290: C472–C483, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Kodentsova VM, Vrzhesinskaia OA, Sokol'nikov AA, Kharitonchik LA, Spirichev VB. [Metabolism of B group vitamins in patients with insulin-dependent and non-insulin dependent forms of diabetes mellitus]. Vopr Med Khim 39: 26–29, 1993 [PubMed] [Google Scholar]
- 17. Law LK, Tang NL, Hui J, Fung SL, Ruiter J, Wanders RJ, Fok TF, Lam CW. Novel mutations in ETFDH gene in Chinese patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Clin Chim Acta 404: 95–99, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Liang WC, Ohkuma A, Hayashi YK, Lopez LC, Hirano M, Nonaka I, Noguchi S, Chen LH, Jong YJ, Nishino I. ETFDH mutations, CoQ10 levels, and respiratory chain activities in patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Neuromuscul Disord 19: 212–216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marchant JS, Subramanian VS, Parker I, Said HM. Intracellular trafficking and membrane targeting mechanisms of the human reduced folate carrier in Mammalian epithelial cells. J Biol Chem 277: 33325–33333, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Muth TR, Ahn J, Caplan MJ. Identification of sorting determinants in the C-terminal cytoplasmic tails of the gamma-aminobutyric acid transporters GAT-2 and GAT-3. J Biol Chem 273: 25616–25627, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Pangrekar J, Krishnaswamy K, Jagadeesan V. Effects of riboflavin deficiency and riboflavin administration on carcinogen-DNA binding. Food Chem Toxicol 31: 745–750, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Rosenthal WS, Adham NF, Lopez R, Cooperman JM. Riboflavin deficiency in complicated chronic alcoholism. Am J Clin Nutr 26: 858–860, 1973 [DOI] [PubMed] [Google Scholar]
- 23. Said HM, Arianas P. Transport of riboflavin in human intestinal brush border membrane vesicles. Gastroenterology 100: 82–88, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Said HM, Hollander D, Mohammadkhani R. Uptake of riboflavin by intestinal basolateral membrane vesicles: a specialized carrier-mediated process. Biochim Biophys Acta 1148: 263–268, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Said HM, Ma TY. Mechanism of riboflavin uptake by Caco-2 human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 266: G15–G21, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Said HM, Mohammadkhani R. Uptake of riboflavin across the brush border membrane of rat intestine: regulation by dietary vitamin levels. Gastroenterology 105: 1294–1298, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Said HM, Mohammadkhani R, McCloud E. Mechanism of transport of riboflavin in rabbit intestinal brush border membrane vesicles. Proc Soc Exp Biol Med 202: 428–434, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Subramanian VS, Marchant JS, Boulware MJ, Ma TY, Said HM. Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells. Am J Physiol Cell Physiol 296: C663–C671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Subramanian VS, Marchant JS, Boulware MJ, Said HM. A C-terminal region dictates the apical plasma membrane targeting of the human sodium-dependent vitamin C transporter-1 in polarized epithelia. J Biol Chem 279: 27719–27728, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Subramanian VS, Marchant JS, Parker I, Said HM. Cell biology of the human thiamine transporter-1 (hTHTR1). Intracellular trafficking and membrane targeting mechanisms. J Biol Chem 278: 3976–3984, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Subramanian VS, Marchant JS, Parker I, Said HM. Intracellular trafficking/membrane targeting of human reduced folate carrier expressed in Xenopus oocytes. Am J Physiol Gastrointest Liver Physiol 281: G1477–G1486, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Subramanian VS, Marchant JS, Said HM. Targeting and trafficking of the human thiamine transporter-2 in epithelial cells. J Biol Chem 281: 5233–5245, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Subramanya SB, Subramanian VS, Kumar SJ, Hoiness R, Said HM. Inhibition of intestinal biotin absorption by chronic alcohol feeding: Cellular and molecular mechanisms. Am J Physiol Gastrointest Liver Physiol 300: G494–G501, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun AQ, Salkar R, Sachchidanand, Xu S, Zeng L, Zhou MM, Suchy FJ. A 14-amino acid sequence with a beta-turn structure is required for apical membrane sorting of the rat ileal bile acid transporter. J Biol Chem 278: 4000–4009, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Tanaka K, Zhou F, Kuze K, You G. Cysteine residues in the organic anion transporter mOAT1. Biochem J 380: 283–287, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tu BP, Ho-Schleyer SC, Travers KJ, Weissman JS. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science 290: 1571–1574, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Wang LD, Zhou FY, Li XM, Sun LD, Song X, Jin Y, Li JM, Kong GQ, Qi H, Cui J, Zhang LQ, Yang JZ, Li JL, Li XC, Ren JL, Liu ZC, Gao WJ, Yuan L, Wei W, Zhang YR, Wang WP, Sheyhidin I, Li F, Chen BP, Ren SW, Liu B, Li D, Ku JW, Fan ZM, Zhou SL, Guo ZG, Zhao XK, Liu N, Ai YH, Shen FF, Cui WY, Song S, Guo T, Huang J, Yuan C, Huang J, Wu Y, Yue WB, Feng CW, Li HL, Wang Y, Tian JY, Lu Y, Yuan Y, Zhu WL, Liu M, Fu WJ, Yang X, Wang HJ, Han SL, Chen J, Han M, Wang HY, Zhang P, Li XM, Dong JC, Xing GL, Wang R, Guo M, Chang ZW, Liu HL, Guo L, Yuan ZQ, Liu H, Lu Q, Yang LQ, Zhu FG, Yang XF, Feng XS, Wang Z, Li Y, Gao SG, Qige Q, Bai LT, Yang WJ, Lei GY, Shen ZY, Chen LQ, Li EM, Xu LY, Wu ZY, Cao WK, Wang JP, Bao ZQ, Chen JL, Ding GC, Zhuang X, Zhou YF, Zheng HF, Zhang Z, Zuo XB, Dong ZM, Fan DM, He X, Wang J, Zhou Q, Zhang QX, Jiao XY, Lian SY, Ji AF, Lu XM, Wang JS, Chang FB, Lu CD, Chen ZG, Miao JJ, Fan ZL, Lin RB, Liu TJ, Wei JC, Kong QP, Lan Y, Fan YJ, Gao FS, Wang TY, Xie D, Chen SQ, Yang WC, Hong JY, Wang L, Qiu SL, Cai ZM, Zhang XJ. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet 42: 759–763, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Xia X, Wang G, Peng Y, Jen J. Cys351 and Cys361 of the Na+/glucose cotransporter are important for both function and cell-surface expression. Arch Biochem Biophys 438: 63–69, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Yamamoto S, Inoue K, Ohta KY, Fukatsu R, Maeda JY, Yoshida Y, Yuasa H. Identification and functional characterization of rat riboflavin transporter 2. J Biochem 145: 437–443, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Yao Y, Yonezawa A, Yoshimatsu H, Masuda S, Katsura T, Inui K. Identification and comparative functional characterization of a new human riboflavin transporter hRFT3 expressed in the brain. J Nutr 140: 1220–1226, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Yonezawa A, Masuda S, Katsura T, Inui K. Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am J Physiol Cell Physiol 295: C632–C641, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.