Abstract
Curcumin, the major phenolic compound in the spice turmeric, exhibits numerous biological effects, including lowering plasma cholesterol and preventing diet-induced hypercholesterolemia. The mechanisms underlying the hypocholesterolemic effect of curcumin are not fully understood. In this regard, intestinal Niemann-Pick C1-like 1 (NPC1L1) cholesterol transporter, the molecular target of intestinal cholesterol absorption inhibitor ezetimibe, plays an essential role in the maintenance of cholesterol homeostasis. The current studies were designed to investigate the effect of curcumin on NPC1L1 function, expression, and promoter activity in intestinal Caco-2 monolayers. NPC1L1 function was evaluated by the measurement of ezetimibe-sensitive [3H]cholesterol esterification. Relative abundance of NPC1L1 mRNA and protein was evaluated by real-time PCR and Western blotting, respectively. Luciferase assays were used to measure NPC1L1 promoter activity. Our results showed that curcumin significantly inhibited ezetimibe-sensitive cholesterol esterification in a dose-dependent manner with a maximum decrease (by 52% compared with control) occurring at 50 μM concentration. Curcumin treatment of Caco-2 monolayers also significantly decreased NPC1L1 mRNA and protein expression. Similarly, the promoter activity of the NPC1L1 gene was inhibited significantly (55%) by 50 μM curcumin. The decrease in NPC1L1 promoter activity by curcumin was associated with a reduction in the expression and the DNA-binding activity of the sterol response element-binding protein 2 (SREBP2) transcription factor. Furthermore, the overexpression of active SREBP2 protected NPC1L1 from the inhibitory effect of curcumin. Our studies demonstrate that curcumin directly modulates intestinal NPC1L1 expression via transcriptional regulation and the involvement of SREBP2 transcription factor.
Keywords: Niemann Pick type C1-like 1, sterol response element-binding protein 2, polyphenols
the niemann pick type c1-like 1 (NPC1L1) transporter is responsible for intestinal cholesterol absorption and is the molecular target for ezetimibe (3, 18), the recently discovered cholesterol-lowering drug (4, 14). NPC1L1 protein is predominantly expressed in the liver and the small intestine where it has been localized to the canalicular membrane of the hepatocytes and the brush-border membrane of intestinal epithelial cells (6). NPC1L1 shares high homology with the intracellular cholesterol transporter NPC1 (the protein defective in Niemen-Pick type C1 cholesterol storage disorder) and contains a sterol-sensing domain common in proteins involved in cholesterol homeostasis (6). Studies in knockout animals demonstrated a significant inhibition in intestinal cholesterol absorption in mice lacking the expression of NPC1L1 indicating its essential role in cholesterol absorption and the processes of cholesterol homeostasis (3, 13). Recent studies have shown the increase in NPC1L1 expression in hypercholesterolemia associated with diseases such as diabetes mellitus (24, 25). Moreover, NPC1L1 inhibition or deficiency was shown to protect against diet-induced hypercholesterolemia and hepatic steatosis (13, 15). Despite the current advances and the use of NPC1L1 inhibitor in the treatment of hypercholesterolemia, lowering plasma cholesterol to levels dictated by stringent guidelines in diseases such as diabetes mellitus remains a challenge (35, 36). Therefore, immense interest has been generated to investigate the molecular mechanisms involved in the regulation of NPC1L1 and cholesterol absorption to unravel potential targets for better treatment of hypercholesterolemia.
Several cellular mechanisms have been shown to be involved in altering the intestinal expression of NPC1L1. For example, we have recently shown that the sterol response element-binding protein (SREBP) 2 induces the basal activity of NPC1L1 promoter and mediates the modulation of NPC1L1 expression by cholesterol (1). Also, the stimulation of the peroxisome proliferator activator receptor PPARα by fenofibrate, the drug used to lower plasma triglycerides, has been shown to cause a reduction in NPC1L1 (34). Furthermore, recent reports demonstrated the potential inhibitory roles for beneficial dietary supplements such as diosgenin and curcumin in the inhibition of cholesterol absorption and NPC1L1 function and expression (16, 32). However, the mechanisms involved in NPC1L1 modulation by these dietary supplements such as curcumin are not fully understood.
Dietary curcumin, the major component of the spices turmeric and curry, has been long known to reduce plasma cholesterol, to protect against hypercholesterolemia induced by cholesterol-enriched diet, and to decrease high plasma cholesterol associated with diabetes mellitus (5, 27). Also, curcumin was shown to inhibit the development of atherosclerotic lesions in a apolipoprotein E/LDLR double-knockout mouse model of atherosclerosis (26). Despite these reported effects, the systemic bioavailability of curcumin has been shown to be relatively low because of generally poor absorption (31). Therefore, it is suggested that the gastrointestinal tract is a major site where curcumin mediates its effects on biological processes in the body, including lowering plasma cholesterol. Indeed, previous studies have suggested a decrease in intestinal cholesterol absorption by curcumin (28). However, the molecular mechanisms via which curcumin directly influences cholesterol absorption are not known. Our data utilizing human intestinal Caco-2 monolayers as a model for the intestinal epithelium demonstrated that curcumin significantly reduced ezetimibe-sensitive cholesterol esterification in Caco-2 cells concomitant with a decrease in NPC1L1 mRNA and protein expression. NPC1L1 promoter activity was also reduced by curcumin, indicating transcriptional modulation. Curcumin-induced reduction of NPC1L1 promoter activity appears to be mediated by the SREBP2 transcription factor. Our findings provide novel insights into the molecular mechanisms by which a beneficial dietary compound such as curcumin lowers plasma cholesterol.
MATERIALS AND METHODS
Cell culture and materials.
Caco-2 cells were obtained from ATCC (Manassas, VA) and were grown routinely in T-75-cm2 plastic flasks at 37°C in a 5% CO2-95% air environment. The culture medium consisted of minimum essential medium, 20% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Cells from passages between 25 and 50 were plated in six-well Falcon plates (treated by vacuum gas plasma; Becton-Dickinson, Franklin Lakes, NJ) at a density of 2 × 104 cells/cm2 and were fed with fresh incubation media every alternate day. Confluent monolayers were then used for the transport experiments at day 10 postplating. Curcumin was obtained from Sigma (St. Louis, MO). Ezetimibe was a generous gift from Schering-Plough (Kenilworth, NJ). [3H]cholesterol was obtained from Perkin-Elmer (Waltham, MA). Silica plates for the thin-layer chromatography were obtained from ANALTECH (Newark, DE).
[3H]cholesterol uptake and esterification.
[3H]cholesterol micelle solution was prepared as previously described with minor modifications (17). Appropriate volumes of each component from ethanol stock solutions were added to a glass tube, evaporated under nitrogen, and then dissolved in DMEM supplemented with 5% of lipoprotein-deficient calf serum (Sigma) by vigorous stirring until the solution was clear. The final concentrations of the micelle solution are as follows: 5 mM taurocholic acid, 0.3 mM oleic acid, 10 μM of l-phosphatidylcholine, and 5 μM of [3H]cholesterol. Cells were then incubated with the micelle solution for the indicated time and then harvested in hypotonic buffer (5 mM Tris, pH 7.4), and protein concentration was measured by the method of Bradford (8). An aliquot was used to measure the total radioactivity and normalized to the amount of protein in the sample to represent total cholesterol uptake. Lipids were extracted by the methods of Bligh and Dyer (7) and then were spotted on a silica plate of thin-layer chromatography to separate free cholesterol from cholesteryl esters using hexane-ethylacetate (70:30, vol/vol). The bands for cholesterol and cholesteryl ester were localized by authentic standards and then scraped, and the associated radioactivity was counted. The ratio between the counts of cholesteryl ester to the counts of free cholesterol from each sample was calculated as a measure for the rate of cholesterol esterification.
RNA extraction and real-time RT-PCR analysis.
Total RNA was prepared from Caco-2 cells using an RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Equal amounts of RNA from both treated and control samples were reverse transcribed and amplified in one step reaction using a Brilliant SYBR Green QRT-PCR Master Mix Kit (Stratagene). Real-time PCR was performed using Mx3000 (Stratagene) with gene-specific primers as previously described (1). Primers for human NPC1L1 are as follows: sense primer, 5′-TATCTTCCCTGGTTCCTGAACGAC-3′; antisense primer, 5′-CCGCAGAGCTTCTGTGTAATCC-3′. Actin was amplified as an internal control using gene-specific primers (sense primer, 5′-CATGTTTGAGACCTTCAACAC-3′; antisense primer, 5′-CCAGGAAGGAAGGCTGGAA-3′). Human SREBP2 mRNA was amplified using the following primers: 5′-CCCTGGGAGACATCGACGA-3′ and 5′-CGTTGCACTGAAGGGTCCA-3′.
Western blotting.
Total protein was extracted by suspending the cell pellet in a cell lysis buffer containing 50 mM Tris·HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, and 1 mM EDTA supplemented with protease inhibitor cocktail from Roche (Indianapolis, IN). The Bradford (8) assay was used to determine the protein concentration. Protein (100 μg) from both control and treated samples was subjected to 6% SDS-PAGE. The resolved proteins in the gel were transferred to a nitrocellulose membrane electrophoretically. The membrane was incubated first with anti-NPC1L1 antibody (1:250) from Santa Cruz (Santa Cruz, CA) or anti-actin antibodies (Sigma) and then with goat anti-rabbit secondary antibody (1:2,000) conjugated with horseradish peroxidase followed by ECL detection from Bio-Rad (Hercules, CA).
Transient transfection and luciferase assay.
Transient transfection and luciferase assay were performed as previously described by us (1). Briefly, Caco-2 cells (2 × 105) were seeded into 24-well plates and cotransfected while still in suspension with one of the human NPC1L1 promoter-luciferase constructs and pCMVβ, β-galactosidase mammalian expression vector (BD Biosciences Clontech, Palo Alto, CA), using FuGENE reagent from Roche. The latter plasmid served as an internal control for transfection efficiency. A total of 3 μg DNA/well, at a ratio of 5:1 for experimental vs. pCMVβ, was used for each transfection. In some experiments, Caco-2 cells were transfected using the Amaxa Nucleofector System (Amaxa) according to the manufacturer’s instructions. Briefly, ∼2 × 106 cells were harvested and then were electroporated in 100 μl of solution T (supplied by Amaxa) along with 8 μg of NPC1L1 promoter construct, 2 μg of pCMVβ, and various amounts of mammalian expression vector for SREBP2 that was obtained from ATCC. In some samples, cells were cotransfected with mammalian expression vector for c-fos transcription factor (a generous gift from Dr. Nancy Colburn; NCI, Frederick, MD). The cells were then transferred to full media and plated on 8 wells of a 24-well plate. After 24 h, cells were washed with PBS and lysed using a kit from Promega (Madison, WI). The activities of both firefly luciferase and β-galactosidase were measured by luminometer according to the manufacturer’s instructions by using kits from Promega and Clontech (Mountain View, CA), respectively. The promoter activity was expressed as a ratio of luciferase to β-galactosidase activity in each sample.
Nuclear protein extraction and gel shift assay.
Nuclear extracts from Caco-2 cells were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents from Pierce (Rockford, IL) according to the manufacturer’s instructions. The sequence of the double-stranded oligo used as a probe for the gel shift assay was designed as previously described by us (5′-TCGAGTCATCGAAGGGGAGGAGGCTGCCTTAAT-3′). Gel shift assay was performed using a DIG gel shift kit from Roche according to the manufacturer’s instructions. The double-stranded oligonucleotide was end labeled with digoxigenin-11, and binding reactions were carried out using 15 μg of nuclear extract. DNA-protein complexes were separated by electrophoresis in a nondenaturing 6% polyacrylamide gel at 4°C. The complexes were then electrotransferred onto a positively charged nylon membrane followed by detection with anti-digoxigenin antibody.
Statistical analysis.
Results are expressed as means ± SE. Student’s t-test was used for statistical analysis. A P value of 0.05 or less was considered statistically significant.
RESULTS
Ezetimibe-sensitive cholesterol uptake and esterification in Caco-2 cells.
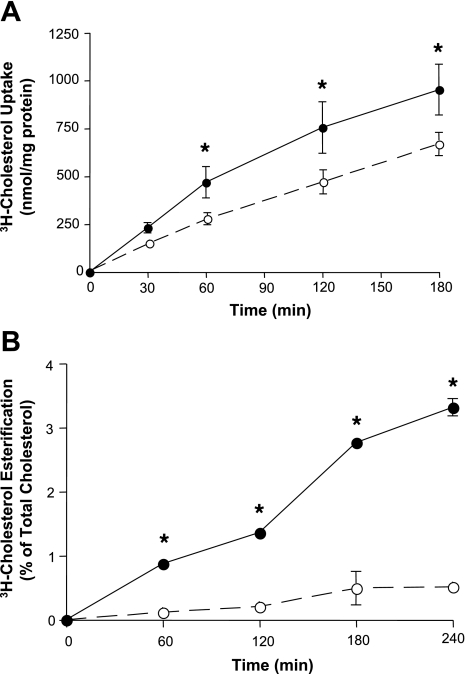
NPC1L1 has been shown to sense cholesterol in the brush-border membrane and then shuttle the absorbed cholesterol to the endoplasmic reticulum (ER) for esterification and packaging into chylomicrons to complete the absorption process (12, 17, 19, 38). Hence, NPC1L1 activity may be assessed by measuring the amount of total cholesterol uptake or by determining the rate of cholesterol esterification, since this represents the delivery of cholesterol by NPC1L1 to the ER. We investigated the effects of ezetimibe on both cholesterol uptake and esterification in human intestinal Caco-2 monolayers. As shown in Fig. 1A, cholesterol uptake was linear as a function of time up to 2 h and was significantly but modesty inhibited (∼20%) by 50 μM of ezetimibe. In contrast, ezetimibe significantly reduced cholesterol esterification by ∼90% at all the time points examined (Fig. 1B). These data clearly indicate that ezetimibe is a more potent inhibitor of cholesterol esterification compared with cholesterol uptake in human intestinal Caco-2 cells.
Fig. 1.
Inhibition of cholesterol uptake and esterification by ezetimibe. Caco-2 cells were preincubated for 30 min with (○) or without (●) 50 μM of ezetimibe and then incubated with solution containing micelles with 5 μM of [3H]cholesterol. Cells were then washed, and total radioactivity associated with the cells was measured as indicated in materials and methods (A). In other set of experiments (B), total lipids were extracted from the cells and then separated by thin-layer chromatography. Bands corresponding to free cholesterol and cholesteryl ester were scraped, and the associated radioactivity was counted. Ezetimibe-sensitive cholesterol esterification was then calculated as described in materials and methods. Data are presented (in B and A) as means ± SE from 4–6 values obtained from at least 3 separate occasions. Error bars are not shown when they are smaller than the symbol. *P < 0.05 or less compared with cells without ezetimibe in A and B.
Effect of curcumin on cholesterol uptake and esterification.
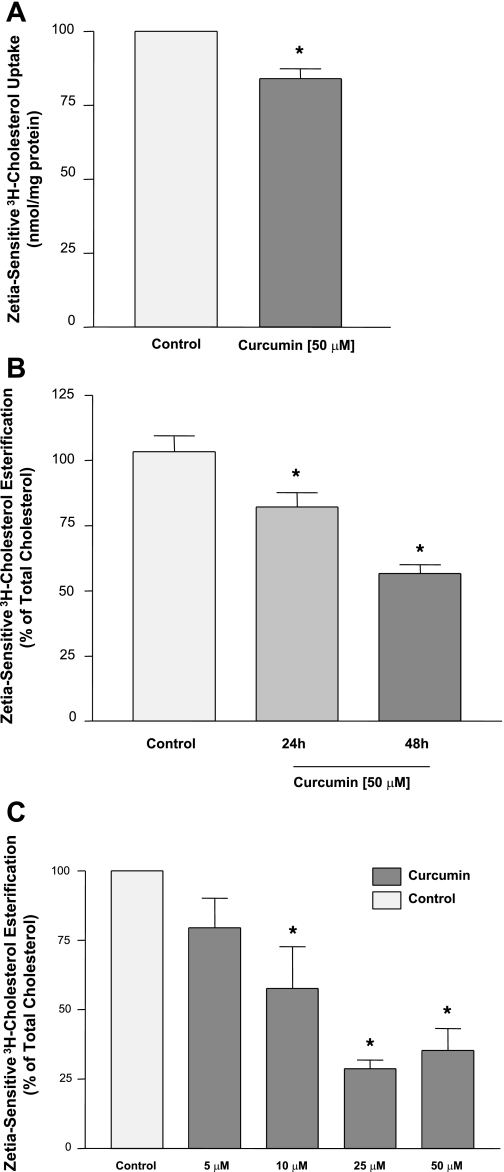
To assess the effects of curcumin on NPC1L1 function, we next examined its effects on cholesterol uptake and esterification in Caco-2 cells. As shown in Fig. 2A, ezetimibe-sensitive cholesterol uptake was moderately inhibited by ∼20% in response to 48 h incubation with 50 μM curcumin. However, incubation with 50 μM curcumin significantly reduced the ezetimibe-sensitive cholesterol esterification in a time-dependent manner with significantly higher inhibition at 48 h (Fig. 2B). Also, 48 h incubation with curcumin showed a concentration-dependent decrease in the rate of cholesterol esterification (Fig. 2C). These results show that the effect of curcumin on the ezetimibe-sensitive cholesterol esterification is more pronounced compared with its effect on ezetimibe-sensitive cholesterol uptake.
Fig. 2.
Effects of curcumin on cholesterol uptake and esterification. A: Caco-2 cells were incubated for 48 h with 50 μM of curcumin, and ezetimibe-sensitive cholesterol uptake was then measured as described in the legend for Fig. 1. B: Caco-2 monolayers were incubated with 50 μM curcumin for 24 or 48 h, and then ezetimibe-sensitive cholesterol esterification was assessed as described in the legend for Fig. 1. In C, cholesterol esterifiaction was measured after 48 h incubation with different concentrations of curcumin. Data presented are %control and are the mean ± SE of 6–8 values obtained from at least 3 separate occasions. *P < 0.05 or less compared with control.
Curcumin reduces NPC1L1 expression.
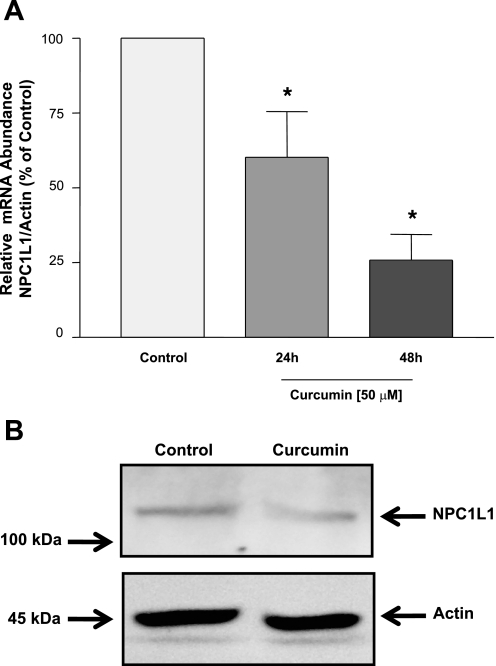
We next investigated the effect of curcumin on the expression of NPC1L1 expression in Caco-2 cells. As shown in Fig. 3A, NPC1L1 mRNA was significantly reduced by incubation with 50 μM curcumin in a time-dependent manner as assessed with real-time PCR. Western blot analysis also demonstrated that NPC1L1 protein was decreased by 48 h incubation with 50 μM curcumin (Fig. 3B). The results showed that, corresponding to the decrease in NPC1L1 function, NPC1L1 mRNA and protein expression are decreased by curcumin in intestinal epithelial cells.
Fig. 3.
Niemann-Pick C1-like 1 (NPC1L1) mRNA and protein expression is reduced by curcumin. A: Caco-2 cells were incubated for 24 or 48 h with 50 μM of curcumin, and total RNA was extracted. The relative expression of NPC1L1 mRNA normalized to actin mRNA was assessed by real-time RT-PCR. Data are presented as %control and represent the mean ± SE of 6–8 values obtained from at least 3 separate occasions. *P < 0.05 or less compared with control. B: Caco-2 monolayers were incubated with 50 μM curcumin for 48 h, and proteins were then extracted. Equal amounts of protein were separated on a 6% SDS-PAGE and electrotransferred to nitrocellulose membranes. Blots were then probed with anti-NPC1L1 or anti-actin antibodies, and bands were visualized as described in materials and methods. A representative of 3 different experiments is shown.
NPC1L1 promoter activity is reduced by curcumin.
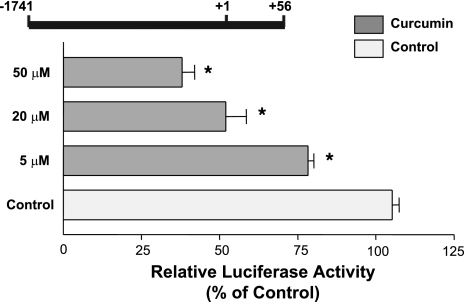
Because curcumin decreased NPC1L1 mRNA expression, we then investigated whether the reduction was mediated via transcriptional regulation. We have previously cloned promoter fragments of human NPC1L1 gene and analyzed their activities in Caco-2 cells (1). We first investigated the influence of curcumin on the activity of NPC1L1 promoter flanking the region between −1,741/+56 in the NPC1L1 gene (+1 represents the transcription initiation site). Caco-2 cells were transiently transfected with the promoter construct and were then treated for 24 h with different concentrations of curcumin (5, 20, and 50 μM), and the NPC1L1 promoter activity was determined by the firefly luciferase assay and the measurement of β-galactosidase to normalize for the transfection efficiency. As shown in Fig. 4, the NPC1L1 promoter activity was decreased significantly by curcumin in a concentration-dependent manner with a maximal inhibition (∼55%) occurring at 50 μM concentration. These results show that curcumin decreases NPC1L1 expression via the modulation of the promoter activity of the NPC1L1 gene.
Fig. 4.
Curcumin reduces NPC1L1 promoter activity. Intestinal Caco-2 cells were transiently transfected with human NPC1L1 promoter along with pCMVβ for β−galactosidase to normalize for the transfection efficiency. Twenty four hours posttransfection, cells were treated for 24 h with different concentrations of curcumin and harvested for firefly luciferase and β-glactosidase assays to assess the promoter activity. Results are shown as %control and represent the mean ± SE of 6–9 values obtained from at least 3 separate occasions. *P < 0.05 or less compared with control.
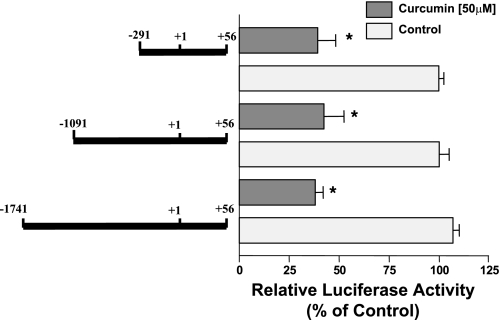
Mapping of curcumin response elements on the NPC1L1 promoter.
We next aimed to identify the curcumin response elements mediating the inhibitory effect of curcumin on NPC1L1 promoter activity. Caco-2 cells were transiently transfected with various constructs representing progressive 5′-deletions in the NPC1L1 promoter. Cells were then treated with curcumin (50 μM), and the promoter activity was measured by the luciferase assay. As shown in Fig. 5, curcumin treatment decreased the activity of the three promoter constructs, indicating that the cis-elements mediating the inhibition by curcumin are located in the shortest fragment flanking the region between −291 and +56 of the NPC1L1 promoter.
Fig. 5.
Mapping of curcumin response region in NPC1L1 promoter. Intestinal Caco-2 cells were transiently cotransfected with different deletion constructs of human NPC1L1 promoter and pCMVβ for β−galactosidase to normalize for the transfection efficiency. Twenty four hours posttransfection, cells were treated for 24 h with 50 μM curcumin and harvested for firefly luciferase and β-glactosidase assays to measure the promoter activity. Results are shown as %control and represent the mean ± SE of 6–9 values obtained from at least 3 separate occasions. *P < 0.05 or less compared with respective control.
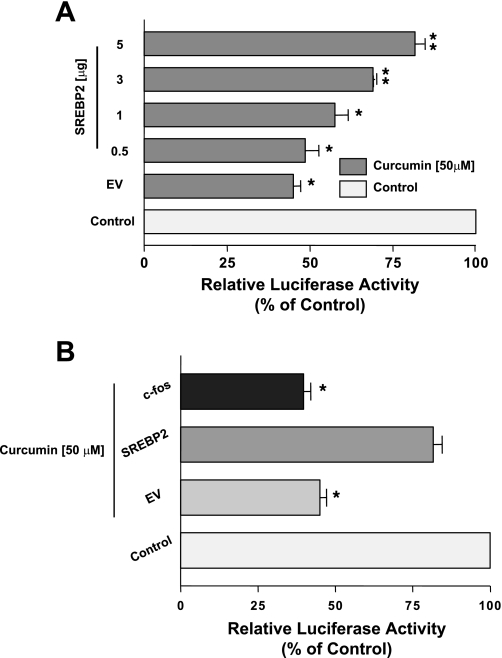
SREBP2 protects against the inhibitory effects of curcumin on NPC1L1 promoter activity.
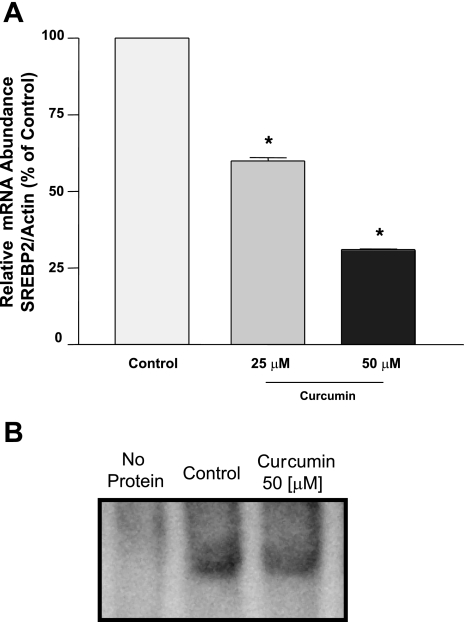
Previous studies from our laboratory showed that the −291/+56 region of the NPC1L1 gene harbors a binding site for the SREBP2 that is essential for the basal activity of the NPC1L1 promoter as well as the modulation by cholesterol (1). Previous studies have shown that curcumin inhibits SREBP2 expression (22). Therefore, we sought to determine whether the modulation of SREBP2 expression is involved in the inhibition of NPC1L1 promoter activity by curcumin. First, we examined changes in SREBP2 mRNA expression by curcumin in Caco-2 cells. As shown in Fig. 6A, treatment with curcumin significantly decreased SREBP2 mRNA expression in a dose-dependent manner. Furthermore, Fig. 6B shows that the DNA-binding activity of SREBP2 to the sterol-binding cis element of the NPC1L1 promoter is also decreased by curcumin treatment as judged by the gel shift assay analysis. We next examined the effect of curcumin on NPC1L1 promoter activity in the presence of constitutively active SREBP2. Previous studies from our laboratory have shown that NPC1L1 promoter activity is stimulated by the overexpression of active SREBP2 (1). Similarly, our current studies also showed an increase in the basal activity of NPC1L1 promoter by the overexpression of active SREBP2 transcription factor (8.2 ± 0.5- and 14.2 ± 0.7-fold increase by the cotransfection with 1 and 5 μg of SREBP2 expression vector, respectively). As depicted in Fig. 7A, increasing amounts of active SREBP2 overexpressed in Caco-2 cells reduced the curcumin-induced inhibition of NPC1L1 promoter activity. The protection against curcumin was specific, since the overexpression of unrelated transcription factor (c-fos) did not block the inhibitory effect of curcumin on NPC1L1 promoter activity (Fig. 7B). These data strongly suggest that curcumin modulates NPC1L1 expression by decreasing SREBP2 in intestinal Caco-2 cells.
Fig. 6.
Sterol response element-binding protein 2 (SREBP2) mRNA expression and DNA-binding activity are decreased by curcumin. A: intestinal Caco-2 monolayers were incubated for 48 h with different concentrations of curcumin, and total RNA was extracted. The relative expression of SREBP2 mRNA normalized to actin mRNA was assessed by real-time RT-PCR. Data are presented as %control and represent the mean ± SE of 6–8 values obtained from 3 separate occasions. *P < 0.05 or less compared with control. B: Caco-2 cells were treated with 50 μM curcumin for 48 h, and nuclear proteins were extracted. The nuclear extracts were incubated with DIG-labeled oligonucleotide representing the sterol response element (SRE) (−36/−26) of the NPC1L1 promoter. DNA-protein complexes from control and treated cells were examined by the gel mobility shift assay and are shown.
Fig. 7.
Active SREBP2 abrogates the inhibition of NPC1L1 promoter activity by curcumin. Caco-2 cells were transiently cotransfected by electroporation (Amaxa) with human NPC1L1 promoter along with different amounts of empty vector (EV) or mammalian expression vector for active SREBP2 (A) or mammalian expression vector for c-fos (B) with pCMVβ for β-galactosidase to normalize for the transfection efficiency. Cells were then treated with 50 μM curcumin for 24 h and harvested for firefly luciferase and β-glactosidase assays to assess the promoter activity. Results are shown as %control and represent the mean ± SE of 6–9 determinations performed on 3 separate occasions. *P < 0.05 or less compared with respective control. **P < 0.05 or less compared with empty vector.
DISCUSSION
The inhibition of intestinal cholesterol absorption efficiently lowers the level of plasma low density lipoprotein cholesterol (11, 35). Targeting the function of intestinal NPC1L1 by the recently discovered drug ezetimibe has been shown to be useful as a treatment for hypercholesterolemia (11). Current studies explored the regulatory mechanisms via which beneficial dietary supplement such as curcumin decreases NPC1L1 function and expression to unravel additional potential targets for the treatment of a high level of cholesterol. Our findings show that NPC1L1 function, expression, and promoter activity are reduced significantly by curcumin. The effect of curcumin on NPC1L1 promoter activity is associated with reduced expression and binding activity of the SREBP2. We have also shown that the overexpression of constitutively active SREBP2 blocked the inhibitory effects of curcumin, indicating that the inhibitory effect of curcumin is mediated via alterations in SREBP2 transcription factor.
Intestinal absorption of cholesterol is first initiated by its incorporation into the apical membrane of epithelial cells (35). Cholesterol is then shuttled to the ER for esterification by the acyl-CoA cholesterolacyltransferase and is then packaged into chylomicrons that exit the enterocytes to complete the absorption process (35). A number of studies have shown a significant (>50%) inhibition of total cellular cholesterol uptake by ezetimiibe in several cell culture models, suggesting that NPC1L1 mediates the cellular uptake of free cholesterol into the cell (9, 12, 37, 38). Our results, however, showed only modest (∼20%) inhibition of total cholesterol uptake into intestinal Caco-2 cells. The discrepancy between our results and the findings of others, with respect to the magnitude of ezetimibe inhibition of cholesterol uptake, might be contributed to the use of different cell types (hepatic vs. intestinal) (9, 17, 38) or to the difference between investigating the endogenously expressed NPC1L1 in Caco-2 cells vs. exogenously expressed NPC1L1 (17, 37). In contrast, our data showed that ezetimibe remarkably inhibited the rate of cholesterol esterification similar to those previously reported (17). Because ezetimibe specifically inhibits NPC1L1 but not ACAT2 (10, 17, 29), it is, therefore, conceivable to conclude that measuring ezetimibe-sensitive cholesterol esterification rather than measuring total cholesterol uptake represents a valid method to assess NPC1L1 activity. This conclusion is consistent with previous studies of Field et al. (17) in Caco-2 cells and is completely in agreement with recent findings demonstrating that NPC1L1 is indeed essential for microtubule-dependent shuttling of cholesterol from the apical plasma membrane.
Because NPC1L1 plays a pivotal role in mediating intestinal cholesterol absorption (6), we argued that a beneficial dietary supplement such as curcumin may directly influence its function. In this regard, curcumin and its derivatives are the major phenolic compounds found in curcuma plant species and are the main components of turmeric and curry spices (20). Curcumin has been shown to exhibit a variety of biological effects, including anti-diabetic, anti-inflammatory, and anti-cancer properties (20, 27). Curcumin has been shown to lower total plasma cholesterol and to prevent diet-induced hypercholesterolemia as well as high plasma cholesterol associated with diabetes mellitus (5, 27). The diversity in its biological actions arises from the divergent molecular effects of curcumin at the cellular level, including modulation of transcription factors (30). Because curcumin is poorly absorbed from the intestinal lumen (31), a direct modulation of intestinal epithelial cells by curcumin may be important for eliciting its beneficial properties. Previous studies have shown that curcumin feeding increased cholesterol excretion in the stool, suggesting a decrease in intestinal cholesterol absorption (28). Our findings clearly show the direct effect of curcumin on cholesterol handling in intestinal epithelial cells. Interestingly, incubation with 50 μM of curcumin for 48 h resulted in a remarkable decrease in the ezetimibe-sensitive rate of cholesterol esterification. The dose and duration of curcumin treatment are compatible with previous studies in various cell culture models (27, 39). This effect of curcumin on cholesterol esterification was concomitant with a decrease in NPC1L1 mRNA and protein expression in intestinal Caco-2 cells. Interestingly, curcumin caused a modest reduction (∼20%) in ezetimibe-sensitive cholesterol uptake, albeit NPC1L1 expression was reduced by >50%. These findings confirm the notion reported by others that NPC1L1, at least in ileal brush-border membranes, may not be essential for the initial incorporation of cholesterol in the plasma membrane, but it mediates intracellular cholesterol trafficking to the ER (17, 23).
The decrease in NPC1L1 expression by curcumin may result from a modulation of gene transcription. Our data showed that the promoter activity of the NPC1L1 gene is significantly inhibited to the same extent as the decrease in the mRNA expression by curcumin, suggesting the involvement of transcriptional regulation. Our data further mapped the curcumin-response elements on the NPC1L1 gene to the fragment flanking the region between −291/+56 bp of the NPC1L1 gene. We have previously shown that this region of the NPC1L1 gene harbors a sterol response element (SRE) that binds to the SREBP2 transcription factor and mediates the modulation of NPC1L1 promoter activity by cholesterol. Recent studies have shown that curcumin effects could be mediated via SREBP2 transcription factor (22). Our results clearly showed a decrease in SREBP2 expression by curcumin in Caco-2 cells associated with a reduction in its DNA-binding activity to the SRE of the NPC1L1 promoter as judged by the gel shift assay. The fact that the inhibitory effect of curcumin was abrogated by overexpressing SREBP2 strongly indicates the involvement of SREBP2 in mediating the inhibition of NPC1L1 function and expression by curcumin.
During the preparation of the current manuscript, Feng et al. (16) published a report showing the inhibition of NPC1L1 expression by curcumin in Caco-2 cells. Our data further confirm the inhibitory effect of curcumin and provide novel insights on the molecular mechanisms underlying the decrease of NPC1L1-mediated cholesterol absorption by curcumin. In this regard, previous studies from our laboratories and others have shown that SREBP2 transcription factor modulates the expression of intestinal NPC1L1 cholesterol transporter and intestinal apical sodium-dependent bile acid transporter in the same manner in response to alterations in the level of cholesterol (1, 2, 21, 33). Our current studies further establish the essential role of the SREBP2 in mediating the inhibition of intestinal cholesterol absorption by the beneficial dietary supplement curcumin. These novel findings not only unravel the molecular mechanisms involved in the cholesterol-lowering effect of curcumin but also reveal an additional important role for the intestinal SREBP2 pathway in modulating cholesterol balance in the body. It may be interesting in future studies to further scrutinize the roles of intestinal SREBP2 in other regulatory pathways in the intestine and to determine its contribution to the maintenance of cholesterol homeostasis in the body.
GRANTS
These studies were supported by grants from the Department of Veteran Affairs (W. A. Alrefai and P. K. Dudeja), National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-71596 (W. A. Alrefai), DK-54016, DK-81858, and P01 DK-067887 (P. K. Dudeja), and DK-74458 (R. K. Gill), and the Crohn’s and Colitis Foundation of America CCFA Ref. no. 1942 (S. Saksena).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Alrefai WA, Annaba F, Sarwar Z, Dwivedi A, Saksena S, Singla A, Dudeja PK, Gill RK. Modulation of human Niemann-Pick C1-like 1 gene expression by sterol: role of sterol regulatory element binding protein 2. Am J Physiol Gastrointest Liver Physiol 292: G369–G376, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Alrefai WA, Sarwar Z, Gill RG. Modulation of human ileal bile acid transporter by 25-hydroxycholesterol in Caco2 cells (Abstract). Gastroenterology 126: A297, 2004 [Google Scholar]
- 3. Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 303: 1201–1204, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, Paisley S, Chilcott J. Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation. Health Tech Assess 12: 1–212, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Arafa HM. Curcumin attenuates diet-induced hypercholesterolemia in rats. Med Sci Monit 11: BR228–B234, 2005 [PubMed] [Google Scholar]
- 6. Betters JL, Yu L. NPC1L1 and cholesterol transport. FEBS Lett 584: 2740–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 8. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 9. Brown JM, Rudel LL, Yu L. NPC1L1 (Niemann-Pick C1-like 1) mediates sterol-specific unidirectional transport of non-esterified cholesterol in McArdle-RH7777 hepatoma cells. Biochem J 406: 273–283, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burnett DA, Caplen MA, Davis HR, Jr, Burrier RE, Clader JW. 2-Azetidinones as inhibitors of cholesterol absorption. J Med Chem 37: 1733–1736, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Chan J, Kushwaha RS, Vandeberg JF, Vandeberg JL. Effect of ezetimibe on plasma cholesterol levels, cholesterol absorption, and secretion of biliary cholesterol in laboratory opossums with high and low responses to dietary cholesterol. Metab Clin Exp 57: 1645–1654, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu BB, Ge L, Xie C, Zhao Y, Miao HH, Wang J, Li BL, Song BL. Requirement of myosin Vb. Rab11a Rab11-FIP2 complex in cholesterol-regulated translocation of NPC1L1 to the cell surface. J Biol Chem 284: 22481–22490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem 280: 12710–12720, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Davis HR, Veltri EP. Zetia: inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to reduce intestinal cholesterol absorption and treat hyperlipidemia. J Atheroscl Thromb 14: 99–108, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Deushi M, Nomura M, Kawakami A, Haraguchi M, Ito M, Okazaki M, Ishii H, Yoshida M. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett 581: 5664–5670, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Feng D, Ohlsson L, Duan RD. Curcumin inhibits cholesterol uptake in Caco-2 cells by down-regulation of NPC1L1 expression (Abstract). Lipids Health Dis 9: 40, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Field FJ, Watt K, Mathur SN. Ezetimibe interferes with cholesterol trafficking from the plasma membrane to the endoplasmic reticulum in CaCo-2 cells. J Lipid Res 48: 1735–1745, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR, Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O’Neill KA, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci USA 102: 8132–8137, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ge L, Wang J, Qi W, Miao HH, Cao J, Qu YX, Li BL, Song BL. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab 7: 508–519, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 65: 1631–1652, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iwayanagi Y, Takada T, Suzuki H. HNF4alpha is a crucial modulator of the cholesterol-dependent regulation of NPC1L1. Pharm Res 25: 1134–1141, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Kang Q, Chen A. Curcumin inhibits srebp-2 expression in activated hepatic stellate cells in vitro by reducing the activity of specificity protein-1. Endocrinology 150: 5384–5394, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Labonte ED, Howles PN, Granholm NA, Rojas JC, Davies JP, Ioannou YA, Hui DY. Class B type I scavenger receptor is responsible for the high affinity cholesterol binding activity of intestinal brush border membrane vesicles. Biochim Biophys Acta 1771: 1132–1139, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lally S, Owens D, Tomkin GH. Genes that affect cholesterol synthesis, cholesterol absorption, and chylomicron assembly: the relationship between the liver and intestine in control and streptozotosin diabetic rats. Metab Clin Exp 56: 430–438, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Lally S, Tan CY, Owens D, Tomkin GH. Messenger RNA levels of genes involved in dysregulation of postprandial lipoproteins in type 2 diabetes: the role of Niemann-Pick C1-like 1, ATP-binding cassette, transporters G5 and G8, and of microsomal triglyceride transfer protein. Diabetologia 49: 1008–1016, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Olszanecki R, Jawien J, Gajda M, Mateuszuk L, Gebska A, Korabiowska M, Chlopicki S, Korbut R. Effect of curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J Physiol Pharmacol 56: 627–635, 2005 [PubMed] [Google Scholar]
- 27. Peschel D, Koerting R, Nass N. Curcumin induces changes in expression of genes involved in cholesterol homeostasis. J Nutr Biochem 18: 113–119, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Rao DS, Sekhara NC, Satyanarayana MN, Srinivasan M. Effect of curcumin on serum and liver cholesterol levels in the rat. J Nutr 100: 1307–1315, 1970 [DOI] [PubMed] [Google Scholar]
- 29. Salisbury BG, Davis HR, Burrier RE, Burnett DA, Bowkow G, Caplen MA, Clemmons AL, Compton DS, Hoos LM, McGregor DG. Hypocholesterolemic activity of a novel inhibitor of cholesterol absorption, SCH 48461. Atherosclerosis 115: 45–63, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Shishodia S, Singh T, Chaturvedi MM. Modulation of transcription factors by curcumin. Adv Exp Med Biol 595: 127–148, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Suresh D, Srinivasan K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Ind J Med Res 131: 682–691, 2000 [PubMed] [Google Scholar]
- 32. Temel RE, Brown JM, Ma Y, Tang W, Rudel LL, Ioannou YA, Davies JP, Yu L. Diosgenin stimulation of fecal cholesterol excretion in mice is not NPC1L1 dependent. J Lipid Res 50: 915–923, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas C, Landrier JF, Gaillard D, Grober J, Monnot MC, Athias A, Besnard P. Cholesterol-dependent down-regulation of mouse and human apical sodium-dependent bile acid transporter (ASBT) gene expression: molecular mechanism and physiological consequences. Gut, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Valasek MA, Clarke SL, Repa JJ. Fenofibrate reduces intestinal cholesterol absorption via PPARalpha-dependent modulation of NPC1L1 expression in mouse. J Lipid Res 48: 2725–2735, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Wang DQ. Regulation of intestinal cholesterol absorption. Ann Rev Physiol 69: 221–248, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Weingartner O, Lutjohann D, Bohm M, Laufs U. Relationship between cholesterol synthesis and intestinal absorption is associated with cardiovascular risk. Atherosclerosis 210: 362–365, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Yamanashi Y, Takada T, Suzuki H. Niemann-Pick C1-like 1 overexpression facilitates ezetimibe-sensitive cholesterol and beta-sitosterol uptake in CaCo-2 cells. J Pharmacol Exp Ther 320: 559–564, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Yu L, Bharadwaj S, Brown JM, Ma Y, Du W, Davis MA, Michaely P, Liu P, Willingham MC, Rudel LL. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J Biol Chem 281: 6616–6624, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Yuan HY, Kuang SY, Zheng X, Ling HY, Yang YB, Yan PK, Li K, Liao DF. Curcumin inhibits cellular cholesterol accumulation by regulating SREBP-1/caveolin-1 signaling pathway in vascular smooth muscle cells. Acta Pharm Sin 29: 555–563, 2008 [DOI] [PubMed] [Google Scholar]