Abstract
The role of mitogen-activated protein kinases (MAPK) in the mechanism of EGF-mediated prevention of acetaldehyde-induced tight junction disruption was evaluated in Caco-2 cell monolayers. Pretreatment of cell monolayers with EGF attenuated acetaldehyde-induced decrease in resistance and increase in inulin permeability and redistribution of occludin, zona occludens-1 (ZO-1), E-cadherin, and β-catenin from the intercellular junctions. EGF rapidly increased the levels of phospho-ERK1/2, phospho-p38 MAPK, and phospho-JNK1. Pretreatment of cell monolayers with U-0126 (inhibitor of ERK activation), but not SB-202190 and SP-600125 (p38 MAPK and JNK inhibitors), significantly attenuated EGF-mediated prevention of acetaldehyde-induced changes in resistance, inulin permeability, and redistribution of occludin and ZO-1. U-0126, but not SB-202190 and SP-600125, also attenuated EGF-mediated prevention of acetaldehyde effect on the midregion F-actin ring. However, EGF-mediated preservation of junctional distribution of E-cadherin and β-catenin was unaffected by all three inhibitors. Expression of wild-type or constitutively active MEK1 attenuated acetaldehyde-induced redistribution of occludin and ZO-1, whereas dominant-negative MEK1 prevented EGF-mediated preservation of occludin and ZO-1 in acetaldehyde-treated cells. MEK1 expression did not alter E-cadherin distribution in acetaldehyde-treated cells in the presence or absence of EGF. Furthermore, EGF attenuated acetaldehyde-induced tyrosine-phosphorylation of occludin, ZO-1, claudin-3, and E-cadherin. U-0126, but not SB-202190 and SP-600125, prevented EGF effect on tyrosine-phosphorylation of occludin and ZO-1, but not claudin-3, E-cadherin, or β-catenin. These results indicate that EGF-mediated protection of tight junctions from acetaldehyde requires the activity of ERK1/2, but not p38 MAPK or JNK1/2, and that EGF-mediated protection of adherens junctions is independent of MAPK activities.
Keywords: tight junction, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase, acetaldehyde, epidermal growth factor, c-Jun NH2-terminal kinase, occludin, zona occludens-1, claudin
gastrointestinal (gi) mucosal barrier function prevents the diffusion of toxins, allergens, and pathogens from the lumen into the mucosal tissue. Tight junctions (TJs) are the epithelial structures that act as physical and functional barrier against the paracellular penetration of macromolecules from the lumen (3, 5). Immediately basal to the TJ is the adherens junction (AJ), which is responsible for intercellular adhesion between neighboring cells. Although they do not form a physical barrier for diffusion of macromolecules, AJs indirectly regulate the integrity of TJs. Therefore, disruption of AJs leads to disruption of TJs (38). Both TJs and AJs associate with perijunctional actin cytoskeleton and signaling molecules through multiprotein complexes to form an integrated functional unit (25). The TJ is assembled by the organization of at least three types of transmembrane proteins, occludin, claudins, and junctional adhesion molecules, which interact with cytoplasmic scaffold protein such as zona occludens (ZO)-1, ZO-2, and ZO-3 (3).
Increased intestinal permeability and endotoxemia play crucial roles in the pathogenesis of alcoholic liver disease (27, 33). Acetaldehyde, a highly mutagenic and carcinogenic molecule, is produced in the intestinal lumen at high concentrations (17, 34, 43). High levels of alcohol dehydrogenase and low levels of aldehyde dehydrogenase in enteric bacteria are responsible for such an accumulation of acetaldehyde in the colonic lumen (22). It was demonstrated that acetaldehyde production is involved in the ethanol-induced increase in gut permeability in rats in vivo (15). This study also showed the role of acetaldehyde in barrier disruption in rat intestine in vitro in Ussing chambers. Our laboratory's previous studies showed that acetaldehyde (100–500 μM) disrupts TJs and increases paracellular permeability in Caco-2 cell monolayers by a tyrosine kinase-dependent mechanism (4, 30, 36, 40). Acetaldehyde induces tyrosine-phosphorylation of occludin, ZO-1, E-cadherin, and β-catenin and dissociates these proteins from the actin-rich, detergent-insoluble fractions (30, 39).
Epidermal growth factor (EGF), a GI mucosal protective factor, is secreted in saliva and other GI secretions (31). EGF protects the GI mucosa from a variety of insults (19, 23, 28, 29). Our laboratory's previous study demonstrated that EGF prevents acetaldehyde-induced disruption of TJs in Caco-2 cell monolayers (36). EGF-mediated protection of TJs from acetaldehyde was mediated by PLC-γ-induced activation of PKC-βI and PKC-ε (42). Another signaling pathway that is well known to be involved in EGF activity is mitogen-activated protein kinase (MAPK) pathway.
Three well-characterized MAPK pathways in different cells are ERK, p38 MAPK, and JNK signaling pathways (11). ERK-MAPKs are activated by several extracellular stimuli, mainly growth factors, cytokines, and oxidative stress. JNKs or stress-activated protein kinases respond to different types of stress to the cells. The p38 MAPK is activated in response to inflammatory mediators. These different MAPK cascades show a high degree of functional specificity (20, 24). Our laboratory's previous study indicated the ERK plays a role in EGF-mediated protection of TJs from hydrogen peroxide (6).
In the present study, we determined the role of MAPKs in EGF-mediated protection of TJs from acetaldehyde in Caco-2 cell monolayers. These results show that ERK, but not p38 MAPK or JNK, plays a role in EGF-mediated protection of TJs from acetaldehyde.
MATERIALS AND METHODS
Chemicals.
Cell culture media and related reagents, including precast SDS-polyacrylamide gels, were purchased from Invitrogen (Carlsbad, CA). Acetaldehyde, cyanamide, fluorescein isothiocyanate (FITC)-inulin, leupeptin, aprotinin, bestatin, pepstatin A, phenylmethylsulfonyl fluoride (PMSF), Triton X-100, EGF, and vanadate were purchased from Sigma Chemical (St. Louis, MO). SP-600125, SB-202190, and U-0126 were purchased from Calbiochem (San Diego, CA). Other chemicals of analytic grade were purchased from Fisher Scientific (Tustin, CA).
Antibodies.
Rabbit polyclonal anti-JNK1/2 (pTpY183/185) and anti-green fluorescent protein (GFP) antibodies were purchased from Biosource/Invitrogen (Carlsbad, CA). Rabbit polyclonal anti-ERK (pTpY202/204) and anti-p38 MAPK (pTpY180/182) were purchased from Cell Signaling Technology (Beverly, MA). Rabbit polyclonal anti-JNK/SAPK1 was purchased from Upstate Biotechnology (Lake Placid, NY). Mouse monoclonal anti-E-cadherin and anti-β-catenin antibodies were purchased from BD Transduction Laboratories (San Jose, CA). Horseradish peroxidase (HRP)-conjugated anti-mouse IgG, HRP-conjugated anti-rabbit IgG, and anti-β-actin antibodies were obtained from Sigma Chemical (St. Louis, MO). Rabbit polyclonal anti-ZO-1 and anti-occludin antibodies, and mouse monoclonal anti-ZO-1 and anti-ERK1/2 antibodies, were from Zymed Laboratories (San Francisco, CA). Alexa Fluor-488-conjugated anti-mouse IgG, Cy3-conjugated anti-rabbit IgG, and Alexa Fluor-488-conjugated phalloidin were purchased from Molecular Probes (Eugene, OR). Mouse anti-GFP antibody was purchased from Clontech (Mountain View, CA).
Cell culture.
Caco-2 cells purchased from American Type Cell Collection (Rockville, MD) were grown under standard cell culture conditions, as previously described (32). Cells were grown on polyester membranes in Transwell inserts (6.5-, 12-, or 24-mm diameter; Costar, Cambridge, MA), and experiments were conducted on days 11–13 (6.5 mm), days 13–15 (12 mm), and days 17–19 (24-mm Transwells) postseeding.
Acetaldehyde and inhibitor treatment.
Acetaldehyde was administered by exposing cell monolayers bathed in phosphate-buffered saline (Dulbecco's saline containing 1.2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 0.6% bovine serum albumin) to vapor phase acetaldehyde, as described previously (4, 30), to achieve acetaldehyde concentration of 300 μM in the buffer bathing the cell monolayer. Briefly, cell monolayers were treated with vapor phase acetaldehyde by placing stock acetaldehyde solution (0.3%) in the reservoir wells and sealing the lid to the plate with tapes. EGF and inhibitors were administered to the apical and basal compartments. Cell monolayers were incubated with MAPK inhibitors 10 μM U-0126 or 10 μM SB-202190 or 1 μM SP-600125 for 50 min before EGF administration. EGF (30 nM) was administered to both apical and basal wells 10 min before acetaldehyde treatment. This concentration of EGF is well within the physiological level of EGF in salivary and GI secretions (31).
MEK constructs.
Tetracycline responsive element (TRE) containing expression vector with AcGFP gene was prepared in our laboratory, as described recently (2). Briefly, commercially available pTRE2hyg vector (Clontech) was digested at SalI and EcoRV sites, and the GFP gene, PCR-cloned from pAcGFP1-N1 plasmid (Clontech, Mountain View, CA), was inserted to generate pTRE2hyg-GFP-N1. MEK1 sequences, wild type, dominant negative (MEK1K97M), and constitutively active (MEK1S218E,S220D) with cohesive BamHI and MluI sites were inserted into pAcGFP1-N1 vector, as described recently (2). The plasmids were sequenced, and the expression of various mutants of MEK upon their transfection into the cells was analyzed by RT-PCR.
Cloning of Tet-On Caco-2 cell line and transfection of MEK constructs.
Caco-2 cells at 60–70% confluence were transfected with commercially available pTet-on vector (Clontech, Mountain View, CA), using lipofectamine LTX and plus reagent. The cells were then selected with G0418 sulfate (700 μg/ml). After 14 days, the colonies of cells were cloned, cultured, and maintained in a lower concentration (350 μg/ml) of G0418 sulfate. These clones were characterized by immunoblotting the whole cell lysate for Tet-R protein, and by luciferase assay for doxycycline-induced expression. MEK1 constructs were transfected to pTet-On Caco-2 cells, as described above, and the transfected cells were selected by incubation with hygromycin. Expression of MEK1 was induced by incubation with doxycycline for 6–18 h. Expression was assessed by analysis for GFP by immunofluorescence staining.
Measurement of transepithelial electrical resistance.
Transepithelial electrical resistance (TER) was measured as described previously (28) using a Millicell Electrical Resistance System (Millipore, Bedford, MA). TER was calculated as ohm times centimeter squared (Ω·cm2) by multiplying it with the surface area of the monolayer. The TER of the polyester membrane in Transwells (∼30 Ω·cm2) was subtracted from all readings.
Unidirectional flux of inulin.
Transwells with the cell monolayers were incubated under different experimental conditions in the presence of FITC-inulin (0.5 mg/ml) in the basal well. At 5 h after acetaldehyde treatment, 100 μl of apical and basal media were withdrawn, and fluorescence was measured using a fluorescence plate reader (BioTEK Instruments, Winooski, VT). The flux into the apical well was calculated as the percentage of total fluorescence administered into the basal well per hour per centimeter squared surface area.
Preparation of detergent-insoluble fractions.
Triton-insoluble fractions were prepared as previously described (7). Cell monolayers in Transwells (12 or 24 mm) were washed twice with ice-cold PBS and incubated for 5 min with cold lysis buffer-CS (20 mM Tris buffer containing 1.0% Triton X-100, 2 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml bestatin, 10 μg/ml pepstatin A, 1 mM vanadate, and 1 mM PMSF) at 4°C for 10 min. Cell lysates were centrifuged at 15,600 g for 4 min at 4°C to sediment the high-density actin cytoskeleton-rich fraction. Supernatant collected was Triton-soluble fraction. The pellet was resuspended in 200 μl of lysis buffer-D (0.3% SDS in 10 mM Tris buffer, pH 7.4, 10 mM sodium fluoride, 1 mM PMSF, 1 mM vanadate, and 10 μl/ml protease inhibitor cocktail). Protein contents in different fractions were measured by BCA method (Pierce Biotechnology, Rockford, IL). Triton-insoluble and Triton-soluble fractions were mixed with equal volume of Laemmli's sample buffer (2× concentrated), heated at 100°C for 5 min, and stored until immunoblot analysis.
Confocal immunofluorescence microscopy.
Under various experimental conditions, cell monolayers (6.5 mm/12 mm) were quickly washed in cold PBS and fixed in 3% paraformaldehyde (pH 7) at room temperature for 15 min. Cell monolayers were blocked in 4% nonfat milk or 1% BSA in TBS-Tween (20 mM Tris, pH 8.0, containing 150 mM NaCl and 0.5% Tween 20) and incubated for 1 h with primary antibodies, mouse monoclonal anti-occludin and rabbit polyclonal anti-ZO-1 antibodies/mouse monoclonal anti-E-cadherin and rabbit polyclonal anti-β-catenin antibodies, followed by incubation for 1 h with secondary antibodies, Alexa Fluor 488-conjugated anti-mouse IgG, and Cy3-conjugated anti-rabbit IgG antibodies. Alexa Fluor 488-conjugated phalloidin was used to stain F-actin. The fluorescence was examined by using a Zeiss LSM 5 laser scanning confocal microscope, and images from x-y sections (1 μm) were collected using LSM 5 Pascal software. Images were stacked using the software, Image J (National Institutes of Health) and processed by Adobe Photoshop (Adobe Systems, San Jose, CA).
Immunoprecipitation.
Cell monolayers in Transwells (12 or 24 mm) were washed twice with ice-cold PBS, and proteins were extracted with hot lysis buffer-D by heating at 100°C for 10 min, as described previously (32). Phospho-tyrosine was immunoprecipitated from the extracts, as described previously (32), using biotin conjugated anti-phospho-tyrosine antibody. Immunocomplexes were isolated using streptavidin-agarose and immunoblotted for different TJ and AJ proteins using specific antibodies.
Immunoblot analysis.
Cell extracts heated in Laemmli's sample buffer were separated by SDS-polyacrylamide gel (7% gradient) electrophoresis and transferred to polyvinylidene diflouride membranes. Membranes were blotted for phosphorylated (p)-ERK, p-JNK, ERK1/2, JNK1/2, p38 MAPK, and β-actin using specific antibodies in combination with HRP-conjugated anti-mouse IgG or HRP-conjugated anti-rabbit IgG antibodies. The blots were developed using ECL chemiluminescence method (Amersham, Arlington Heights, IL).
Statistics.
Comparison between two groups was made by Student's t-tests for grouped data. Significance in all tests was set at 95% or greater confidence level.
RESULTS
EGF activates different MAPKs in Caco-2 cell monolayers.
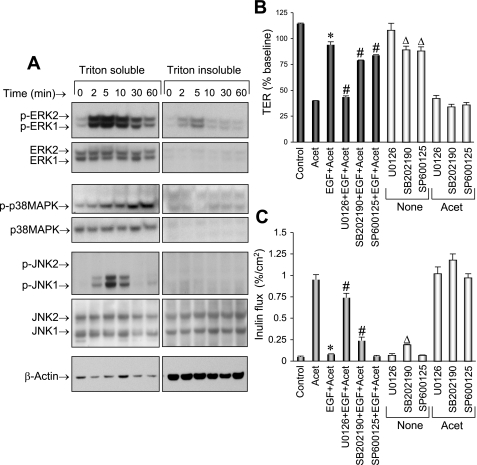
To determine the effect of EGF on activation of MAPKs, protein extracts prepared from cells incubated with EGF for varying times were immunoblotted for p-ERK1/2, p-JNK1/2, and p-p38 MAPK (activated MAPKs). EGF treatment rapidly increased the levels of p-ERK1/2, p-JNK1, and p-p38 MAPK (Fig. 1A) predominantly in the detergent-soluble fractions. ERK activation was much more rapid than JNK1 and p38 MAPK activation. Peak levels of p-ERK, p-p38 MAPK, and p-JNK were recorded at 2, 5, and 30 min, respectively. Activations of ERK and JNK1 were transient, with the activity fading by ∼30 min, while p38 MAPK activation sustained at least until 60 min. Significant level of p-ERK was present in the detergent-insoluble fraction, while p-p38 MAPK and p-JNK were undetectable in detergent-insoluble fraction.
Fig. 1.
EGF attenuates acetaldehyde (Acet)-induced barrier disruption by a MAPK-dependent mechanism. A: Caco-2 cell monolayers were incubated with EGF for varying times, and protein extracts were immunoblotted for phosphorylated (p) and total ERK, p38 MAPK, and JNK. B and C: Caco-2 cell monolayers were preincubated with or without MAPK inhibitors U-0126 (10 μM), SB-202190 (10 μM), or SP-600125 (1 μM) for 50 min, followed by incubation with or without EGF (30 nM). Ten minutes after EGF administration, cell monolayers were incubated with or without acetaldehyde, as described in material and methods. Tight junction (TJ) integrity was evaluated by measuring transepithelial electrical resistance (TER; B) and unidirectional inulin flux (C). Control experiments were performed by evaluating the effect of different inhibitors on TER (B) and inulin flux (C) in the presence or absence of acetaldehyde. Values are means ± SE (n = 6). *Values that are significantly different (P < 0.05) from corresponding value for acetaldehyde group. #Values that are significantly different (P < 0.05) from corresponding values for EGF + acetaldehyde group. Δ Values that are significantly different (P < 0.05) from corresponding values for control group.
Activity of ERK, but not p38 MAPK or JNK, mediates prevention of acetaldehyde-induced barrier dysfunction by EGF.
Previous studies showed that EGF prevents acetaldehyde-induced increase in paracellular permeability in Caco-2 monolayers (40). The present study confirms this effect of EGF on acetaldehyde-induced decrease in TER (Fig. 1B) and increase in inulin permeability (Fig. 1C). The present study also shows that pretreatment of cell monolayers with U-0126 (ERK inhibitor) significantly attenuates EGF-mediated prevention of acetaldehyde-induced changes in TER and inulin permeability. Pretreatment of cells with SP-600125 (JNK inhibitor) or SB-202190 (p38 MAPK inhibitor) produced only a minor influence on EGF-mediated prevention of acetaldehyde-induced changes in TER and inulin permeability. Control experiments showed that SB-202190 and SP-600125 slightly but significantly affected TER and/or inulin flux in the absence or presence of acetaldehyde. While the effect of U-0126 was dose dependent, the minor effect of SP-600125 or SB-202190 on TER and inulin flux was not dose dependent (data not shown), indicating that the minor effects seen with SP-600125 and SB-202190 are nonspecific responses. EGF alone did not produce a significant change in TER or inulin permeability, although a tendency for slight enhancement of barrier function was often observed.
ERK activation is involved in EGF-mediated protection of TJs from acetaldehyde.
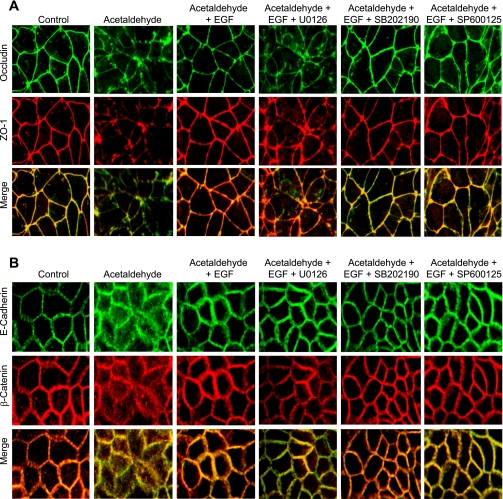
Confocal immunofluorescence microscopy showed that acetaldehyde treatment results in the redistribution of occludin and ZO-1 from the intercellular junctions into the intracellular compartments (Fig. 2A). Treatment of cell monolayers with EGF ameliorates this effect of acetaldehyde. Pretreatment of cell monolayers with U-0126, but not SB-202190 or SP-600125, attenuated EGF-mediated prevention of acetaldehyde-induced redistribution of occludin and ZO-1 from the intercellular junctions (Fig. 2A). EGF treatment also attenuated acetaldehyde-induced redistribution of E-cadherin and β-catenin from the intercellular junctions into the intracellular compartments (Fig. 2B). However, U-0126, SB-202190, or SP-600125 did not alter EGF-mediated preservation of junctional distribution of E-cadherin and β-catenin in acetaldehyde-treated cell monolayers (Fig. 2B).
Fig. 2.
Role of MAPK in EGF-mediated attenuation of acetaldehyde-induced disruption of TJ and adherens junction (AJ). Caco-2 cell monolayers were preincubated with or without MAPK inhibitors U-0126 (10 μM), SB-202190 (10 μM), or SP-600125 (1 μM) for 50 min, followed by incubation with or without EGF (30 nM). Ten minutes after EGF administration, cell monolayers were incubated with or without acetaldehyde, as described in materials and methods. Cell monolayers were fixed and stained for occludin and zona occludens-1 (ZO-1; A) or E-cadherin and β-catenin (B). Fluorescence images were collected by using a confocal laser scanning microscope.
EGF prevents acetaldehyde-induced reorganization of actin cytoskeleton by an ERK-dependent mechanism.
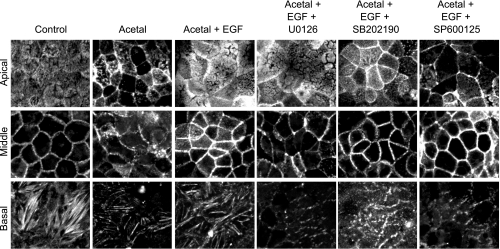
Our laboratory's previous study (40) showed that acetaldehyde induces reorganization of actin cytoskeleton, and EGF pretreatment prevented this effect of acetaldehyde. The present study confirms that EGF protects the actin cytoskeletal organization at the apical (microvilli), middle (actomyosin ring), and basal (stress fibers) regions of Caco-2 cells. In addition, the present study shows that pretreatment of cells with U-0126 prevents EGF-mediated preservation of actin cytoskeletal structure at the midregion of cells (Fig. 3). U-0126, however, did not prevent EGF effect on the actin cytoskeleton at the apical region of the cells. SB-202190 did not influence the EGF effect on actin cytoskeleton at all three regions. SP-600125 did prevent the EGF effect on actin cytoskeletal organization at the apical and basal parts of the cell. On the other hand, SP-600125 may have enhanced the actin organization at the midregion of cells (Fig. 3).
Fig. 3.
EGF attenuates acetaldehyde-induced remodeling of actin cytoskeleton by a MAPK-dependent mechanism. Caco-2 cell monolayers were preincubated without or with MAPK inhibitors U-0126 (10 μM), SB-202190 (10 μM), or SP-600125 (1 μM) for 50 min, followed by incubation with or without EGF (30 nM). Ten minutes after EGF administration, cell monolayers were incubated with or without acetaldehyde, as described in materials and methods. Cell monolayers were fixed and stained for F-actin. Fluorescence images were collected by using a confocal laser scanning microscope. Images from 2-μm-thick laser sections from the apical, middle, and basal regions of cell were processed.
Regulated expression of wild-type and constitutively active MEK1 ameliorates acetaldehyde-induced disruption of TJs, whereas dominant-negative MEK1 attenuates EGF-mediated protection of TJs.
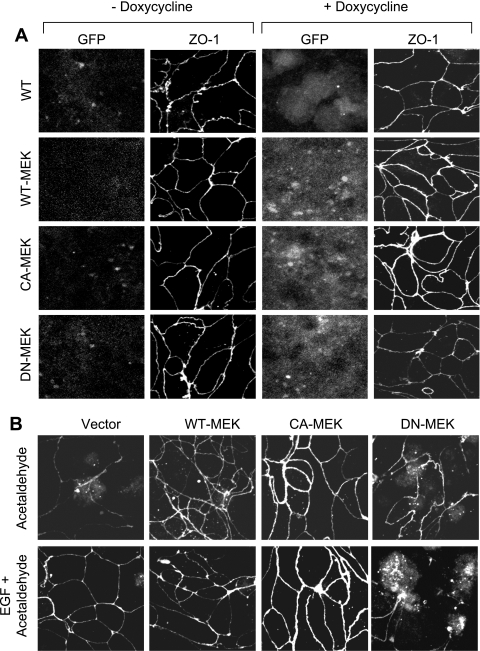
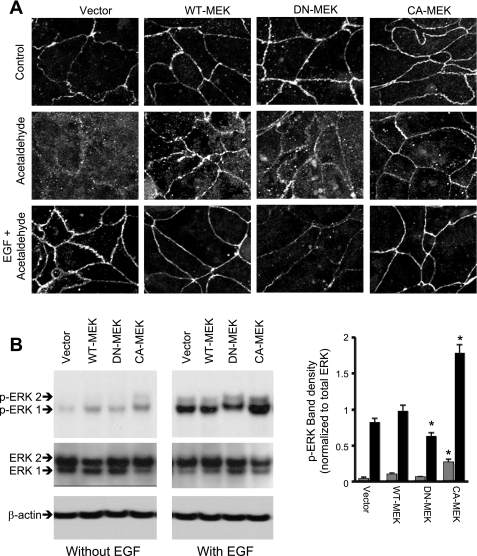
The above-described observation indicates that ERK, but not p38 MAPK or JNK, is involved in the mechanism of EGF-mediated protection of TJs from acetaldehyde in Caco-2 cell monolayers. MEK1, the immediate upstream signal, is known to activate ERK by dual phosphorylation on Tyr and Thr residues. To further confirm the role of ERK-dependent MAPK pathway in EGF-mediated protection of TJs from acetaldehyde, we expressed WT-MEK (wild type), DN-MEK (MEK1K97M, dominant negative), or CA-MEK (MEK1S218E,S220D, constitutively active) as GFP-fusion proteins in Caco-2 cells by a doxycycline-regulated mechanism. Expression of MEK in cell lines that stably express pTet-ON was confirmed by immunofluorescence staining for GFP before and 18 h after addition of doxycycline (Fig. 4A). Confocal microscopy revealed that expression of WT-MEK and CA-MEK resulted in enhanced junctional localization of ZO-1, compared with cells expressing vector alone or DN-MEK.
Fig. 4.
Expression of active MEK1 ameliorates acetaldehyde-induced loss of junctional distribution of ZO-1, and inactive MEK1 attenuates EGF-mediated protection of ZO-1 distribution. A: Caco-2 cells stably expressing Tet-On regulator were transfected with green fluorescent protein (GFP) or GFP-tagged MEK1 [wild type (WT), dominant negative (DN), or constitutively active (CA)] in pTRE2hyg-GFP-N1 vector, and their expression was induced by doxycycline administration. Expression of GFP before and after doxycycline was visualized by immunofluorescence staining and confocal microscopy for GFP and ZO-1. B: Caco-2 cells expressing WT-MEK, DN-MEK, or CA-MEK were preincubated with or without EGF (30 nM). Ten minutes after EGF administration, cell monolayers were incubated with or without acetaldehyde, as described in materials and methods. Cell monolayers were fixed and stained for GFP and ZO-1 (A). Fluorescence images were collected by using a confocal laser scanning microscope.
Acetaldehyde treatment resulted in redistribution of ZO-1 (Fig. 4B) from the intercellular junctions in cells expressing empty vector or DN-MEK (Fig. 4B), whereas junctional distribution of ZO-1 (Fig. 4B) was preserved in acetaldehyde-treated cells expressing WT-MEK and CA-MEK. EGF treatment ameliorated acetaldehyde-induced disruption of junctional organization of ZO-1 in vector-transfected cells, whereas EGF failed to prevent acetaldehyde-induced redistribution of ZO-1 in cells expressing DN-MEK (Fig. 4B). Similarly, expression of WT-MEK and CA-MEK preserved junctional distribution of occludin in acetaldehyde-treated cell monolayers, while the expression of DN-MEK prevented EGF-mediated protection of occludin distribution from acetaldehyde (Fig. 5A). Expression of constitutively active MEK1 showed significantly higher level of p-ERK in the control and EGF-treated cell monolayers (Fig. 5B). The moderate difference in the p-ERK levels between groups is likely due to homeostatic mechanisms within the cell to maintain ERK activity at low levels.
Fig. 5.
Expression of active MEK1 ameliorates acetaldehyde-induced loss of junctional distribution of occludin, and inactive MEK1 attenuates EGF-mediated protection of occludin distribution. A: Caco-2 cells expressing WT-MEK, DN-MEK, or CA-MEK were preincubated with or without EGF (30 nM). Ten minutes after EGF administration, cell monolayers were incubated with or without acetaldehyde, as described in materials and methods. Cell monolayers were fixed and stained for occludin. Fluorescence images were collected by using a confocal laser scanning microscope. B: cells transfected with different MEK constructs were incubated with or without EGF for 5 min. Proteins extracted from these cells were immunoblotted for p-ERK and total ERK. Bands for p-ERK were evaluated by densitometric analysis and normalized by dividing with density for corresponding band of total ERK. Values are means ± SE (n = 3). *Values that are significantly different (P < 0.05) from corresponding value for vector group.
EGF prevents acetaldehyde-induced redistribution of E-cadherin and β-catenin by a mechanism independent of ERK.
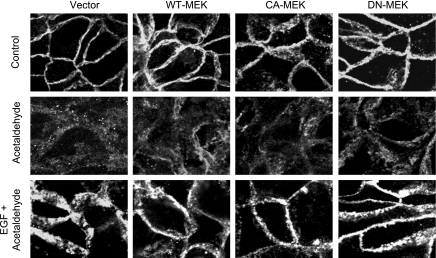
Unlike occludin and ZO-1, E-cadherin was distributed at the intercellular junctions similarly in vector-transfected cell monolayers and those that express WT-MEK, CA-MEK, or DN-MEK (Fig. 6). Acetaldehyde induced redistribution of E-cadherin in cell monolayers expressing different forms of MEK. Furthermore, EGF treatment ameliorated acetaldehyde-induced redistribution of E-cadherin in vector, DN-MEK, WT-MEK, or CA-MEK expressing cell monolayers, and no significant difference was observed in the EGF effect on E-cadherin distribution in cells expressing different forms of MEK (Fig. 6).
Fig. 6.
MEK1 expression does not affect the junctional distribution of E-cadherin. Caco-2 cells expressing WT-MEK, DN-MEK, or CA-MEK were preincubated with or without EGF (30 nM). Ten minutes after EGF administration, cell monolayers were incubated with or without acetaldehyde, as described in materials and methods. Cell monolayers were fixed and stained for E-cadherin. Fluorescence images were collected by using a confocal laser scanning microscope.
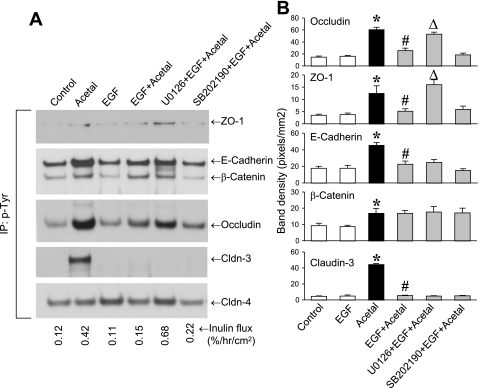
EGF attenuates acetaldehyde-induced tyrosine-phosphorylation of occludin and ZO-1 by an ERK-dependent mechanism.
Our laboratory's previous study has showed that acetaldehyde induces tyrosine-phosphorylation of ZO-1, E-cadherin, and β-catenin (4). In the present study, we investigated whether EGF prevents such tyrosine-phosphorylation of junctional proteins and determined whether ERK plays a role in this effect of EGF. The results show that EGF treatment attenuates acetaldehyde-induced increase in tyrosine-phosphorylation of occludin, ZO-1, E-cadherin, and claudin-3, but tyrosine-phosphorylation of β-catenin was unaffected (Fig. 7). Pretreatment of cells with U-0126, but not SB-202190, attenuated EGF-mediated prevention of tyrosine-phosphorylation of occludin and ZO-1 (Fig. 7B). U-0126, however, did not prevent the EGF effect on tyrosine-phosphorylation of E-cadherin and claudin-3.
Fig. 7.
EGF attenuates acetaldehyde-induced tyrosine-phosphorylation of TJ proteins by a MAPK-dependent mechanism. A: Caco-2 cell monolayers were preincubated with or without MAPK inhibitors for 50 min, followed by incubation with or without EGF (30 nM). Ten minutes later, cell monolayers were incubated with or without acetaldehyde, as described in materials and methods. Phospho-tyrosine was immunoprecipitated (IP) from denatured protein extracts and immunoblotted for TJ and AJ proteins. B: quantitative analysis of different bands by densitometry is presented. Values are means ± SE (n = 3). *Values that are significantly different (P < 0.05) from corresponding control values. Values that are significantly different (P < 0.05) from corresponding values for #acetaldehyde or Δ acetaldehyde + EGF groups.
DISCUSSION
A significant body of evidence indicates that EGF protects the GI epithelium from various types of insults (31). EGF protects the epithelial barrier function and TJs in Caco-2 cell monolayers from oxidative stress and acetaldehyde-induced injury (28, 40). The present study shows that EGF protects the epithelial barrier function by preventing acetaldehyde-induced TJ disruption in Caco-2 cell monolayers by a MAPK-dependent mechanism. The activity of ERK, but not p38 MAPK or JNK, is required for EGF-mediated protection of TJs from acetaldehyde. The role of ERK in protection of TJ was confirmed by a regulated expression of active or inactive MEK1, the immediate upstream regulator of ERK1/2.
EGF is known to activate different MAPKs in various cell systems. The present study shows that EGF activates all three MAPKs in differentiated Caco-2 cell monolayers, however, with some distinct temporal differences. ERK activation was most rapid, and p38 MAPK activation was slow. Activation of ERK and JNK1 was transient, but p38 MAPK activation sustained at least until 60 min. Interestingly, EGF did not activate JNK2. Our laboratory's recent study demonstrated that activation of JNK2 disrupts TJs in the intestinal epithelium (35). Attenuation of EGF-mediated prevention of acetaldehyde-induced TJ disruption and barrier dysfunction by U-0126, but not by SB-202190 or SP-600125, indicates that activation of ERK, but not p38 MAPK and JNK, is required for EGF-mediated protection of TJs from acetaldehyde. Previous studies showed that ERK activation plays a role in EGF-mediated protection of intestinal mucosa from various types of insults (6, 9, 13), whereas p38 MAPK activation is involved in disruption of TJs in the intestinal epithelia (12, 44). A recent study showed that JNK1 is involved in TJ disruption in T84 cell monolayers (26). However, our laboratory's recent study indicated that JNK2, but not JNK1, disrupts TJs in Caco-2 cell monolayers (35). The present study suggests that ERK activation can be TJ protective, even in the face of p38 MAPK and JNK activation.
Prevention of acetaldehyde-induced redistribution of E-cadherin and β-catenin indicates that EGF prevents acetaldehyde-induced disruption of AJs in Caco-2 cell monolayers. Although AJs do not form a physical barrier to the diffusion of macromolecules, they indirectly regulate the integrity of TJs. Disruption of AJs by removal of extracellular calcium has been repeatedly demonstrated to disrupt the TJs as well (37, 41). Protection of AJs by EGF was previously shown in Caco-2 cell monolayers treated with hydrogen peroxide or acetaldehyde (8, 36). Interestingly, the present study shows that EGF-induced prevention of redistribution of E-cadherin and β-catenin was unaffected by U-0126, SP-600125, or SB-202190. This observation indicates that EGF-mediated preservation of AJs was independent of MAPK activities.
Our laboratory's previous study showed that exposure to acetaldehyde causes reorganization of the actin cytoskeletal architecture, and that EGF pretreatment preserves the actin cytoskeleton structure in acetaldehyde-treated cell monolayers (40). In the differentiated intestinal epithelial cells, such as Caco-2 cells, the organization of actin cytoskeleton is distinctly different at different levels of cell height. At the apical region, F-actin filaments are organized as microvilli and terminal web, in the midregion of cells it is organized to form actomyosin ring and the circumferential cortical network, and, in the basal region, actin is organized into stress fibers. Acetaldehyde treatment results in remodeling of actin cytoskeleton at all regions, and EGF preserves the native architecture of actin cytoskeleton in the acetaldehyde-treated cells. However, the EGF effect was more prominent in the midregion, actomyosin ring, while its influence on preservation of stress fibers was moderate. Additionally, EGF appears to modulate the microvillar organization of actin, and its significance is unclear at this point. TJ integrity is known to be dependent on the structure of actomyosin ring, and, therefore, preservation of actomyosin structure by EGF is one of the likely mechanisms by which it prevents acetaldehyde-induced barrier disruption. The present study shows that inhibition of ERK activation by U-0126 attenuates EGF effect on the actin cytoskeleton at the middle and basal regions of the cell, but it did not alter the EGF-mediated preservation of actin cytoskeleton at the apical region of the cell. Regulation of the actomyosin ring is likely to be mediated by EGF-induced ERK activation and thus leading to the preservation of the barrier function. Inhibition of p38 MAPK did not alter EGF effects on the actin cytoskeleton at all levels, but inhibition of JNK prevented the EGF effect on actin organization at the apical and basal regions of cell, but not at the actomyosin ring. JNK inhibition appears to enhance actin organization at the midregion. Our recent study indicated that JNK activity leads to destabilization of actin cytoskeleton. Therefore, in the present study, it is likely that inhibition of JNK activity lead to enhancement of cytoskeletal integrity.
MEK1 selectively activates ERK1/2 without any influence on p38 MAPK or JNK1/2. Our present study demonstrates that overexpression of MEK1 or expression of CA-MEK1 attenuates acetaldehyde-induced redistribution of occludin and ZO-1 in the absence of EGF. This observation indicates that prolonged activation of MEK1 can substitute for EGF in protecting the TJ integrity in acetaldehyde-treated Caco-2 cell monolayers. Furthermore, our data also demonstrate that expression of DN-MEK1 attenuates EGF-mediated protection of TJ integrity from acetaldehyde, further confirming that ERK signaling pathway is required for EGF-mediated preservation of TJ integrity in acetaldehyde-treated cell monolayers. Our laboratory's previous study (42) demonstrated that PLC-γ and intracellular calcium-mediated activation of PKC isoforms also play a role in the protection of TJs from acetaldehyde. Therefore, EGF appears to trigger activation of multiple signaling pathways and influence different cellular targets that all converge at the TJs. There are several ways to protect TJ integrity, such as enhancing the organization of actin ring, tightening AJs, affecting TJs directly, or intervening at various points in acetaldehyde-induced signaling pathways. Further studies are necessary to determine whether these signaling pathways are interconnected or they act independently on TJ integrity.
In our laboratory's previous study, we showed that acetaldehyde induces tyrosine-phosphorylation of TJ and AJ proteins and that tyrosine kinase inhibitors prevent acetaldehyde-induced barrier disruption (4). The present study confirms that acetaldehyde elevates tyrosine-phosphorylation of occludin, ZO-1, E-cadherin, claudin-3, and β-catenin. Additionally, the present study demonstrates that EGF significantly attenuates acetaldehyde-induced increase in tyrosine-phosphorylation of occludin, ZO-1, claudin-3, and E-cadherin, but not β-catenin. Attenuation of EGF-mediated prevention of tyrosine-phosphorylation of occludin and ZO-1 by MEK inhibitor U-0126 indicated that ERK mediates the effect of EGF on occludin and ZO-1 phosphorylation. Previous studies demonstrated that tyrosine-phosphorylation of occludin on Y398 and Y402 attenuates its interaction with ZO-1 and sensitizes Caco-2 cell monolayers to hydrogen peroxide-induced TJ disruption (14). The present study showing a prevention of occludin tyrosine-phosphorylation by EGF by an ERK-dependent mechanism is, therefore, complimentary to our previous observation.
The EGF-mediated attenuation of acetaldehyde-induced tyrosine-phosphorylation of E-cadherin was unaffected by U-0126, suggesting that EGF effect on E-cadherin phosphorylation is independent of ERK activity. This is in agreement with the above-described data showing that EGF-mediated prevention of acetaldehyde-induced redistribution of E-cadherin and β-catenin was independent of ERK activity. In the present study, we also show that acetaldehyde induces a robust increase in tyrosine-phosphorylation of claudin-3, and EGF pretreatment attenuates this effect of acetaldehyde. However, U-0126, SB-202190, or SP-600125 failed to alter the effect of EGF on claudin-3 tyrosine-phosphorylation, suggesting that EGF effect on tyrosine-phosphorylation of claudin-3 is also independent of MAPK activity. Acetaldehyde or EGF did not alter the tyrosine-phosphorylation of claudin-4. Claudin-3 and claudin-4 are among the major claudins expressed in the intestinal epithelium that play a crucial role in the assembly and integrity of TJs. The present study indicates that acetaldehyde-induced tyrosine-phosphorylation of claudin-3 may play a role in the disruption of barrier function, and prevention of claudin-3 tyrosine-phosphorylation by EGF may be involved in the mechanism of TJ protection.
MAPK pathway is a major intracellular signaling pathway involved in EGF-receptor-mediated Ras/MAPK signaling. It mediates the promotion of cell proliferation, growth, and differentiation (1). Previous study showed that activation of Ras in Madin-Darby canine kidney cells disrupts the TJ (10). ERK activity was also found to be involved in the disruption of TJ by hydrogen peroxide in endothelial cell monolayers (21) and by phorbol ester in corneal epithelium (45). On the contrary, ERK activity is involved in the stabilization of TJ integrity in a differentiated, nonproliferating Caco-2 cell monolayer (2, 6). MAPK activity is required for the EGF-induced protection of TJs from hydrogen peroxide. In the present study, we demonstrate that MEK1 and ERK activities are involved in EGF-mediated protection of TJs from acetaldehyde. ERK activity is also seen to be required in growth factor-mediated TJ protective function in pig thyrocytes (16), T84 epithelial cells (18), and glucocorticoid-stimulated TJ sealing in mammary tumor cells (46).
This study, therefore, demonstrates that the mechanism of EGF-mediated protection of TJs from acetaldehyde in Caco-2 cell monolayers involves MEK1 and ERK-mediated signaling, and that p38 MAPK or JNK1 is not involved in this effect of EGF. Furthermore, EGF-mediated protection of AJs is independent of MAPK activities in acetaldehyde-treated cells.
GRANTS
This study was supported by National Institutes of Health Grants AA12307 and DK55532.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal 14: 649–654, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Aggarwal S, Suzuki T, Taylor WL, Bhargava A, Rao R. Contrasting effects of ERK on tight junction integrity in differentiated and under-differentiated Caco-2 cell monolayers. Biochem J 433: 51–63, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol Gastrointest Liver Physiol 269: G467–G475, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol 280: G1280–G1288, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Balda MS, Matter K. Tight junctions. J Cell Sci 111: 541–547, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Basuroy S, Seth A, Elias B, Naren AP, Rao R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J 393: 69–77, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM, Rao RK. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem 278: 11916–11924, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Basuroy S, Sheth P, Mansbach CM, Rao RK. Acetaldehyde disrupts tight junctions and adherens junctions in human colonic mucosa: protection by EGF and l-glutamine. Am J Physiol Gastrointest Liver Physiol 289: G367–G375, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Blikslager AT, Rhoads JM, Bristol DG, Roberts MC, Argenzio RA. Glutamine and transforming growth factor-alpha stimulate extracellular regulated kinases and enhance recovery of villous surface area in porcine ischemic-injured intestine. Surgery 125: 186–194, 1999 [PubMed] [Google Scholar]
- 10. Chen Y, Lu Q, Schneeberger EE, Goodenough DA. Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol Biol Cell 11: 849–862, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. MAP kinases. Chem Rev 101: 2449–2476, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Costantini TW, Peterson CY, Kroll L, Loomis WH, Eliceiri BP, Baird A, Bansal V, Coimbra R. Role of p38 MAPK in burn-induced intestinal barrier breakdown. J Surg Res 156: 64–69, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology 129: 609–625, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, Rao R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem 284: 1559–1569, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrier L, Berard F, Debrauwer L, Chabo C, Langella P, Bueno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol 168: 1148–1154, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grande M, Franzen A, Karlsson JO, Ericson LE, Heldin NE, Nilsson M. Transforming growth factor-beta and epidermal growth factor synergistically stimulate epithelial to mesenchymal transition (EMT) through a MEK-dependent mechanism in primary cultured pig thyrocytes. J Cell Sci 115: 4227–4236, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Homann N, Tillonen J, Meurman JH, Rintamaki H, Lindqvist C, Rautio M, Jousimies-Somer H, Salaspuro M. Increased salivary acetaldehyde levels in heavy drinkers and smokers: a microbiological approach to oral cavity cancer. Carcinogenesis 21: 663–668, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Howe KL, Reardon C, Wang A, Nazli A, McKay DM. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am J Pathol 167: 1587–1597, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ishikawa S, Cepinskas G, Specian RD, Itoh M, Kvietys PR. Epidermal growth factor attenuates jejunal mucosal injury induced by oleic acid: role of mucus. Am J Physiol Gastrointest Liver Physiol 267: G1067–G1077, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298: 1911–1912, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Kevil CG, Oshima T, Alexander B, Coe LL, Alexander JS. H2O2-mediated permeability: role of MAPK and occludin. Am J Physiol Cell Physiol 279: C21–C30, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Koivisto T, Salaspuro M. Aldehyde dehydrogenases of the rat colon: comparison with other tissues of the alimentary tract and the liver. Alcohol Clin Exp Res 20: 551–555, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Konturek PK, Brzozowski T, Konturek SJ, Dembinski A. Role of epidermal growth factor, prostaglandin, and sulfhydryls in stress-induced gastric lesions. Gastroenterology 99: 1607–1615, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Lin A. Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays 25: 17–24, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Madara JL, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol 102: 2125–2136, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naydenov NG, Hopkins AM, Ivanov AI. c-Jun N-terminal kinase mediates disassembly of apical junctions in model intestinal epithelia. Cell Cycle 8: 2110–2121, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 50: 638–644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rao R, Baker RD, Baker SS. Inhibition of oxidant-induced barrier disruption and protein tyrosine phosphorylation in Caco-2 cell monolayers by epidermal growth factor. Biochem Pharmacol 57: 685–695, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Rao R, Porreca F. Epidermal growth factor protects mouse ileal mucosa from Triton X-100-induced injury. Eur J Pharmacol 303: 209–212, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Rao RK. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res 22: 1724–1730, 1998 [PubMed] [Google Scholar]
- 31. Rao RK. Biologically active peptides in the gastrointestinal lumen. Life Sci 48: 1685–1704, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J 368: 471–481, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rao RK, Seth A, Sheth P. Recent advances in alcoholic liver disease. I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 286: G881–G884, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Salaspuro MP. Acetaldehyde, microbes, and cancer of the digestive tract. Crit Rev Clin Lab Sci 40: 183–208, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Samak G, Suzuki T, Bhargava A, Rao RK. c-Jun NH2-terminal kinase-2 mediates osmotic stress-induced tight junction disruption in the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol 299: G572–G584, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seth A, Basuroy S, Sheth P, Rao RK. l-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol 287: G510–G517, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Seth A, Sheth P, Elias BC, Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem 282: 11487–11498, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Sheth P, Samak G, Shull JA, Seth A, Rao R. Protein phosphatase 2A plays a role in hydrogen peroxide-induced disruption of tight junctions in Caco-2 cell monolayers. Biochem J 421: 59–70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sheth P, Seth A, Atkinson KJ, Gheyi T, Kale G, Giorgianni F, Desiderio DM, Li C, Naren A, Rao R. Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem J 402: 291–300, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sheth P, Seth A, Thangavel M, Basuroy S, Rao RK. Epidermal growth factor prevents acetaldehyde-induced paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res 28: 797–804, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Stuart RO, Nigam SK. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci U S A 92: 6072–6076, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suzuki T, Seth A, Rao R. Role of phospholipase C gamma-induced activation of protein kinase C epsilon (PKC epsilon) and PKC beta I in epidermal growth factor-mediated protection of tight junctions from acetaldehyde in Caco-2 cell monolayers. J Biol Chem 283: 3574–3583, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Visapaa JP, Tillonen J, Salaspuro M. Microbes and mucosa in the regulation of intracolonic acetaldehyde concentration during ethanol challenge. Alcohol Alcohol 37: 322–326, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Wang S, Huang Q, Guo X, Brunk UT, Han J, Zhao K, Zhao M. The p38 alpha and p38 delta MAP kinases may be gene therapy targets in the future treatment of severe burns. Shock 34: 176–182, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, Zhang J, Yi XJ, Yu FS. Activation of ERK1/2 MAP kinase pathway induces tight junction disruption in human corneal epithelial cells. Exp Eye Res 78: 125–136, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Woo PL, Ching D, Guan Y, Firestone GL. Requirement for Ras and phosphatidylinositol 3-kinase signaling uncouples the glucocorticoid-induced junctional organization and transepithelial electrical resistance in mammary tumor cells. J Biol Chem 274: 32818–32828, 1999 [DOI] [PubMed] [Google Scholar]