Abstract
Objective
Levels of serum amyloid A (SAA), an acute phase protein carried on HDL, increase in inflammatory states and associates with increased risk of cardiovascular disease. HDL co-localizes with vascular proteoglycans in atherosclerotic lesions. However, its major apolipoprotein, apoA-I, has no proteoglycan-binding domains. Therefore, we investigated whether SAA, which has proteoglycan-binding domains, plays a role in HDL retention by proteoglycans
Methods and Results
HDL from control mice and mice deficient in both SAA1.1 and SAA2.1 (SAAKO mice) injected with bacterial lipopolysaccharide (LPS) was studied. SAA mRNA expression in the liver and plasma levels of SAA increased dramatically in C57BL/6 mice after LPS administration, although HDL-cholesterol did not change. FPLC analysis showed most of the SAA to be in HDL. Mass spectrometric analysis indicated that HDL from LPS-injected control mice had high levels of SAA1.1/2.1 and reduced levels of apoA-I. HDL from LPS-injected control mice demonstrated high affinity binding to biglycan relative to normal mouse HDL. In contrast, HDL from LPS-injected SAAKO mice showed very little binding to biglycan, consistent with SAA facilitating the binding of HDL to vascular proteoglycans.
Conclusions
SAA enrichment of HDL under inflammatory conditions plays an important role in the binding of HDL to vascular proteoglycans.
Keywords: Lipoproteins, apolipoproteins, inflammation, amyloid, proteoglycans
Introduction
Atherosclerosis is a maladaptive inflammatory response initially to lipoproteins retained in the artery wall 1, 2. Inflammatory cells such as T-lymphocytes and macrophages, as well as inflammatory cytokines are detectable in atherosclerotic lesions 3. In addition, chronically elevated levels of inflammatory proteins such as C-reactive protein and serum amyloid A (SAA) are associated with an increased risk of cardiovascular disease 4–9. An independent relationship exists between SAA and future cardiovascular events, similar to that found for hs-CRP 5. Although SAA was independently but moderately associated with angiographic CAD, this association was not found for hs-CRP 5. Whether these proteins have any causal relationship with cardiovascular disease or are simply markers of inflammation remains unclear.
SAA is produced mainly in the liver in response to inflammatory stimuli 10 and is transported in plasma primarily associated with high density lipoprotein (HDL) 11. Circulating SAA levels increase up to 1000-fold during the acute phase response following bacterial infection, tissue damage and acute inflammatory stimuli 12, 13. More modest elevations are seen in chronic inflammatory conditions such as obesity, and are associated with an increased risk of cardiovascular disease 5, 7.
LDL and other apoB- and apoE-containing lipoproteins bind to proteoglycans with high affinity due to ionic interactions between positively charged amino acids on apoB and apoE, and negatively charged sulfate and carboxylic acid groups on the glycosaminoglycan side chains of proteoglycans 14, 15. Lipoproteins retained in the arterial intima undergo oxidative and enzymatic modifications, which generate products that can be atherogenic 16. Since HDL particles do not contain apoB nor do they normally contain large amounts of apoE, i.e., apolipoproteins with positively charged proteoglycan binding domains, HDL should not bind proteoglycans effectively. ApoA-I, the major HDL apolipoprotein, co-localizes with proteoglycans in atherosclerotic lesions in both mice 17, 18 and humans 19. Hence it is possible that additional HDL apolipoproteins might target some HDL particles to vascular proteoglycans. Since SAA contains positively charged proteoglycan binding domains, we hypothesized that acute inflammation due to the injection of lipopolysaccharide (LPS), would increase SAA levels and facilitate the binding of SAA-enriched HDL particles to vascular proteoglycans, thus explaining the co-localization of apoA-I with SAA in vascular lesions. The recent availability of SAAKO mice 20 allowed us to definitively show a role for SAA in the binding of SAA-containing HDL to vascular proteoglycans.
Methods
Mice
C57BL/6J male mice (8 weeks) of age were obtained from The Jackson Laboratory (Bar Harbor, ME) and housed under specific pathogen-free conditions in static microisolator cages. LPS (O111:B4, lot #20137A3) (100 μg/mouse) or PBS were administered via intraperitoneal injection, 24 hours prior to sacrifice. After a 4 hour fast, blood was collected and plasma was prepared immediately. All procedures were performed in accordance with the guidelines for animal welfare of University of Washington and the protocols were approved by the Animal Welfare Committee of the university.
Mice that lack both major acute phase SAAs (SAAKO mice) 20 were injected with LPS 24 hours prior to sacrifice for preparing HDL from plasma. Wild type (WT) littermates received PBS. These procedures were performed at the University of Kentucky in accordance with the guidelines for animal welfare at that institution. The HDL was then shipped on ice to the University of Washington.
Lipoprotein Preparation
HDL (d=1.063–1.210 g/mL) was isolated from plasma of control and LPS injected mice by ultracentrifugation. No SAA was detected in the lipoprotein deficient fraction of plasma. Because apoE can bind to proteoglycans, for proteoglycan binding experiments apoE-containing HDL was removed from aliquots of the HDL preparations using an antibody to apoE bound to magnetic beads (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Removal of apoE was confirmed by immunoblot. Human LDL (d=1.019–1.063 g/mL) and HDL (d=1.063–1.210 g/mL) also were isolated from plasma of healthy human volunteers by ultracentrifugation. All lipoproteins were extensively dialyzed against 150 mmol/L NaCl with 1 mmol/L EDTA.
Plasma levels of lipids and SAA
Plasma levels of total cholesterol, HDL-cholesterol (Genzyme, Cambridge, MA) and triglyceride (Roche, Indianapolis, IN) were determined using enzymatic kits. Plasma levels of SAA were measured by enzyme-linked immunosorbent assay (ELISA) using a goat anti-mouse SAA1 antibody (AF2948, R&D Systems, MN), as described previously 21, 22.
Real-Time PCR
Please see online supplement for details of RT-PCR.
Mass spectrometric analysis
Please see online supplement for details of the mass spectrometric analysis of plasma and HDL.
Gel Mobility Shift Assay
[35S]SO4-labeled biglycan synthesized by cultured human aortic smooth muscle cells was interacted with lipoproteins using a gel shift mobility assay as described previously 23. Additional details of the biglycan isolation and gel mobility shift assay are provided in the online supplement.
Statistical analysis
Statistical significance was determined either by Student’s t-tests or ANOVA.
Results
LPS injection increases expression of SAA in liver and plasma
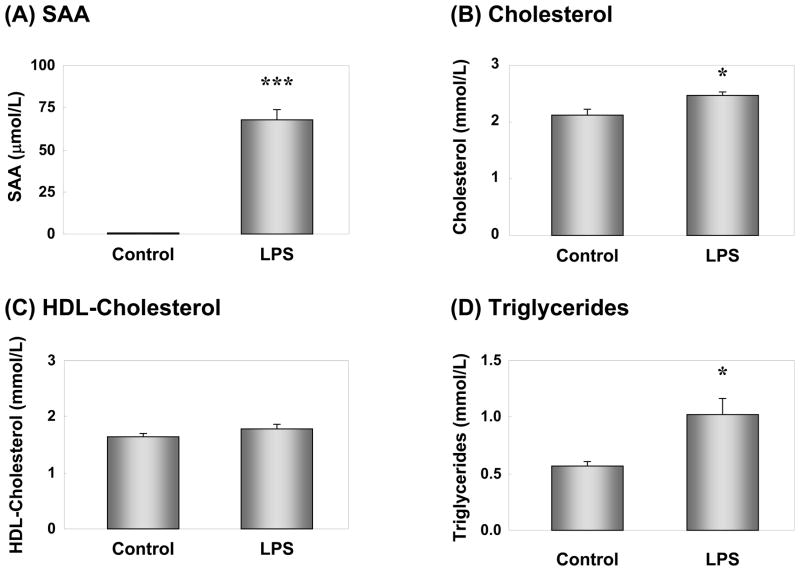
SAA mRNA expression in the liver increased dramatically following LPS injection (Supplemental Fig. 1A). Conversely, apoA-I mRNA expression was slightly decreased (Supplemental Fig. 1B). These results were consistent with our previous observations using cytokines 24. Plasma SAA also increased dramatically after LPS injection (Fig. 1A), consistent with the increased mRNA expression in the liver. Plasma levels of cholesterol and triglycerides increased significantly following LPS injection (Fig. 1B & D). However, there were no significant changes in HDL-cholesterol levels between control and LPS-injected mice (Fig. 1C). Very little SAA could be detected by FPLC and ELISA in association with any lipoprotein particles in control mice (Supplemental Fig. 2A). The increase in cholesterol and triglycerides in the LPS-injected mice was due to an increase in the VLDL-IDL fraction (Supplemental Fig. 2B). The majority of SAA eluted in the HDL particle size range in LPS injected mice (Supplemental Fig. 2B).
Figure 1. Effect of LPS-injection on plasma SAA and lipid levels.
Plasma from control or LPS-injected mice were evaluated for SAA by ELISA(A). Plasma levels of cholesterol (B), HDL-cholesterol (C), and triglyceride (D) were measured enzymatically. Results represent the mean ± SEM; n=12, Control; n=11, LPS. *P<0.05, ***P<0.001 vs. Control.
HDL from LPS-injected mice is enriched in SAA
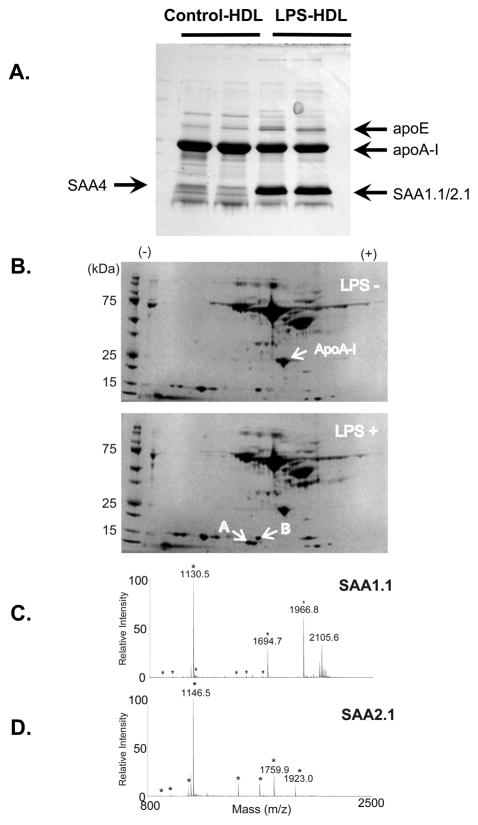
To evaluate apolipoproteins, HDL from both control and LPS injected mice plasma was separated by 10 – 20% gradient SDS-PAGE. SDS-PAGE in conjunction with MALDI-TOF analysis showed that inflammatory HDL had a lower level of apoA-I than those of control HDL (Fig. 2A). In addition, apoE was increased and SAA was dramatically increased in HDL from LPS-injected mice. Only SAA4, the constitutive isoform of SAA, was detected in control HDL by MALDI-TOF, whereas most of the SAA in the LPS-injected animals was SAA1.1 and SAA2.1, which are inducible forms of SAA made by the liver. The identification was further confirmed by tandem MS/MS of major peaks in each spectrum. To further address the distribution of apoA-I and SAA in HDL particles, plasma from control mice and LPS-injected mice were separated by two-dimensional electrophoresis, prior to MALDI-TOF analysis (Fig. 2B). Two-dimensional electrophoresis could separate SAA1.1 (Fig. 2C) from SAA2.1 (Fig. 2D), and indicated that both isoforms were increased.
Figure 2. MALDI-TOF analysis.
HDL (d=1.063–1.210 g/mL) was isolated by ultracentrifugation from plasma of control and LPS-injected mice. HDL (20 μg protein) was separated by SDS-PAGE (10 – 20 % gradient gel) and the gel was stained with Coomassie Brilliant Blue. Each gel band corresponding to the apparent molecular weight of SAA1.1/2.1, SAA4, apoA-I and apoE was cut out, digested with trypsin, and the peptide digest was extracted for tandem mass spectrometric analysis by MALDI-TOF. The arrows indicate bands that were identified by MALDI-TOF and database searching that contained peptides unique to SAA1.1/2.1, SAA4, apoA-I, and apoE (Figure 2A).
Albumin-depleted plasma samples (20 μl) from control (A) and LPS-injected (B) mice were separated by two dimensional electrophoresis (first dimension: IEF pH 3–10, second dimension: 10% SDS-PAGE) and the gel was stained with a silver stain. Selected spots from 2D gels were identified by in-gel tryptic digest and MALDI-TOF analysis. The small arrows indicate bands that were identified by tandem MS/MS MALDI-TOF and database searching that contained peptides unique to SAA1.1, SAA2.1, and apoA-I (Figure 2B). The spot designated as SAA2.1 was identified as such with a MASCOT MOWSE score of 374 (CI 100% - Figure 2C), and the adjacent spot was identified as SAA1.1 with a MOWSE score of 403 (CI 100% - Figure 2D). The asterisks indicate peaks corresponding to SAA peptides.
HDL from LPS-injected mice binds biglycan
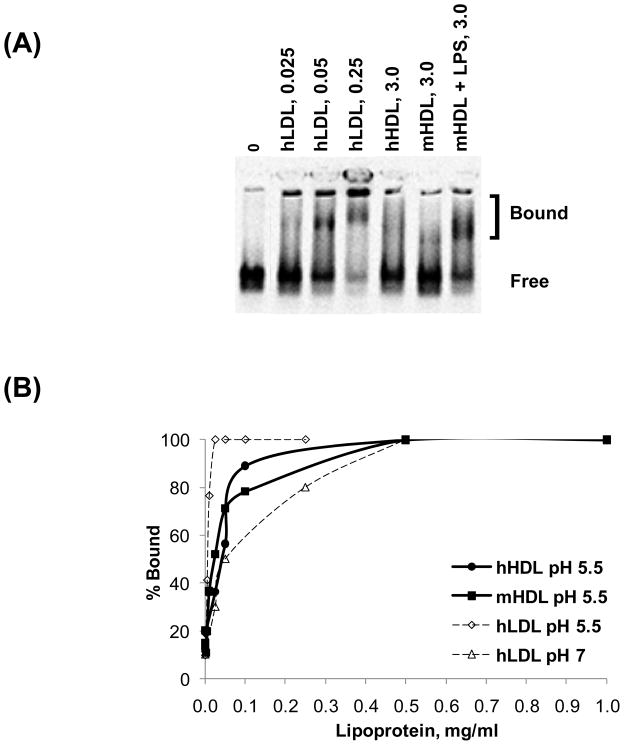
HDL-biglycan binding interactions were evaluated by gel mobility shift assay. This assay was performed at pH 7 or 5.5, modeling the pH of normal physiological interactions or that of the acidic microenvironment of the arterial wall 25, respectively. Whereas human LDL binds to biglycan with high affinity (50% maximal binding at ~0.05 mg/mL;) 26 (Fig. 3A), neither human nor control mouse HDL binds to biglycan even at concentrations up to 3 mg/mL at pH 7 (Fig. 3A). However, HDL prepared from LPS-injected mice binds to biglycan, with ~80% total biglycan bound at 3 mg/mL HDL (Fig. 3A). This result was not due to the presence of LPS itself in these particles, as treatment of this HDL with polymixin B did not affect its biglycan-binding properties and addition of LPS to control HDL did not improve its inability to bind to biglycan (data not shown). These results suggest that, unlike normal HDL, HDL from LPS-injected mice does have proteoglycan-binding properties at physiological pH.
Figure 3. HDL from LPS-injected mice has high affinity for biglycan.
(A) HDL-biglycan binding was evaluated by gel mobility shift assay at physiological pH. A constant amount of biglycan was incubated with no lipoproteins (0), increasing concentrations of human LDL (0.025, 0.05, 0.25 mg/mL), human HDL (3 mg/mL) or HDL from control and LPS-treated mice (3 mg/mL) and electrophoresed. Figure shown is representative of three independent experiments. (B) HDL-biglycan binding was quantified as % Bound using Opti-Quant software(Packard). Representative binding curves are shown.
Recent studies have described lipoprotein-proteoglycan interactions occurring at low pH, modeling the acidic microenvironment of atherosclerotic lesions 25. Thus, we also evaluated the ability of LDL and HDL to bind to biglycan at pH 5.5. For LDL, ~50% maximal binding was detected at ~0.005 mg/mL at pH 5.5 (vs 0.05 mg/mL at pH 7) (Fig. 3B). For either human or mouse HDL, ~50% maximal binding was detected at 0.025–0.05 mg/mL (vs no binding at up to 3 mg/mL at pH 7) (Fig. 3B). Thus, both LDL and HDL bind to biglycan with higher affinity at acidic pH than at physiological pH.
SAA facilitates binding of HDL to biglycan
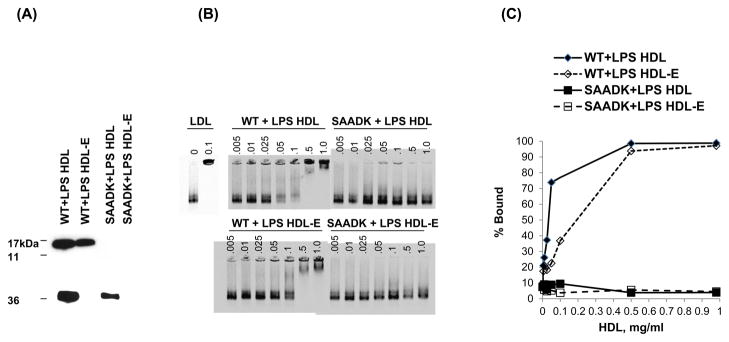
To determine the role of SAA in facilitating the binding of HDL from LPS-injected mice to biglycan, we compared the biglycan-binding ability at pH 5.5 of HDL from SAAKO mice following LPS injection with that of HDL from LPS-treated wild type mice. As expected, SAA levels were undetectable in HDL from LPS-treated SAAKO mice, but SAA was strongly expressed in HDL from LPS-treated wild type mice (Fig. 4A). HDL from LPS-treated mice contains some apoE, with SAAKO mice containing less apoE than wild type mice. The presence of apoE could confound the potential role of SAA in mediating HDL binding to proteoglycans, as a subclass of apoE-containing HDL particles binds to biglycan 27. Therefore, to determine the effect of SAA independent of apoE, apoE-containing HDL was removed from the preparations using an antibody to apoE bound to magnetic beads prior to subjecting HDL from these mice to the gel mobility shift assay (Fig. 4A). Apo E-free HDL from LPS-treated wild type mice bound biglycan with ~50% maximal binding at 0.25–0.5 mg/mL, whereas essentially no proteoglycan binding was detected with HDL from LPS-treated SAA KO mice (Fig. 4B and C). Even without removal of the apoE containing particles, very little biglycan binding was detected using HDL from LPS-injected SAAKO mice (data not shown). The presence of apoE on HDLfrom LPS-injected wild type mice increased binding by a maximum of 20%, but the marked difference between binding of HDL from LPS-treated SAAKO mice and LPS-treated wild type mice was still present (data not shown). These findings thus indicate an important independent role for SAA in facilitating the binding of HDL to vascular proteoglycans under inflammatory conditions.
Figure 4. HDL from LPS-injected SAA knockout mice does not bind biglycan.
(A) HDL from wild-type and SAAKO mice treated with LPS were evaluated for SAA by Western immunoblot analysis. ApoE was removed using an antibody bound to magnetic beads, and resulting HDL lacking apoE (HDL-E) preparations were evaluated for apoE content by Western immunoblot analysis. (B) Binding of apoE-free HDL from LPS-injected wild-type and SAAKO mice to biglycan was evaluated at acidic pH. A constant amount of biglycan was incubated with no lipoproteins (0), human LDL (0.1 mg/mL), or increasing concentrations of HDL (0–1 mg/mL) from LPS-treated wild type mice, or from LPS-treated SAAKO mice and electrophoresed. (C) HDL-biglycan binding was quantified as % Bound using Opti-Quant software(Packard). Representative binding curves are shown.
Discussion
SAA is well recognized as an inflammatory marker that is synthesized mainly in the liver and transported through plasma predominantly bound to HDL. Modest elevations of SAA levels such as are seen in obesity, insulin resistance, and diabetes 28–30 associate with an increased risk of cardiovascular disease 5, 8, 31. We previously have reported that addition of cholesterol to a high-saturated fat diet increased plasma levels of SAA and accelerated atherosclerosis in LDL receptor-deficient mice independent of its effect on plasma of cholesterol level 21, 22. In these and other studies, the extent of atherosclerosis correlates with SAA levels and not with circulating lipids and lipoproteins 21, 22, 32. These findings raise the question of whether SAA might be a mediator rather than a marker of atherosclerosis. In the present study we demonstrate that SAA levels increase dramatically in HDL after administration of LPS, and that SAA facilitates the binding of HDL to the vascular proteoglycan, biglycan.
ApoB and apoE bind vascular proteoglycans via ionic interactions between positively charged lysine and arginine residues on the apolipoproteins with negatively charged sulfate and carboxylic acid groups on the glycosaminoglycan side chains of vascular proteoglycans 14, 15. Once retained in the arterial intima, components of these lipoproteins undergo oxidative modification and modification by enzymes such as secretory phospholipase A2, which generate atherogenic toxic products that can injure the artery wall 16, 33. The major atherogenic lipoproteins are believed to be apoB- and apoE-containing lipoproteins such as LDL, VLDL and their remnants, and Lp(a). However, HDL also is present in atherosclerotic lesions in both humans and experimental animals 18, 34, 35 and we have shown that its major apolipoprotein, apoA-I, co-localizes with apoB, apoE, SAA and proteoglycans in atherosclerotic lesions 17–19, 21. Since apoA-I does not have a proteoglycan binding domain, these observations raise the question of whether apolipoproteins such as apoE and SAA might target some HDL particles to be retained by vascular proteoglycans. We previously have demonstrated that the presence of apoE in a subclass of HDL particles can facilitate proteoglycan binding27. In this study we now demonstrate that SAA, a HDL apolipoprotein that is increased in inflammatory states, has a similar effect. For this proof of principle study we used LPS to induce large changes in the SAA content of HDL, such that a large percentage of the HDL particles would contain SAA and hence could be tested for proteoglycan binding in a gel shift assay. Much lower levels of SAA are found in both mouse models and human subjects in whom SAA is associated with atherosclerosis. Nonetheless, retention of even a much smaller number of SAA-containing HDL particles by vascular proteoglycans could with time lead to their buildup in the arterial intima.
We used a gel mobility shift assay to demonstrate that SAA-containing HDL binds to biglycan, a vascular proteoglycan that co-localizes with lipoproteins in human atherosclerotic lesions. The recent availability of SAAKO mice20 allowed us to definitively evaluate the role of SAA in the binding of HDL to proteoglycans after invoking an inflammatory response by injecting LPS. By using HDL from mice deficient in the 2 major circulating inducible isoforms of SAA, we were able to unequivocally demonstrate an important role for SAA in facilitating the binding of HDL to proteoglycans in the inflammatory state. The increased apoE content of HDL in the SAAKO animals after LPS treatment might have contributed to the binding of HDL to biglycan. Therefore, to directly evaluate the role of SAA, we removed apoE-containing HDL particles by immunoaffinity chromatography. HDL particles that did not contain apoE from wild-type mice bound effectively to proteoglycans, particularly at pH5.5, whereas HDL that was devoid of apoE obtained from LPS-injected SAAKO mice showed negligible proteoglycan binding. These observations provide definitive evidence for a role for SAA in HDL binding to proteoglycans. Even though apoE-containing HDL particles might contribute to HDL retention by vascular proteoglycans in some circumstances 19, 27, the findings in the present study demonstrate a definitive additional role for SAA. After LPS injection, there is a large abundance of apoA-I relative to apoE in HDL 36, as confirmed in the present paper (see Fig 4). From the relative molecular weights of apoA-I and apoE, it can be calculated that only a small percentage of HDL particles contain apoE. However, after LPS injection, the very elevated concentration of SAA in HDL suggests that a considerably higher percentage of HDL particles contain SAA than apoE. These findings are consistent with our observation that removal of apoE-containing HDL particles had a minor effect on their ability to bind biglycan, whereas deficiency of SAA essentially eliminated the ability of HDL to bind proteoglycans.
It was widely believed that SAA can displace apoA-I from HDL particles following inflammatory stimuli 37, 38. Subsequent studies using adenoviral vector overexpression of SAA in the absence of an acute phase response indicated that apoA-I levels were not influenced by SAA 39. Recent data using SAA knockout mice unequivocally shows that SAA does not impact apoA-I clearance 20. The reduction in apoA-I and the increase in the SAA content of HDL is thus likely the result of the co-ordinate and reciprocal regulation by inflammatory cytokines of hepatocytes24. Due to the polydispersity of human HDL, it is difficult to estimate the number of apoA-I and SAA particles on acute phase HDL. Mouse HDL is more homogeneous, with acute phase particles (9.3nm) containing three apoA-I molecules and 3 – 5 SAA molecules.
Apo-I does not bind to proteoglycans. The presence of SAA with its well characterized proteoglycan binding site10 is likely the most important determinant of proteoglycan binding by HDL. This is consistent with our data that indicates that SAA-containing HDL particles bind biglycan with high affinity. Lipoproteins generally bind proteoglycans as a result of interaction of positively charged residues on apolipoproteins with negatively charged residues on the glycosaminoglycan side chains of proteoglycans. Acute phase SAA-containing HDL migrates differently on agarose gels to HDL not containing SAA20. Interpreting the charge effect that the presence of SAA could have on whole HDL particles is complex due to important compositional changes, particularly relating to lipids. During the acute phase reaction, HDL is enriched in surface components where on the other hand the core cholesteryl ester content is decreased20. Such changes in the lipid composition could render oval shaped particles, which would migrate with a larger Stokes radius in non-denaturing gels. Alternatively the apolipoproteins could be re-arranged on the surface of the HDL so as to migrate as an apparently larger particle. Further, weak SAA interactions with agarose can retard migration in non-denaturing gels. Thus, emphasis should be placed on the proteoglycan binding site of SAA that is critical for amyloid formation40. The relevance of this interaction has resulted in it being successfully targeted in therapeutic assault on amyloid formation40.
The near absence of proteoglycan binding of HDL from LPS-injected SAAKO mice suggests that SAA -containing HDL can be bound and retained by vascular proteoglycans such as biglycan in humans 19, 41 or perlecan in mice 17, 18, and that SAA plays a critical role in its binding. These retained HDL particles could then be susceptible to oxidative and enzymatic modifications that render them more atherogenic 16, 42, 43.
For the current studies we administered LPS to acutely induce a large increase in the SAA content of HDL. Whether similar changes occur with the more modest changes in HDL composition seen in chronic inflammatory states such as obesity remains to be determined. Moreover, some SAA is transported on lipoproteins other than HDL in chronic inflammatory states associated with systemic inflammation, obesity and insulin resistance 21, 22, or in HDL deficiency states associated with the absence of apoA-I 44. In these circumstances it is possible that SAA might enhance the proteoglycan binding and vascular retention of apoB and apoE-containing lipoproteins.
In conclusion, inflammatory SAA-containing HDL binds biglycan with high affinity, potentially reducing the atheroprotective functions of HDL and converting it from an anti-atherogenic to a pro-atherogenic lipoprotein.
Acknowledgments
Individual acknowledgements – none
Sources of funding: Supported in part by NIH grants HL94352, HL18645, HL086670, and DK35816, and a VA Merit Review for FCDB. Mass spectrometric analysis was performed at Mass spectrometry resource of the Department of Medicine, University of Washington, supported by the Diabetes and Endocrinology Research Center (DK017047).
Footnotes
Disclosures: There are no real or apparent conflicts of interest.
References
- 1.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. 2004;116(Suppl 6A):9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, Pepine CJ, Sharaf B, Bairey Merz CN, Sopko G, Olson MB, Reis SE. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:726–732. doi: 10.1161/01.CIR.0000115516.54550.B1. [DOI] [PubMed] [Google Scholar]
- 6.Chen GL, Liu LW, Xie S, Liu H, Liu YQ, Li YS. High-density lipoprotein associated factors apoA-I and serum amyloid A in Chinese non-diabetic patients with coronary heart disease. Chin Med J (Engl) 2010;123:658–663. [PubMed] [Google Scholar]
- 7.Jousilahti P, Salomaa V, Rasi V, Vahtera E, Palosuo T. The association of c-reactive protein, serum amyloid a and fibrinogen with prevalent coronary heart disease--baseline findings of the PAIS project. Atherosclerosis. 2001;156:451–456. doi: 10.1016/s0021-9150(00)00681-x. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 9.Erren M, Reinecke H, Junker R, Fobker M, Schulte H, Schurek JO, Kropf J, Kerber S, Breithardt G, Assmann G, Cullen P. Systemic inflammatory parameters in patients with atherosclerosis of the coronary and peripheral arteries. Arterioscler Thromb Vasc Biol. 1999;19:2355–2363. doi: 10.1161/01.atv.19.10.2355. [DOI] [PubMed] [Google Scholar]
- 10.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 11.Coetzee GA, Strachan AF, van der Westhuyzen DR, Hoppe HC, Jeenah MS, de Beer FC. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J Biol Chem. 1986;261:9644–9651. [PubMed] [Google Scholar]
- 12.Malle E, Steinmetz A, Raynes JG. Serum amyloid A (SAA): an acute phase protein and apolipoprotein. Atherosclerosis. 1993;102:131–146. doi: 10.1016/0021-9150(93)90155-n. [DOI] [PubMed] [Google Scholar]
- 13.Malle E, De Beer FC. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur J Clin Invest. 1996;26:427–435. doi: 10.1046/j.1365-2362.1996.159291.x. [DOI] [PubMed] [Google Scholar]
- 14.Camejo G, López A, López F, Quiñones J. Interaction of low density lipoproteins with arterial proteoglycans: The role of charge and sialic acid content. Atherosclerosis. 1985;55:93–105. doi: 10.1016/0021-9150(85)90169-8. [DOI] [PubMed] [Google Scholar]
- 15.Camejo G, Rosengren B, Olson U, Lopez F, Olofson SO, Westerlund C, Bondjers G. Molecular basis of the association of arterial proteoglycans with low density lipoproteins: Its effect on the structure of the lipoprotein particle. European Heart Journal. 1990;11:164–173. doi: 10.1093/eurheartj/11.suppl_e.164. [DOI] [PubMed] [Google Scholar]
- 16.Williams KJ, Tabas I. The response-to-retention hypothesis of atherogenesis reinforced. Curr Opin Lipidol. 1998;9:471–474. doi: 10.1097/00041433-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Kunjathoor VV, Chiu D, O’Brien KD, LeBoeuf RC. Accumulation of biglycan and perlecan, but not versican, in lesions of murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:462–468. doi: 10.1161/hq0302.105378. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien KD, McDonald TO, Kunjathoor V, Eng K, Knopp EA, Lewis K, Lopez R, Kirk EA, Chait A, Wight TN, deBeer FC, LeBoeuf RC. Serum amyloid A and lipoprotein retention in murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:785–790. doi: 10.1161/01.ATV.0000158383.65277.2b. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien KD, Olin KL, Alpers CE, Chiu W, Ferguson M, Hudkins K, Wight TN, Chait A. Comparison of apolipoprotein and proteoglycan deposits in human coronary atherosclerotic plaques: colocalization of biglycan with apolipoproteins. Circulation. 1998;98:519–527. doi: 10.1161/01.cir.98.6.519. [DOI] [PubMed] [Google Scholar]
- 20.de Beer MC, Webb NR, Wroblewski JM, Noffsinger VP, Rateri DL, Ji A, van der Westhuyzen DR, de Beer FC. Impact of serum amyloid A on high density lipoprotein composition and levels. J Lipid Res. 2010;51:3117–3125. doi: 10.1194/jlr.M005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis KE, Kirk EA, McDonald TO, Wang S, Wight TN, O’Brien KD, Chait A. Increase in serum amyloid a evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation. 2004;110:540–545. doi: 10.1161/01.CIR.0000136819.93989.E1. [DOI] [PubMed] [Google Scholar]
- 22.Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A, 3rd, Kirk EA, O’Brien KD, Chait A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:685–691. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olin KL, Potter-Perigo S, Barrett HR, Wight TN, Chait A. Lipoprotein lipase enhances the binding of native oxidized low density lipoproteins to versican and biglycan synthesized by cultured arterial smooth muscle cells. Journal of Biological Chemistry. 1999;274:34629–34636. doi: 10.1074/jbc.274.49.34629. [DOI] [PubMed] [Google Scholar]
- 24.Han CY, Chiba T, Campbell JS, Fausto N, Chaisson M, Orasanu G, Plutzky J, Chait A. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol. 2006;26:1806–1813. doi: 10.1161/01.ATV.0000227472.70734.ad. [DOI] [PubMed] [Google Scholar]
- 25.Oorni K, Kovanen PT. Enhanced extracellular lipid accumulation in acidic environments. Curr Opin Lipidol. 2006;17:534–540. doi: 10.1097/01.mol.0000245259.63505.c2. [DOI] [PubMed] [Google Scholar]
- 26.Borén J, Olin K, Lee I, Chait A, Wight TN, Innerarity TL. Identification of the principal proteoglycan-binding site in LDL: A single point mutation in apolipoprotein B100 severely affects proteoglycan interaction without affecting LDL receptor binding. Journal of Clinical Investigation. 1998;101:2658–2664. doi: 10.1172/JCI2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olin-Lewis K, Benton J, Rutledge J, Baskin D, Wight T, Chait A. Apolipoprotein E mediates the retention of high-density lipoproteins by mouse carotid arteries and cultured arterial smooth muscle cell extracellular matrices. Circ Res. 2002;90:1333–1339. doi: 10.1161/01.res.0000024691.82864.f0. [DOI] [PubMed] [Google Scholar]
- 28.Yang RZ, Lee MJ, Hu H, Pollin TI, Ryan AS, Nicklas BJ, Snitker S, Horenstein RB, Hull K, Goldberg NH, Goldberg AP, Shuldiner AR, Fried SK, Gong DW. Acute-phase serum amyloid A: an inflammatory adipokine and potential link between obesity and its metabolic complications. PLoS Med. 2006;3:e287. doi: 10.1371/journal.pmed.0030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tannock LR, O’Brien KD, Knopp RH, Retzlaff B, Fish B, Wener MH, Kahn SE, Chait A. Cholesterol feeding increases C-reactive protein and serum amyloid A levels in lean insulin-sensitive subjects. Circulation. 2005;111:3058–3062. doi: 10.1161/CIRCULATIONAHA.104.506188. [DOI] [PubMed] [Google Scholar]
- 30.Leinonen ES, Hiukka A, Hurt-Camejo E, Wiklund O, Sarna SS, Mattson Hulten L, Westerbacka J, Salonen RM, Salonen JT, Taskinen MR. Low-grade inflammation, endothelial activation and carotid intima-media thickness in type 2 diabetes. J Intern Med. 2004;256:119–127. doi: 10.1111/j.1365-2796.2004.01350.x. [DOI] [PubMed] [Google Scholar]
- 31.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hama S, Reddy ST, Fogelman AM. Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J Lipid Res. 2007;48:2344–2353. doi: 10.1194/jlr.M700138-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hama S, Reddy ST, Fogelman AM. Lipoprotein inflammatory properties and serum amyloid a levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J Lipid Res. 2007 doi: 10.1194/jlr.M700138-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Sartipy P, Camejo G, Svensson L, Hurt-Camejo E. Phospholipase A(2) modification of low density lipoproteins forms small high density particles with increased affinity for proteoglycans and glycosaminoglycans. J Biol Chem. 1999;274:25913–25920. doi: 10.1074/jbc.274.36.25913. [DOI] [PubMed] [Google Scholar]
- 34.Mackness B, Hunt R, Durrington PN, Mackness MI. Increased immunolocalization of paraoxonase, clusterin, and apolipoprotein A-I in the human artery wall with the progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:1233–1238. doi: 10.1161/01.atv.17.7.1233. [DOI] [PubMed] [Google Scholar]
- 35.Smith EB, Ashall C, Walker JE. High density lipoprotein (HDL) subfractions in interstitial fluid from human aortic intima and atherosclerotic lesions. Biochemical Society Transactions. 1984;12:843–844. [Google Scholar]
- 36.Liao W, Rudling M, Angelin B. Endotoxin suppresses mouse hepatic low-density lipoprotein-receptor expression via a pathway independent of the toll-like receptor 4. Hepatology. 1999;30:1252–1256. doi: 10.1002/hep.510300524. [DOI] [PubMed] [Google Scholar]
- 37.Clifton PM, Mackinnon AM, Barter PJ. Effects of serum amyloid A protein (SAA) on composition, size, and density of high density lipoproteins in subjects with myocardial infarction. J Lipid Res. 1985;26:1389–1398. [PubMed] [Google Scholar]
- 38.Husebekk A, Skogen B, Husby G. Characterization of amyloid proteins AA and SAA as apolipoproteins of high density lipoprotein (HDL). Displacement of SAA from the HDL-SAA complex by apo AI and apo AII. Scand J Immunol. 1987;25:375–381. doi: 10.1111/j.1365-3083.1987.tb02203.x. [DOI] [PubMed] [Google Scholar]
- 39.Hosoai H, Webb NR, Glick JM, Tietge UJ, Purdom MS, de Beer FC, Rader DJ. Expression of serum amyloid A protein in the absence of the acute phase response does not reduce HDL cholesterol or apoA-I levels in human apoA- I transgenic mice. J Lipid Res. 1999;40:648–653. [PubMed] [Google Scholar]
- 40.Dember LM, Hawkins PN, Hazenberg BP, Gorevic PD, Merlini G, Butrimiene I, Livneh A, Lesnyak O, Puechal X, Lachmann HJ, Obici L, Balshaw R, Garceau D, Hauck W, Skinner M. Eprodisate for the treatment of renal disease in AA amyloidosis. N Engl J Med. 2007;356:2349–2360. doi: 10.1056/NEJMoa065644. [DOI] [PubMed] [Google Scholar]
- 41.Nakashima Y, Wight TN, Sueishi K. Early atherosclerosis in humans: role of diffuse intimal thickening and extracellular matrix proteoglycans. Cardiovasc Res. 2008;79:14–23. doi: 10.1093/cvr/cvn099. [DOI] [PubMed] [Google Scholar]
- 42.Aviram M. Modified forms of low density lipoprotein and atherosclerosis. Atherosclerosis. 1993;98:1–9. doi: 10.1016/0021-9150(93)90217-i. [DOI] [PubMed] [Google Scholar]
- 43.Sartipy P, Bondjers G, Hurt-Camejo E. Phospholipase A2 type II binds to extracellular matrix biglycan: modulation of its activity on LDL by colocalization in glycosaminoglycan matrixes. Arterioscler Thromb Vasc Biol. 1998;18:1934–1941. doi: 10.1161/01.atv.18.12.1934. [DOI] [PubMed] [Google Scholar]
- 44.Cabana VG, Reardon CA, Wei B, Lukens JR, Getz GS. SAA-only HDL formed during the acute phase response in apoA-I+/+ and apoA-I−/− mice. J Lipid Res. 1999;40:1090–1103. [PubMed] [Google Scholar]