Abstract
Preeclampsia (PE) impacts 8 million mother-infant pairs worldwide each year. This human pregnancy–specific disease characterized by hypertension and proteinuria accounts for significant maternal and neonatal morbidity and mortality. The current theory of the pathogenesis of PE as reviewed by Drs. Christopher Redman and Ian Sargent is thought to occur as a 2-stage process with poor placentation in the first half of pregnancy resulting in the maternal response in the second half of pregnancy. Our studies have focused on understanding the placental contribution to this serious disease by examining the gene expression profile of the deciduas basalis or basal plate, the region of the placenta involved in the “poor placentation”. In this review we present summaries of our microarray datasets both of normal placentation and those gene expression changes resulting in the context of PE. Additionally, we have taken this opportunity to combine the data sets to provide a more comprehensive view of this region of the placenta. As defects in the basal plate are, in part, at the root of the disease process, we believe that understanding the pathobiology that occurs in this region will increase our ability to alter the development and/or course of PE.
Keywords: placenta, maternal-fetal interface, basal plate, preeclampsia
Normal Human Placental Development
Survival and growth of the fetus requires normal development of the placenta, which in humans involves the formation of a transient organ with both maternal and fetal contributions. During cytotrophoblast (CTB) differentiation, progenitors assume one of two fates. In floating villi, they fuse to form multinucleate syncytiotrophoblasts (STBs), whose primary functions are transport and hormone production. In anchoring villi, mononuclear CTBs acquire tumor-like properties that enable them to invade the decidua, the endometrium of pregnancy, and the adjacent third of the myometrium (interstitial invasion). They also breach the small uterine vessels they encounter, intercalating within the muscular walls and completely replacing the resident maternal endothelial lining (endovascular invasion). As a result, high-resistance spiral arterioles are transformed into low-resistance, high-capacitance vessels that divert uterine blood flow to the floating villi. This invasion process is most active during 10–20 wk of gestation. This region where maternal and fetal cells coexist is termed the basal plate or maternal-fetal interface, and its proper formation and function are required for normal pregnancy outcome (Fig. 1).
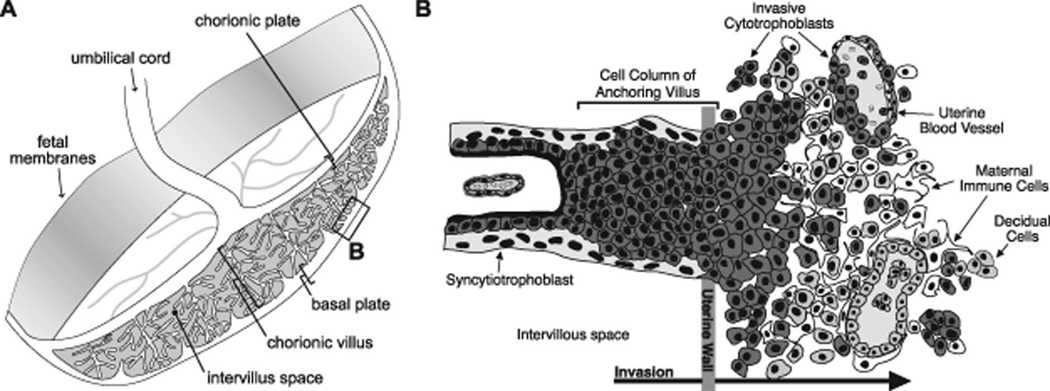
Figure 1. Diagram of the human maternal-fetal interface.
(A) Representation of the human placenta after delivery. The placental surface that was adjacent to the uterine wall is termed the basal plate. The boxed area denotes the region biopsied for these studies. (B) View of the basal plate at the cellular level. This chimeric region of the placenta is composed of both maternal and fetal cells: extravillous (invasive) cytotrophoblasts (dark grey), decidual cells (light grey), remodeled vasculature (both invasive CTBs and maternal endothelium) and maternal immune cells (white). (Reproduced with permission from Endocrinology (13)).
At a molecular level, many unusual processes occur in this area. For example, invasive CTBs execute a novel epithelial-to-mesenchymal transition that enables vascular mimicry, required for establishing blood flow to the placenta (1, 2). Perhaps most remarkably, the maternal immune system tolerates the invasion of the hemi-allogeneic fetal cells for the duration of pregnancy.
Over the past several decades, a great deal of information has been gained about placental development by taking a candidate molecule approach (3). For example, the fact that endovascular CTBs function as endothelial cells prompted investigators to study the role of vasculogenic/angiogenic molecules, including adhesion receptors, at the maternal-fetal interface (4, 5). As in many tumors, CTBs use matrix metalloproteinases for the purpose of invasion (6, 7). Interestingly, CTBs express several molecules involved in leukocyte function, including TOLL-like receptors, which are involved in the response to infections, and L-selectin, which mediates a novel type of rolling adhesion (8, 9).
However, there are also numerous examples of seemingly novel mechanisms that are unique to placental development. For example, trophoblasts in all locations lack major histocompatibility class (MHC) II expression, and upon allocation to the invasive pathway, CTBs upregulate HLA-G, a nonclassical MHC class I molecule, in the absence of HLA-A and B expression (10, 11). In addition, many lines of evidence suggest that CTBs have unusual responses to hypoxia, likely a reflection of the fact that the fetus resides in a physiologic state of lower oxygen tension (12). Furthermore, very little is known about gene expression during the embryonic and fetal stages of human development. Accordingly, unbiased analyses, such as microarray approaches, are also crucial for obtaining new insights into the mechanisms that are required for normal basal plate formation and function during pregnancy. Determining the gene expression profile of this critical region also provides an important foundation for investigations of the basal plate region in pathologic conditions such as preeclampsia.
Gene Expression Profiling at the Human Maternal-Fetal Interface Over Gestation
Basal plate biopsy specimens were obtained from 36 placentas (14–40 weeks) from women who had normal pregnancies. RNA was isolated, processed and hybridized to HG-U133A and HG-U133B Affymetrix GeneChips. Surprisingly, the expression of very few genes was modulated during the 14- to 24-week interval. In contrast, hundreds of genes, including those already known to be regulated over gestation, were modulated between mid-pregnancy (14–24 weeks) and term. These data allowed us to identify molecules that play potentially important roles in the formation of the maternal-fetal interface during the second trimester or in preparation of this area for parturition.
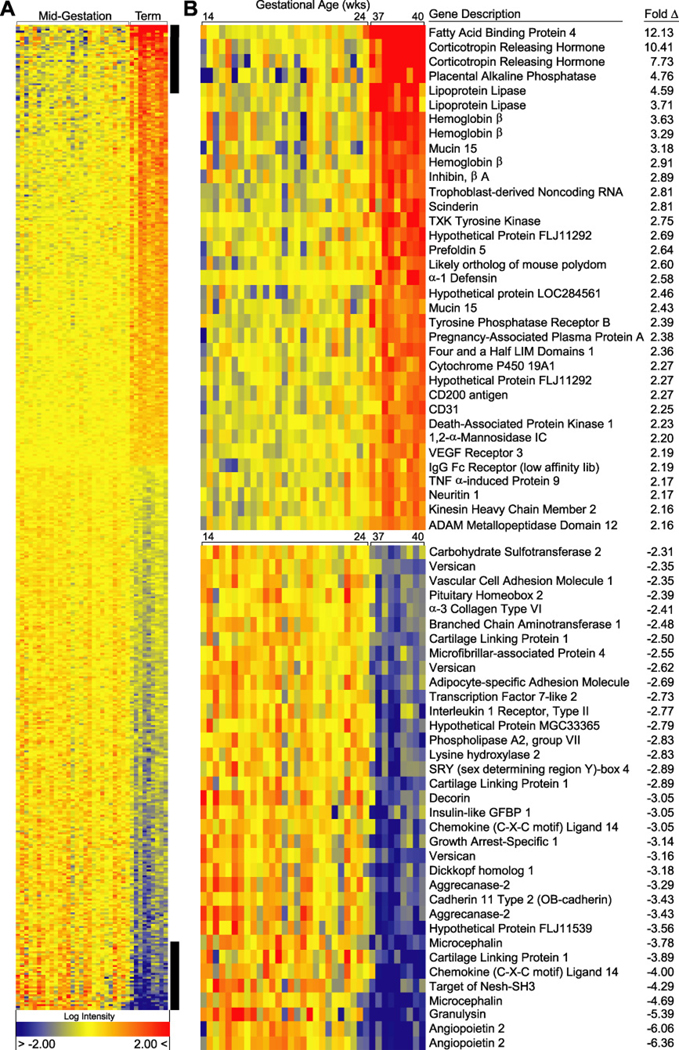
Our analysis revealed a total of 418 genes/expressed sequence tags that were differentially regulated between term and mid-gestation. A heat map of the top 25 up and down regulated genes is shown [Fig. 2; complete heat map and data (13)]. Based on GO annotations, the differentially expressed genes were involved in a variety of biological processes. At least one sixth were expressed sequence tags or hypothetical proteins and thus lacked annotations. Of the known differentially expressed genes, 17 were related to lipid metabolism, 10 were involved with formation or regulation of the extracellular matrix (ECM), 21 were immune effectors or modulators, 24 were transcription factors, and 6 had angiogenic/vasculogenic functions.
Figure 2. Heat map of the most highly up regulated (upper panel) and down regulated (lower panel) differentially expressed genes in the basal plate region at term in normal pregnancy.
The normalized log intensity values were centered to the median value of each probe set and colored on a range of −2 to +2. Red denotes up regulated, yellow denotes intermediate, and blue denotes down regulated expression levels as compared with the median value. Columns contain data from a single basal plate specimen, and rows correspond to a single probe set. Samples are arranged from left to right, ordered by increasing gestational age. Rows are ranked by fold change (mean term value [n = 9] divided by mean midgestation value [n = 27]). (Reproduced with permission from Endocrinology (13))
Ingenuity Pathway Analysis (IPA) software was used to further evaluate the participation of the differentially expressed genes in metabolic and signaling pathways. Analysis of genes with at least a twofold change highlighted two metabolic pathways—folate biosynthesis and N-glycan degradation involving mannose-containing structures. With regard to signaling, eleven of the differentially expressed genes mapped to the Wnt-β-catenin pathway. We also used the IPA software to map networks of the differentially expressed genes. The largest network contained genes that were involved in cell motility, cell-to-cell signaling/interaction and tissue development.
We were also interested to find that genes encoding molecules that are involved in immune defense are highly regulated. For example, defensin alpha 1 was upregulated about threefold at the RNA level at term as compared with the second trimester. Production of this antimicrobial peptide is constitutive in some cells (e.g., neutrophils) and induced in others (e.g., monocytes and CD8 T lymphocytes) in response to proinflammatory mediators (14). The presence of defensins in human term placental tissue has been previously reported (15). Increased expression at term could occur in preparation for labor and placental separation, which increase the risk of infection. In contrast, the expression of another antimicrobial molecule, granulysin, which localizes to the cytolytic granules of T cells, natural killer (NK) cells (16) and certain dendritic cells (17), is downregulated at term. We speculate that the decreased granulysin expression we observed parallels the decrease in T cell and NK cell numbers at the maternal-fetal interface at term (18). The downregulation of Ly96 expression, another NK-cell-specific molecule, provides further support for this concept. Although the mechanisms that lead to the eventual disappearance of decidual leukocytes from the maternal-fetal interface are not known, the observed concurrent decrease in expression of chemotactic molecules, such as chemokine-like factor superfamily 6 (CKLFSF6) and secreted phosphoprotein 1 (SPP1), could be a related phenomenon.
A trophoblast-derived noncoding RNA (TncRNA) was one of the most interesting of the highly upregulated differentially expressed genes in the immune function category. This transcript, which directly suppresses MHC class II expression by interacting with the MHC IITA-PIII transactivator, likely accounts for the lack of trophoblast MHC class II expression (19). As such, this molecule could play an important role in promoting maternal immunotolerance of the hemi-allogeneic fetus. Why TncRNA expression increases at term is unclear, but this phenomenon could be related to the continuing need to suppress MHC class II expression in trophoblasts, particularly as they are shed into maternal blood at the time of delivery. In this regard, it is interesting to note that expression of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), which plays a role in regulating decidual immune responses, is also upregulated at term (20).
Additionally, our group has also been interested in the functions of the myriad angiogenic factors that are produced at the maternal-fetal interface (3, 21). In broad terms, we know that these molecules have at least three targets—the intrinsic placental vasculature, the maternal vasculature, and the CTB subpopulation that executes an unusual epithelial-to-endothelial transition as the cells invade the uterine wall and remodel the maternal vasculature in this region. Thus, we anticipated that molecules involved in vasculogenesis/angiogenesis would be upregulated during the active phases of placentation, i.e., in the second trimester rather than at term, which is what we found. Consistent with our previously published work, the downregulated genes included angiopoietin (ANGPT)-2 (22).
As with every microarray analysis, we made a number of interesting observations that warrant additional follow-up. For example, the cluster analysis showed a striking co-downregulation at term of ANGPT-2 and microcephalin (MCPH1). Interestingly, ANGPT-2 and MCPH1 genes are transcribed from opposite strands of the same region (chromosome 8p23.1). Their tight coexpression suggests that transcription from this area could be silenced at term, perhaps by local chromatin modifications or the recruitment of inhibitory protein complexes to the same promoter element. It will be interesting to determine if the pattern of co-expression of ANGPT-2 and MCPH1 occurs in other tissues or is specific to our data set. It is known that MCPH1 controls brain size in humans by regulating the proliferative and, hence, differentiative capacity of neuroblasts, ultimately exerting its effects through cell cycle regulators (23, 24). Furthermore, during human evolution there is evidence that strong genetic selection has been exerted on MCPH1 (25). While the most obvious consequence is brain size, another interesting possibility is that placental form and function have been affected as well.
In summary, we found that gene expression patterns in the basal plate region change dramatically between the second trimester and term. Thus, it is important to control for this variable when studying the effect of pregnancy complications that occur during this timeframe. For our purposes, understanding the normal development and formation of the maternal-fetal interface is an important first step toward understanding PE-related changes.
Human Placental Development in Preeclampsia
PE, a pregnancy complication, is manifested by the onset of hypertension and proteinuria in the second half of pregnancy. PE is relatively common (4–8% of pregnancies), with potentially deadly consequences for the mother and/or her offspring. Currently, the only definitive treatment for this condition is delivery of the placenta, and therefore the infant, accounting for 15% of all preterm births in the U.S. Despite decades of research, a full understanding of the pathogenesis of PE remains elusive. Nevertheless, it is clear that the placenta plays a central role: the signs of PE can occur in molar pregnancies, which lack a fetus, and the disease resolves once the placenta is delivered.
During the last several years, a clearer picture of the pathogenesis of PE has begun to emerge. A two-stage model has been proposed in which the initiating event, poor placentation, is thought to occur early in gestation (26). This concept is supported by several studies that document the association between reduced blood flow to the placenta before 20 wk of gestation, as determined by color Doppler ultrasound evaluation of uterine artery blood flow, and a greatly increased risk of developing PE (27, 28). Anatomic examination shows that the specific area of the placenta most affected by this syndrome is the basal plate, the site of CTB invasion. Interstitial CTB invasion is often shallow, and endovascular invasion does not proceed beyond the terminal portions of the spiral arterioles. Thus, the maternal vessels do not undergo the complete spectrum of physiological changes that normally occur (e.g. loss of endothelial lining and musculoelastic tissue); the mean external diameter of the myometrial vessels is less than half that of equivalent vessels from uncomplicated pregnancies (29–31). In addition, fewer vessels show evidence of CTB invasion (32). Thus, their architecture precludes an adequate response to gestation-related fetal demands for increased blood flow.
The second stage of PE is thought to be the maternal response to abnormal placentation. Systemic endothelial dysfunction appears to be an important common denominator (26, 33, 34). Recent data point to an imbalance in circulating factors with angiogenic/vasculogenic functions, such as soluble vascular endothelial growth factor receptor-1 (VEGFR-1, sFlt-1), placental growth factor, and the transforming growth factor-beta receptor endoglin (35–40).
Gene Expression at the Maternal-Fetal Interface Impacted by Preeclampsia
As in our studies of gestation-related change in gene expression at the maternal-fetal interface, we used an unbiased approach to analyze basal plate biopsies from pregnancies afflicted by PE. We focused on preeclampsia that presented in the preterm period (24–36 weeks) as this disease is thought to be more severe and also associated with the greatest morbidity. In this regard, we exploited our observation that preterm labor (PTL) without signs of inflammation is associated with normal CTB differentiation/invasion (41). Thus, basal plate specimens from these patients served as gestational age-matched controls.
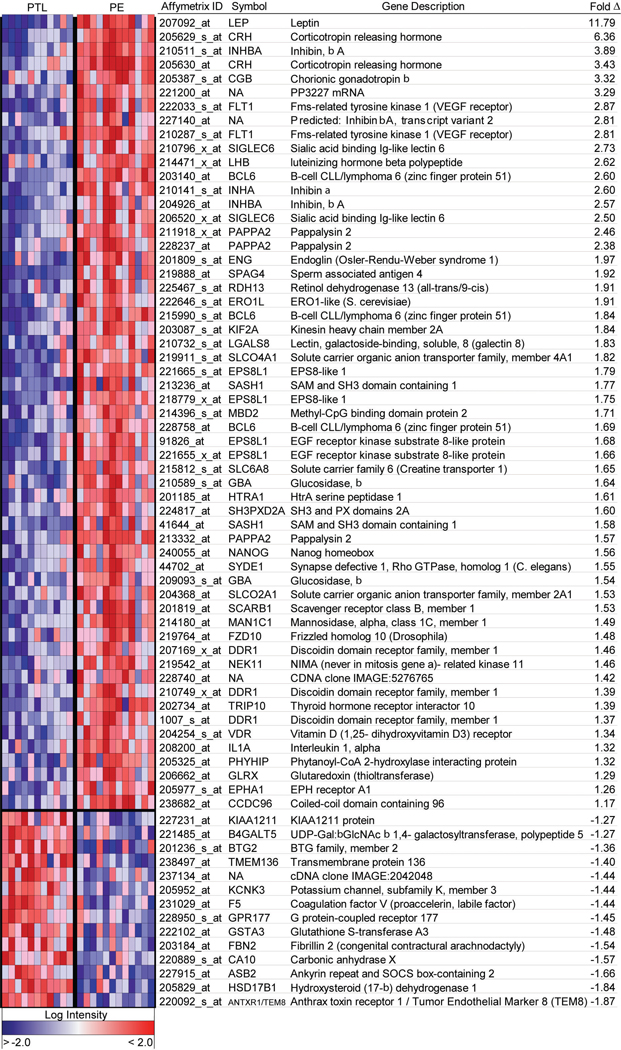
The microarray analysis revealed 55 differentially expressed genes, of which the majority were not previously known to be dysregulated in PE [Fig. 3, (42)]. This list includes molecules that were previously reported to be present at higher than normal levels in maternal serum, chorionic villi and/or cord blood in pregnancies complicated by PE, a finding that gives added confidence to the novel genes that we identified as similarly regulated. However, even for these previously reported molecules, in most cases this was the first description of their increased expression in the basal plate region of the placenta.
Figure 3. Heat map of differentially expressed genes in basal plates of PE placentas as compared to controls.
The normalized log intensity values for 71 differentially expressed probe sets were centered to the median value of each probe set and colored on a range of −2.5 to +2.5. Red denotes upregulated and blue denotes downregulated expression levels as compared with the median value. Columns contain data from a single basal plate specimen, and rows correspond to a single probe set. Samples are arranged from left to right, ordered by increasing gestational age within each category. Rows are ranked by fold change (mean PE value [n = 12] divided by mean PTL value [n = 11]). (Reproduced with permission from Endocrinology (42)).
For example, our data demonstrated increased leptin expression in the basal plate of PE placentas as compared to control tissue. Numerous investigators have reported a PE-associated increase in circulating levels of leptin (43–51), and a leptin gene polymorphism has been linked to an increased risk of developing this pregnancy complication (52). However, a clear picture of how an increase in leptin expression is linked to the pathophysiology of PE has yet to emerge. Interestingly, although the classic leptin receptors were not differentially expressed, we observed elevated levels of the mRNA that encodes Siglec-6, a transmembrane protein that also binds leptin. These findings suggest that this molecule may play an important role as a placental leptin receptor, and that increased Siglec-6 levels could contribute to the pathogenesis of PE. While the cloning strategy for Siglec-6 was based on its ability to interact with leptin, the other Siglec family members bind sialic acid-containing glycans. Siglec-6 has binding specificity for the sialyl-Tn epitope (Siaα2-6Gal-NAcα1-O-R, where R is a serine or threonine). Published data suggest that, in the placenta, leptin is a Siglec-6 ligand, but the endogenous binding partners have yet to be identified (53). Additionally, Siglec-6 expression has other interesting features. For example, in humans, it is restricted to the placenta and B-lymphocytes. In other species, including non-human primates, placental cells lack Siglec-6 expression, which B cells retain (54). The fact that Siglec-6 is expressed only in human placentas and not in non-human primate placentas (54) is intriguing, as PE is thought to be a uniquely human disease; spontaneous PE has not been reported in other animals, even non-human primates (55).
A PE-associated increase in the expression of pappalysin (PAPP-A2) was another novel observation that emerged from our work. PAPP-A2, which has 46% sequence identity with PAPP-A, is a metalloproteinase that cleaves insulin-like growth factor (IGF) binding protein-5 (IGFBP-5) (56). In a fibroblast model, an increase in IGFBP-5 proteolysis attenuates IGF-I stimulatory effects on cell migration (57). If CTBs respond in an analogous manner, then the observed PE-associated increase in PAPP-A2 levels could inhibit CTB invasion by mechanisms that include an increase in IGFBP-5 proteolysis. These two interesting novel observations, enhanced Siglec-6 and PAPP-A2 expression, were validated at both the RNA and the protein level. In toto, these results suggest fundamental alterations in important biological processes including pathways that are regulated by leptin and IGF signals.
The results of this work highlight the complex pathophysiology of PE and the many pathways it impacts. Understanding the pregnancy-related functions of the differentially expressed genes in PE will likely lead to a better understanding of the pathogenesis of this human-specific condition, the crucial first step in the rational design of treatments (both preventative and therapeutic) that address the causes, rather than the consequences, of this pregnancy complication.
Combined Analysis of Gene Expression Profiles at the Human Maternal-Fetal Interface
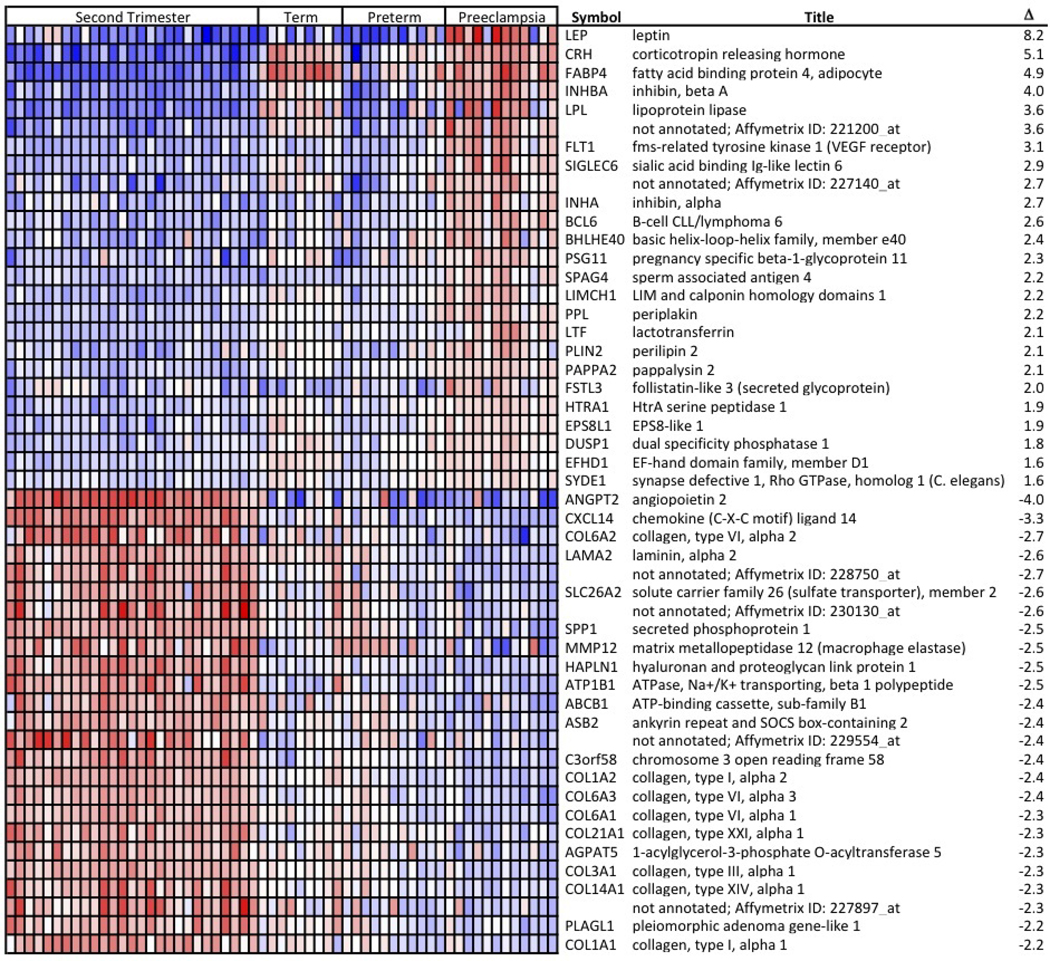
To develop a more global view of the maternal-fetal interface we combined the gestational age and preeclampsia microarray datasets. The sample characteristics are summarized in Fig. 4. Intensity measures from the Affymetrix U133 A and B chips for each basal plate biopsy were stored as CEL files and probe level normalization performed using robust multichip averaging. The two classes, preeclampsia and “other” (second trimester, PTL and term), were compared using the linear analysis of microarrays (limma; Bioconductor) and Comparative Marker Selection (GenePattern). Genes considered significant had a Bonferonni-corrected p<0.01 and an absolute fold change of ≥1.5. This analysis resulted in the identification of 33 genes as increased and 89 genes as decreased in PE. A heat map of the top 25 up and down regulated genes is presented (Fig. 5; complete heat map Supplemental Fig. S1).
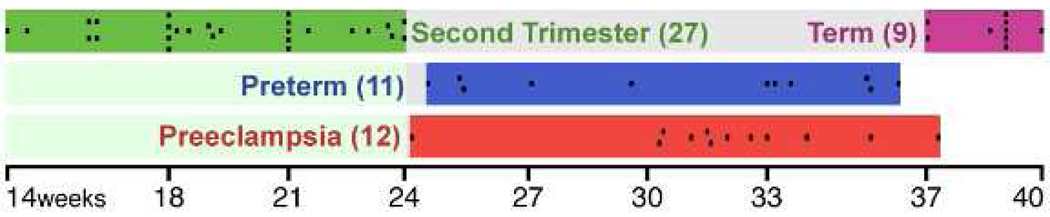
Figure 4. Gestational Timeline of Basal Plate Biopsies Used in the Composite Analysis.
The microarray data from human basal plate biopsies were used in a combined analysis. Each black box represents one individual placenta and is listed by gestational age and condition.
Figure 5. Heat map of the most highly up regulated and down regulated differentially expressed genes in basal plates of PE placentas as compared to the second trimester, term and preterm labor samples.
The normalized log intensity values for the differentially expressed probe sets were centered to the median value of each probe set and colored on a range of −2.5 to +2.5. Red denotes up regulated and blue denotes down regulated expression levels as compared with the median value. Columns contain data from a single basal plate specimen, and rows correspond to a single probe set. Samples within each catergory are arranged from left to right, ordered by increasing gestational age. Rows are ranked by fold change. The complete heat map is shown in supplemental Fig. S1.
When considering this combined analysis there a few clinical features to keep in mind. First, the second trimester and term samples were from non-labored deliveries while all the PTL and the majority of the PE subjects were from labored deliveries. Interestingly, labor had relatively little impact. Also the clinical outcome for the second trimester samples was not known. Given the ~5% incidence of PE, it is likely that 1–2 samples included in the analysis were from pregnancies that would have been impacted by PE. It is tempting to consider the few samples that clearly have different expression patterns that correspond to PE-specific changes (e.g., leptin, FSTL3, coagulation factor 5 and semaphoring 6D) as being in this category.
Several interesting patterns emerge from this analysis. Some genes that are up re regulated in PE and term samples as compared to second trimester and PTL. These included corticotropin releasing hormone (CRH), fatty acid binding protein 4 (FABP4) and lipoprotein lipase (LPL). Interestingly, these are also the genes that tightly clustered in the gestational age dataset discussed above suggesting co-regulation. Conversely, matrix metallopeptidase 12 (MMP12) and several of the collagens are examples of genes that were down regulated in both PE and term samples. This analysis provides a glimpse at what may be the molecular profile of premature placental aging in PE. However, it is also clear that this is not the only process involved in PE, as some genes are distinctly dysregulated by this disease process as compared to all other samples regardless of gestational age or condition. Examples of these PE-specific genes are leptin, Flt-1 (the parent molecule of sFlt-1), PAPPA2 and inhibin A (INHA), which are up regulated in PE and laminin alpha 2 (LAMA2), secreted phosphoprotein 1 (SPP1) and ankyrin repeated and SOCS box-containing 2 (ASB2), which are down regulated in PE. Interestingly, although there is a strong correlation of elevated leptin and sFlt-1 with PE, these molecules are clearly not elevated in several of the subjects despite a clear clinical diagnosis of PE upon review of the clinical data. This is additional evidence that PE is a syndrome and may be the end result of divergent pathogenesis pathways. In fact, we observed substantial variability in gene expression for both the PTL and PE groups as compared to the ostensibly normal samples, a reflection of the heterogeneity of the underlying pathologies. Our data suggest that molecular signatures could be used to better classify these disease entities.
A third group of genes were distinctly expressed in the second trimester samples. These include ANGPT2, chemokine (C-X-C motif) ligand 14 (CXCL14) and (hyaluronoan and proteoglycan link protein 1 (HAPLN1). These may be genes with critical activities during the active process of CTB interstitial and endovascular invasion, which forms the maternal-fetal interface.
Further analyses using IPA showed that the most differentially expressed genes mapped to the PPAR (as was seen with the gestational age dataset) and the neuronal guidance pathways. The latter result was particularly interesting given the importance of neuronal guidance molecules, EPH and Ephrins, in CTB invasion and vascular remodeling (58). These differences may be related at the molecular level to the impaired invasion observed in the basal plate regions of PE placentas.
In summary, the maternal-fetal interface is a remarkable chimeric tissue that holds the answers to many interesting biological questions regarding invasion, vasculogenesis/angiogenesis and immunotolerance. It may also hold the answer to understanding the early pathogenesis of PE. Our studies provide a global analysis of the expression patterns of genes that are involved in these and other processes. Further, the results of these studies provide reference data sets of gene expression profiles against which changes that occur in a variety of other pregnancy complications such as recurrent miscarriage, intrauterine growth restriction and placenta accreta can be compared. Now we are in a position to differentiate changes in gene expression that occur in these conditions with the hope of providing sufficient insight to develop ways to impact the clinical course of a variety of obstetrical complications.
Supplementary Material
Acknowledgements
The field of obstetrics and particularly preeclampsia owes a great deal to the life work of Prof. Chris Redman. His contributions will continue to guide research toward finding diagnostic markers, predictive markers as well as preventative and curative strategies for the treatment of preeclampsia. His work will benefit the health of mothers and children around the world and inspire generations of researchers to come.
Funding: Work was supported by NIH grants 5K12HD00849, 2K12HD001271 and R01 HD60723 (VDW); R01 HL 64597 and R01 HD 30367 (SJF); 5-MO1-RR 00083 (General Clinical Research Center at San Francisco General Hospital) and HL 072301 (University of California, San Francisco, National Heart, Lung and Blood Institute Shared Microarray Facility). Support also from the American Board of Obstetrics and Gynecology/American Association of Obstetricians and Gynecologists Foundation (VDW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Virginia D. Winn, Department of Obstetrics and Gynecology, University of Colorado School of Medicine, Aurora, Colorado 80045.
Matthew Gormley, Departments of Obstetrics, Gynecology and Reproductive Sciences and Cell and Tissue Biology, University of California, San Francisco, San Francisco, California 94143.
Susan J. Fisher, Departments of Obstetrics, Gynecology and Reproductive Sciences and Cell and Tissue Biology, University of California, San Francisco, San Francisco, California 94143.
References
- 1.Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, et al. Integrin switching regulates normal trophoblast invasion. Development. 1994 Dec;120(12):3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, et al. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997 May 1;99(9):2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004 Sep;114(6):744–754. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark DE, Smith SK, Licence D, Evans AL, Charnock-Jones DS. Comparison of expression patterns for placenta growth factor, vascular endothelial growth factor (VEGF), VEGF-B and VEGF-C in the human placenta throughout gestation. J Endocrinol. 1998 Dec;159(3):459–467. doi: 10.1677/joe.0.1590459. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002 Apr;160(4):1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staun-Ram E, Goldman S, Gabarin D, Shalev E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol Endocrinol. 2004 Aug 4;2:59. doi: 10.1186/1477-7827-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Librach CL, Feigenbaum SL, Bass KE, Cui TY, Verastas N, Sadovsky Y, et al. Interleukin-1 beta regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J Biol Chem. 1994 Jun 24;269(25):17125–17131. [PubMed] [Google Scholar]
- 8.Abrahams VM, Mor G. Toll-like receptors and their role in the trophoblast. Placenta. 2005 Aug;26(7):540–547. doi: 10.1016/j.placenta.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, et al. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003 Jan 17;299(5605):405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- 10.McMaster MT, Librach CL, Zhou Y, Lim KH, Janatpour MJ, DeMars R, et al. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol. 1995 Apr 15;154(8):3771–3778. [PubMed] [Google Scholar]
- 11.Mattsson R. The non-expression of MHC class II in trophoblast cells. Am J Reprod Immunol. 1998 Dec;40(6):383–384. doi: 10.1111/j.1600-0897.1998.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 12.Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000 Mar–Apr 21; Suppl A:S25–S30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- 13.Winn VD, Haimov-Kochman R, Paquet AC, Yang YJ, Madhusudhan MS, Gormley M, et al. Gene Expression Profiling of the Human Maternal-Fetal Interface Reveals Dramatic Changes between Midgestation and Term. Endocrinology. 2007 Mar;148(3):1059–1079. doi: 10.1210/en.2006-0683. [DOI] [PubMed] [Google Scholar]
- 14.Oppenheim JJ, Biragyn A, Kwak LW, Yang D. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann Rheum Dis. 2003 Nov;62 Suppl 2:ii17–ii21. doi: 10.1136/ard.62.suppl_2.ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svinarich DM, Gomez R, Romero R. Detection of human defensins in the placenta. Am J Reprod Immunol. 1997 Oct;38(4):252–255. doi: 10.1111/j.1600-0897.1997.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 16.Krensky AM. Granulysin: a novel antimicrobial peptide of cytolytic T lymphocytes and natural killer cells. Biochem Pharmacol. 2000 Feb 15;59(4):317–320. doi: 10.1016/s0006-2952(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 17.Raychaudhuri SP, Jiang WY, Raychaudhuri SK, Krensky AM. Lesional T cells and dermal dendrocytes in psoriasis plaque express increased levels of granulysin. J Am Acad Dermatol. 2004 Dec;51(6):1006–1008. doi: 10.1016/j.jaad.2003.10.679. [DOI] [PubMed] [Google Scholar]
- 18.King A, Loke YW, Chaouat G. NK cells and reproduction. Immunol Today. 1997 Feb;18(2):64–66. doi: 10.1016/s0167-5699(97)01001-3. [DOI] [PubMed] [Google Scholar]
- 19.Geirsson A, Paliwal I, Lynch RJ, Bothwell AL, Hammond GL. Class II transactivator promoter activity is suppressed through regulation by a trophoblast noncoding RNA. Transplantation. 2003 Jul 27;76(2):387–394. doi: 10.1097/01.TP.0000073612.04525.46. [DOI] [PubMed] [Google Scholar]
- 20.Markel G, Wolf D, Hanna J, Gazit R, Goldman-Wohl D, Lavy Y, et al. Pivotal role of CEACAM1 protein in the inhibition of activated decidual lymphocyte functions. J Clin Invest. 2002 Oct;110(7):943–953. doi: 10.1172/JCI15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol. 2005 Jun;48(2):372–386. doi: 10.1097/01.grf.0000160313.82606.d7. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Bellingard V, Feng KT, McMaster M, Fisher SJ. Human cytotrophoblasts promote endothelial survival and vascular remodeling through secretion of Ang2, PlGF, and VEGF-C. Dev Biol. 2003 Nov 1;263(1):114–125. doi: 10.1016/s0012-1606(03)00449-4. [DOI] [PubMed] [Google Scholar]
- 23.Wyatt SM, Kraus FT, Roh CR, Elchalal U, Nelson DM, Sadovsky Y. The correlation between sampling site and gene expression in the term human placenta. Placenta. 2005 May;26(5):372–379. doi: 10.1016/j.placenta.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Lee J, Stern DF. Microcephalin is a DNA damage response protein involved in regulation of CHK1 and BRCA1. J Biol Chem. 2004 Aug 13;279(33):34091–34094. doi: 10.1074/jbc.C400139200. [DOI] [PubMed] [Google Scholar]
- 25.Evans PD, Gilbert SL, Mekel-Bobrov N, Vallender EJ, Anderson JR, Vaez-Azizi LM, et al. Microcephalin, a gene regulating brain size, continues to evolve adaptively in humans. Science. 2005 Sep 9;309(5741):1717–1720. doi: 10.1126/science.1113722. [DOI] [PubMed] [Google Scholar]
- 26.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001 Jan 6;357(9249):53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 27.Gomez O, Martinez JM, Figueras F, Del Rio M, Borobio V, Puerto B, et al. Uterine artery Doppler at 11–14 weeks of gestation to screen for hypertensive disorders and associated complications in an unselected population. Ultrasound Obstet Gynecol. 2005 Oct;26(5):490–494. doi: 10.1002/uog.1976. [DOI] [PubMed] [Google Scholar]
- 28.Hershkovitz R, de Swiet M, Kingdom J. Mid-trimester placentation assessment in high-risk pregnancies using maternal serum screening and uterine artery Doppler. Hypertens Pregnancy. 2005;24(3):273–280. doi: 10.1080/10641950500280995. [DOI] [PubMed] [Google Scholar]
- 29.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- 30.Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol. 1981 Sep;88(9):876–881. doi: 10.1111/j.1471-0528.1981.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 31.Moodley J, Ramsaroop R. Placental bed morphology in black women with eclampsia. S Afr Med J. 1989 Apr 15;75(8):376–378. [PubMed] [Google Scholar]
- 32.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986 Oct;93(10):1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 33.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989 Nov;161(5):1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 34.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16(1):5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 35.Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003 Jan;188(1):177–182. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 36.Friedman SA, Schiff E, Emeis JJ, Dekker GA, Sibai BM. Biochemical corroboration of endothelial involvement in severe preeclampsia. Am J Obstet Gynecol. 1995 Jan;172(1 Pt 1):202–203. doi: 10.1016/0002-9378(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 37.Tyurin VA, Liu SX, Tyurina YY, Sussman NB, Hubel CA, Roberts JM, et al. Elevated levels of S-nitrosoalbumin in preeclampsia plasma. Circ Res. 2001 Jun 8;88(11):1210–1215. doi: 10.1161/hh1101.092179. [DOI] [PubMed] [Google Scholar]
- 38.Clark BA, Halvorson L, Sachs B, Epstein FH. Plasma endothelin levels in preeclampsia: elevation and correlation with uric acid levels and renal impairment. Am J Obstet Gynecol. 1992 Mar;166(3):962–968. doi: 10.1016/0002-9378(92)91372-h. [DOI] [PubMed] [Google Scholar]
- 39.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006 Sep 7;355(10):992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 40.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003 Mar;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y, Bianco K, Huang L, Nien JK, McMaster M, Romero R, et al. Comparative analysis of maternal-fetal interface in preeclampsia and preterm labor. Cell Tissue Res. 2007 Jun 5; doi: 10.1007/s00441-007-0428-0. [DOI] [PubMed] [Google Scholar]
- 42.Winn VD, Gormley M, Paquet AC, Kjaer-Sorensen K, Kramer A, Rumer KK, et al. Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology. 2009 Jan;150(1):452–462. doi: 10.1210/en.2008-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laivuori H, Gallaher MJ, Collura L, Crombleholme WR, Markovic N, Rajakumar A, et al. Relationships between maternal plasma leptin, placental leptin mRNA and protein in normal pregnancy, pre-eclampsia and intrauterine growth restriction without pre-eclampsia. Mol Hum Reprod. 2006 Sep;12(9):551–556. doi: 10.1093/molehr/gal064. [DOI] [PubMed] [Google Scholar]
- 44.Laivuori H, Kaaja R, Koistinen H, Karonen SL, Andersson S, Koivisto V, et al. Leptin during and after preeclamptic or normal pregnancy: its relation to serum insulin and insulin sensitivity. Metabolism. 2000 Feb;49(2):259–263. doi: 10.1016/s0026-0495(00)91559-2. [DOI] [PubMed] [Google Scholar]
- 45.Li RH, Poon SC, Yu MY, Wong YF. Expression of placental leptin and leptin receptors in preeclampsia. Int J Gynecol Pathol. 2004 Oct;23(4):378–385. doi: 10.1097/01.pgp.0000139647.40620.c8. [DOI] [PubMed] [Google Scholar]
- 46.Lu D, Yang X, Wu Y, Wang H, Huang H, Dong M. Serum adiponectin, leptin and soluble leptin receptor in pre-eclampsia. Int J Gynaecol Obstet. 2006 Nov;95(2):121–126. doi: 10.1016/j.ijgo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 47.McCarthy JF, Misra DN, Roberts JM. Maternal plasma leptin is increased in preeclampsia and positively correlates with fetal cord concentration. Am J Obstet Gynecol. 1999 Mar;180(3 Pt 1):731–736. doi: 10.1016/s0002-9378(99)70280-2. [DOI] [PubMed] [Google Scholar]
- 48.Mise H, Sagawa N, Matsumoto T, Yura S, Nanno H, Itoh H, et al. Augmented placental production of leptin in preeclampsia: possible involvement of placental hypoxia. J Clin Endocrinol Metab. 1998 Sep;83(9):3225–3229. doi: 10.1210/jcem.83.9.5117. [DOI] [PubMed] [Google Scholar]
- 49.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Divergent metabolic and vascular phenotypes in pre-eclampsia and intrauterine growth restriction: relevance of adiposity. J Hypertens. 2004 Nov;22(11):2177–2183. doi: 10.1097/00004872-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 50.Tommaselli GA, Pighetti M, Nasti A, D'Elia A, Guida M, Di Carlo C, et al. Serum leptin levels and uterine Doppler flow velocimetry at 20 weeks' gestation as markers for the development of pre-eclampsia. Gynecol Endocrinol. 2004 Sep;19(3):160–165. doi: 10.1080/09513590400007267. [DOI] [PubMed] [Google Scholar]
- 51.Vitoratos N, Chrystodoulacos G, Kouskouni E, Salamalekis E, Creatsas G. Alterations of maternal and fetal leptin concentrations in hypertensive disorders of pregnancy. Eur J Obstet Gynecol Reprod Biol. 2001 May;96(1):59–62. doi: 10.1016/s0301-2115(00)00401-2. [DOI] [PubMed] [Google Scholar]
- 52.Muy-Rivera M, Ning Y, Frederic IO, Vadachkoria S, Luthy DA, Williams MA. Leptin, soluble leptin receptor and leptin gene polymorphism in relation to preeclampsia risk. Physiol Res. 2005;54(2):167–174. [PubMed] [Google Scholar]
- 53.Patel N, Brinkman-Van der Linden EC, Altmann SW, Gish K, Balasubramanian S, Timans JC, et al. OB-BP1/Siglec-6. a leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J Biol Chem. 1999 Aug 6;274(32):22729–22738. doi: 10.1074/jbc.274.32.22729. [DOI] [PubMed] [Google Scholar]
- 54.Brinkman-Van der Linden E, Hurtado-Ziola N, Hayakawa T, Wiggleton L, Benirschke K, Varki A, et al. Human-Specific Expression of Siglec-6 in the Placenta. Glycobiology. 2007 Jun 22; doi: 10.1093/glycob/cwm065. [DOI] [PubMed] [Google Scholar]
- 55.Chez RA. Nonhuman primate models of toxemia of pregnancy. Perspect Nephrol Hypertens. 1976;5:421–424. [PubMed] [Google Scholar]
- 56.Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factorbinding protein-5 proteinase. J Biol Chem. 2001 Jun 15;276(24):21849–21853. doi: 10.1074/jbc.M102191200. [DOI] [PubMed] [Google Scholar]
- 57.Xu Q, Yan B, Li S, Duan C. Fibronectin binds insulin-like growth factor-binding protein 5 and abolishes Its ligand-dependent action on cell migration. J Biol Chem. 2004 Feb 6;279(6):4269–4277. doi: 10.1074/jbc.M311586200. [DOI] [PubMed] [Google Scholar]
- 58.Red-Horse K, Kapidzic M, Zhou Y, Feng KT, Singh H, Fisher SJ. EPHB4 regulates chemokine-evoked trophoblast responses: a mechanism for incorporating the human placenta into the maternal circulation. Development. 2005 Sep;132(18):4097–4106. doi: 10.1242/dev.01971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.