Highlights
▸ We examine development of syntactic processing in children using multi-modal neuroimaging methods. ▸ We examine brain activity with fMRI, cortical thickness with sMRI, and language functioning. ▸ We describe structural and functional correlations in the inferior frontal gyrus. ▸ Syntactic development is related to functional and structural brain changes. ▸ Syntactic development is a prolonged process with increased specialization by age 10.
Keywords: Syntax, language, Typical development, Lateralization, fMRI, Multimodal
Abstract
Development of syntactic processing was examined to evaluate maturational processes including left language lateralization functions and increased specialization of brain regions important for syntactic processing. We utilized multimodal methods, including indices of brain activity from fMRI during a syntactic processing task, cortical thickness measurements from structural MRI, and neuropsychological measures. To evaluate hypotheses about increasing lateralization and specialization with development, we examined relationships between cortical thickness and magnitude and spatial activation extent within the left inferior frontal gyrus (IFG) and its right hemisphere homologue. We predicted that increased activation in the left and decreased activation in the right IFG would be associated with increased syntactic proficiency. As predicted, a more mature pattern of increased thickness in the right pars triangularis was associated with decreased activation intensity and extent in the right IFG. These findings suggest a maturational shift towards decreased involvement of the right IFG for syntactic processing. Better syntactic skills were associated with increased activation in the left IFG independent from age, suggesting increased specialization of the left IFG with increased proficiency. Overall, our findings show relationships between structural and functional neurodevelopment that co-occur with improved syntactic processing in critical language regions of the IFG in typically developing children.
1. Introduction
By the time children begin to attend school at around age six, they are capable of producing grammatically correct and well-formed sentences about numerous topics in conversations with family and friends, and on the surface it may appear that they have mastered the subtleties of the syntactic rules of their native language (Scott, 2004). While the understanding of individual word meanings and expansion of vocabulary is essential to the development of further language skills, the grammatical or syntactic structure in which individual words are contained convey essential information as well. Syntactic capacity is a necessary prerequisite for sentence comprehension that allows the listener to understand “who is doing what to whom” through grammatical relationships in sentence elements (Friederici and Kotz, 2003). Early behavioral studies have suggested that semantic and syntactic processing are interdependent early in childhood (age 5), and that syntactic processing gradually becomes independent through childhood by about age 10 (Friederici, 1983). However, more recent studies have shown continued improvement of syntactic skills through childhood, adolescence and into early adulthood (ages 20–29), stabilizing only in middle age (Nippold et al., 2005). Essentially, this protracted development is associated with mastery of less frequent, more demanding linguistic structures taking longer to acquire than structures that are more frequently encountered (Leech et al., 2007). Consistent with this idea, behavioral studies across a wider age-range of typically developing children and adolescents show that children gradually become faster and more accurate at interpreting and producing complex sentences, as well as in detecting grammatical violations in these sentences (Berman, 2004, Nippold et al., 2005, Wulfeck et al., 2004).
Neuroimaging studies are beginning to shed light on the gradual process of specialization and lateralization of syntactic development in the brain. Event-related potential (ERP) studies in children provide further evidence that syntactic processing is not fully established neurally in early childhood but gradually develops toward adult-like processing during late childhood (reviewed in Friederici, 2006, Hahne et al., 2004). fMRI studies with adults have suggested that semantic and syntactic processing are neurally distinct at least in part, and that the left pars opercularis (Brodmann area 44) may be particularly implicated in syntactic processing with other portions of the IFG also involved depending on the task (reviewed in Bookheimer, 2002, Friederici, 2002). Syntactic development has been investigated with fMRI in young (ages 5–6) typically developing children (Brauer and Friederici, 2007), and methods for assessing syntactic processing have included rule violation tasks and sentence comprehension tasks. In general, studies with children have not demonstrated a fully developed level of specialization or lateralization for syntactic processing. For example, Brauer and Friederici (2007) evaluated differences in functional activation between children ages 5–6 and adults for semantic and syntactic aspects of sentence processing with a rule violation paradigm. They found that adults demonstrated syntax-specific activation in the superior temporal gyrus (STG) and pars opercularis while children had significant overlap for semantic and syntactic processing, engaging additional areas of the left and right inferior frontal gyrus (IFG), which are known to support resource demanding processes.
Along with functional changes in syntactic processing in childhood, dynamic changes in brain structure occur simultaneously. Many brain regions undergo cortical thinning through maturation, including the dorsal cortices of frontal and parietal lobes, whereas the IFG (Broca's area) and posterior perisylvian cortices (Wernicke's area) undergo cortical thickening (Shaw et al., 2008, Sowell et al., 2004a). Cortical thinning is thought to be associated with the pruning of underutilized synapses and increased myelination related to increased efficiency in these neural systems (reviewed in Sowell et al., 2004b). Cortical thickening in the IFG has been associated with improved phonological processing skills within individuals studied longitudinally (Lu et al., 2007). In addition, structural brain asymmetries in perisylvian brain language regions have been observed perinatally and throughout childhood, suggesting that brain lateralization begins early in development and continues later in childhood (Amunts et al., 2003, Chi et al., 1977, Shaw et al., 2009, Wada et al., 1975). These structural changes are likely related to functional activation changes in the brain that have been observed during the same age range. For example, increased hemispheric language lateralization with increased age has been demonstrated in developmental fMRI studies, and there is evidence for some degree of lateralization and specialization early in childhood that may vary among those with different levels of language skills (Holland et al., 2001, Szaflarski et al., 2006). To our knowledge, no studies have directly explored these relationships using multimodal brain imaging methods to examine developmental changes in the neural correlates of syntactic processing or other language abilities.
Using an fMRI paradigm previously shown to yield different activation patterns between semantic and syntactic processing of sentences in adults (Dapretto and Bookheimer, 1999), here, structural and functional correlates of syntactic processing were examined in a group of school-aged children and young adolescents. The sentence comprehension paradigm employed is more complex than rule violation tasks used in previous studies, and allows evaluation of syntactic processing in this wider age range of children. In addition, we evaluated brain–behavior relationships with neuropsychological measures. First, we hypothesized that there would be no differential activation between the syntactic and semantic conditions of this task across the entire age rage studied, as behavioral and ERP studies suggest a late independent emergence of syntactic processing, but we predicted that we would see increased activation in the left IFG, primarily pars opercularis of the IFG (BA 44), for the syntactic condition than the semantic condition for a subset of older children. We expected that brain activation patterns would show left lateralization with decreased reliance on right hemisphere homologous regions and increased specialization of the left IFG in children with better syntactic skills, regardless of chronological age. In other words, we predicted that inter-individual variability in syntactic skill would better predict activation patterns than age alone such that more proficient individuals would have increased left specialization and lateralization. Further, we predicted that increased thickness (observed in older relative to younger children in previous studies in the age range studied here, Sowell et al., 2004a, Sowell et al., 2004b, Shaw et al., 2008) in bilateral IFG would be associated with increased magnitude (z values) and spatial extent of activation in the left IFG, and decreased activation intensity and extent in the right IFG.
2. Materials and methods
2.1. Participants
Participants were healthy and typically developing children (n = 19) from the Los Angeles region who were recruited to participate in a study of normal brain development. Using UCLA IRB approved procedures, participants’ parents consented to participation in the study, and children provided both verbal and written assent. According to parental report during a structured background and developmental interview, participants had no history of medical or neurological conditions (e.g., low birth weight, epilepsy, head injury with loss of consciousness), psychiatric or behavioral disorders (e.g., attention deficit hyperactivity disorder, schizophrenia), developmental disability (e.g. mental retardation or autism), developmental language disorders, learning disabilities, or significant prenatal exposure to teratogens such as alcohol. They were all native English speakers. Gender was distributed evenly (females = 10), and their age ranged from 7.2 to 15.8 years with a mean age of 11.1 years (s.d. = 2.6). One participant was left-handed. Inspection of this participant's individual activation map did not suggest that this participant was an outlier, and these data were retained in the final analysis. Children were also administered a comprehensive battery of neuropsychological measures, including IQ testing with the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler, 2003) and subtests of the Clinical Evaluation of Language Fundamentals, Fourth Edition (CELF-4; Semel et al., 2003). The Sentence Assembly subtest of the CELF-4 was of most interest in the current study, as it is a direct measure of syntactic skill in which examinees are asked to create two distinct sentences using jumbled phrases that are provided. The CELF-4 is a well-validated measure, and the Sentence Assembly subtest is believed to tap syntactic processing as it requires application of syntactic rules. Only raw scores (with possible range between 0 and 19) were used because of limited normative data across the age group studied here.
2.2. Experimental stimuli and activation paradigm
During fMRI, participants performed a sentence comprehension and judgment task in which they were asked to listen to pairs of sentences and decide if they had the same meaning. They indicated their responses by pressing buttons in the scanner. Unknown to the participants, there were two conditions presented in a blocked design: semantic and syntactic. In the semantic condition, sentence pairs were identical, and meaning was varied by replacing one word with a different word or maintaining the meaning by replacing a word with a synonym. For example, children heard through earphones in the scanner, “The car is in the garage” and “The auto is in the garage.” In the syntactic condition, sentences were either in a different form (e.g., active vs. passive voice), or word order differed (e.g., preposed vs. postposed prepositional phrases). For example, children heard “The school is south of the park” and “South of the school is the park” and were asked if the two sentences meant the same thing. The number of sentences in the active and passive forms was the same in both conditions, as was the number of preposed and postposed phrases. Thus, both overall syntactic complexity and working memory demands were comparable between both conditions. Recorded sentences were read by a native English speaker with a standard American accent at the rate of one sentence pair every 7.5 s. There were eight trials (2 sentences in each trial, total of 16 sentences in each block) in each of the two 60-s blocks, and initial presentation of the semantic or syntactic condition was counterbalanced evenly across subjects. In each condition, half of the sentence pairs had the same meaning, and the other half had a different meaning. A 30-s rest block was placed before the first block, between blocks, and after the last block. Stimuli were presented with MacStim 3.2 psychological experimentation software (CogState, West Melbourne, Victoria, Australia). Both accuracy and response time were recorded.
2.3. Data acquisition
fMRI data were acquired on a 3 Tesla, Siemens Allegra magnet. A gradient echo EPI sequence was used, with TE = 25 ms, 90° flip angle, TR = 2.5 s, slice thickness = 3 mm/1 mm skip, 36 total slices, FOV = 200 mm. The acquisition matrix was 64 × 64 voxels, with in-plane resolution = 3 mm× 3 mm. T2-weighted anatomical volumes were acquired during the same scanning session for each subject, with TE = 33 ms, 90° flip angle, TR = 5 s, slice thickness = 3 mm/1 mm skip, 36 total slices (whole brain coverage), FOV = 200 mm. The anatomical volume was utilized for registration of the functional data for each subject, and functional data were registered to anatomical volumes with a 6 DOF linear transformation, allowing registration to standard space (MNI-152 space) with a 12 DOF linear transformation. Both functional and structural data were acquired at alignment to the anterior and posterior commissures which facilitated registration to MNI-152 space.
High-resolution T1-weighted structural MRI images were acquired on a 1.5 Tesla, Siemens Sonata. Both functional and structural scans were acquired on the same day. A T-1 weighted 3 dimensional (3D) magnetization prepared rapid gradient echo (MPRAGE) protocol was used with the following parameters: TR = 1900 ms, TE = 4.38 ms, flip angle = 15°, voxel size = 1 mm × 1 mm × 1 mm, acquisition time = 8:08 min. Up to four scans per child were obtained in order to improve the signal-to-noise ratio. Two of the 19 participants with usable fMRI data did not have useable structural scans due to excessive motion.
2.4. Data processing
2.4.1. fMRI
FSL, 4.1 (FMRIB Analysis Group, www.fmrib.ox.ac.uk/fsl) was used to analyze all fMRI data, and detailed assumptions and methods are described elsewhere (Smith et al., 2004, Woolrich et al., 2009). Preprocessing was carried out with MCFLIRT (the motion correction tool in FSL), to obtain 6 parameter estimates of motion, which were entered into the general linear model at the individual subject level as individual explanatory variables (EVs) with a temporal derivative to statistically control for motion. The addition of the temporal derivative to motion parameter EVs effectively models relative motion, and time points with very high levels of motion are down-weighted in the model. A trained research assistant visually inspected raw fMRI data for all subjects to assess for signal drop out artifact related to excessive motion that could not be corrected statistically. No subjects were excluded based on these inspections. In addition, motion parameter values for each subject were inspected, and in order to ensure adequate statistical power, subjects were only included in the analysis if they had 15 images for each condition with less than 2 mm of motion from the center image. No subjects were excluded based on this criterion. In order to increase the signal to noise ratio, all fMRI data were smoothed using a 6 mm Gaussian kernel. Individual functional data were registered to subjects’ T2-weighted structural image with a 6 DOF linear registration, utilizing FMRIB's Linear Image Registration Tool, v 5.5 (FLIRT). For group analyses, images were normalized into MNI-152 space with a 12 DOF linear registration using FLIRT. Utilizing adult-based atlases for registration of child data has been demonstrated to be valid for children 7 years of age and older (e.g., Burgund et al., 2002, Kang et al., 2003).
Contrasts at the individual level included syntactic > rest, semantic > rest, semantic > syntactic, and syntactic > semantic. Group means for each contrast were examined, and Z-statistic images were thresholded using clusters determined at either Z > 1.7 or Z > 2.3. To the extent that we focused our analyses on a priori regions of interest (ROIs) in the inferior frontal gyrus, we did not correct for multiple comparisons across the entire brain volume for most analyses. For the group analysis, mean activation for syntactic > rest was the primary contrast examined, and the relationship between activation, CELF-4 SA raw score, and age (in months) were examined using the general linear model. An ROI was created for the group mean syntax > rest and the syntax > semantic analysis for the older age subset, using regions with a 10% probability of including the IFG (regions 4, 5, 6, 7, 33, 41, 42) from the Harvard-Oxford proabablistic atlas provided in FSL (FMRIB Analysis Group, www.fmrib.ox.ac.uk/fsl). This resulted in a liberal ROI that takes into account variability in IFG location across individuals while also restricting the analysis to frontal language regions instead of a whole brain analysis.
2.4.2. sMRI
Preprocessing and definition of cortical and subcortical gray matter regions on structural images were conducted in the UCLA Laboratory of Neuro Imaging (LONI) Pipeline Processing Environment (Dinov et al., 2009, Rex et al., 2003) and using FreeSurfer's automated segmentation software (FreeSurfer 4.0.2, http://surfer.nmr.mgh.harvard.edu), as described in the work of Fischl and Dale (Dale et al., 1999, Fischl et al., 2002, Fischl et al., 1999). During preprocessing, two MPRAGE acquisitions for each participant were averaged to improve signal to noise ratios. The resultant images were then brain extracted and gray/white matter boundaries were automatically delineated. A surface of connected white matter voxels was refined to create sub millimeter voxel resolution in the gray/white matter boundary (Dale et al., 1999, Fischl et al., 1999). The gray/white matter boundary was then deformed outward to estimate the pial surface with the following constraints; the surface needed to be smooth and maintain the natural topology of the brain.
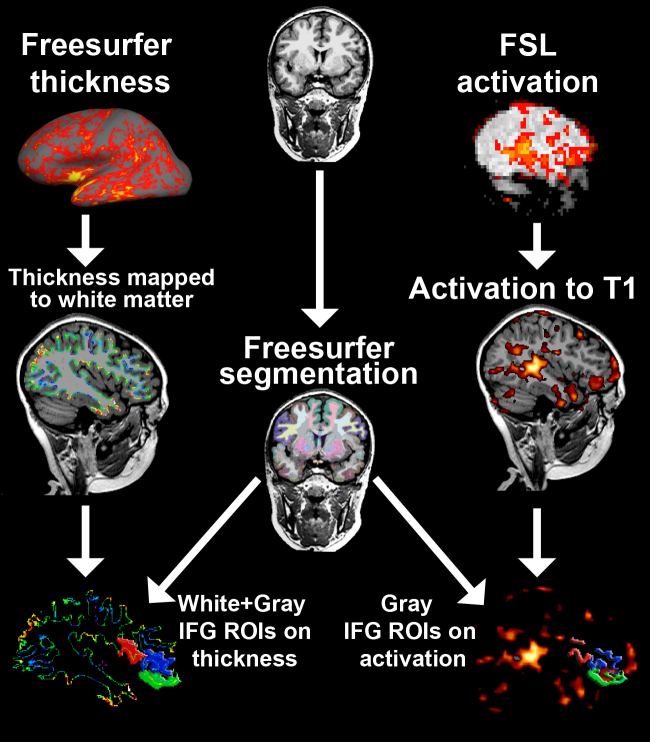
Procedures for the automatic quantification of gray matter thickness for a variety of brain structures are detailed by Fischl and colleagues (Fischl et al., 2002), and include the hypothesis-driven regions investigated here within the IFG (left and right Pars Opercularis, Pars Triangularis, Pars Orbitalis). Briefly, thickness of the cortical ribbon was computed for each subject across both hemispheres. The thickness is defined as the shortest distance between the white and pial surface models, which provide estimates of sub millimeter differences. All subjects were aligned to a common surface template using a high-resolution surface-based averaging technique that aligned cortical folding patterns. Gyral based regions of interest (ROIs; described in Desikan et al., 2006) on a standard brain provided in the FreeSurfer package (2007 standard) were then mapped back to each subject's native space T1-weighted image. The cortical thickness surface values were then converted into a 3D volume based on the FreeSurfer-generated white matter surface (i.e. each voxel in the new volume was assigned the thickness value for cortex at that x, y, z coordinate). White and gray segmentations for each of the IFG ROIs were combined to ensure that all thickness value voxels were captured by the ROI (see Fig. 1). Finally, mean thickness values of cortical gray matter in each FreeSurfer-generated ROI were calculated.
Fig. 1.
Summary of methods for obtaining structural (i.e. segmentation, cortical thickness) and functional (segmentation-based ROI mean activation and volume) measurements. The T1-weighted image is processed through FreeSurfer's recon-all command to obtain a thickness surface map and segmentation labels of anatomical regions. Thickness values were first converted into a 3D volume that follows the contours of the white matter surface, and then the ROIs were applied to get average thickness values within the IFG. For thickness, a combination of both white and gray matter segmentations for each ROI was used to ensure that all values in the white surface-based thickness ribbon were captured. FSL-processed activation maps were first registered to T1-weighted activation maps and then the ROIs were applied to the resultant transformed image to get average thickness and extent values within the IFG ROIs. Gray matter segmentations for each ROI were used to capture values on the cortical surface.
2.4.3. fMRI activation values for ROIs
Activation images from FSL were aligned to the MPRAGE images for each subject using the following steps: (1) T2-weighted high-resolution structural images from the functional run were aligned to the T1-weighted MPRAGE image used in the FreeSurfer analyses described above, with a full-affine registration in FSL's FLIRT program. (2) The resultant transformation file was then applied to the volume for the thresholded activation contrast (syntax > rest) to bring the activation map into T1 MPRAGE space. Activation results from FSL were utilized to obtain mean activation and extent of activation measures in the FreeSurfer cortical ROIs (i.e. IFG). The number of suprathreshold activation voxels that fell into each ROI was then summed to get the “extent of activation” for each subject (see Fig. 1). Mean activation intensity of suprathreshold voxels (Z values) in the same ROIs was also calculated.
Data processing procedures for ROI-based cortical thickness and activation measures (mean intensity and activation volume) are illustrated in Fig. 1. All activation measures were output in tabular format and merged with thickness measures and neurobehavioral data for each subject. This table was then exported into SPSS 17 for statistical analysis, including correlation analyses with Bonferroni correction for multiple comparisons.
3. Results
3.1. Neuropsychological testing
Mean full scale IQ was 112.7 (s.d. = 14.7, range 88–147), and mean verbal comprehension index score, a composite measure of overall verbal abilities, was 116.0 (s.d. = 18.4, range 83–148). Mean raw score for the CELF-4 SA was 12.0 (s.d. = 5.4) with a range between 2 and 19. The correlation between CELF-4 SA raw scores and age was moderately high at trend level (r = 0.44, p = 0.06).
3.2. Task behavior
Mean accuracy for the semantic condition was 83% (s.d. = 0.11), and for the syntactic condition mean accuracy was 88% (s.d. = 0.11). Accuracy did not differ significantly between conditions (t = −1.72, p = 0.10). Mean response time for the semantic condition (5.1 s, s.d. = 0.36) and syntactic condition (4.9 s, s.d. = 0.45) did not differ significantly (t = 1.37, p = 0.19). Mean syntactic accuracy was not correlated with age (r = 0.24, p = 0.32), but there was a trend level age correlation with mean semantic accuracy (r = 0.43, p = 0.07). Response time was not correlated with age in either the syntactic (r = 0.22, p = 0.38) or semantic (r = −0.18, p = 0.47) condition. Correlations between in-scanner task performance and neuropsychological test results did not indicate any significant relationships.
3.3. fMRI
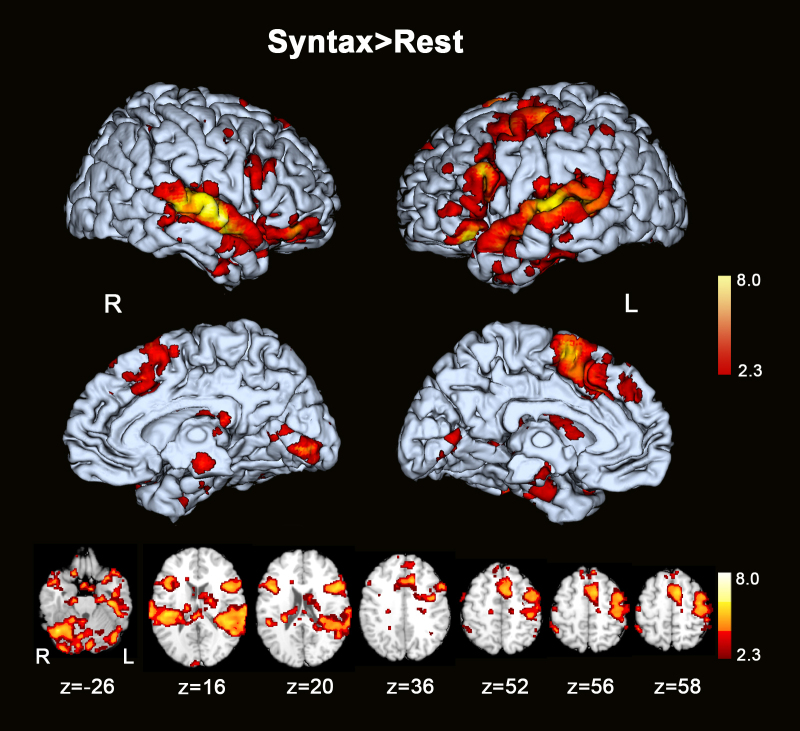
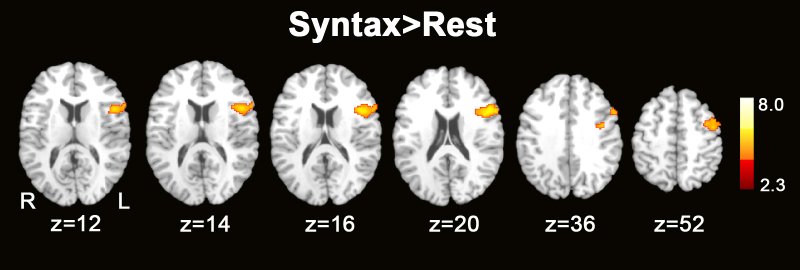
Group mean activation for the syntax > rest contrast included suprathreshold (uncorrected) activation in bilateral primary auditory cortex, left temperoparietal cortex, left superior frontal gyrus (SFG), left middle frontal gyrus (MFG), left inferior frontal gyrus (IFG), left supplementary motor area (SMA), and right cerebellum (see Fig. 2). Results are summarized in a table with peak coordinates and z-values for regions showing activation in the syntax > rest analysis (Table 1). An ROI (prefrontal language regions) analysis (corrected, z > 2.3, p = 0.05) included group mean activation in discrete and continuous left prefrontal language regions, including left IFG (see Fig. 3).
Fig. 2.
Syntax task: group average activation maps. Lateral and medial whole brain maps with activation on the surface of an individual brain registered to MNI-152 space. Axial sections displaying group average activation maps during the syntax vs. rest contrast. Regions of significant activation (Z > 2.3 and a uncorrected cluster significance threshold of p = 0.05) for the syntax > rest contrast. Z values indicate z coordinate in MNI-152 space.
Table 1.
Coordinates in MNI space and peak activation values for the syntactic condition > rest contrast.
| Syntactic condition > Rest | |||||
|---|---|---|---|---|---|
| Anatomical regions | x | y | z | z-Stat. | |
| Parietal lobe | L | −64 | −38 | 20 | 2.99 |
| L | −64 | −40 | 20 | 2.76 | |
| Superior parietal lobe | L | −48 | −34 | 56 | 2.38 |
| L | −46 | −30 | 56 | 1.95 | |
| Supplementary motor area | L | −8 | 6 | 58 | 4.18 |
| L | −6 | 4 | 58 | 3.88 | |
| R | 2 | 8 | 58 | 2.62 | |
| R | 2 | 4 | 58 | 2.38 | |
| Superior frontal gyrus | L | −4 | 12 | 52 | 3.42 |
| L | −6 | 14 | 52 | 3.20 | |
| R | 2 | 12 | 52 | 2.82 | |
| R | 4 | 16 | 52 | 1.90 | |
| Middle frontal gyrus | L | −54 | 14 | 34 | 2.88 |
| L | −52 | 16 | 36 | 2.70 | |
| Inferior frontal gyrus | L | −44 | 14 | 16 | 3.83 |
| L | −50 | 12 | 16 | 3.50 | |
| R | 44 | 22 | 16 | 3.38 | |
| R | 46 | 16 | 16 | 3.29 | |
| Pars opercularis | L | −52 | 14 | 22 | 3.74 |
| R | 46 | 14 | 22 | 3.83 | |
| Pars triangularis | L | −54 | 26 | 8 | 2.59 |
| R | 50 | 20 | 4 | 2.24 | |
| Pars orbitalis | L | −54 | 26 | −10 | 3.28 |
| R | 52 | 18 | −10 | 2.53 | |
| Cerebellum | L | −40 | −76 | −26 | 3.42 |
| R | 42 | −62 | −26 | 4.68 | |
| R | 40 | −64 | −26 | 3.95 | |
Fig. 3.
Syntax task ROI analysis: group average activation maps. Axial sections displaying group average activation maps during the syntax vs. rest contrast. Regions of significant activation in the left IFG (Z > 2.3 and a corrected cluster significance threshold of p = 0.05) for the syntax > rest contrast. Z values indicate z coordinate in MNI-152 space.
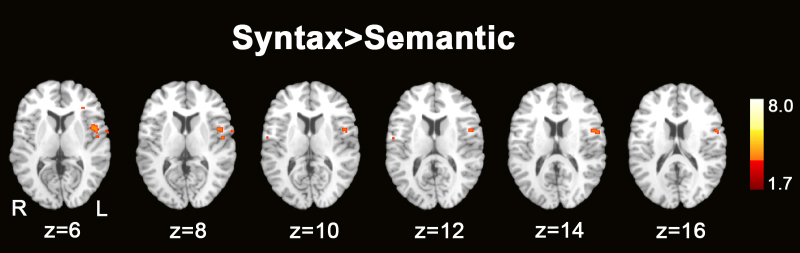
As noted previously, differences between the semantic and syntactic conditions were not predicted across the entire age range studied (ages 7–15), given results of previous behavioral and ERP studies that suggest a late independent emergence of syntactic processing in children. Using a median split (median age = 10.7) to identify older subjects in the sample (n = 10), an ROI analysis indicated differential activation between the two conditions in this subset of older subjects (see Fig. 4), with the syntactic condition showing increased activation relative to the semantic condition in left IFG (uncorrected, z > 1.7), specifically pars opercularis of the IFG (BA 44). The subgroup of younger children did not have any differential activation between the syntactic and semantic conditions (results not shown). There were no statistically significant differences in the semantic > rest condition (results not shown), and no specific hypotheses were made about this condition.
Figure 4.
Syntax > semantic conditions ROI analysis: condition contrast activation maps. Axial sections displaying condition contrast maps for the syntax > semantic contrast. Regions of significant activation (Z > 1.7 and an uncorrected cluster significance threshold of p = 0.05) for the syntax > rest contrast. Z values indicate z coordinate in MNI-152 space.
3.4. fMRI and syntactic skill performance
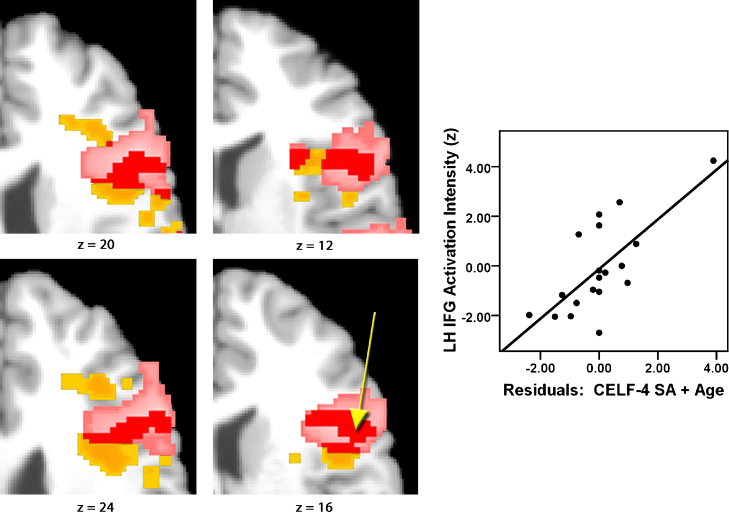
Simultaneous multiple regression analyses that included CELF-IV SA raw and age as explanatory variables revealed significant relationships between test scores and activation in left hemisphere SFG, MFG, and IFG independent of age. To examine the contribution of individuals’ data on the regression analyses and to rule out spurious effects possibly caused by outliers, activation values (Z) at a specific point within the left IFG (MNI-152 coordinates x = -54, y = 12, z = 16) were plotted against age-residualized values for CELF-4 Sentence Assembly raw scores (see Fig. 5).
Fig. 5.
Axial maps illustrate overlapping values in the IFG for voxels that were more active during syntax than rest (from Fig. 2), and/or voxels where activation during syntax vs. rest were correlated with performance on the CELF-4 sentence assembly (SA) subtest (pink = group mean activation, yellow = voxels where activation was correlated with CELF-4 SA performance independent from age, red = overlapping regions). Yellow arrow indicates location of values (Z) in the IFG (x = −54, y = 12, z = 16 in MNI-152 space) plotted against age-residualized values for CELF-4 SA raw scores in the graph. Only significant voxels within the IFG are shown; IFG mask applied to the image.
Correlation analyses between activation in the left hemisphere language regions and other neuropsychological measures (FSIQ and VIQ) did not yield significant results, suggesting either that activation in the left IFG is not related to overall intellectual functioning (FSIQ) or overall verbal abilities (VCI), or that we lacked statistical power to find these effects.
3.5. Cortical thickness and activation correlations
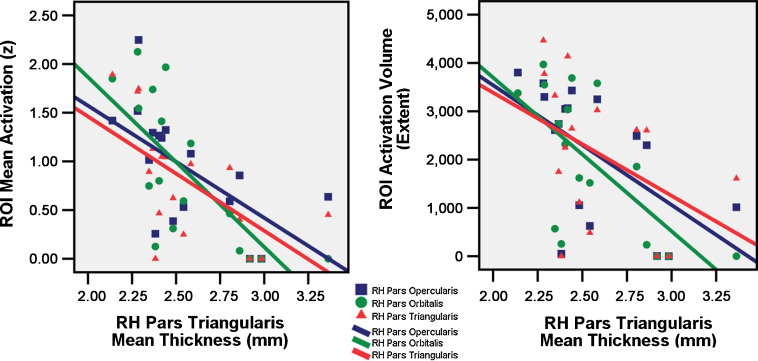
Correlations between cortical thickness in bilateral pars triangularis and activation intensity and extent in the pars opercularis, pars triangularis and pars orbitalis are summarized in Table 2. Data for 17 participants were part of this analysis, as two participants did not have useable structural imaging data as a result of excessive motion. When using a strict Bonferonni correction (p < 0.05) across the 24 statistical tests (p < 0.002), the negative correlation between activation intensity in right pars orbitalis and cortical thickness in right pars triangularis was the only test to survive (r = −0.724, p = 0.001). Nevertheless, other correlations of considerable interest are discussed given our a priori hypotheses. As predicted, mean cortical thickness in the right pars triangularis was negatively correlated with activation intensity (r = −0.724, p = 0.001) and activation extent (r = −0.676, p = 0.003) in the right pars orbitalis, activation intensity (r = -0.613, p = 0.009) and activation extent (r = −0.577, p = 0.015) in the right pars opercularis, and activation intensity (r = −0.616, p = 0.009) in the right pars triangularis, with a trend level correlation in activation extent for this region (r = −0.464, p = 0.061). In other words, increased thickness in a subregion of the RH IFG was associated with decreased activation in all RH IFG subregions. See Fig. 6 for plots of right hemisphere (RH) pars triangularis mean thickness (mm) and mean activation and activation volume (extent) in the three RH ROIs of the IFG. There was only a trend level negative correlation between right pars triangularis mean thickness and activation intensity (r = −0.471, p = 0.056) and extent (r = −0.407, p = 0.105) in the left pars opercularis with no other correlations between right thickness and left activation/extent. Also consistent with our predictions, though only at trend level significance, were negative correlations observed between left hemisphere pars triangularis thickness and activation intensity and extent in all RH IFG subregions (see Table 2). Correlations between cortical thickness in pars orbitalis and pars opercularis and activation extent and intensity were not significant for left or right hemisphere inferior frontal regions of interest.
Table 2.
Correlations between pars triangularis (PT) cortical thickness and intensity and volume of activation (extent) during syntactic processing (syntax > rest) in left and right hemisphere inferior frontal gyrus regions of interest.
| Activation Intensity (z-values) |
Volume of Activation (extent) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Left |
Right |
Left |
Right |
|||||
| r | p | r | p | r | p | r | p | |
| Right PT thickness | ||||||||
| Pars opercularis | −0.471 | 0.056 | −0.613 | 0.009 | −0.407 | 0.105 | −0.577 | 0.015 |
| Pars triangularis | −0.232 | 0.371 | −0.616 | 0.009 | 0.078 | 0.765 | −0.464 | 0.061 |
| Pars orbitalis | −0.347 | 0.172 | −0.724 | 0.001 | −0.246 | 0.342 | −0.676 | 0.003 |
| Left PT thickness | ||||||||
| Pars opercularis | −0.262 | 0.311 | −0.452 | 0.068 | −0.242 | 0.350 | −0.386 | 0.126 |
| Pars triangularis | −0.206 | 0.427 | −0.452 | 0.068 | 0.024 | 0.926 | −0.453 | 0.068 |
| Pars orbitalis | −0.188 | 0.470 | −0.456 | 0.066 | −0.123 | 0.637 | −0.474 | 0.055 |
Fig. 6.
RH pars triangularis mean thickness (mm) plotted against mean activation (z) and activation volume (extent) in three RH ROIs of the IFG (pars orbitalis, pars opercularis, and pars triangularis) for each individual.
In order to rule out correlations between activation and structure that could simply reflect general changes in cognition with brain maturation, additional correlation analyses were done between bilateral thickness and activation/extent in another ROI with group activation and located outside the IFG, the superior frontal area. Results did not indicate any significant or trend level correlations (results not shown), suggesting that correlations in the IFG are not solely related to overall brain maturation.
4. Discussion
The results of the current study reveal relationships between inter-individual variability in brain morphology and functional brain activity suggesting increasing specialization and lateralization of language functions in typically developing children. Specifically, we found negative correlations between cortical thickness and activation intensity and extent in the right IFG during a syntactic processing task. Previous structural imaging studies have shown that cortex in the perisylvian regions of the frontal and temporal lobes increase in thickness during the age range studied here (Shaw et al., 2008, Sowell et al., 2004a). Although we cannot make assumptions about causality, results of the current study suggest that children with thicker cortex in the right IFG rely less on right hemisphere language areas to process verbal linguistic information and rely more on left hemisphere structures for syntactic processing than children with thinner cortex in the same brain regions. Further, we report positive correlations between activation in the left IFG during a syntactic processing and proficiency of syntactic skills independent from age, suggesting that increased syntactic proficiency impacts brain activation during sentence-level language processing.
Activation patterns for the syntactic component of the task in the current study were consistent with the temporo-frontal network known to be associated with syntactic processing (Friederici and Kotz, 2003), but as predicted based on earlier behavioral studies, the degree of brain specialization for syntactic processing does not appear to be fully established in school aged children and early adolescents (Nippold et al., 2005). Supporting a qualitative shift in syntactic processing at around age 10 (Leech et al., 2007), a direct contrast between the syntactic and semantic task showed differential activation in the left IFG for the syntactic condition for an older subset of children (over age 10.7) but not across the whole age ranged studied (7–15). Overall syntactic complexity, working memory demands, and general task demands (i.e., “do both sentences have the same meaning?”) were comparable between both conditions, and thus we believe differences in activation can be attributed to syntactic processing. Significant variability in localization of language tasks has been reported (Burton et al., 2001), and it was not expected that the current study would demonstrate a specific syntactic processing region in children across the whole age range studied. Rather, we (Lu et al., 2009), and others (Gaillard et al., 2000, Holland et al., 2001, Schlaggar et al., 2002) have speculated that inter-individual variability in activation of inferior frontal regions would relate to brain structural maturation processes that include cortical thickening in IFG and perisylvian brain–language regions throughout childhood and adolescence. Our findings of negative thickness–activation correlations provide support for this notion. Both age and proficiency were related to activation in the IFG, and this suggests the importance of both physical maturity and continued exposure to language in brain development. The relationship with proficiency on the syntactic measure and brain activation patterns, which was statistically independent from age, is important because it suggests that proficiency on a measure of syntactic processing involves dynamic inter-relationships between brain structure and function through development. In other words, children with better syntactic processing skills have different, perhaps more left-lateralized, structure-function brain relationships than children of the same age with poorer syntactic processing skills.
Along with the pars opercularis, the pars triangularis is believed to be important for language comprehension and it has also been implicated in syntactic processing (Bookheimer, 2002). Recent studies have shown that electrical stimulation in one region of the IFG results in activity in other regions, and a high degree of connectivity between the different regions of the IFG has been demonstrated although the functional significance is unknown (Greenlee et al., 2007). It is unclear from the current study why thickness in the pars triangularis had the strongest relationships with neural activity in other parts of the IFG. Future studies would benefit from focusing on the role of the pars triangularis in general and in syntactic processing, as well as its structural and functional trajectory in development. Researchers have also suggested that the involvement of the IFG in sentence comprehension may reflect a more generalized cognitive control mechanism (Novick et al., 2005), and future studies would benefit from including cognitive control as a variable of interest when studying language processing and the role of the IFG. Future studies might also include larger sample sizes so that additional statistical analyses that assess the role of this variable while directly comparing differences (e.g., ANCOVA) could be utilized.
Results of this study are consistent with other developmental language processing studies that indicate strong language lateralization in children over age 6 with a continuing process of regional specialization related to maturity (Gaillard et al., 2002, Gaillard et al., 2001, Holland et al., 2001, Schlaggar et al., 2002). Holland et al. (2001) found, that lateralization changes were more closely tied to the period of acquisition of language tasks than to general maturation. Moreover, the largest changes in lateralization occurred for skills such as verb generation that are acquired over a longer period of development. The lateralization shifts in our study support novel behavioral studies that indicate increased independence of syntactic processing through age 10 and continued development of syntactic skills through early adulthood (Friederici, 1983, Nippold et al., 2005).
Hypotheses about increased cortical thickness and increased activation intensity/extent in the left hemisphere were only partially supported by our data. Some degree of left lateralization for language is known to be present very early (Dehaene-Lambertz et al., 2002, Dehaene-Lambertz et al., 2006), and it is conceivable that left hemisphere networks are well established early with maintenance of right hemisphere language in place in order to maintain neural redundancy. With increased development, as the brain becomes more efficient, its redundancy and potential for plasticity decreases. Furthermore, there may be physiological limits on the extent of intensity of activation in the left IFG that have already been met in the age range studied.
This study utilized multiple methods, including functional MRI, structural MRI, and neuropsychological measurement, to demonstrate relationships between functional and structural brain maturation and the development of language processing skills. Here, we illustrate the independent and prolonged development of syntactic processing in brain activation and structure throughout childhood that had previously been shown only with behavioral studies. Furthermore, our results suggest that cortical activation is related to both age and proficiency with syntactic processing. Finally, the present findings illustrate the ongoing structural development of the brain through cortical thinning and thickening in childhood and its relationship with how the brain functions.
Acknowledgements
This research was supported by the following organizations: National Institute of Child Health and Human Development (R01 HD053893), National Institute on Drug Abuse (R01 DA017831), National Institutes of Alcoholism and Alcohol Abuse (U01 AA017122), National Center for Research Resources (U54 RR021813), and March of Dimes (6-FY2008-50). Portions of the analysis were also conducted using the UCLA Laboratory of Neuro Imaging Pipeline processing environment. The authors report no conflicts of interest.
References
- Amunts K., Schleicher A., Ditterich A., Zilles K. Broca's region: cytoarchitectonic asymmetry and developmental changes. J. Comp. Neurol. 2003;465:72–89. doi: 10.1002/cne.10829. [DOI] [PubMed] [Google Scholar]
- Berman R.A. Between emergence and mastery: the long developmental route of language acquisition. In: Berman R.A., editor. Language Development across Childhood and Adolescence. John Benjamins Publishing Company; Amsterdam/Philadelphia: 2004. pp. 9–34. [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu. Rev. Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Brauer J., Friederici A.D. Functional neural networks of semantic and syntactic processes in the developing brain. J. Cogn. Neurosci. 2007;19:1609–1623. doi: 10.1162/jocn.2007.19.10.1609. [DOI] [PubMed] [Google Scholar]
- Burgund E.D., Kang H.C., Kelly J.E., Buckner R.L., Snyder A.Z., Petersen S.E., Schlaggar B.L. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Burton M.W., Noll D.C., Small S.L. The anatomy of auditory word processing: individual variability. Brain Lang. 2001;77:119–131. doi: 10.1006/brln.2000.2444. [DOI] [PubMed] [Google Scholar]
- Chi J.G., Dooling E.C., Gilles F.H. Left-right asymmetries of the temporal speech areas of the human fetus. Arch. Neurol. 1977;34:346–348. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dapretto M., Bookheimer S.Y. Form and content: dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24:427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Dehaene S., Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Hertz-Pannier L., Dubois J. Nature and nurture in language acquisition: anatomical and functional brain-imaging studies in infants. Trends Neurosci. 2006;29:367–373. doi: 10.1016/j.tins.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dinov I.D., Van Horn J.D., Lozev K.M., Magsipoc R., Petrosyan P., Liu Z., Mackenzie-Graham A., Eggert P., Parker D.S., Toga A.W. Efficient, distributed and interactive neuroimaging data analysis using the LONI pipeline. Front. Neuroinform. 2009;3:22. doi: 10.3389/neuro.11.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. Children's sensitivity to function words during sentence comprehension. Linguistics. 1983;21:717–739. [Google Scholar]
- Friederici A.D. Towards a neural basis of auditory sentence processing. Trends Cogn. Sci. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The neural basis of language development and its impairment. Neuron. 2006;52:941–952. doi: 10.1016/j.neuron.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Kotz S.A. The brain basis of syntactic processes: functional imaging and lesion studies. Neuroimage. 2003;20(Suppl 1):S8–S17. doi: 10.1016/j.neuroimage.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Gaillard W.D., Ahmad Z., Balsamo L., Sachs B., Xu B. Language networks underlying auditory comprehension in young children identified with fMRI. Ann. Neurol. 2002;52:S114. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- Gaillard W.D., Hertz-Pannier L., Mott S.H., Barnett A.S., LeBihan D., Theodore W.H. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gaillard W.D., Pugliese M., Grandin C.B., Braniecki S.H., Kondapaneni P., Hunter K., Xu B., Petrella J.R., Balsamo L., Basso G. Cortical localization of reading in normal children: an fMRI language study. Neurology. 2001;57:47–54. doi: 10.1212/wnl.57.1.47. [DOI] [PubMed] [Google Scholar]
- Greenlee J.D., Oya H., Kawasaki H., Volkov I.O., Severson M.A., 3rd, Howard M.A., 3rd, Brugge J.F. Functional connections within the human inferior frontal gyrus. J. Comp. Neurol. 2007;503:550–559. doi: 10.1002/cne.21405. [DOI] [PubMed] [Google Scholar]
- Hahne A., Eckstein K., Friederici A.D. Brain signatures of syntactic and semantic processes during children's language development. J. Cogn. Neurosci. 2004;16:1302–1318. doi: 10.1162/0898929041920504. [DOI] [PubMed] [Google Scholar]
- Holland S.K., Plante E., Weber Byars A., Strawsburg R.H., Schmithorst V.J., Ball W.S., Jr. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Kang H.C., Burgund E.D., Lugar H.M., Petersen S.E., Schlaggar B.L. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Leech R., Aydelott J., Symons G., Carnevale J., Dick F. The development of sentence interpretation: effects of perceptual, attentional and semantic interference. Dev. Sci. 2007;10:794–813. doi: 10.1111/j.1467-7687.2007.00628.x. [DOI] [PubMed] [Google Scholar]
- Lu L., Leonard C., Thompson P., Kan E., Jolley J., Welcome S., Toga A., Sowell E. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb. Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- Lu L.H., Dapretto M., O’Hare E.D., Kan E., McCourt S.T., Thompson P.M., Toga A.W., Bookheimer S.Y., Sowell E.R. Relationships between brain activation and brain structure in normally developing children. Cereb. Cortex. 2009;19:2595–2604. doi: 10.1093/cercor/bhp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nippold M.A., Hesketh L.J., Duthie J.K., Mansfield T.C. Conversational versus expository discourse: a study of syntactic development in children, adolescents, and adults. J. Speech Lang. Hear. Res. 2005;48:1048–1064. doi: 10.1044/1092-4388(2005/073). [DOI] [PubMed] [Google Scholar]
- Novick J.M., Trueswell J.C., Thompson-Schill S.L. Cognitive control and parsing: reexamining the role of Broca's area in sentence comprehension. Cogn. Affect Behav. Neurosci. 2005;5:263–281. doi: 10.3758/cabn.5.3.263. [DOI] [PubMed] [Google Scholar]
- Rex D.E., Ma J.Q., Toga A.W. The LONI pipeline processing environment. Neuroimage. 2003;19:1033–1048. doi: 10.1016/s1053-8119(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Schlaggar B.L., Brown T.T., Lugar H.M., Visscher K.M., Miezin F.M., Petersen S.E. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Scott C.M. Syntactic ability in children and adolescents with language and learning disabilities. In: Berman R.A., editor. Language Development across Childhood and Adolescence. John Benjamins Publishing Co; Amsterdam: 2004. pp. 111–133. [Google Scholar]
- Semel E., Wiig E.H., Secord W.A. fourth ed. (CELF-4) Harcourt Assessment, Inc.; San Antonio, TX: 2003. Clinical Evaluation of Language Fundamentals. [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L., Giedd J.N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Lalonde F., Lepage C., Rabin C., Eckstrand K., Sharp W., Greenstein D., Evans A., Giedd J.N., Rapoport J. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry. 2009;66:888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Leonard C.M., Welcome S.E., Kan E., Toga A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Toga A.W. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Szaflarski J.P., Schmithorst V.J., Altaye M., Byars A.W., Ret J., Plante E., Holland S.K. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann. Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada J.A., Clarke R., Hamm A. Cerebral hemispheric asymmetry in humans. Cortical speech zones in 100 adults and 100 infant brains. Arch. Neurol. 1975;32:239–246. doi: 10.1001/archneur.1975.00490460055007. [DOI] [PubMed] [Google Scholar]
- Wechsler D. fourth ed. The Psychological Corporation; San Antonio, TX: 2003. The Wechsler Intelligence Scale for Children. [Google Scholar]
- Woolrich M.W., Jbabdi S., Patenaude B., Chappell M., Makni S., Behrens T., Beckmann C., Jenkinson M., Smith S.M. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Wulfeck B., Bates E., Krupa-Kwiatkowski M., Saltzman D. Grammaticality sensitivity in children with early focal brain injury and children with specific language impairment. Brain Lang. 2004;88:215–228. doi: 10.1016/S0093-934X(03)00100-7. [DOI] [PubMed] [Google Scholar]