Abstract

Botulinum neurotoxin (BoNT), the etiological agent that causes the neuroparalytic disease botulism, has become a highly studied drug target in light of the potential abuse of this toxin as a weapon of bioterrorism. In particular, small molecule inhibitors of the light chain metalloprotease of BoNT serotype A have received significant attention and a number of small molecule and biologic inhibitors have been reported. However, all small molecules reported have been identified from either primary screens or medicinal chemistry follow-up studies, and the pharmacokinetic profiles of these compounds have not been addressed. In this study, we have removed the pharmacologic liabilities of one of the best compounds reported to date, 2,4-dichlorocinnamate hydroxamic acid, and in the process uncovered a related class of benzothiophene hydroxamic acids that are significantly more potent inhibitors of the BoNT/A light chain, while also possessing greatly improved ADME properties, with the best compound showing the most potent inhibition of BoNT/A light chain reported (Ki = 77 nM). Using a strategy of incorporating traditional drug development filters early into the discovery process, potential liabilities in BoNT/A lead compounds have been illuminated and removed, clearing the path for advancement into further pharmacologic optimization and in vivo efficacy testing.
Keywords: Botulinum neurotoxin, ADME, benzothiophene, competitive inhibitor, zinc metalloprotease
Botulinum neurotoxins (BoNTs) produced by the soil bacteria Botulinum clostridium are extremely potent toxins causing the life-threatening neuroparalytic disease, botulism.1 Indeed, these proteins are the most potent toxins known to man with an estimated lethality of ∼1 ng/kg.2 All seven identified serotypes of BoNT (serotypes A−G) are heterodimeric proteins of similar fold consisting of heavy and light chain subunits. The heavy chain of BoNT is responsible for binding of the toxin to cell surface receptors and subsequent internalization of the toxin into the cells. The light chain domain (LC) is a zinc-dependent endoprotease that cleaves specific proteins of the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex, which is critical for the release of the neurotransmitter acetylcholine from synaptic terminals of neuronal cells at the neuromuscular junction.3 Inhibition of acetylcholine release by BoNT leads to flaccid paralysis and may eventually lead to death of organism caused typically by heart and/or respiratory failure.4
Current treatment of botulism consists of injection with antitoxin (e.g., equine neutralizing polyclonal antibodies) soon after intoxication to prevent the toxin from entering neuronal cells.5 Due to this mechanism of action, this type of treatment cannot reverse paralysis once the toxin has entered target cells. Additionally, equine antitoxin can lead to severe allergic reactions6 and its availability in the case of a larger scale intervention, such as after a bioterrorist attack, is severely limited.7 Development of small molecule drugs that ease or reverse BoNT intoxication as an alternative to antitoxins is therefore of great interest, and a number of molecules that modulate different steps of the intoxication process have been reported.8,9 Inhibitors of the light chain protease are the most widely studied type of BoNT inhibitors, and this protein is considered to be a very promising therapeutic target as inhibition could result in attenuated intoxication, even after internalization of the protease into neuronal cells.8
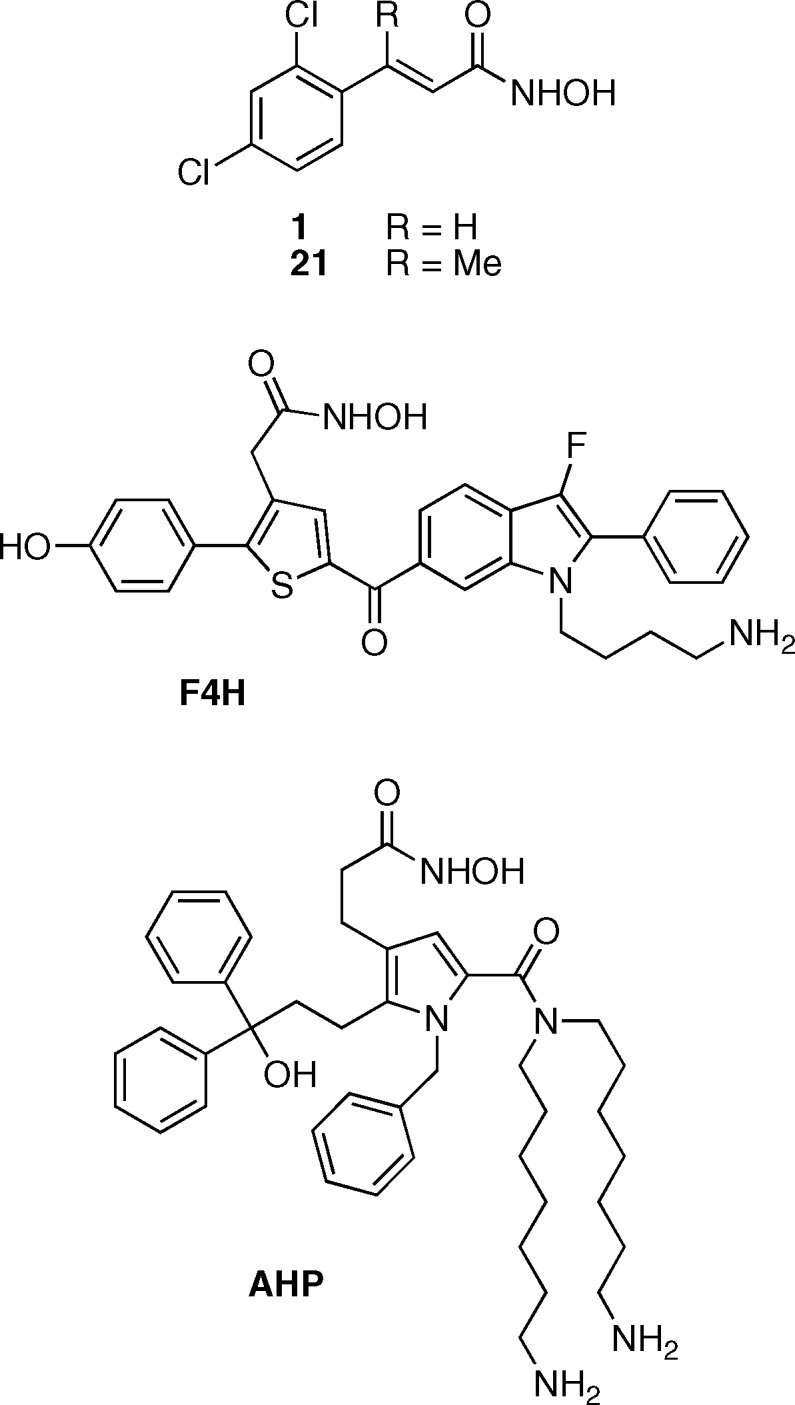
Most of the research efforts have been focused on the LC of BoNT serotype A, since this serotype is the most toxic to humans with the longest lasting effect on the organism.10 One of the most effective reported small molecule inhibitors of BoNT/A LC is 2,4-dichlorocinnamic hydroxamate 1 (Figure 1) with Ki < 1 μM and marginal in vivo activity.5,11,12 This molecule was used as a lead in several studies with the goal of further improving its inhibitory activity by either varying substituents on the aromatic ring or by modifying the linker between the aromatic ring and hydroxamate; however, only limited improvement was achieved.11,13−15 Even with these improvements, 1 as well as most of its derivative compounds have two major structural features undesirable in a potential candidate for in vivo studies. First, the highly hydrophobic 2,4-dichlorophenyl ring both compromises the solubility of the compound and also is unable to take advantage of potential hydrophilic interactions in the active site of the enzyme. The cinnamate provides a second liability of the structure, as this moiety can undergo possibly deleterious Michael addition16 making 1 potentially unstable in vivo.
Figure 1.
Examples of the most potent BoNT/A LC inhibitors: 2,4-dichlorocinnamic hydroxamate 1,11corresponding methyl analog 21,15F4H,17 and AHP.18
In our laboratory, we have sought to use high throughput screening and medicinal chemistry principles to identify inhibitors of BoNT/A that possess suitable inhibitory potency as well as favorable pharmacological properties for advancement into animal models of BoNT intoxication. Compound 1 has been an excellent tool for the in vitro study of BoNT inhibition, but it requires significant optimization for further advancement. To improve upon the pharmacological properties of 1 without significantly sacrificing its efficacy, we undertook an iterative process to sequentially remove both major drug development liabilities described above.
Initially, our studies focused on the replacement of the 2,4-dichlorophenyl ring with a heterocyclic scaffold. Heterocycles have several potential advantages over the substituted phenyl ring, including the possibility of hydrophilic/hydrogen bond interactions with the enzyme and better solubility. A small library of 23 heterocyclic hydroxamates (Table S1, Supporting Information) derived from 1 by replacement of phenyl ring with various substituted five-membered, six-membered or bicyclic heterocycles was designed and synthesized from commercially available aldehydes (Scheme 1). Diversity of the scaffolds represented in the library included heterocycles such as benzothiophenes, thiophenes, indoles, furans, pyrazoles, thiazoles, isoxazoles, and pyridines. To prepare these molecules, aldehydes 2a−w were first converted into the corresponding methyl acrylates 4a−w in a Horner−Wadsworth−Emmons reaction with phosphonate 3. The resulting methyl acrylates were treated with O-tetrahydropyran (THP)-protected hydroxylamine in the presence of a base to give THP-protected hydroxamates 5a−w. Acidic cleavage of THP then afforded the desired hydroxamates 6a−w in 5−37% overall yield.
Scheme 1. Synthesis of Hydroxamate Library.
R = heterocycles. See Table S1 in the Supporting Information for complete list of synthesized structures.
The inhibitory activity of isolated hydroxamates 6a−w was tested, and directly compared to 1, in the SNAPtide assay, a widely implemented assay used for the identification of BoNT/A LC inhibitors which consists of cleavage of fluorogenic substrate by the LC of BoNT/A.11 This assay relies on kinetic measurements of fluorescence changes produced by cleavage of a synthetic 13 amino acid peptide that contains a fluorophore/quencher pair separated by the BoNT/A LC cleavage site. Given the absence of a cell-based model for BoNT/A intoxication that accurately predicts in vivo activity, molecules identified through the SNAPtide assay are considered good candidates for in vivo testing.12 Three out of the 23 hydroxamates tested had IC50 < 10 μM. Two of these most active derivatives, compounds 6a and 6b, possessed IC50 values of 0.34 and 0.63 μM, respectively (Table 1), with both being more potent inhibitors than the parent hydroxamate 1 (IC50 = 0.67 μM). Interestingly, both of these compounds were based on the 3-substituted benzothiophen-2-yl scaffold and the third most active compound, 6c (IC50 = 5.9 μM), also was a structurally similar bicyclic heterocycle with a 3-substituted thiophen-2-yl motif (Table 1). An effect of the bicyclic system on the BoNT/A inhibition is apparent from direct comparison of bicyclic 3-methyl benzothiophen-2-yl derivative 6b and monocyclic 3-methyl thiophen-2-yl derivative 6d. The first compound is an approximately 38-fold more potent inhibitor, presumably as a consequence of enhanced stacking interactions in an active site of the LC. Furthermore, the connectivity of the double bond to the benzothiophene heterocycle is also of great importance. Both benzothiophen-2-yl derivatives 6a and 6b are significantly more potent inhibitors than benzothiophen-3-yl derivative 6e, suggesting that the connection of double bond to position 2 of benzothiophene is favored over position 3.
Table 1. IC50 and Ki Values of Selected Heterocyclic Hydroxamates.
 |
For the IC50 values of all compounds tested, refer to Table S1 in the Supporting Information.
IC50 values were determined using the SNAPtide assay.11 IC50 values were calculated from at least three experiments if standard error of mean is given.
Ki values were determined using a 66-mer SNAP-25[141−206] substrate LCMS assay.19
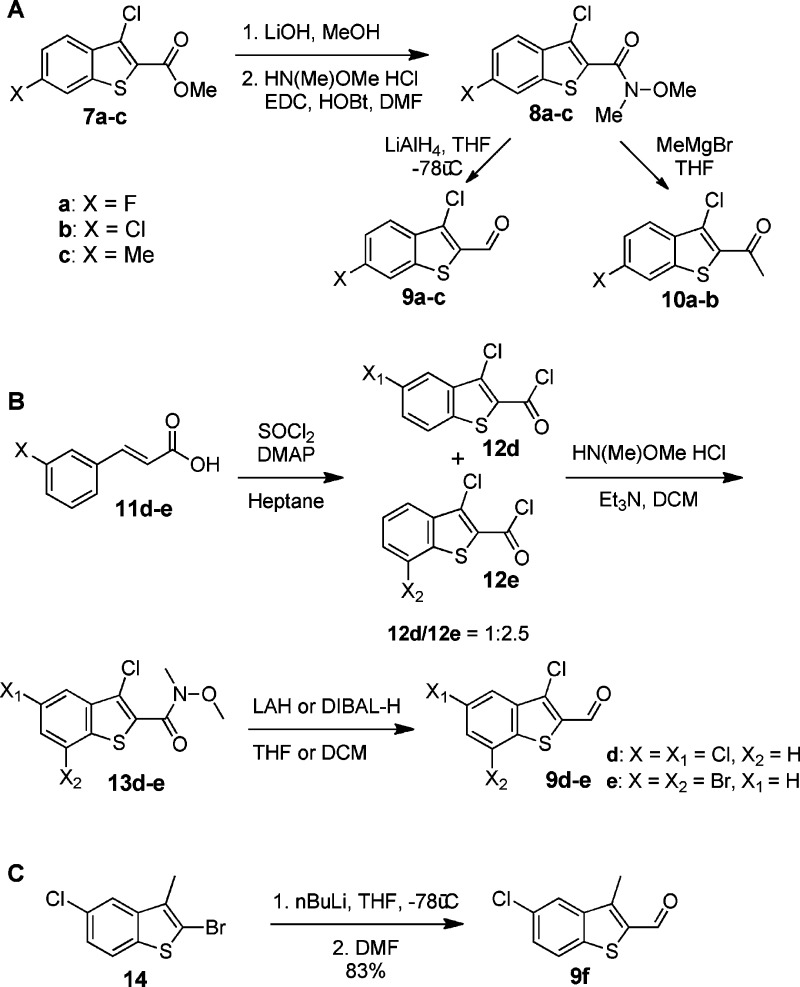
No other library member displayed an IC50 value less than the desired 10 μM threshold; therefore, we focused exclusively on leads with the thiophen-2-yl scaffold, and in particular we wished to examine the effect of stereoelectronic changes to the thiophen-2-yl scaffold. Six derivatives of 6a and 6b were synthesized, all with additional substituents on the benzothiophene in position 5, 6, or 7 (Table 1). The corresponding aldehydes necessary for synthesis of hydroxamates 15a−f were not commercially available and therefore were prepared synthetically. Aldehydes leading to 15a−c were synthesized from methyl benzothiophen-2-yls 7a−c (Scheme 2A). These molecules were first converted into the corresponding Weinreb amides 8a−c in a sequence of methyl ester saponification followed by amide bond formation with N,O-dimethylhydroxylamine. Subsequent reduction of the Weinreb amide led to the desired aldehydes 9a−c. For the preparation of 15d and 15e, the benzothiophene system was built from cinnamic acid 11d and 11e in one step via treatment with SOCl2 (Scheme 2B). This ring closing reaction produces two products, the 3,5-dihalo- and 3,7-dihalobenzothiophene formate chlorides, which were separated and converted into N,O-dimethyl-protected hydroxamates 13d−e. Selective reduction of these compounds yielded aldehydes 9d−e. The aldehyde required for the synthesis of 15f was prepared by lithiation of benzothiophen-2-yl bromide 14 and subsequent treatment of the intermediate lithium benzothiophene with DMF (Scheme 2C). With the required aldehydes in hand, hydroxamates 15a−f were synthesized using the procedure used previously for synthesis of the initial hydroxamate library (Scheme 1).
Scheme 2. Preparation of Thiophen-2-yl Aldehydes.
Synthetic yields for each compound can be found in the Supporting Information.
It is clear from the IC50 values determined using the SNAPtide assay (Table 1) that introduction of fluorine (15a) or chlorine (15b) in position 6 of the thiophene ring significantly improves inhibitory activity. On the other hand, introduction of a methyl group (15c), which is similar in size to the chlorine, has the opposite effect, rendering the compound a less efficient inhibitor. We hypothesize that it is the electron withdrawing effect of the halogens that changes the electronic properties of the aromatic ring rather than direct interaction of the halogen with the LC active site that results in the improved inhibitory properties. Alternatively, when chlorine is introduced into position 5 of the benzothiophene, the activity is dependent on the substituent in position 3; while 3-methyl-5-chloro-benzothiophene 15f is about 3-fold more potent than the parental compound 6b, 3,5-dichlorobenzothiophene 15d and 3-chlorobenzothiophene 6a are comparable in potency. Introduction of bromine into position 7 of the benzothiophene scaffold (15e) had a profoundly negative effect on the activity most likely due to disfavored steric interactions with the enzyme.
Replacement of the phenyl ring was only one of the liabilities of dichlorocinnamic hydroxamate 1. The second liability of these structures, the cinnamate double bond, is arguably easier to mitigate. Introduction of a methyl substituent on the double bond in the position susceptible to nucleophilic attack or reduction of the double bond are the two most straightforward approaches to inactivate these molecules as Michael acceptors. Both of these approaches have been applied to cinnamic hydroxamate 1 in the past in the context of activity optimization. Reduction of the double bond of 1 leads to a greater than 7-fold decrease in the activity (IC50 = 3.1 μM).11 On the other hand, the introduction of methyl substituent onto the β position of the double bond of 1, leading to compound 21 (Figure 1), did not significantly alter its inhibitory properties.15
We hypothesized that the application of these double bond replacements to the lead benzothiophene structures would yield compounds with the needed potency and pharmacologic stability for further in vivo study. To obtain saturated hydroxamate analog 18, the double bond of methyl acrylate 16a was hydrogenated in the presence of platinum oxide catalyst. The use of platinum oxide was found to be essential in order to avoid reduction of the heteroaryl halogens. Saponification of the saturated intermediate 17 followed by amide coupling with THP-protected hydroxylamine and subsequent THP deprotection afforded desired hydroxamate 18 (Scheme 3). The methylated analogs 20a and 20b were synthesized in the same fashion as the other hydroxamates but with methyl ketone prepared from Weinreb amides 10a−b (Scheme 2A) instead of an aldehyde as the starting material (Scheme S1, Supporting Information). Using this route, a mixture of Z and E isomers was formed upon conversion of the methyl ester into the corresponding THP-protected hydroxamate (Scheme S1, Supporting Information). These isomers were separated and both deprotected and tested for BoNT/A LC inhibition; the Z isomer was a significantly less active inhibitor of BoNT/A LC then the E isomer (Table S1, Supporting Information).
Scheme 3. Synthesis of Hydroxamate 18.
Gratifyingly, the inhibitory activity of 18 with a saturated linker between hydroxamate and the benzothiophene was only approximately 3.5-fold lower than that of parental 15a, a smaller difference than resulted from the double bond reduction of hydroxamate 1.11 However, unlike hydroxamate 1 where introduction of methyl group onto the double bond did not change inhibitory activity, a 2−3 fold decrease in inhibitory activity was observed with benzothiophene inhibitors 20a-(E) and 20b-(E) (Table 1).
The kinetics of the LC inhibition by the most active benzothiophene 15a and two of its analogues, reduced double bond 18 and methyl substituted double bond 20a-(E), were examined in more detail using a LCMS-based assay that uses a substrate consisting of the 66 amino acid long C-terminal domain of SNAP-25.19 Unlike the short fluorogenic substrate used in the SNAPtide assay, this substrate contains all of the amino acids known to interact with the LC and is therefore more representative of the native substrate in kinetic models. As expected, kinetic parameters of all three molecules confirmed that they are competitive inhibitors of BoNT/A LC (Figure S1, Supporting Information). Inhibitor 15a with Ki = 77 nM is one of the most potent small molecule BoNT/A inhibitors reported (6-fold more potent than dichlorocinnamic acid 1 which possessed a Ki = 460 nM determined under the same conditions). Its two derivatives, 18 and 20a-(E), with double bond reduced and methyl substituted, respectively, are also potent BoNT/A inhibitors with Ki values of 270 and 160 nM, respectively (Table 1). We anticipated that this 3.5- and 2-fold decrease in potency in comparison to the parental compound would be overcome by an increase in their metabolic stability or improvement in other pharmacologic properties.
Consequently, five compounds were advanced into an initial battery of in vitro pharmacologic tests to ascertain the effects of the rational structural modifications. The strategy here was to prioritize compounds that give sustained exposure in vivo to both determine if our discovery approach yielded efficacious compounds as well as to obtain the data necessary to progress lead compounds into IND-enabling toxicology studies and, ultimately, human dosing. All compounds were tested for aqueous solubility, metabolic stability in liver microsomes, and percent protein binding in serum (Table 2). These specific assays were chosen as they directly impact formulation, in vivo exposure, and volume of distribution, respectively; each of these factors can be critical in the advancement of a lead candidate into proof-of-concept testing both preclinically and clinically. Furthermore, compounds were tested in the context of human, rat, and mouse liver microsomal metabolic stability, due to potential species differences in liver metabolism as well as to inform future toxicology and clinical study designs. Not surprisingly, given the relatively hydrophobic nature of the tested hydroxamates, all compounds were highly protein bound in serum (>84%). However, because of the increased potency of these compounds (15a, 18, and 20-(E)), significant protein binding is not anticipated to cause any issues in downstream in vivo studies. As expected, methylation of the double bond in 1 to generate 21 resulted in a compound with a markedly improved half-life, presumably as a consequence of the reduced propensity to undergo Michael addition. We were gratified to observe that parent benzothiophene 15a discovered in this study also possessed a long half-life when exposed to human liver microsomes, comparable to 21. It is important to note that significant differences were observed between species in terms of microsomal stability, highlighting the need to examine compounds in a species appropriate for future studies (compounds 15a, 18, and 21). However, we were surprised to note that both modifications of 15a aimed at removing the presumed metabolic liabilities resulted in compounds with reduced liver microsomal stability; indeed, compound 20a-(E) had a dramatically short half-life in human, rat, and mouse microsomes. Nonetheless, 15a possesses the requisite combination of target inhibition and favorable ADME properties for further study. Given its increased potency (6-fold) and half-life (2.5-fold) relative to the parent benchmark hydroxamate 1, this compound represents a viable lead for advancement into in vivo pharmacokinetics and studies of BoNT/A inhibition.
Table 2. In Vitro ADME Properties of Lead Hydroxamates.
| human |
rat |
mouse |
||||||
|---|---|---|---|---|---|---|---|---|
| compd | metabolic rate (μM/min·mg protein) | half-life (min) | metabolic rate (μM/min·mg protein) | half-life (min) | % protein binding | metabolic rate (μM/min·mg protein) | half-life (min) | aqueous solubility, pH 6.8 (μM) |
| 1 | 0.08 | 72.4 | 0.13 | 36.0 | 85.7 | 0.46 | 11.8 | 24.9 |
| 21 | 0.03 | 182.9 | 0.02 | 311.2 | 79.2 | 0.06 | 90.0 | >500 |
| 15a | 0.03 | 183.3 | 0.10 | 49.1 | 89.8 | 0.36 | 15.2 | <10 |
| 18 | 0.16 | 35.3 | 0.03 | 142.1 | 95.3 | 1.36 | 4.0 | 270 |
| 20a-(E) | 0.27 | 20.3 | 0.45 | 10.8 | 83.9 | 0.42 | 13.0 | <10 |
In the discovery of small molecule therapeutics, it is essential to consider parameters beyond simple in vitro enzymatic inhibition.20 This is particularly important for targets that have potent toxicity and long half-life, and are not readily amenable to clinical trials, such as botulinum neurotoxin. In this study, we have benchmarked one of the most potent compounds characterized as a BoNT/A light chain inhibitor and demonstrated its pharmacologic liabilities that could prevent this molecule from becoming an effective therapeutic. Through rational design, the pharmacologic stability of this compound has been markedly improved, and furthermore, the most potent small molecule inhibitor of BoNT/A reported to date was identified in this process. There are interesting similarities between the molecules identified in this study (15a, 18, 20a-(E)) and the previously reported F4H molecule (Figure 1),17 arguing that despite the presence of significant flexibility in the BoNT/A light chain21 there are common pharmacophores that can be used in the design of small molecule inhibitors. Also, the benzothiophenes reported here suggest that despite the large sequence required for efficient substrate cleavage by BoNT/A a molecule with relatively small molecular surface area can serve as a potent inhibitor worthy of further in vivo study. The specific pharmacokinetic profile and in vivo activity of these compounds is currently under investigation and will be reported in due course.
Methods
Preparation of Hydroxamates
To a stirring solution of the propenoate ester (0.50 mmol, 4a−w and 16a−f) and O-(2-tetrahydropyranyl) hydroxylamine (0.50 mmol) in tetrahydrofuran (2 mL) was added sodium bis(trimethylsilyl)amide (0.50 mL, 1.0 mmol, 2 M in tetrahydrofuran). After shaking for 30 min, hydrochloric acid (0.5 M, 2 mL) was added to the reaction mixture, followed by ethyl acetate (1 mL). After shaking and allowing the layers to separate, the top layer was removed and the organic addition and extraction was repeated. The organic layers were combined and concentrated under reduced pressure to give the desired crude protected hydroxamate (5a−w and 17a−f).
To a stirred solution of the protected hydroxamate (0.5 mmol, 5a−w and 17a−f) in methanol (1.5 mL) was added hydrogen chloride (0.25 mL, 4 N in 1,4-dioxane). After stirring for 4 h, the reaction mixture was concentrated under reduced pressure. Methanol (1 mL) was added to the crude product, and the mixture was again concentrated under reduced pressure. The crude product was purified by silica gel chromatography (4 g cartridge) eluting with ethyl acetate/methanol to give the desired hydroxamic acid (6a−w and 15a−f) with purity ≥ 95% by HPLC.
SNAPtide Assay
The SNAPtide assay was performed as reported elsewhere with minor modifications.11 Reaction of BoNT/A LC (7 nM) with SNAPtide substrate (5 μM, List Biological Laboratories) in the presence of small molecule inhibitor in 40 mM HEPES buffer pH 7.4, 0.01% Tween 20 and 2% DMSO, performed at room temperature was followed by measuring fluorescence in 1 min increments over 30 min. Fluorescence measurements were collected using a SynergyMx (BioTek) plate reader with excitation at 490 nm and emission at 523 (each with 9 nm window). Enzyme velocities used for calculation of IC50 values were determined from the linear portion of the assay response curve, typically from data collected between 2 and 20 min.
SNAP-25[141−206] LCMS Assay
LCMS assay for cleavage of SNAP-25[141−206] was performed as described elsewhere with minor modifications.19 Reaction of BoNT/A LC (150 pM) with substrate (SNAP-25 C-terminal amide, amino acids 141−206) and small molecule inhibitor in 40 mM HEPES buffer pH 7.4 and 2% DMSO was performed at room temperature and quenched by addition of 15% TFA (3 μL into 25 μL of reaction mixture) after 30 min. The quenched reaction mixture was analyzed by LCMS and cleavage product quantified using 13C-labeled internal standard (H-Arg-Ala(13C)-Thr-Lys-Met-Leu(13C)-Gly(13C)-Ser-Gly(13C)-OH).19 Enzyme velocities were calculated as the ratio of concentration of product formed over reaction time (product formation was linear over initial 30 min). The KM (2.0 μM) and kcat (66 min−1) values were determined by fit of the Michaelis−Menten equation from dependence of enzyme velocities on substrate concentrations. The inhibition constants Ki were determined from a nonlinear least-squares global fit of the competitive inhibition equation to the initial rates of product formation.
In Vitro Microsomal Stability
Metabolic stability of lead compounds was assessed in vitro by the method of Ackley et al.22 Briefly, liver microsome preparations (BD Genetest Products and Services, Woburn, MA) were isolated from humans (pooled from 10 male donors), Sprague−Dawley rats (male rats, 8−10 weeks of age), or CD-1 mice (pooled from 10 male mice, 11 weeks of age). Assays were conducted using 0.125 mg/mL protein concentration and 1.0 μM drug concentration under incubation conditions of 37 °C. Metabolic stability was determined following 0, 5, 15, 30, and 60 min of incubation time. The samples were analyzed by reversed phase LC using a triple quadrupole mass spectrometer. Compound specific transitions of parent ion to product ion were monitored and percent remaining calculated based on peak area of 15−60 min time points (relative to time zero). Half-life calculations were determined using the formula t1/2 = −ln(2)/k, where k (min−1) is the turnover rate constant (the slope) estimated from a log−linear regression of the percentage compound remaining versus time.
Acknowledgments
We gratefully acknowledge K. Lacombe for technical assistance with ADME studies.
Supporting Information Available
Additional information including experimental details, supporting figures, and supporting tables. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by the National Institute of Health (AI082190 to T.J.D.).
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Dembek Z. F.; Smith L. A.; Rusnak J. M. (2007) Botulism: cause, effects, diagnosis, clinical and laboratory identification, and treatment modalities. Disaster Med. Public Health Prep. 1, 122–134. [DOI] [PubMed] [Google Scholar]
- Schantz E. J.; Johnson E. A. (1992) Properties and use of botulinum toxin and other microbial neurotoxins in medicine. Microbiol. Rev. 56, 80–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G.; Matteoli M.; Montecucco C. (2000) Neurotoxins affecting neuroexocytosis. Physiol. Rev. 80, 717–766. [DOI] [PubMed] [Google Scholar]
- Kongsaengdao S.; Samintarapanya K.; Rusmeechan S.; Wongsa A.; Pothirat C.; Permpikul C.; Pongpakdee S.; Puavilai W.; Kateruttanakul P.; Phengtham U.; Panjapornpon K.; Janma J.; Piyavechviratana K.; Sithinamsuwan P.; Deesomchok A.; Tongyoo S.; Vilaichone W.; Boonyapisit K.; Mayotarn S.; Piya-Isragul B.; Rattanaphon A.; Intalapaporn P.; Dusitanond P.; Harnsomburana P.; Laowittawas W.; Chairangsaris P.; Suwantamee J.; Wongmek W.; Ratanarat R.; Poompichate A.; Panyadilok H.; Sutcharitchan N.; Chuesuwan A.; Oranrigsupau P.; Sutthapas C.; Tanprawate S.; Lorsuwansiri J.; Phattana N. (2006) An outbreak of botulism in Thailand: clinical manifestations and management of severe respiratory failure. Clin. Infect. Dis. 43, 1247–1256. [DOI] [PubMed] [Google Scholar]
- Capkova K.; Salzameda N. T.; Janda K. D. (2009) Investigations into small molecule non-peptidic inhibitors of the botulinum neurotoxins. Toxicon 54, 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. E.; Gunn R. A. (1980) Hypersensitivity reactions associated with botulinal antitoxin. Am. J. Med. 69, 567–570. [DOI] [PubMed] [Google Scholar]
- Arnon S. S.; Schechter R.; Inglesby T. V.; Henderson D. A.; Bartlett J. G.; Ascher M. S.; Eitzen E.; Fine A. D.; Hauer J.; Layton M.; Lillibridge S.; Osterholm M. T.; O’Toole T.; Parker G.; Perl T. M.; Russell P. K.; Swerdlow D. L.; Tonat K. (2001) Botulinum toxin as a biological weapon: medical and public health management. JAMA, J. Am. Med. Assoc. 285, 1059–1070. [DOI] [PubMed] [Google Scholar]
- Dickerson T. J.; Janda K. D. (2006) The use of small molecules to investigate molecular mechanisms and therapeutic targets for treatment of botulinum neurotoxin A intoxication. ACS Chem. Biol. 1, 359–369. [DOI] [PubMed] [Google Scholar]
- Li B.; Peet N. P.; Butler M. M.; Burnett J. C.; Moir D. T.; Bowlin T. L. (2011) Small molecule inhibitors as countermeasures for botulinum neurotoxin intoxication. Molecules 16, 202–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. M.; Blumenthal J. R.; Merson M. H.; Lombard G. L.; Dowell V. R.; Gangarosa E. J. (1981) Clinical features of types A and B food-borne botulism. Ann. Intern. Med. 95, 442–445. [DOI] [PubMed] [Google Scholar]
- Boldt G. E.; Kennedy J. P.; Janda K. D. (2006) Identification of a potent botulinum neurotoxin a protease inhibitor using in situ lead identification chemistry. Org. Lett. 8, 1729–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubanks L. M.; Hixon M. S.; Jin W.; Hong S.; Clancy C. M.; Tepp W. H.; Baldwin M. R.; Malizio C. J.; Goodnough M. C.; Barbieri J. T.; Johnson E. A.; Boger D. L.; Dickerson T. J.; Janda K. D. (2007) An in vitro and in vivo disconnect uncovered through high-throughput identification of botulinum neurotoxin A antagonists. Proc. Natl. Acad. Sci. U.S.A. 104, 2602–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capkova K.; Yoneda Y.; Dickerson T. J.; Janda K. D. (2007) Synthesis and structure-activity relationships of second-generation hydroxamate botulinum neurotoxin A protease inhibitors. Bioorg. Med. Chem. Lett. 17, 6463–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe G. N.; Silhar P.; Hixon M. S.; Silvaggi N. R.; Allen K. N.; Moe S. T.; Jacobson A. R.; Barbieri J. T.; Janda K. D. (2010) Chirality holds the key for potent inhibition of the botulinum neurotoxin serotype a protease. Org. Lett. 12, 756–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan B.; Potian J. G.; Garcia C. C.; Hognason K.; Capkova K.; Moe S. T.; Jacobson A. R.; Janda K. D.; McArdle J. J. (2010) Effects of hydroxamate metalloendoprotease inhibitors on botulinum neurotoxin A poisoned mouse neuromuscular junctions. Neuropharmacology 58, 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.; Hanefeld U. (2011) The selective addition of water to C=C bonds; enzymes are the best chemists. Chem. Commun. 47, 2502–2510. [DOI] [PubMed] [Google Scholar]
- Pang Y. P.; Davis J.; Wang S. H.; Park J. G.; Nambiar M. P.; Schmidt J. J.; Millard C. B. (2010) Small molecules showing significant protection of mice against botulinum neurotoxin serotype A. PLoS One 5, e10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y. P.; Vummenthala A.; Mishra R. K.; Park J. G.; Wang S. H.; Davis J.; Millard C. B.; Schmidt J. J. (2009) Potent new small-molecule inhibitor of botulinum neurotoxin serotype A endopeptidase developed by synthesis-based computer-aided molecular design. PLoS One 4, e7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capkova K.; Hixon M. S.; McAllister L. A.; Janda K. D. (2008) Toward the discovery of potent inhibitors of botulinum neurotoxin A: development of a robust LC MS based assay operational from low to subnanomolar enzyme concentrations. Chem. Commun. 3525–3527. [DOI] [PubMed] [Google Scholar]
- Gleeson M. P.; Hersey A.; Montanari D.; Overington J. (2011) Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat. Rev. Drug Discovery 10, 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvaggi N. R.; Boldt G. E.; Hixon M. S.; Kennedy J. P.; Tzipori S.; Janda K. D.; Allen K. N. (2007) Structures of Clostridium botulinum neurotoxin serotype A light chain complexed with small-molecule inhibitors highlight active-site flexibility. Chem. Biol. 14, 533–542. [DOI] [PubMed] [Google Scholar]
- Ackley D. C.; Rockich K. T.; Baker T. R. (2004) In Optimization in Drug Discovery. In Vitro Methods (Yan Z., Caldwell G. W., Eds.), p 151, Humana Press, Totowa, NJ. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.