Abstract
Individuals with Down syndrome (DS) exhibit a cholinergic deficiency similar to that found in Alzheimer’s disease. Cholinesterase inhibitors, used to treat Alzheimer’s disease, may improve cognitive function in individuals with DS. This is the first investigation of the safety and efficacy of rivastigmine (an acetyl and butyryl cholinesterase inhibitor) on specific cognitive domains in pediatric DS. Eleven subjects with DS (ages 10–17 years) were treated with a liquid formulation of rivastigmine. Four subjects experienced no adverse events (AEs). Seven subjects reported AEs that were mild, transient and consistent with adverse events typically noted with cholinesterase inhibitors. Significant improvements were found in over-all adaptive function (Vineland Adaptive Behavior Scales and Clinician’s Interview-Based Impression of Change), attention (Leiter Attention Sustained tests A and B), memory (NEPSY: Narrative and Immediate Memory for Names subtests) and language (Test of Verbal Expression and Reasoning and Clinical Evaluation of Language Fundamentals–Preschool) domains. Improved language performance was found across all functional levels. These results underscore the need for larger, controlled studies employing a carefully constructed test battery capable of measuring the full scope of performance across multiple domains and a wide range of functional levels.
INTRODUCTION
Down Syndrome (DS) is the most common chromosomal condition associated with mental retardation in infants and children (Gardner and Sutherland 2004), occurring in 1 of 800 births (Korenberg et al. 1993). The genetic defect is based on a chromosomal aneuploidy (trisomy 21), and the consequent gene dosage imbalance is believed to be the main cause of the phenotype. Although the IQs of most individuals with DS fall in the mild to moderate range of mental retardation (40–70), they exhibit an uneven profile of skills. Cognitive deficits are more prominent in specific domains, such as learning and memory, which probably reflects the disproportionate impairment of the hippocampus and the prefrontal cortex (Nadel 2003; Pennington et al. 2003). Difficulties in the language domain are also common and thought to be independent from learning and memory problems (Nadel 2003). Typically, individuals with DS require special accommodations at home and school to maximize their potential. Even with these support systems in place, there is a substantial unmet need and interest in new strategies to promote independence and to maximize over-all performance.
Cholinesterase inhibitors (ChEIs), including donepezil hydrochloride and rivastigmine, have been shown to be effective in the symptomatic treatment of mild-to-moderate Alzheimer disease (AD)-type dementia and other dementias with known cholinergic deficiency (McKeith et al. 2000; Emre et al. 2004). These agents may be effective in the treatment of cognitive deficits in individuals with DS, due to underlying brain similarities between DS and AD and the cholinergic deficit present in both.
Kishnani et al. (1999) published the first report on the use of a ChEI (donepezil hydrochloride) in 4 adults with DS (2 with dementia, 2 without). When treated with donepezil for a minimum of 26 weeks, these adults (ages 24–64 years) showed improvements in communication, attention, and mood as measured by care taker report. Improved language performance in 8 adults with DS and no dementia was also reported by Heller et al. (2003) in a 24-week, open-label study of donepezil and by Johnson et al. (2003) in a 12-week, double-blind, placebo-controlled study of donepezil in 19 adults with DS. Recently, Kondoh (2005) reported remarkable improvements in the quality of life and in the verbal communication abilities in 2 adults with DS and severe mental retardation (IQ < 20) following done-pezil treatment.
To date, studies of ChEIs in the pediatric population have been limited. Heller et al. (2004) published the first study of donepezil in 7 children with DS (ages 8–13 years). Improved language performance at the end of this 22-week trial was reported. To our knowledge, the current study is the first report of the safety and efficacy of rivastigmine in pediatric DS. Prasher et al. (2005) reported in a retrospective treatment analysis that adults with DS and AD had less of a decline in global and adaptive function over 24 weeks as compared to an untreated group; however, this difference failed to reach statistical significance.
Rivastigmine and donepezil are both acetyl-cholinesterase inhibitors (AChEIs); however, rivastigmine has the additional action of butyrylcholinesterase inhibition. Additionally, rivastigmine is available in liquid form. The liquid form facilitates dose titration and allows individuals who are unable to swallow pills to participate in the research.
On the basis of reports of ChEIs in DS, we hypothesized that: (1) rivastigmine would be safe and well tolerated by adolescents with DS, (2) subjects would demonstrate an improvement in adaptive function as measured by the Vineland Adaptive Behavior Composite, and (3) subjects would demonstrate improved expressive language and memory while on medication as compared to baseline measures.
METHODS
Subjects
A total of 11 adolescents (8 males, 3 females), ages 10–17 years (median age = 12 years, 5 months) with DS (10 subjects had trisomy 21 and one had mosaicism for trisomy 21) and their primary caregivers participated in this 20-week trial approved by the Institutional Review Board (IRB) and conducted at the General Clinical Research Center at Duke University Medical Center. Subjects were recruited through a Duke IRB-approved advertisement posted in the Duke Comprehensive Down Syndrome Clinic and in local DS support group newsletters.
All subjects were verbal, intelligible, and able to hear speech at a conversational level. These attributes were included as part of the prescreening process for subject selection and reviewed informally when the subject came for his or her first visit, a screening visit. None of the subjects had active thyroid disease, vitamin B12 deficiency, clinically confirmed pregnancy, or clinically significant systemic disorders (e.g., cardiac disease, insulin-dependent diabetes mellitus, active peptic ulcer, celiac disease, significant reactive airways, or seizure disorder) at the time of study entry. They had not ingested ChEIs or any other investigational or alternative therapies used to treat the symptoms of DS in the 30 days prior to or during the trial. Seven subjects were taking concomitant oral medications, including fexofenadine (Allegra), minocycline (Minocin), methylphenidate (Concerta), levothyroxine (Synthroid), and amphetamine and dextroamphetamine (Adderall XR) for at least 3 months prior to enrollment and throughout the trial. All subjects lived at home with at least 1 parent and attended school on a regular basis. Each participant gave verbal assent for participation and written informed consent was obtained from the parent or guardian. One subject withdrew from the study approximately 2 weeks following the baseline visit (the reason for withdrawal is provided in the Safety/Compliance section below). The safety data include all 11 enrolled subjects and the efficacy data include the 10 subjects who completed the trial.
Study design
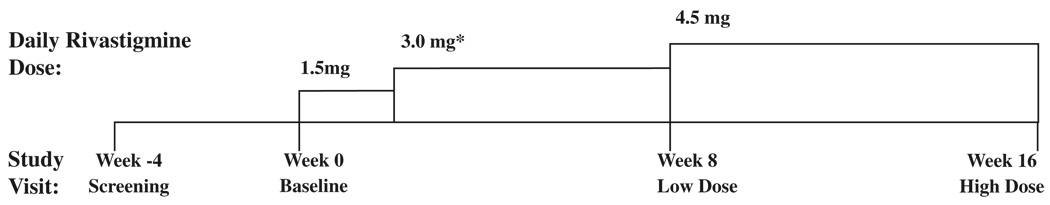
The subjects attended four sessions (Fig. 1): Screening (week −4), Baseline (week 0), Low-Dose Treatment (week 8), and Full-Dose Treatment (week 16). Language and neuropsychological testing were completed at each session. A physical examination was completed at the Baseline, week-8, and week-16 visits. At the completion of the Baseline visit, rivastigmine in a liquid suspension (2 mg/mL) was dosed orally each day for 16 weeks.
FIG. 1.
Study timeline and titration schedule.
*Rivastigminc dose increased from 1.5 mg to 3.0 mg at week 2 (dose increase not associated with a study visit).
Medication dosage and monitoring
The starting dose of 1.5 mg/day, 0.75 mg twice a day (bid) was maintained for 2 weeks. After that period, the dose was titrated, based on tolerability, to 3 mg/day (1.5 mg bid) for 6 weeks. At week 8, the dose was increased further to a maximum of 4.5 mg/day (divided bid as 1.5 mg and 3.0 mg; approximately ½ of the adult dose) (Prasher 2004). This dosing regimen was adopted on the basis of our experience (unpublished) with 2 12-year-old individuals with DS who had multiple adverse events (including emotional lability, agitation, stomachache/ nausea, diarrhea, vomiting, trouble sleeping, decreased appetite, and weight loss) on a faster titration rate and a higher dose (doubling the starting dose of 1.5 mg/day every 2 weeks to a maximum of 6.0 mg/day).
Descriptive measures at screening
Academic performance and overall behavior were measured by the Wide Range Achievement Test 3 (WRAT 3; Wilkinson 1993) and the Child Behavior Checklist (CBCL; Achenbach and Rescorla 2001), respectively.
Safety/compliance
At Screening, laboratory studies, including complete blood count (CBC), thyroid profile, and pregnancy testing for females, and an electrocardiogram (ECG) were performed. Physical examination (including vital signs, height, and weight) and a review of systems were completed at the Baseline, week-8, and week-16 visits. An ECG was repeated at the week-16 visit. Adverse events were compiled and reviewed at and between study visits. Compliance was monitored by written medication diaries and by regular phone calls. Parents reported any adverse events or missed doses to the study coordinator and returned unused study medication at the termination visit.
Anxiety, aggressive reaction, and depression are less frequent side effects of rivastigmine in adults with AD (Novartis 2004). Psychosocial behavior, as measured by the Conners’ Parent Rating Scales–Revised: Long Version (CPRS-R; Conners 1997) was analyzed to identify psychosocial behavioral side effects in this study. Two indexes, the Conners’ Global Index (composed of the Emotional Lability and Restless-Impulsive factors) and the ADHD Index, and seven subscales (Oppositional, Cognitive Problems, Hyperactivity, Anxious-Shy, Perfectionism, Social Problems, and Psychosomatic) were examined.
Efficacy
Efficacy was measured in the following domains: Adaptive function, language, attention, memory, and associative processing. All but two of the measures (Clinician’s Interview-Based Impression of Severity/Change (CIBIS/CIBIC), a nonstandardized revision of The Clinical Global Impression Scale (CGI; NIMH 1985), and the Test of Verbal Expression and Reasoning (TOVER; Heller et al. 2000) used in this study were diagnostic tests standardized on a normally developing population.
The selection of an appropriate test battery for children with cognitive disabilities is problematic. Typically, the performance of these children on age-appropriate tests falls at the lower extreme of the test, an area particularly insensitive to performance change. Additionally, children with cognitive disabilities can demonstrate an atypical profile of strengths and weaknesses that extend beyond the limits of standardized tests. To date, the Duke Down Syndrome Research Team has used mostly standardized test measures that target the subjects’ skill levels in particular cognitive and language domains. To capture the full range of performance change, raw scores rather than standardized scores are reported and analyzed for most measures. Additionally for this study, preschool language and attention measures were used in an attempt to demonstrate performance effects with even the lower functioning subjects.
Adaptive function was measured by the Vineland Adaptive Behavior Scales, Interview Edition (VABS; Sparrow et al., 1984) and the CIBIS/CIBIC, yielding CIBIS and CIBIC scores. The CIBIS/CIBIC is a semistructured, interview-based rating scale adapted for individuals with DS from the CGI Scale (NIMH 1985). The CIBIS (severity) score is obtained by rating the level of impairment in adaptive function at each visit on a seven point scale. Effects of experimental drug treatment can be determined by comparing the severity rating at Baseline versus the severity rating at the end of the experimental treatment. The CIBIC (change) score is obtained using a seven-point scale based on the clinician’s impression of change in adaptive function at each visit relative to baseline. The scale is bimodal; i.e., scores from 1 (marked improvement in adaptive function as compared to baseline performance) to 4 (no change in adaptive function between the baseline and final visit at the end of the experimental treatment) reflect a gradient of performance improvement, whereas scores from 4 (no change relative to baseline) to 7 (marked adaptive functional loss between the final treatment visit and baseline) indicate functional decline.
Language was measured by the TOVER and the Clinical Evaluation of Language Fundamentals–Preschool (CELF-P; Wiig et al. 1992). Attention, memory, and associative processing were measured by subtests of the NEPSY: A Developmental Neuropsychological Assessment (Korkman et al. 1998) and the Leiter International Performance Scale–Revised (Leiter-R; Roid and Miller 1997).
Efficacy analyses
Two types of analyses were completed on the efficacy measures. Initially, statistical analyses were completed on Baseline versus week-16 visit performances to identify treatment effects. Performance change was assessed by repeated measure t-tests or Wilcoxin Sign Rank tests, depending on the normality of the Baseline and week-16 visit score distributions. Due to the preliminary nature of the study, there was no correction for multiple comparisons. Two-tailed p-values less than 0.05 were viewed as significant.
Second, an analysis of the clinical significance of the performance change was made on those measures demonstrating a significant treatment effect. The clinical significance of the performance change was determined by comparing age equivalencies (from the respective test manuals) for the Baseline and the week-16 visits and determining the magnitude of the test score change (in months) between the two sessions.
RESULTS
Screening
WRAT-3 Reading and Spelling standard scores [mean = 100; standard deviation (SD) = 15] were widely distributed (<45 to 80); Arithmetic scores were consistently low [only 1 subject (57) scored above the <45 level]. Although median performance was more than 3 SD below the mean for age on the three WRAT-3 subtests, 3 subjects had WRAT Reading or Spelling scores within 1.5 SD of the mean for age. All CBCL behavioral profiles (measuring clinically significant behavioral factors, such as aggression, anxiety, depression, attention problems, and social problems) were within normal limits.
Compliance/safety
There were 16 adverse events (AEs) related or possibly related to the study medication. Twelve AEs occurred in the first 8 weeks of the treatment (7 at the 1.5-mg and 5 at the 3-mg levels) and four occurred in the second 8 weeks of treatment at the 4.5 mg level (Table 1). None of the AEs was unexpected.
TABLE 1.
Number Of Subjects Reporting A Given Adverse Event Per Dose Level (N = 11)
| Adverse event | 1.5-mg dose |
3.0-mg dose |
4.5-mg dose |
|---|---|---|---|
| Diarrhea | 2 | 1 | 0 |
| Vomiting | 1 | 2 | 0 |
| Stomachache/nausea | 1 | 0 | 1 |
| Decreased appetite | 1 | 0 | 0 |
| Headache | 1 | 0 | 0 |
| Fatigue | 0 | 0 | 2 |
| Trouble sleeping | 0 | 1 | 0 |
| Defiance | 0 | 1 | 0 |
| Moody | 1 | 0 | 0 |
| Emotional lability | 0 | 0 | 1 |
| Total | 7 | 5 | 4 |
Four subjects reported no AEs and 5 subjects reported one to three mild, transient AEs, including vomiting, diarrhea, stomachache, fatigue, trouble sleeping, and an instance of using “defiant, sassy language” at school. None of these subjects experienced bladder or bowel incontinence, AEs that were reported with donepezil treatment in older subjects with DS (Hemingway-Eltomey and Lerner 1999). Two subjects accounted for more than one half of the 16 recorded AEs. One subject began experiencing stomachaches, headaches, vomiting, and moody behavior within 2 days after the medication was administered at the Baseline visit (at a dose of 1.5 mg/day). The parents reported that although their child was able to attend school, she continued to experience stomach problems for the week following the Baseline visit. Thirteen days after the initial administration of the medication, the parents were advised to discontinue its use and return for medical review. The parents reported that the AEs resolved after the medication was discontinued. They withdrew from the study 2 weeks after the Baseline visit.
Another subject exhibited transient diarrhea at the 1.5-mg dose, which resolved without intervention. Emotional lability, decreased appetite, and fatigue were reported on the 4.5-mg dose at the week-16 visit. At the end of her week-16 visit, the dose was lowered to 3.0 mg and the medication was continued for an additional 9 weeks. The emotional lability, decreased appetite, and fatigue resolved at the lowered dose. Across the 16 weeks of treatment, the subjects did not experience a significant change in weight, ratings of behavior (CPRS-R), or ECG parameters.
Efficacy
Significant medication effects were noted in measures of adaptive function, language, memory, and attention (Table 2). No change in performance from Baseline was noted in associative processing.
TABLE 2.
Comparison Of Baseline Andweek-16 Raw Scores (Sd) For Each Efficacy Domain/Measure
| Domain/measure | Baseline | Week 16 | Difference | p-value |
|---|---|---|---|---|
| Adaptive function | ||||
| Vineland Adaptive Behavior Scale (VABS) | ||||
| Adaptive Behavior Composite | 132.8 (27.9) | 140.6 (31.7) | −7.8 (10.5) | 0.04a |
| Communication Domain | 88.7 (13.8) | 94.3 (13.8) | −5.6 (4.1) | 0.01b |
| Daily Living Skills Domain | 96.4 (14.4) | 101.8 (13.4) | −5.4 (6.8) | 0.03a |
| Socialization Domain | 77.2 (12.5) | 79.7 (12.2) | −2.5 (5.9) | 0.21 |
| Maladaptive Behavior Domain (Part I) | 7.5 (5.2) | 7.2 (4.8) | 0.3 (4.3) | 0.83 |
| Maladaptive Behavior Domain (Part II) | 1.2 (1.6) | 0.8 (0.9) | 0.4 (1.1) | 0.31 |
| Clinical Interview-Based Impression of Severity (CIBIS) | 4.4(1.0) | 4.6 (1.2) | −0.2(0.4) | 0.50 |
| Language | ||||
| Clinical Evaluation of Language | ||||
| Fundamentals–Preschool (CELF-P) | ||||
| Total Language Score | 79.7 (31.2) | 86.8 (31.1) | −7.1 (6.8) | 0.01b |
| Expressive Language Score | 42.6 (21.5) | 48.5 (22.0) | −5.9 (7.4) | 0.03a |
| Receptive Language Score | 37.1 (10.6) | 38.3 (11.5) | −1.2 (6.0) | 0.49 |
| Test of Verbal Expression and Reasoning (TOVER) | 17.5 (9.8) | 22.7 (13.1) | −5.2 (5.8) | 0.02a |
| Attention | ||||
| Leiter-R Attention Sustained A | 50.7 (10.6) | 56.6 (8.2) | −5.9 (5.9) | 0.01b |
| Leiter-R Attention Sustained B | 42.5 (9.9) | 50.5 (11.5) | −8.0 (8.9) | 0.02a |
| NEPSY Visual Attention | 6.7 (3.7) | 10.0 (6.5) | −3.3 (6.4) | 0.14 |
| Memory | ||||
| Leiter-R Forward Memory | 10.6 (7.9) | 13.9 (4.9) | −3.3 (5.8) | 0.11 |
| Leiter-R Immediate Recognition | 8.5 (5.8) | 7.0 (2.7) | 1.5 (3.9) | 0.26 |
| NEPSY Narrative Memory | 7.5 (6.3) | 12.2 (10.0) | −4.7 (5.2) | 0.02a |
| NEPSY Immediate Memory for Names | 8.1 (4.3) | 13.9 (5.0) | −5.8 (5.2) | 0.01b |
| Associative Processing | ||||
| Leiter-R Associated Pairs | 15.6 (4.2) | 16.1 (5.6) | −0.5 (4.3) | 0.72 |
| NEPSY Verbal Fluency—Food/Drink | 6.7 (3.7) | 10.0 (6.5) | −3.3 (6.4) | 0.87 |
| NEPSY Verbal Fluency—Animals | 5.2 (2.6) | 6.9 (3.7) | −1.7 (4.2) | 0.23 |
p ≤ 0.05.
p ≤ 0.01
Adaptive function
Significant improvements in adaptive function while on medication were found on the Vineland Adaptive Behavior Scales (VABS) overall Adaptive Behavior Composite (p = 0.04) and on the Communication (p = 0.01) and Daily Living Skills (p = 0.03) domains. On average, there was a 5.4 point (6%) increase on the Daily Living Skills domain corresponding to a 4-month gain in daily living skills and a 5.6 point (6%) increase on the Communication domain, corresponding to a 7-month gain in communication skills (Table 3). The Adaptive Behavior Composite, derived from the Communication, Daily Living Skills, and Socialization scores, also increased 6% from Baseline. No change from Baseline was noted on the Socialization and Maladaptive Behavior domains of the VABS.
TABLE 3.
Average Change In Age-Equivalent Performance For Measures Demonstrating A Statistically Significant Performance Change Between Baseline And Full Dose (Week 16) Visits
| Baseline (months) |
Week 16 (months) |
Average gain |
|
|---|---|---|---|
| VABS Communication | 67 | 74 | 7 Months |
| VABS DLS | 63 | 67 | 4 Months |
| CELF-P Total | 40 | 42 | 2 Monthsa |
| Leiter-R Attention Sustained B |
63 | 71 | 8 Months |
| NEPSY Immediate Memory |
66 | 93 | 27 Months |
| NEPSY Narrative Memory |
46 | 55 | 9 Months |
Average age equivalent gain in language performance may have been constrained by test ceiling effects.
VABS = Vineland Adaptive Behavior Scales; VABS DLS = VABS Daily Living Skills; CELF-P = Clinical Evaluation of Language Fundamentals–Preschool.
No change was found in the assessment of the severity of functional level (CIBIS) between Baseline and week-16 performance. Similarly, no change to a minimal functional decrease was found in the rating of change (CIBIC) between the Baseline and week-8 visits (mean CIBIC score was 4.6). However, a clinically meaningful and statistically significant change in day-to-day living was noted in the week-16 versus the week-8 visits. At the week-16 visit, the average CIBIC score was 1.3 (indicating a marked-to-moderate improvement in adaptive function between Baseline and week-16 and a significant change in CIBIC score between week 8 and week 16 (average CIBIC score change = 3.3; p = 0.04). This improvement was consistent across 9 of the 10 subjects (the CIBIC score of 1 subject did not change).
Language
Significant language effects were noted on both the TOVER expressive language test and the CELF-P. On the TOVER, performance at the week-16 visit showed a significant gain (p = 0.02) of 5.2 points (30% increase) from Baseline. No age-equivalent scores are available for this measure. On the CELF-P, overall language performance at the week-16 visit showed a significant gain (p = 0.01) of 7.1 points (9% increase) from Baseline. Gains of 5.9 (14%) and 1.2 (3%) points, respectively, were found on the expressive language and receptive language components at the week-16 versus Baseline visits. The gain in expressive language was statistically significant (p = 0.03), whereas the gain in receptive language performance was not significant (p = 0.49). On the CELF-P, the relative overall language gain was small, i.e., a 2-month gain in 4 months of treatment (Table 3). However, the magnitude of the language gain was likely limited by test ceiling effects. All subjects failed to reach a test ceiling on at least 2 of 6 subtests at Baseline and 3 of 6 subtests at the week-16 visits. Three subjects failed to reach a test ceiling on almost all (5 of 6) subtests at Baseline and at the week-16 visits.
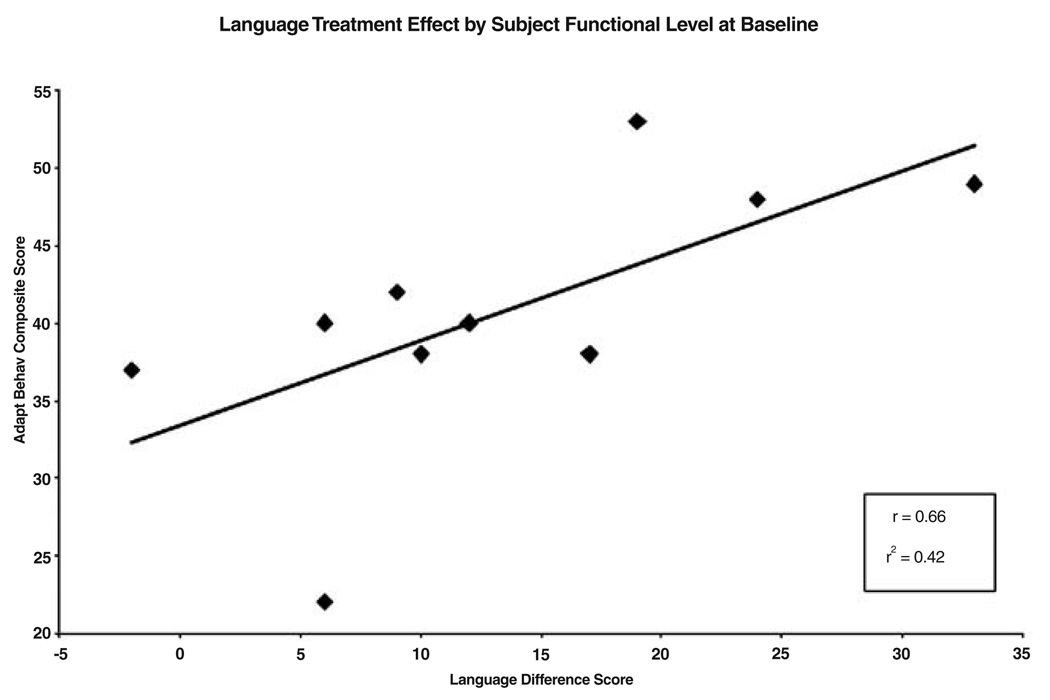
In light of the report by Heller et al. (2003) that little improvement in language functioning was found in subjects with IQs less than 55, the relationship between the degree of language improvement and subject functional level was investigated. The overall language treatment effect (sum of the TOVER and CELF-P week-16 versus Baseline difference scores) was correlated with the Adaptive Behavior Composite score of the VABS at Baseline. The VABS Adaptive Behavior Composite was used as a measure of functional level because recent IQ testing was unavailable for most subjects. The VABS Adaptive Behavior Composite is highly correlated with IQ in the general population (Sparrow et al. 1984) and has been found to be highly correlated with IQ (r = 0.87) in previous subjects with DS at Duke (unpublished). The correlation between functional level at Baseline and language improvement following rivastigmine was high (r = 0.66) and almost all subjects demonstrated a language gain (Fig. 2). We were able to focus on the lower end of the spectrum of language gain across functional level through the use of standardized language tests for younger (preschool) children than the school-age language tests used in Heller et al. (2003). However, the decision to use preschool versus school-age language tests with the adolescent DS sample came with a tradeoff. Although preschool language tests are sensitive to small changes in language ability, they are subject to ceiling effects with higher functioning individuals. School-age language tests highlight large performance gains, especially in the highest-functioning individuals, but are subject to basal effects and are insensitive to the small gains achieved by the lowest functioning individuals.
FIG. 2.
Language treatment effect by subject functional level at Baseline.
Memory
Statistically significant gains were found on the two NEPSY memory measures, Narrative Memory, targeting children ages 3–12 years, (p = 0.02) and Immediate Memory for Names, targeting children ages 5–12 years, (p = 0.01). A 63% increase in performance, from 7.5 at Base-line to 12.2 at week 16 was noted in Narrative Memory and a 72% increase in performance, from 8.1 at Baseline to 13.9 at week 16 was noted in Immediate Memory for Names. This corresponded to a 6-month gain in Narrative Memory and a 27-month gain in Immediate Memory for Names (Table 3). No significant change was noted on the two Leiter-R memory measures, Forward Memory, targeting children ages 2–20 years (p = 0.11), and Immediate Recognition, targeting children ages 4–10 years (p = 0.26). As addressed in the language testing results, multiple factors, including test design factors such as the targeted functional level, contribute to performance differences between tests. In this context, it is important to note that the two NEPSY tests assessed verbal memory in children from 3 to 12 and from 5 to 12 years, whereas the Leiter-R focused on memory for visual information in children from 2 to 20 and 4 to 10 years. Thus, both sets of tests seem to be measuring comparable skill levels. This suggests that the verbal memory versus nonverbal memory distinction may be an important factor to explore in additional studies.
Attention
The subjects demonstrated significantly improved attention on the Leiter-R Attention Sustained tests A(p = 0.01) and B (p = 0.02). Performance on the Attention A test (standardized for children ages 2–3 years) increased 12% from a mean of 50.7 at Baseline to 56.6 at week 16. The performance of 3 subjects approached the test maximum score of 64 at Baseline and at the two treatment visits. Performance on the Attention B test (standardized for children ages 4–5 years) increased 19% from a mean of 42.5 at Baseline to 50.5 at week 16. (No ceiling effects were apparent, test maximum score = 74.) This corresponded to an age-equivalent gain of 8 months in the 16-week treatment phase of the study (Table 3). No gain in visual attention was found on the NEPSY Visual Attention subtest (p = 0.14), a test standardized on children ages 5–12 years. Whereas these results suggest a significant treatment effect on attention, they also reinforce the importance of test battery selection for adolescents with DS. It is particularly important to select measures that target the range of skill levels demonstrated by this group. While a significant treatment effect was found for both Leiter Sustained Attention Tests (A and B), a ceiling effect with the highest functioning subjects was found on the lower-level Attention Test A. Similarly, no effect was found for the NEPSY Visual Attention subtest, a test targeting this skill in typically developing 5–12 year olds. On the basis of the variability in targeted functional level of the different tests, it is impossible to determine if the subjects did not demonstrate a treatment effect on the NEPSY Visual Attention subtest because no treatment effect was experienced or because the skill level measured by subtest was at too high to detect performance change in this group of subjects.
Associative processing
No change in performance was found on any of the Associative Processing measures [p = 0.87, NEPSY Verbal Fluency (Food/Drink); p = 0.23 NEPSY Verbal Fluency (Animals), both targeting children ages 3–6 years, and p = 0.72, Leiter-R Associated Pairs, targeting children ages 2–20 years] (Table 2).
DISCUSSION
Rivastigmine was found to be relatively safe when slowly titrated from an initial dose of 1.5 mg/day to a maximum dose of 4.5 mg/day over an 8-week period. Safety and improved language performance have been associated with rivastigmine use in a 12-week open study of 32 children (ages 2.85–12 years) with autism (Chez et al. 2004). Critical factors for ensuring the safe use of rivastigmine in adolescents with DS include: Subject selection, dose level, titration rate, time of administration (after meals), regular monitoring for adverse events, and close observation of the subject when dose changes are made.
As hypothesized, the results of our study suggest that rivastigmine can improve adaptive function, expressive language, and memory in adolescents with DS. In addition, we found that rivastigmine may also improve aspects of attention. Although the open-label nature of this study limits the objectivity of parent and clinician rating scales, it is important to note that significant improvements in adaptive function were found on both the VABS (obtained via parent interview) and the CIBIC. Also, the improvements in adaptive function were consistent with improvements found in language, memory, and attention testing.
The results of this study highlight the critical importance of test battery selection in identifying treatment effects in adolescents with DS. The challenge is to select measures that target the wide range of skill levels represented in this population and that are sensitive to changes in performance ability. Errors in targeted skill level selection can lead to a misinterpretation of a potential treatment effect due to ceiling or floor effects. Similarly, differences in targeted level and test sensitivity between measures of different cognitive domains may also confound the identification of the true treatment effect on cognitive function.
Due to the inherent pitfalls of interpreting a potential treatment effect, we suggest the use of a test battery that contains both subjective measures of adaptive function (i.e., parental report and clinician-based impressions of change) and objective measures of specific cognitive domains to validate single-measure results and to best characterize the treatment effect.
Because of the limitations of such a small, exploratory study (i.e., small sample, lack of power for formal statistical control, repeated comparisons across a relatively short time span (8–16 weeks), and lack of an untreated control group), our findings should be viewed as preliminary evidence for additional investigation. With these caveats in mind, the results indicate: (1) That after careful consideration of dose and titration schedule, rivastigmine appears to be relatively safe for use in adolescents with DS, (2) using a carefully selected test battery sensitive to the wide range of performance of adolescents with DS, rivastigmine appears to facilitate performance improvement in multiple cognitive domains, i.e. language, memory, attention, and adaptive function, and (3) the magnitude of the language treatment effect appears to be proportional to the subject’s functional level at Baseline.
Clearly, larger, more carefully controlled studies are required before the therapeutic use of rivastigmine in this population can be recommended. Furthermore, long-term studies are needed to ensure safety and continued efficacy in long-term use. These results add to the growing body of evidence of the potential role of ChEIs in cognitive improvement in DS.
Our data support the establishment of larger, more rigorous studies and the need to use a carefully constructed test battery capable of measuring the full scope of performance across multiple domains and a wide range of functional levels.
ACKNOWLEDGMENTS
This work was possible only through the enthusiasm of the participants and their caregivers. We thank the staff of the Duke General Clinical Research Center (GCRC) for their help in completing this project. Also, we thank the Anna Michelle Merrills Foundation for Down Syndrome Research for their support of the Duke Down Syndrome Research Team.
This study was supported by grants from Novartis Pharmaceuticals and M01-RR30, National Center for Research Resources, General Clinical Research Centers program, and a donation from the Down Syndrome Family Support and Advocacy Group of Michiana, Indiana.
Footnotes
DISCLOSURES
The authors have received research grants and/or honoraria from various companies including: Pfizer, Johnson & Johnson, Eisai, Novartis, Jannsen, Merck, Forest, Lilly, Glaxo-SmithKline and Wyeth. The authors do not own stock in these companies. Investigators Heller, Spiridigliozzi, Doraiswamy, Krishnan, and Kishnani have a patent pending on the use of cholinesterase inhibitors for another disorder.
REFERENCES
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles. Burlington: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Chez MG, Aimonovitch M, Buchanan T, Mrazek S, Tremb RJ. Treating autistic spectrum disorders in children: Utility of the cholinesterase inhibitor rivastigmine tartrate. J Child Neurol. 2004;19:165–169. [PubMed] [Google Scholar]
- Conners CK. Conners’ rating scales-revised, technical manual. North Tonawanda (New York): Multi-Health Systems, Inc.; 1997. [Google Scholar]
- Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, Durif F, Kulisevsky J, van Laar T, Lees A, Poewe W, Robillard A, Rosa MM, Wolters E, Quarg P, Tekin S, Lane R. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351:2509–2518. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- Gardner RJM, Sutherland GR. Chromosome abnormalities and genetic counseling. New York: Oxford University Press; 2004. [Google Scholar]
- Heller JH, Spiridigliozzi GA, Kishnani PS. TOVER: Test of Verbal Expression and Reasoning. Duke University Medical Center; 2000. [Google Scholar]
- Heller JH, Spiridigliozzi GA, Sullivan JA, Doraiswamy PM, Krishnan RR, Kishnani PS. Donepezil for the treatment of language deficits in adults with Down syndrome: A preliminary 24-week open trial. Am J Med Genet A. 2003;116:111–116. doi: 10.1002/ajmg.a.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller JH, Spiridigliozzi GA, Doraiswamy PM, Sullivan JA, Crissman BG, Kishnani PS. Donepezil effects on language in children with Down syndrome: Results of the first 22-week pilot clinical trial. Am J Med Genet. 2004;130A:325–326. doi: 10.1002/ajmg.a.30184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway-Eltomey JM, Lerner AJ. Adverse effects of donepezil in treating Alzheimer’s disease associated with Down’s syndrome. Am J Psychiatry. 1999;156:1470. doi: 10.1176/ajp.156.9.1470. [DOI] [PubMed] [Google Scholar]
- Johnson N, Fahey C, Chicoine B, Chong G, Gitelman D. Effects of donepezil on cognitive functioning in Down syndrome. Am J Ment Retard. 2003;108:367–372. doi: 10.1352/0895-8017(2003)108<367:EODOCF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Sullivan JA, Walter BK, Spiridigliozzi GA, Doraiswamy PM, Krishnan KR. Cholinergic therapy for Down’s syndrome. Lancet. 1999;353:1064–1065. doi: 10.1016/S0140-6736(98)05285-4. [DOI] [PubMed] [Google Scholar]
- Kondoh T, Amamoto N, Doi T, Hamada H, Ogawa Y, Nakashima M, Sasaki H, Aikawa K, Tanaka T, Aoki M, Harada J, Moriuchi H. Dramatic improvement in Down syndrome-associated cognitive impairment with donepezil. Ann Pharmacother. 2005;39:563–566. doi: 10.1345/aph.1E427. [DOI] [PubMed] [Google Scholar]
- Korenberg JR, Pulst SM, Gerwehr S. Advances in the understanding of chromosome 21 and down syndrome. In: Lott IT, McCoy EE, editors. Down Syndrome: Advances in Medical Care. New York: John Wiley & Sons, Inc.; 1993. pp. 3–12. [Google Scholar]
- Korkman M, Kirk U, Kemp S. Nepsy: A developmental neuropsychological assessment, manual. San Antonio (Texas): The Psychological Corporation; 1998. [Google Scholar]
- McKeith I, Del Ser T, Spano P, Emre M, Wesnes K, Anand R, Cicin-Sain A, Ferrara R, Spiegel R. Efficacy of rivastigmine in dementia with lewy bodies: A randomised, double-blind, placebocontrolled international study. Lancet. 2000;356:2031–2036. doi: 10.1016/S0140-6736(00)03399-7. [DOI] [PubMed] [Google Scholar]
- Nadel L. Down’s syndrome: A genetic disorder in biobehavioral perspective. Genes Brain Behav. 2003;2:156–166. doi: 10.1034/j.1601-183x.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- NIMH: National institute of mental health (NIMH): Clinical global impression scale. Psychopharmacology Bulletin. 1985;21:839–844. [Google Scholar]
- Novartis: Exelon (rivastigmine tartrate) capsules and oral solution Rx only prescribing information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2004. [Google Scholar]
- Pennington BF, Moon J, Edgin J, Stedron J, Nadel L. The neuropsychology of Down syndrome: Evidence for hippocampal dysfunction. Child Dev. 2003;74:75–93. doi: 10.1111/1467-8624.00522. [DOI] [PubMed] [Google Scholar]
- Prasher VP. Review of donepezil, rivastigmine, galantamine and memantine for the treatment of dementia in Alzheimer’s disease in adults with Down syndrome: Implications for the intellectual disability population. Int J Geriatr Psychiatry. 2004;19:509–515. doi: 10.1002/gps.1077. [DOI] [PubMed] [Google Scholar]
- Prasher VP, Fung N, Adams C. Rivastigmine in the treatment of dementia in Alzheimer’s disease in adults with Down syndrome. Int J Geriatr Psychiatry. 2005;20:496–497. doi: 10.1002/gps.1306. [DOI] [PubMed] [Google Scholar]
- Roid GH, Miller LJ. Leiter international performance scale-revised. Wood Dale (Illinois): Stoelting; 1997. [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales-interview edition (survey form) Circle Pines: American Guidance Service; 1984. [Google Scholar]
- Wiig E, Secord W, Semel E. Clinical evaluation of language fundamentals-preschool. San Antonio (Texas): The Psychological Corporation; 1992. [Google Scholar]
- Wilkinson GS. Wide range achievement test-3 manual. Wilmington: Wide Range, Inc.; 1993. [Google Scholar]