Abstract
At present, there is no proven pharmacologic treatment for cognitive or language impairments in Down syndrome (DS). Cholinergic deficits have been documented in DS and linked to cognitive deficits. This study is a 24-week open-label clinical trial of donepezil hydrochloride for the treatment of language deficits in adults with DS. To our knowledge, this is the first prospective study to evaluate systematically the effects of donepezil, a cholinesterase inhibitor, on specific language domains in DS. The main finding that emerged was an improvement in expressive language performance following donepezil therapy. Despite the multiple methodological limitations, the results raise important questions regarding the role of the cholinergic system in language function and the specific effect of cholinergic therapy in the treatment of language impairment in DS. The results support the need for large-scale controlled studies of the effects of donepezil treatment on language and on other cognitive domains in DS.
Keywords: language impairment, expressive language, donepezil, cholinergic therapy, Down syndrome
INTRODUCTION
Language problems in children and adults with Down syndrome (DS) are well documented (see Chapman [1995] for a review of the language skills of children and adolescents with DS; see Rondal and Comblain [1996] for a review of the language skills of adults with DS). When matched on mental age, children and adolescents with DS exhibit expressive language skills that lag behind those with other developmental disabilities [Evans, 1977; Marcell et al., 1995] and behind normally developing children and adolescents [Byrne et al., 1995; Chapman, 1997]. On the other hand, studies of DS receptive language (language comprehension) skills often show a split between lexical knowledge and language structural knowledge [Fowler, 1990; Chapman et al., 1991], with lexical knowledge (e.g., vocabulary comprehension) superior to structural (e.g., morphology and syntax comprehension) knowledge. The presence of limited syntactic ability in expressive language as well as in verbal comprehension tasks led Fowler [1990] and others to propose a specific DS language deficit in the structural components of language, i.e., syntax and morphology, across comprehension and production.
However, Chapman et al. [1998] found that the way mental age is assessed affects the interpretation of receptive language performance in children with DS. The inclusion of short-term memory tasks in the assessment of mental age determined whether or not vocabulary comprehension was a relative strength and whether or not syntax comprehension was a relative weakness for the DS subjects. A general expressive language deficit in DS was found regardless of the use of short-term memory tasks for matching mental age. The notion of a general expressive language deficit in English-speaking DS individuals is well supported in the literature [Miller, 1988; Chapman et al., 1998; Capone, 2001]. Similarly, research in other languages suggests that a general expressive language problem in individuals with DS is not limited to English language usage [Rondal and Lambert, 1983; Fabbretti et al., 1997].
Although the specific neurobiological correlates of language deficits in DS are unknown, it has been proposed that cholinergic deficits may contribute to some of the cognitive deficits in DS [Kishnani et al., 1999]. Cholinergic deficits are well documented in patients with Alzheimer disease. Cholinesterase inhibitors (ChEIs) have been shown to benefit some cognitive and functional deficits associated with Alzheimer disease, including language production, word finding, and following commands [Doraiswamy, 1996; Raskind et al., 1997; McLendon and Doraiswamy, 1999]. In an earlier pilot study, we showed that a cholinesterase inhibitor, donepezil, benefited global function in adults with DS [Kishnani et al., 1999]. In the current study, we examined the effects of donepezil on language performance with a specific emphasis on expressive language performance in individuals with DS. Language performance was measured with two objective measures, the Test of Problem Solving [Zachman et al., 1984] and the Clinical Evaluation of Language Fundamentals, Revised [Semel et al., 1986].
MATERIALS AND METHODS
After obtaining institutional review board approval and written informed consent, five males and one female with DS (confirmed by karyotyping) between the ages of 20 and 41 years (mean age, 29 years) were enrolled in the trial (Table I). All subjects were verbal, able to hear speech at conversational level, and able to ingest oral medication. None of the subjects had a clinical diagnosis of dementia (DSM-IV criteria), a recent history of sudden decline in life skills, clinically confirmed pregnancy, or clinically significant systemic disorders, e.g., bradycardia (HR<50), insulin-dependent diabetes mellitus, active peptic ulcer, celiac disease, significant reactive airways, and seizure disorder. All individuals were evaluated for thyroid disorders and for vitamin B12 deficiency within 6 months of entry into the trial. They had not previously participated in a trial using cholinesterase inhibitors or had ingested any other investigational or alternative therapies that are used specifically to treat the symptoms of DS (e.g., mega-vitamins, piracetam, Nutrivene-D, MSB plus) in the 30 days prior to or during the trial. Subject IQs ranged from 40 to 60 (mean IQ, 52). The IQs of four subjects were determined during the baseline visit because testing had not been performed in the last 10 years. The IQs of the two youngest subjects were determined from school records.
TABLE I.
Subject Demographic Data at Study Initiation
| Subject | Age | Gender | IQ | Hearing statusa | Medical diagnoses |
|---|---|---|---|---|---|
| 1 | 37 | Female | 60 | Mild hearing loss, bilaterally | DS |
| 2 | 23 | Male | 60 | Within normal limits, LE; mild-moderate hearing loss, RE | DS |
| 3 | 31 | Male | 53 | Within normal limits, bilaterally | DS, mild ventricular septal defect |
| 4 | 24 | Male | 45 | Mild high-frequency hearing loss, RE; severe hearing loss, LE |
DS, hypothyroidism (treated) |
| 5 | 41 | Male | 53 | Within normal limits, bilaterally | DS |
| 6 | 20 | Male | 40 | Within normal limits, RE; severe hearing loss, LE | DS |
LE, left ear; RE, right ear.
Study Design
This was a 24-week open study at the General Clinical Outpatient Research Unit at Duke University in which subjects attended three sessions, week 0 (baseline), week 12, and week 24. Due to scheduling limitations, the average first treatment visit was at week 13 and the second treatment visit was at week 25. Physical examination and language testing were completed at all sessions. A checklist for medication-related adverse events was performed at week 12 and at study termination. At the completion of the baseline visit, donepezil was dosed orally at 5mg once daily for 6 weeks. The dose was increased to 10 mg (two 5 mg tablets) daily for the remaining weeks if the 5 mg dose was well tolerated. This dosage schedule was based on experience from a previous trial [Kishnani et al., 1999] and the donepezil package insert. Patients were monitored closely for safety and tolerability of the medication by regular phone calls in between scheduled visits. Two language measures were used, the Test of Problem Solving (TOPS) and the Clinical Evaluation of Language Fundamentals-Revised (CELF-R).TOPS was completed at baseline and at weeks 12 and 24 of treatment. CELF-R was completed at baseline and at week 24.
Measures
Test of Problem Solving
TOPS [Zachman et al., 1984] is an expressive language test that measures the ability to verbally identify reasonable solutions to problems presented via pictures. It has been used to assess pragmatic language ability in patients with AD [Ripich et al., 1997]. TOPS consists of 15 pictured scenarios with accompanying questions about each picture. The standard set of questions is designed to have the subject make inferences, determine causes of particular events, determine solutions to pictured problems, and determine strategies for avoiding problems. The instrument was standardized on 1,578 individuals (ages 6 years to 11 years 11 months). Age equivalent scores are provided for ages 3 years 5 months to 15 years 9 months. Validity and reliability data are available. TOPS raw scores for the overall test (as recommended by Powell [1993]) were determined by the average of two independent observers, one scoring onsite and a second scoring from audiotaped recordings of the test sessions.
Clinical Evaluation of Language Fundamentals—Revised
CELF-R [Semel et al., 1986] is a diagnostic language test designed to assess language form and content, aspects of language generally regarded as fundamental to effective oral communication. Standardized expressive, receptive, and overall language scores are derived from the summation of individual subtest scores. Typically, overall expressive and receptive language are evaluated by collapsing the subtest performance into a single receptive or expressive language score. Individual subtest performance is analyzed for specific impairment within the broader expressive and receptive language domains. The instrument was standardized on 2,333 individuals (ages 5 years to 16 years 11 months). Validity and reliability norms are available and adequate for each subtest.
Derivation of language level
Due to the absence of appropriate standardized measures for adults with DS, relative language level was determined by comparing subtest performance (raw scores) to the average performance level for 5-year-olds. The 5-year-old level was selected because it is the lowest age level in which both tests provide normative data.
Clinical significance of change
The clinical significance of the change in language performance was determined by comparing subject performance gain (i.e., treatment vs. baseline performance) with the performance gain expected on each language test as a child increases in age from 5 to 6 years. For TOPS, this index was the difference in scores for children aged 5 years-0 months and children aged 6 years-0 months, whereas for the CELF-R subtests, this index was difference in the average scores for 5-year-olds and 6-year-olds (CELF-R scores are reported only per year of age).
Statistical Analyses
The main comparisons were changes from baseline (week 0) assessed by repeated measures. Because of the preliminary and exploratory nature of the study, we did not correct for multiple comparisons. P values at or below 0.05 (two-tailed) were viewed as significant. Changes on standardized measures were viewed as clinically significant if the magnitude of the observed change was substantial in comparison to the level of performance gain typically achieved by children in the 12-month period between ages 5 and 6. Across all measures, the performance gain between ages 5 and 6 years is the highest rate of language gain recorded for any 12-month period.
RESULTS
Overall, all subjects tolerated donepezil relatively well (Table II). All subjects were increased from the 5 to 10mgdose after 6 weeks. Two of six subjects experienced mild cases of diarrhea at the 5 mg dosage; three of six subjects experienced mild diarrhea at the 10 mg dosage. Each case improved spontaneously. One case of nausea (transient), one case of decreased appetite (transient), one case of cramps (transient), and one episode of hypotension were reported on the 10 mg dosage. All subjects completed the study.
TABLE II.
Summary of Side Effects Related to Donepezil at 5 mg and 10 mg Dosages
| Side effect | 5 mg dose | 10 mg dose |
|---|---|---|
| Diarrhea | 2/6 subjects, transient 1–2 days | 3/6 subjects, transient 1–2 days |
| Nausea | None | 1/6 subjects, transient |
| Decreased appetite | None | 1/6 subjects, transient |
| Cramps | None | 1/6 subjects, 1 day |
| Hypotension | None | 1/6 subjects, one episode |
One subject was excluded from the TOPS analysis because of a missing baseline value and a second subject was excluded from the CELF-R analysis because a different version of the test (CELF-3) was administered inadvertently. At baseline, the subjects scored below the 5-year-old range on most language measures (Table I). They scored within the range on only three of six CELF-R subtests (Sentence Assembly, Oral Directions, and Semantic Relationships).
Following 12 weeks of treatment, the subjects demonstrated significantly improved performance on TOPS (baseline vs. treatment-12 weeks paired samples t=4.5; P=0.0107). No change in TOPS performance was noted after 12 additional weeks of treatment [treatment-12 weeks vs. termination (24 weeks of treatment) paired samples t=0.52; P=0.6313]. The overall TOPS performance gain was 6.5 after 12 weeks and 5.1 after 24 weeks (baseline vs. termination paired samples t=1.10; P=0.0513). In terms of clinical significance, the overall performance gain after treatment was more than one-half of the gain expected by the average 5-year-old in 1 year of development.
Following 24 weeks of donepezil treatment, the subjects showed gains in five of six of the CELF-R subtests (Table III). None of the differences was significantly different from baseline levels. Improvement approached significance (i.e., P=0.15–0.23) in all three expressive subtests and one receptive subtest (Word Classes).
TABLE III.
Comparison of Language Performance at Baseline and at Treatment for Each Language Measure*
| Test | Base line | Treatment | Difference | P-value (two-tailed) | Average score and (range) for 5 year-olds |
Change from 5 to 6 years |
|---|---|---|---|---|---|---|
| TOPS | ||||||
| TOPS, 12 weeks | 14.4 | 20.9 | 6.5 | 0.0107 | 41 (37 to 44) | 8.5 points |
| TOPS, 24 weeks | 14.4 | 19.5 | 5.1 | 0.0513 | 41 (37 to 44) | 8.5 points |
| CELF-R expressive subtestsa | ||||||
| Formulating | 8.2 | 11.2 | 3.0 | 0.2215 | 23–27 (9 to 38) | 13.5 points |
| Sentences | ||||||
| Recalling Sentences | 12.2 | 14.0 | 1.8 | 0.2326 | 43–46 (32 to 58) | 6.5 points |
| Sentence Assembly | 0.4 | 1.0 | 0.6 | 0.2080 | 0 (0 to 3) | 3.5 points |
| CELF-R receptive subtestsa | ||||||
| Oral Directions | 4.3 | 3.3 | −1.0 | 0.6042 | 6–7 (3 to 13) | 5 points |
| Word Classes | 1.2 | 3.2 | 2.0 | 0.1543 | 7–8 (2 to 14) | 13 points |
| Semantic Relationships | 3.0 | 4.0 | 1.0 | 0.5538 | 3 (0 to 9) | 6.5 points |
Baseline and treatment values are group average raw score performance (n=5). The statistical significance of the difference in baseline and treatment performance is determined by a paired t-test. Indexes of clinical significance, i.e., average performance and the range in raw scores for 5-year-olds, and the average improvement in raw score performance as children age from 5 to 6 years old, are provided.
CELF-R subtests administered only at baseline and at 24 weeks.
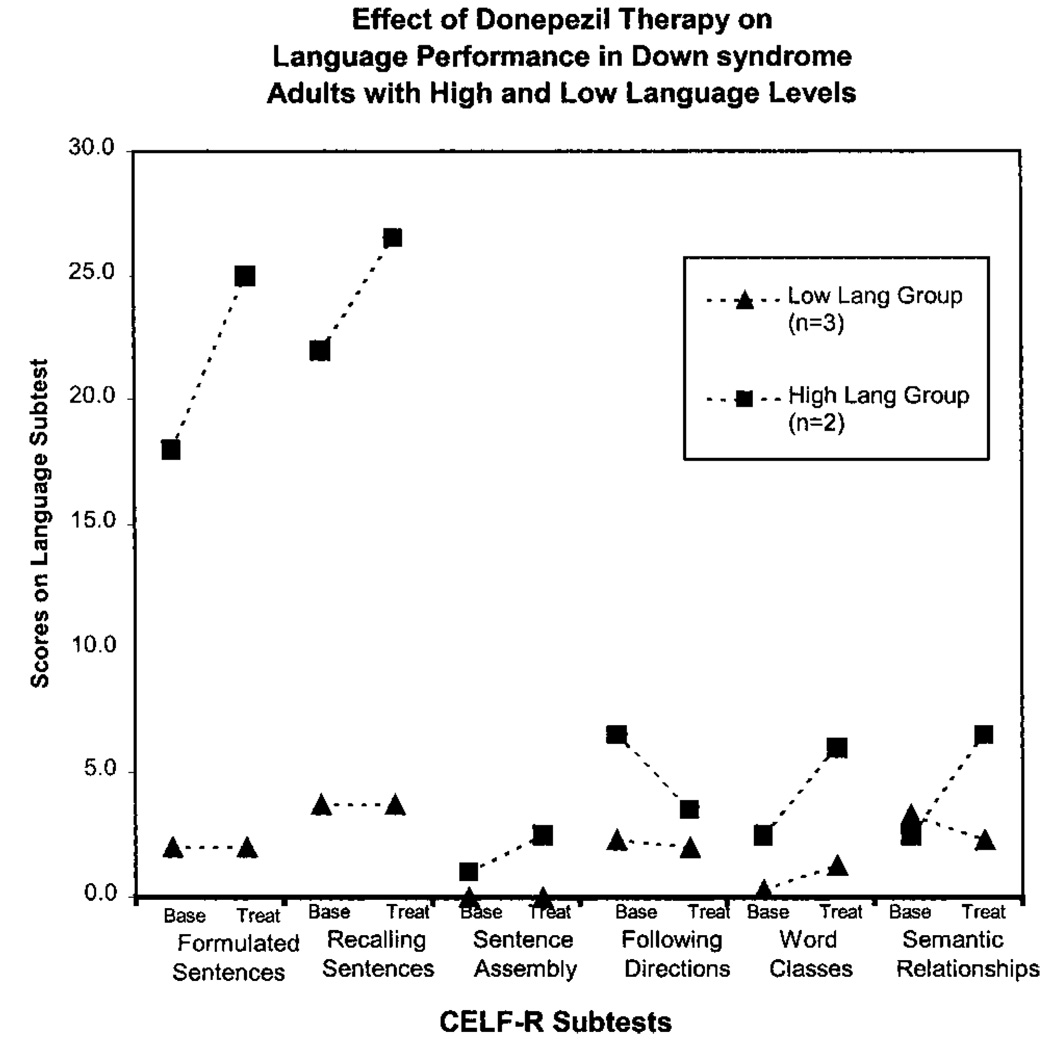
An analysis of individual performance on CELF-R revealed two different language performance patterns (Table IV and Fig. 1). Individuals with higher language skills at baseline (high language group, n=2) tended to show large gains in language performance on the CELF-R subtests following treatment, whereas individuals with lower language skills at baseline (low language group, n=3) showed little gain on the CELF-R language measures. Almost all of the performance gain on the CELF-R subtests reflected in the group data (Table III) can be attributed to two subjects. This was in contrast with the TOPS performance where all subjects showed improvement following treatment.
TABLE IV.
Comparison of High Language (n=2) and Low Language (n=3) Group Performance at Baseline and at 24 Weeks Treatment by Language Measure*
| High language | Low language | Average score | ||||||
|---|---|---|---|---|---|---|---|---|
| and (range) for | Change from | |||||||
| Test | Baseline | Treatment | Difference | Baseline | Treatment | Difference | 5-year-olds | 5 to 6 years |
| CELF-R expressive subtests | ||||||||
| Formulating Sentences | 18.0 | 25.0 | 7.0 | 2.0 | 2.0 | 0.0 | 23–27 (9 to 38) | 13 points |
| Recalling Sentences | 22.0 | 26.5 | 4.5 | 5.7 | 5.7 | 0.0 | 43–46 (32 to 58) | 6.5 points |
| Sentence Assembly | 1.0 | 2.5 | 1.5 | 0.0 | 0.0 | 0.0 | 0 (0 to 3) | 3.5 points |
| CELF-R receptive subtests | ||||||||
| Oral Directions | 6.5 | 3.5 | −3.0 | 2.3 | 3.0 | 0.7 | 6–7 (3 to 13) | 5 points |
| Word Classes | 2.5 | 6.0 | 3.5 | 0.3 | 1.3 | 1.0 | 7–8 (2 to 14) | 13.5 points |
| Semantic Relationships | 2.5 | 6.5 | 4.0 | 3.3 | 2.3 | −1.0 | 3 (0 to 9) | 6.5 points |
Baseline and treatment values are group average raw score performance. Indexes of clinical significance, i.e., average performance and the range in raw scores for 5-year-olds and the average improvement in raw score performance as children age from 5 to 6 years old, are provided.
Fig. 1.
High language (high lang) and low language (low lang) group performance by CELF-R subtest at baseline (base) and at study termination (treat).
DISCUSSION
To our knowledge, this is the first prospective study to evaluate systematically the effects of donepezil on specific language domains in DS over 24 weeks. Because of limitations such as an extremely small sample size, lack of power for formal statistical control, repeated comparisons across a relatively short time span (12–24 weeks), and lack of an untreated control group, our findings should be viewed as preliminary and interpreted appropriately. The primary finding that emerged was an improvement in the expressive language performance (specifically, TOPS) of adults with DS following donepezil therapy. The magnitude of the gain was more than one-half of the typical 1-year improvement in developmental language function experienced by 5-year-olds, a period of rapid language gain.
CELF-R provided comparable results. Of the four subtests yielding treatment effects that approached significance (P=0.15–0.23), three were expressive subtests and only one, Word Classes, was a receptive language measure. It can be argued that the Word Classes subtest may not be a pure measure of receptive language, because it requires a verbal response. Word Classes was the only CELF-R subtest in which both high language and low language groups showed improved performance with treatment.
The discrepancy in treatment effect between the high language and low language groups on all other CELF-R subtests is important to consider in light of reports [Fowler, 1988; Miller, 1988] of differential language performance abilities in children with DS. Miller [1988] identified two patterns of language development in children with DS, a flat profile where cognition, language production, and language comprehension are equivalent, and a profile of specific delay in language production. He suggested that these developmental differences were caused by neurologic differences in the two groups of children. Similarly, Fowler [1988] suggested that IQ plays a substantial role in determining the prognosis of language learning in children with DS. She concluded that an IQ level of greater than 50 is critical to ensuring substantial growth when maturational factors permit it. In the present study, language performance at baseline and the treatment effect appeared to be related to IQ. The IQs of the high language group were both 60, whereas the IQs of the low language group ranged from 40 to 53.
In addition to the methodological limitations noted above, it is important to note that one of the criterion measures, TOPS, has been unfavorably reviewed for its psychometric properties [Bernhardt, 1990; Skarakis-Doyle and Mallet, 1991]. It should be noted that for this study, high interrater agreement (0.96) was obtained from two independent examiners (one obtained from live scoring and the second from scoring via audiotape).
The selection of a language battery capable of sampling important language functions in the adult DS population is problematic. Many measures designed to assess language development at the 5- to 6-year-old level lack content relevant to adults with DS. For example, the Elementary TOPS, Revised [Zachman et al., 1994] has replaced TOPS. However, we have found that our DS subjects misinterpret many of the TOPS-R pictured scenarios, making the language probe irrelevant. Additional development of language measures will be important to isolate pharmacologic effects on language function in the DS population and in other groups with specific language impairments.
Our findings also support our earlier report [Kishnani et al., 2001] that donepezil is relatively well tolerated by DS adults. This finding does contrast with one case study that reported urinary incontinence associated with donepezil in DS patients [Hemingway-Eltomey and Lerner, 1999]. Clearly, larger studies are needed to address this issue more conclusively.
Despite these methodological limitations, the results of this study raise important questions regarding the role of the cholinergic system in language function and the specific effect of cholinergic therapy in the treatment of language impairment in DS. For individuals with DS, improvements in language usage of the magnitude found in this study can lead to important functional gains in activities of daily living. An improvement in the ability to express ideas and verbally interact with others can lead to better reading and writing skills, improved social relationships, improved mood and emotional stability, increased independence, and improved opportunities for employment. Large-scale controlled studies of the effects of donepezil treatment on language and on other cognitive domains in DS are required to address questions regarding the role of the cholinergic system in language function and the specific effect of cholinergic therapy in the treatment of language impairment in DS.
ACKNOWLEDGMENTS
The research was completed at the Duke General Clinical Research Center. We thank its entire staff for their support, including Beth Hauser for her assistance in the statistical analysis and Karen MacKethan for her assistance in the language analysis.
Grant sponsor: National Center for Research Resources; Grant number: M01-RR30; Grant sponsor: National Center for Research Resources; Grant number: 5K23-RR16060 (to P.S.K.).
REFERENCES
- Bernhardt B. A test of the test of problem solving. Language Speech Hearing Services Schools. 1990;21:98–101. [Google Scholar]
- Byrne A, Buckley S, Macdonald J, Bird G. Investigating the literacy, language and memory skills of children with Down’s syndrome. Down’s Syndrome Res Pract. 1995;3:53–58. [Google Scholar]
- Capone GT. Down syndrome: advances in molecular biology and neurosciences. Dev Behav Pediatr. 2001;22:40–58. doi: 10.1097/00004703-200102000-00007. [DOI] [PubMed] [Google Scholar]
- Chapman RS. Language development in children and adolescents with Down syndrome. In: Fletcher P, MacWhinney B, editors. Handbook of child language. Oxford: Blackwell; 1995. pp. 641–663. [Google Scholar]
- Chapman RS. Language development in children and adolescents with Down syndrome. Mental Retard Dev Dis Res Rev. 1997;3:307–312. [Google Scholar]
- Chapman R, Schwartz SE, Kay-Raining Bird E. Language skills of children and adolescents with Down syndrome: I, comprehension. J Speech Hear Res. 1991;34:1106–1120. doi: 10.1044/jshr.3405.1106. [DOI] [PubMed] [Google Scholar]
- Chapman R, Seung HK, Schwartz SE, Kay-Raining Bird E. Language skills of children and adolescents with Down syndrome: II, production deficits. J Speech Lang Hear Res. 1998;41:861–873. doi: 10.1044/jslhr.4104.861. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM. Current cholinergic therapy for symptoms of Alzheimer’s disease. Prim Psychiatry. 1996;3:3–11. [Google Scholar]
- Evans D. The development of language abilities in mongols: a correlational study. J Mental Defic Res. 1977;23:103–117. doi: 10.1111/j.1365-2788.1977.tb00030.x. [DOI] [PubMed] [Google Scholar]
- Fabbretti D, Pizzuto E, Vicari S, Volterra V. A story description task in children with Down’s syndrome: lexical and morphosyntactic abilities. J Int Dis Res. 1997;41:165–179. doi: 10.1111/j.1365-2788.1997.tb00693.x. [DOI] [PubMed] [Google Scholar]
- Fowler AE. Determinants or rate of language growth in children with DS. In: Nadel L, editor. The psychobiology of Down syndrome. Cambridge, MA: MIT Press; 1988. pp. 217–245. [Google Scholar]
- Fowler AE. Language abilities in children with Down syndrome: evidence for a specific syntactic delay. In: Cicchetti D, Beeghly M, editors. Children with Down syndrome: a developmental perspective. New York: Cambridge University Press; 1990. pp. 302–328. [Google Scholar]
- Hemingway-Eltomey JM, Lerner AJ. Adverse effects of donepezil in treating Alzheimer’s disease associated with Down’s syndrome. Am J Psychiatry. 1999;156:1470. doi: 10.1176/ajp.156.9.1470. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Sullivan JA, Walter BK, Spiridigliozzi GA, Doraiswamy PM, Krishnan KRR. Cholinergic therapy for Down’s syndrome. Lancet. 1999;353:1064–1065. doi: 10.1016/S0140-6736(98)05285-4. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Spiridigliozzi GA, Heller JH, Sullivan J, Doraiswamy PM, Krishnan KRR. Donepezil for Down syndrome. Am J Psychiatry. 2001;158:143. doi: 10.1176/appi.ajp.158.1.143. [DOI] [PubMed] [Google Scholar]
- Marcell MM, Ridgeway D, Sewell DH, Whelan ML. Sentence imitation by adolescents and young adults with Down’s syndrome and other intellectual disabilities. J Int Dis Res. 1995;39:215–232. doi: 10.1111/j.1365-2788.1995.tb00504.x. [DOI] [PubMed] [Google Scholar]
- McLendon B, Doraiswamy PM. Defining meaningful change in Alzheimer’s disease trials: the donepezil experience. J Geriatr Psychiatry Neurol. 1999;12:39–48. doi: 10.1177/089198879901200108. [DOI] [PubMed] [Google Scholar]
- Miller JF. The developmental asynchrony of language development in children with Down syndrome. In: Nadel L, editor. The psychobiology of Down syndrome Cambridge. MIT Press; 1988. pp. 167–198. [Google Scholar]
- Powell TW. Factorial validity of the test of problem solving. Percept Motor Skills. 1993;76:753–754. [Google Scholar]
- Raskind MA, Sadowsky CH, Sigmund WR, Beitler PJ, Auster SB. Effect of tacrine on language, praxis and noncognitive behavioral problems in Alzheimer disease. Arch Neurol. 1997;54:836–840. doi: 10.1001/archneur.1997.00550190026010. [DOI] [PubMed] [Google Scholar]
- Ripich DN, Carpenter B, Ziol E. Comparison of African-American and white persons with Alzheimer’s disease on language measures. Neurology. 1997;48:781–783. doi: 10.1212/wnl.48.3.781. [DOI] [PubMed] [Google Scholar]
- Rondal JA, Comblain A. Language in adults with Down syndrome. Down Syndrome Res Pract. 1996;4:3–14. [Google Scholar]
- Rondal JA, Lambert JL. The speech of mentally retarded adults in a dyadic communication situation: some formal and informative aspects. Psychol Belgica. 1983;23:49–56. [Google Scholar]
- Semel E, Wiig E, Secord W. Clinical evaluation of language fundamentals, revised. San Antonio, TX: Psychological Corporation; 1986. [Google Scholar]
- Skarakis-Doyle E, Mallet CA. Test-retest reliability: another evaluation of the test of problem solving. Language Speech Hearing Services Schools. 1991;22:278–279. [Google Scholar]
- Zachman L, Jorgensen C, Huisingh R, Barrett M. Test of problem solving. Moline, IL: Linguisystems; 1984. [Google Scholar]
- Zachman L, Huisingh R, Barrett M, Orman J, LoGiudice C. Elementary test of problem solving, revised. Moline, IL: Linguisystems; 1994. [Google Scholar]