Abstract
The sodium-dependent multivitamin transporter (SMVT) is a major biotin transporter in a variety of tissues including the small intestine. The human SMVT (hSMVT) polypeptide is predicted to have four N-glycosylation sites and two putative PKC phosphorylation sites but their role in the function and regulation of the protein are not known and were examined in this investigation. Our results showed that the hSMVT protein is glycosylated and that this glycosylation is important for its function. Studies utilizing site-directed mutagenesis revealed that the N-glycosylation sites at position Asn138 and Asn489 are important for the function of hSMVT and that mutating these sites significantly reduces the Vmax of the biotin uptake process. Mutating the putative PKC phosphorylation sites Thr286 of hSMVT led to a significant decrease in the PMA-induced inhibition in biotin uptake. The latter effect was not mediated via changes in the level of expression of the hSMVT protein and mRNA or in its level of expression at the cell membrane. These findings demonstrate that the hSMVT protein is glycosylated, and that glycosylation is important for its function. Furthermore, the study shows a role for the putative PKC-phosphorylation site Thr286 of hSMVT in the PKC - mediated regulation of biotin uptake.
Keywords: Biotin, hSMVT, glycosylation, PKC, transport, regulation
1. Introduction
Biotin, a water-soluble vitamin, is required for normal cellular functions, growth and development. It acts as coenzyme for five carboxylases involved in a variety of intermediate metabolic reactions that include fatty acid synthesis, gluconeogenesis, and catabolism of branched chains amino acids [1]. The importance of biotin as an essential micronutrient is underscored by the range of abnormalities that develop as result of its deficiency which include growth retardation, and neurological and dermatological disorder [2]. The incidence of biotin deficiency and sub-optimal levels are common in both developed and developing countries and occurs due to a variety of factors and conditions.
Humans and other mammals cannot synthesize biotin and must obtain the micronutrient from exogenous sources via intestinal absorption. Two sources of biotin are available to the human intestine; a dietary source, which is absorbed in the small intestine, and a bacterial source where the vitamin is synthesized by the normal microflora of the large intestine and is absorbed in that region of the gut [3–7]. Absorption of both sources of biotin occurs by a saturable, Na+- dependent, carrier-mediated mechanism that involves the sodium-dependent multivitamin transporter (SMVT) [8, 9]. The human SMVT (hSMVT) is the product of the SLC5A6 gene, which is located on chromosome 2. Molecular identity of SMVT system has been delineated as a result of cloning from a number of species (human, rabbit, mice, rat) [10–12]. Hydrophobicity analysis predicts the hSMVT protein to have 12 trans-membrane domains (TMD), and that both the N- and C- termini are oriented toward cell interior [11]. The hSMVT protein has also been predicted to have four potential N-glycosylation sites (at Asn138, Asn489, Asn498 and Asn534) and two potential protein kinase C (PKC) phosphorylation sites (Ser283 and Thr286) [11, 13]. Functional, immunological and confocal imaging studies from our laboratory and others have shown that the hSMVT protein is exclusively expressed at the apical membrane domain of polarized intestinal epithelial cells [8, 9, 14]. Other studies, utilizing gene-specific siRNA approach, have shown that the hSMVT system is the main biotin uptake system in human intestinal epithelial cells [15]. Furthermore, modulators of the PKC - mediated regulatory pathway were found to exert a significant effect on intestinal biotin uptake via changes in the number/activity of the biotin uptake carriers with no effect on their affinity [16, 17].
There is little known about the structure-function and structure-regulatory relationships of the hSMVT system. We have begun to address these issues and have recently showed the importance of the conserved positively charged His115 and His254 residues in the function of hSMVT [18]. We have also showed that mutating these histidine residues results in changes in the membrane expression of hSMVT [18]. More work is needed to fully understanding the structure-function and structure- regulatory relationships of the hSMVT system.
As mentioned earlier, the hSMVT polypeptide is predicted to have four potential N-glycosylation sites but it is not known if these sites are actually glycosylated, and what effect such glycosylation has on the physiology of hSMVT. Also not known is whether any of the two conserved putative PKC phosphorylation sites of hSMVT are involved in mediating the PKC regulatory effect on biotin uptake observed in our previous studies [16, 17]. Our aim in this study was to address these issues.
2. Materials and Methods
2.1. Material
[3H] Biotin (Specific activity > 30 Ci/mmol, radiochemical purity > 98%) was obtained from American Radiolabeled Chemicals (ARC, St. Louis, MO). All the other chemicals used in the study were commercially obtained from the vendors and are of analytical/molecular biology grade.
2.2. Cell Culture and transfection
Human retinal pigmented epithelial cells (ARPE19; which are highly efficient in expressing hSMVT; [13]) and human intestinal epithelial cell (Caco-2) were used for uptake and confocal imaging studies. These cells were obtained from ATCC (Manassas, VA), and were cultured in DMEM and MEM medium (Sigma, St. Louise, MO), respectively, at 37°C in a 5% CO2-95% air atmosphere. For optimized growth, the culture medium was supplemented with 10% fetal bovine serum and penicillin (100 units/ml) as well as streptomycin (100 μg/ml). Transient transfection was performed using Lipofectamine 2000 (Invitrogen) following manufacturer’s protocol.
2.3. Transport measurement
Radio-labeled biotin uptake was determined at 37°C in Krebs-Ringer (KR) buffer for initial linear period of 10 min as previously described [17, 18]. Protein concentrations of cell digest were measured in parallel using Dc protein assay kit (Bio-Rad, Hercules, CA). Total biotin uptake mediated by endogenous transporter was determined using ARPE19 cell transfected with pcDNA 3.1(−) vector and carrier-mediated biotin uptake was determined by subtracting the passive diffusion component from total uptake.
2.4. Site-directed mutagenesis
For introducing mutations we used full-length hSMVT constructs cloned either in pcDNA 3.1(−)[18](Invitrogen) or GFP-N3 expression vector (clontech, Mountain View, CA) [14]. Quick Change II Site-directed mutagenesis kit from Stratagen (La Jolla, CA) was used for mutating individual residues using specific mutant primers (Table 1). Briefly, either hSMVT-pcDNA 3.1(−) or hSMVT-GFP constructs were used as template and PCR was performed using specific mutant primers following manufacturer’s instruction. The mutatedclone was identified by sequencing from the isolated plasmid DNA (Laragen, CA).
Table 1.
Combination of primers used for PCR.
| Forward and Reverse Primers (5′3′) | ||
|---|---|---|
| hSMVT-RT | F | TGTCTACCTTCTCCATCATGGA; |
| R | TAGAGCCCAATGGCAAGAGA | |
| β-actin | F | AAATGGGTTCTAGACCGCGGAGA; |
| R | CATGCTCGATGCGGTACTTCA | |
| N138A | F | CTGGAGCTTCGATTCGCTAAAACTGTGCGAGTG; |
| R | CACTCGCACAGTTTTAGCGAATCGAAGCTCCAG | |
| N489A | F | CCACCCTCTCCCTCTGCTGGGTCCAGCTTCTCC; |
| R | GGAGAAGCTGGACCCAGCAGAGGGAGAGGGTGG | |
| N498A | F | TTCTCCCTGCCCACCGCTCTAACCGTTGCCACT ; |
| R | AGTGGCAACGGTTAGAGCGGTGGGCAGGGAGAA | |
| N534A | F | TGGTACAGTGCTCACGCCTCCACCACAGTGATT; |
| R | AATCACTGTGGTGGAGGCGTGAGCACTGTACCA | |
| S283A | F | GTGCAGCGGTACCTCGCTTCCCGCACGGAGAAG; |
| R | CTTCTCCGTGCGGGAAGCGAGGTACCGCTGCAC | |
| T286A | F | TACCTCAGTTCCCGCGCGGAGAAGGCTGCTGTG; |
| R | CACAGCAGCCTTCTCCGCGCGGGAACTGAGGTA | |
“hSMVT-RT” and β-actin denote the primer sequences used for quantitative real-time RT-PCR. The changed nucleotides to mutate hSMVT are shown in bold in respective primer sequence.
2.5. Real-time PCR analysis
Total RNA was isolated after 48 h post transfection using TRizol reagent (Invitrogen) as mentioned in manufacturer’s protocol. To remove the residual DNA contamination RNA samples were treated with DNAse I (Invitrogen) and cDNA was prepared using iScript cDNA synthesis kit (Bio-Rad). The level of hSMVT expression was quantified in CFX 96 real-time PCR system (Bio-Rad) using specific primer [19] and corresponding β-actin expression was used for normalization. The relative expression of hSMVT was quantified by comparative CT method (2 −ΔΔCT method) where ΔCT value was calculated by subtracting CT value of β-actin from hSMVT [20].
2.6. Total protein isolation and Western blotting
To measure the total expression of hSMVT protein, ARPE19 cells (48 h post transfection) were lysed with RIPA buffer (Sigma) supplemented with protease inhibitor cocktail (Roche, NJ). Equal amount of protein (30 μg) was electrophoresed in a NuPAGE 4–12% Bis-Tris gradient minigels (Invitrogen) and electro blotted onto a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ) for Western blot analysis as described before [18]. To detect specific protein, the blot was probed for 90 min with specific antibody [anti-human polyclonal SMVT antibodies (Santa Cruz biotechnology Inc., Santa Cruz, CA) or anti-GFP monoclonal antibody (clontech)] followed by corresponding secondary antibody conjugated with horseradish peroxidase for 1 h at room temperature and the bands were developed by an enhanced chemiluminescence (ECL) kit (Amersham Biosciences). To ensure the equal loading, the same blot was striped with re-striping solution (Chemicon, CA) and probed with anti-human β-actin polyclonal antibodies (Santa Cruz biotechnology Inc.). Intensity level of each band was determined and normalized with human β-actin by UN-SCAN-IT gel software (version 6.1).
2.7. Cell surface biotinylation assay
Total surface protein of transiently transfected cells was biotinylated using EZ-Link Sulfo-NHS-Biotinylation Kit (Thermo Fisher Scientific Inc., Rockford, IL) following the protocol described by the manufacturer. Briefly, after biotinylation the cells were lysed, and the lysate was incubated with avidine-conjugated agarose. Followed by washing the biotinylated protein bound to agarose - avidine bead was eluted in NuPAGE LDS sample loading dye and subjected to Western blot analysis in a NuPAGE 4–12% Bis-Tris gradient minigels (Invitrogen). For normalization, total cell lysate was loaded simultaneously and the level of surface expression of hSMVT was determined using specific antibody followed by HRP tagged secondary antibody. The band intensity of biotinylated hSMVT was normalized with total cellular hSMVT expression.
2.8. Confocal imaging
The live cell imaging was performed by an inverted phase-contrast confocal microscope (Nikon-C1) by growing Caco-2 cells on glass bottomed petri-dishes (MatTek Corp., Ashland, MA). The cells were excited using the 488 nm wavelength from an argon ion-laser, and emission was measured at 530 ±20-nm band-pass filter.
2.9. Statistical Analysis
The uptake data shown here are the mean ± SE of at least three independent experiments and are expressed in terms of fmoles per milligram protein per 10 min. The significance level was determined by the Student’s t-test, and p value of < 0.05 was considered as being significant. Carrier-mediated uptake of 3H-biotin was determined by subtracting the diffusion component (uptake in the presence of 1 mM biotin) from total uptake. For the kinetic study, the diffusion component was determined by multiplying biotin concentration by the slope of the line between uptake at high biotin concentration (1 mM) and the point of origin. The apparent Km and Vmax were determined by applying the Michaelis - Menten equation via using Graph Pad Prism software (Version 5.03). Western blotting, confocal imaging and PCR analysis were all performed on three separate occasions.
3. Results
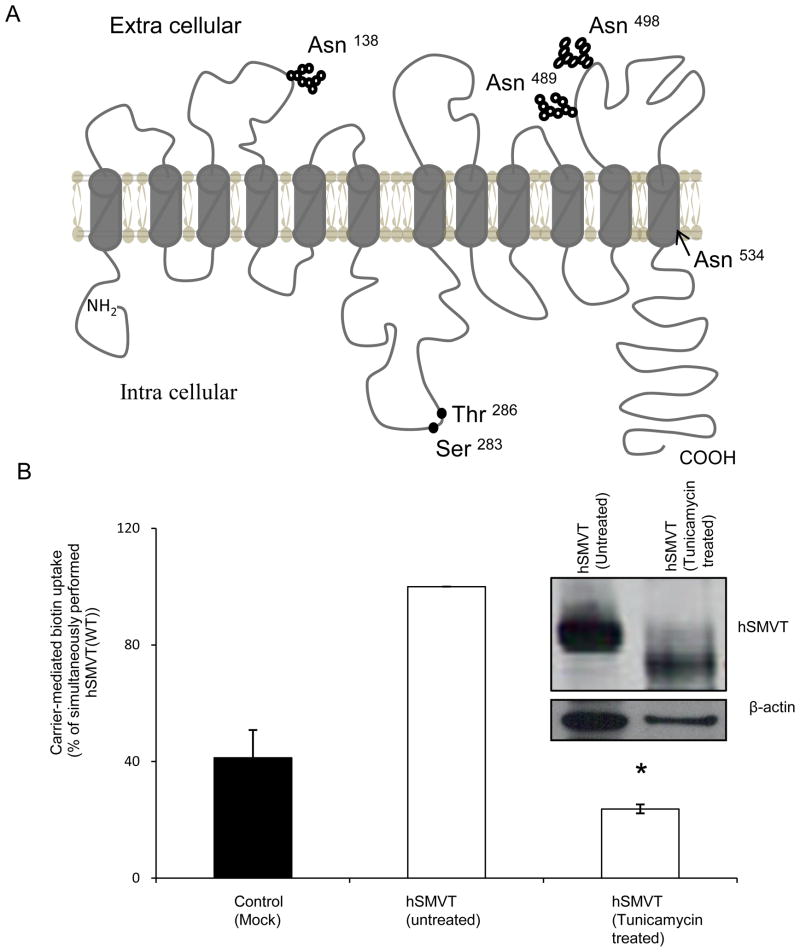
As mentioned earlier, hydrophobicity analysis predicts the hSMVT polypeptide to have 12 TMD (Fig.1A)[11, 13]. In addition, analysis of the amino acid sequence of hSMVT suggests that the polypeptide has four potential N-glycosylation sites (at position Asn138, Asn489, Asn498, and Asn534) and two potential PKC- phosphorylation sites (at position Ser283 and Thr286) (Fig. 1A)[11, 13]. The potential N-glycosylation at Asn138 is unique to hSMVT, while the other three potential N-glycosylation sites are conserved across species (human, mouse, rat and rabbit). It is also predicted that the potential N-glycosylation site at Asn 138 is located in the extracellular domain between the third and fourth TMD of the hSMVT polypeptide, whereas Asn489 and Asn498 are both located in the extracellular sequence between the eleventh and twelfth TMD. The other predicted N-glycosylation site, Asn 534, falls within the twelfth TMD (Fig.1A). With regards to the two potential PKC - phosphorylation sites, both of these sites are predicted to be located in the intracellular loop between the sixth and seventh TMD of the hSMVT polypeptide (Fig. 1A). In addition, while the Ser283 is conserved across species, Thr286 is conserved in human and rabbit but replaced by Ser in mouse and rat.
Fig. 1. hSMVT protein is glycosylated, and glycosylation is important for function.
A: Predicted membrane topology of hSMVT. Theoretical prediction suggests the hSMVT protein to have 12 TMD [11, 13]. The protein is also predicted to have four putative N-glycosylation motifs (Asn138, Asn489 Asn498 and Asn 534) and two potential PKC-phosphorylation sites (Ser283 and Thr286). B: Effect of tunicamycin on carrier-mediated biotin uptake by ARPE19 cells transiently expressing hSMVT. Following pre-treatment with tunicamycin (2 μg/ml for 48 h) biotin uptake was assayed in KR buffer (pH 7.4). Control cells were transfected with empty pcDNA3.1(−) vector. Data are mean ± SE of three independent experiments. * p < 0.01. inset: Western blot showing a shift in the molecular mass of hSMVT-GFP upon tunicamycin pre-treatment. Transiently expressing ARPE19 cells were pre-treated with tunicamycin (2 μg/ml for 48 h) and total protein was isolated as described in “Methods”. Equal amount of protein (30 μg) was resolved in NuPAGE 4–12% Bis-Tris gradient mini gels and subjected to Western blot analysis using anti-GFP monoclonal antibody. As a control, the same blot was probed with anti- β-actin antibody. The image shown is a representative of three independent experiments.
3.1 Glycosylation status of the hSMVT protein and role in hSMVT function
3.1.1. The hSMVT protein is glycosylated
Previous immunological studies have demonstrated that the hSMVT polypeptide has a higher molecular mass than what is predicted from its molecular mass [14, 18, 21]. This suggests that the protein is subjected to a post-translational modification(s). In this study, we aimed at testing whether the hSMVT protein is glycosylated, and if so, to determine the effect of this glycosylation on its function. Thus, we pre-treated ARPE19 cells that are transiently expressing hSMVT-GFP with the glycosylation inhibitor tunicamycin (2 μg/ml for 48 h) then performed Western blot analysis on cellular protein (ARPE19 cells were chosen in these studies because they express hSMVT efficiently and previously used for such investigations;[13, 18]). The result showed a clear shift in the molecular size of the specific hSMVT band in cells treated with tunicamycin were compared to control (untreated) cells (Fig. 1B inset). A similar shift in the molecular size of hSMVT was observed on the Western blot when the total protein isolated from the hSMVT-GFP expressing cells was treated with N-glycosidase F (PNGaseF) (data not shown). These results clearly suggest that the hSMVT polypeptide is glycosylated.
To examine the effect of this glycosylation on the function of hSMVT, we examined the effect of tunicamycin pre-treatment (2 μg/ml for 48 h) on biotin uptake by hSMVT expressing ARPE19 cells. The results showed a significant (p < 0.01) reduction in carrier-mediated biotin (9 nM) uptake in tunicamycin pre-treated cells compared to control (untreated) cells (Fig. 1B).
3.1.2. Effect of mutating the individual potential N-glycosylation sites on hSMVT function
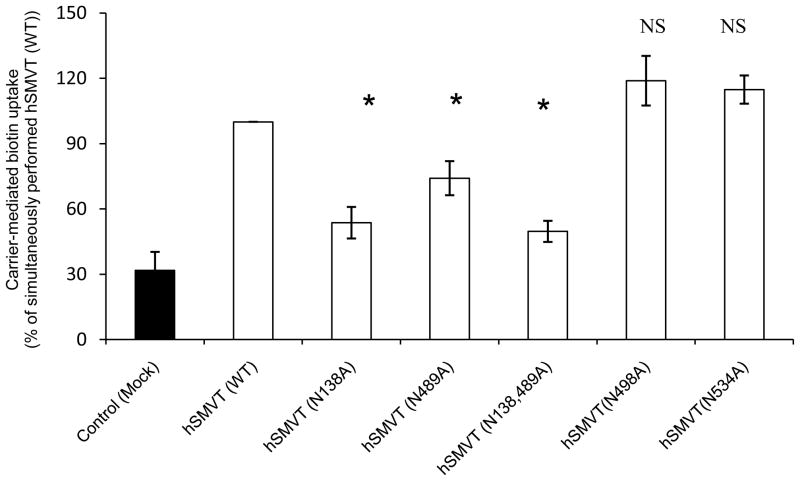
To determine which of the four potential N-glycosylation sites of the hSMVT polypeptide plays a role in the function of the protein in transporting biotin, we mutated these sites individually (to alanine) and examined the effect that mutation on functionality of hSMVT following transient expression in ARPE19 cells. The results showed that while mutating Asn 138 and Asn 489 lead to a significant (p < 0.01 for both) decrease in carrier-mediated biotin uptake, mutating Asn 498 and Asn 534 resulted in no effect (Fig. 2). We also introduced double mutations (at Asn138 and Asn489) in the hSMVT protein and observed even further reduction in biotin uptake.
Fig. 2. Effect of mutating potential N-glycosylation sites on hSMVT functionality.
Carrier-mediated biotin uptake by cells expressing wild - type and mutated hSMVT. ARPE19 cells were transiently transfected with 3 μg of wild-type or mutated hSMVT and biotin uptake was assayed 48 h post transfection. Control (mock) ARPE19 cells were transiently transfected with empty pcDNA 3.1 (−) vector. Data are mean ± SE of at least three independent experiments. * p < 0.01.
3.1.3. Effect of mutating Asn138 and Asn489 of the hSMVT protein on kinetic parameters of biotin uptake
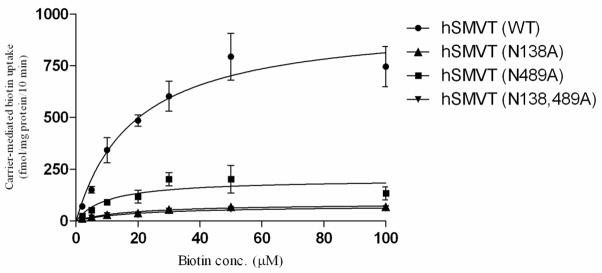
The effect of mutating Asn138 or Asn489 of hSMVT individually or together on kinetic parameters (apparent Km and Vmax) of the biotin uptake process of ARPE19 cells expressing these mutants was examined and the data was compared to uptake kinetic parameter of cells expressing the wild-type hSMVT. The results showed a significant (p < 0.01 for all) reduction in the Vmax of the biotin uptake process by all mutants with no effect on the apparent Km (Fig. 3 and Table 2). The decrease in Vmax, but not apparent Km of the biotin uptake process suggests that the observed loss in hSMVT functionality is mediated via a decrease in the number (and/or activity) of the functional carriers with no change in their affinity.
Fig. 3. Kinetic parameters of carrier-mediated biotin uptake by ARPE19 cells transiently expressing the wild-type and mutated hSMVT.
ARPE19 cells were transfected with equal amount (3 μg) of plasmids and uptake was measured 48h of transfection. Data are mean ± SE of three independent experiments; when not shown, the SE is smaller than the symbol.
Table 2.
Kinetic parameters of the biotin uptake process in ARPE19 cells expressing wild-type and mutated hSMVT.
| Km (μM) | Vmax (fmol/mg protein/10 min) | |
|---|---|---|
| hSMVT | 18.91 ± 3.7 | 967.6 ± 68.69 |
| hSMVT (N138A) | 17.61 ± 2.56 | 84.53 ± 4.29 (p<0.01)* |
| hSMVT (N489A) | 10.26 ± 4.76 | 202 ± 27.2 (p<0.01)* |
| hSMVT (N138A, N489A) | 20.13 ± 3.57 | 74.63 ± 4.85 (p<0.01)* |
Kinetic parameters of the carrier-mediated biotin uptake by ARPE19 cells expressing the wild-type hSMVT and the mutants.
p values were determined by Student-t test.
3.1.4. Effect of Asn138 and Asn489 mutations on level of hSMVT mRNA and protein expression
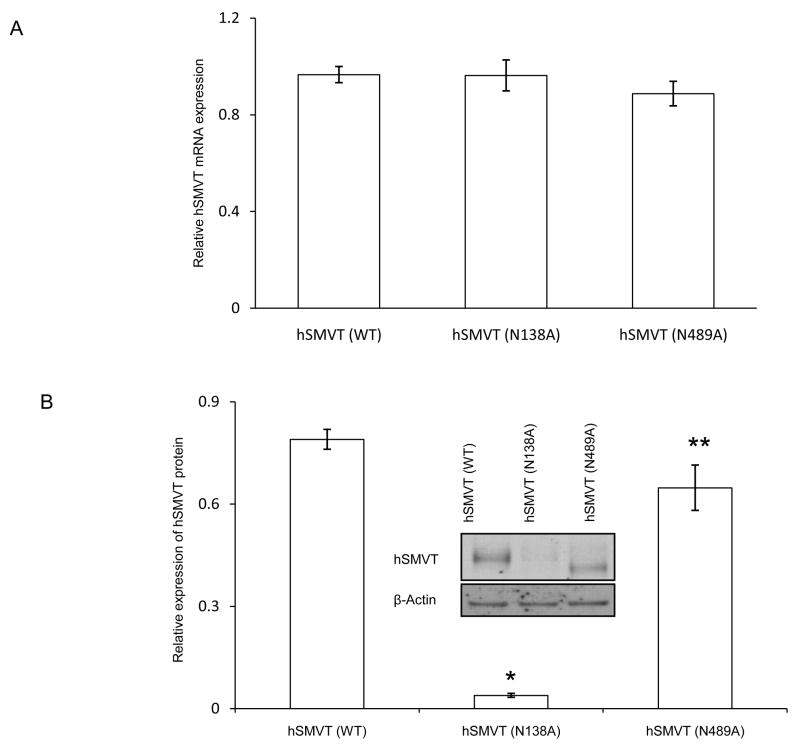
To determine whether mutating the Asn138 and Asn489 sites of hSMVT affected the transport via affecting the mRNA expression, we used quantitative PCR to determine the mRNA level of the different hSMVT mutants as well as that of the wild - type protein after simultaneous transfection of equal amounts (3 μg) of cDNA into ARPE19 cells. Data was normalized relative to β-actin. The results showed no significant difference in the level of mRNA expression between the two mutants to that of wild-type hSMVT (Fig 4A). We also examined the effect of mutating Asn138 or Asn489 residues on the level of hSMVT protein expression. In this study, we again transfected ARPE19 cells with equal amounts of wild-type or mutated hSMVT-GFP constructs (3 μg), then we extracted total protein and subjected the extract to Western blot analysis using monoclonal anti-GFP antibody; level of β-actin was also determined as internal control (Fig. 4B, inset). The results showed that mutating Asn138 leads to an approximately 20 fold reduction in the level of expression of the hSMVT protein (p < 0.01), while mutating Asn 489 lead to a reduction of 1.2 fold (p < 0.05) compared to wild-type hSMVT (Fig. 4B inset).
Fig. 4. Effect of mutating Asn138 and Asn489 of the hSMVT protein on level of expression.
A: Effect on mRNA expression: Real - time PCR was performed using total RNA isolated from ARPE19 cells transiently expressing wild-type or mutated hSMVT. Level of expression of hSMVT was normalized relative to β-actin as described in “Methods”. Data are mean ± SE of three independent experiments. B: Effect on protein expression: ARPE19 cells were transiently transfected with wild-type or mutated hSMVT-GFP constructs and total protein was isolated 48 h post transfection and used for Western blotting. The blot was probed with anti-GFP antibody and expression was normalized relative to β-actin. The graphical representation shows the relative expression of the mutated hSMVT after normalization. * p < 0.01 and ** p < 0.05. The inset shows a representative Western blot image.
3.2. Role of the potential PKC - phosphorylation sites of the hSMVT protein in mediating the PMA-induced inhibition in biotin uptake
Previous studies have suggested the involvement of a PKC - mediated pathway in the regulation of intestinal biotin uptake [16, 17]. Stimulating PKC with phorbol 12-myristate 13-acetate (PMA) was found to lead to significant inhibition in biotin uptake, an effect that was mediated via changes in the Vmax (but not the apparent Km) of the biotin uptake process [17]. The latter observation suggested that the PMA (PKC) effect is mediated via changes in the activity (and/or number) of the biotin transport carriers (i.e., hSMVT) with no change in their affinity. The cellular and molecular mechanism(s) involved in this regulation, however, was not investigated and was therefore addressed in this study.
3.2.1. Effect of PMA pretreatment on level of expression of hSMVT mRNA and protein, and on membrane expression
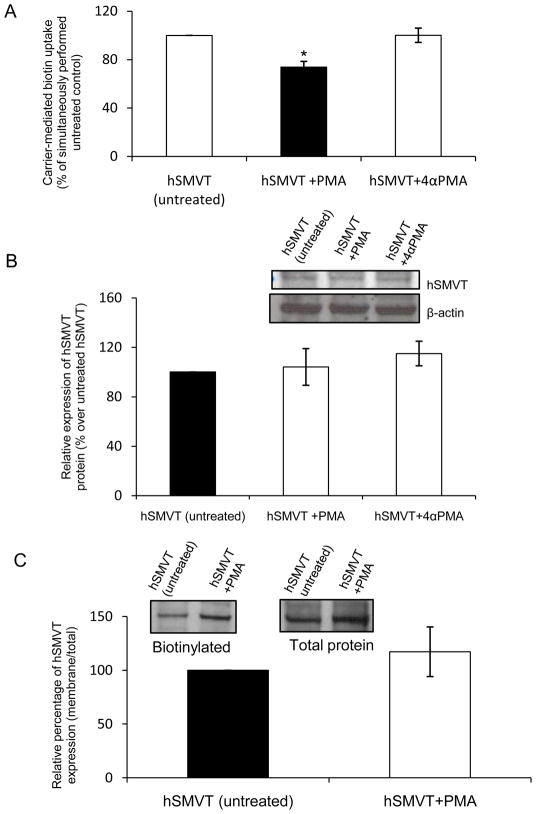
In these studies, we first confirmed that PMA (but not its negative control 4α PMA) pre-treatment (10 μM for 1h) inhibits biotin (9 nM) uptake by ARPE19 cells expressing hSMVT (Fig. 5A). We then asked if this inhibition is due to reduction in the level of expression of the hSMVT mRNA and/or protein using quantitative PCR and Western blot analysis, respectively. Quantative PCR data showed that PMA pre-treatment does not show any effect on the level of hSMVT mRNA expression (Data not shown). Western blotting using total protein also showed no change in hSMVT expression on PMA pre-treatment (Fig. 5B).
Fig. 5. Effect of pre-treatment with phorbol 12-myristate 13-acetate (PMA) on hSMVT functionality and expression.
A: Effect on biotin uptake: ARPE19 cells transiently expressing hSMVT were pre-treated with 10 μM of PMA (or its negative control 4α-PMA) for 1 h, followed by determination of carrier -mediated biotin uptake as described in “Methods”. Data are mean ± SE of three independent experiments. * p < 0.01. B: Effect on total cellular hSMVT protein expression: Total protein was isolated from ARPE19 cells transiently expressing hSMVT-GFP and pretreated with PMA. Western blot analysis was performed using anti-hSMVT antibody as described in “Methods”. Expression was normalized relative to β-actin and data shown are mean ± SE of three experiments. Inset shows representative Western blot image. C: Effect of PMA pre-treatment on membrane expression of hSMVT. Biotinylation of cell surface protein of transiently transfected ARPE19 cells was performed as described in “Methods”. Equal amount (3 μg) of wild-type hSMVT construct was transfected into ARPE19 cells and 48 h after transfection cells were treated for 1 h with PMA. Level of biotinylated membranous hSMVT was normalized relative to total hSMVT. Data are mean ± SE of three independent experiments. Inset: Representative Western blot image from the same blot (adjacent lanes) showing the level of membrane expression of wild-type hSMVT on PMA treatment.
Since PKC regulation of a membrane transporter could involve internalization of the carrier (i.e., removal from cell membrane) [10, 22, 23], we investigated whether this possibility also applies in the case of PMA inhibition of biotin uptake. This was performed using biotinylation assay (see “Methods”). The results showed no change in the intensity of the biotinylated hSMVT (normalized relative to total cellular hSMVT) in PMA - pretreated and control (untreated) cells (Fig. 5C), suggesting equal membrane expression of the protein.
Collectively, the above findings suggest that the inhibitory effect of PMA (PKC modulator) on biotin uptake is not mediated via alterations in the level of expression of hSMVT protein or mRNA, nor in its level of expression at the cell membrane.
3.2.2. Role of the potential PKC phosphorylation sites (Ser283 and Thr286) of the hSMVT polypeptide in mediating the PMA-induced inhibition of biotin uptake
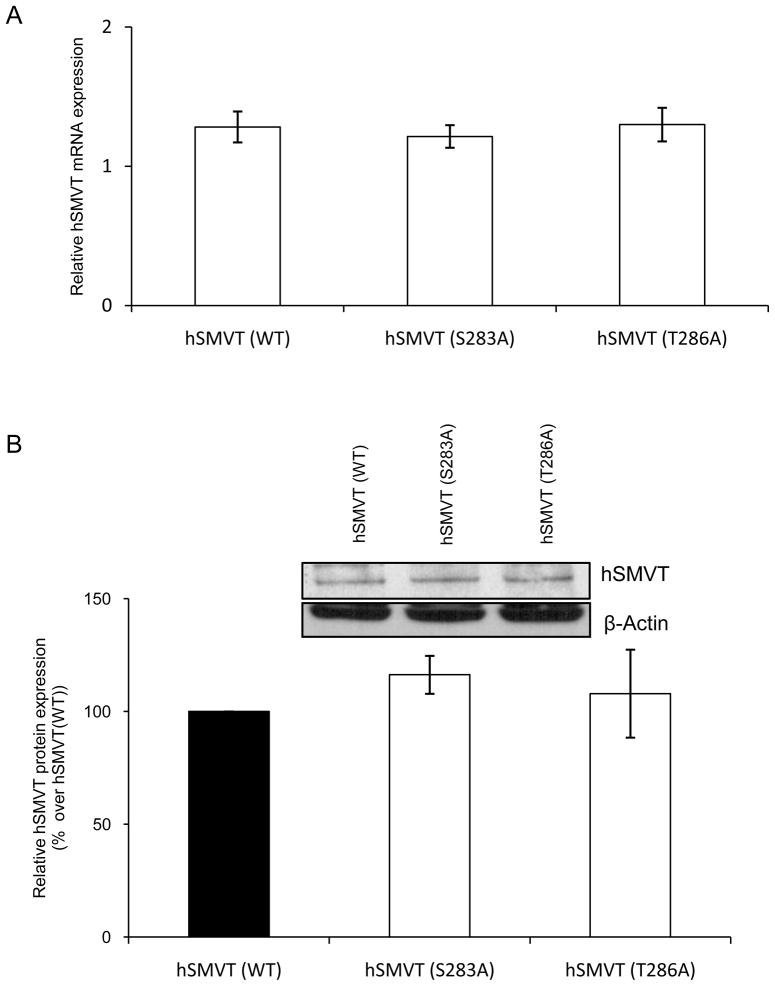
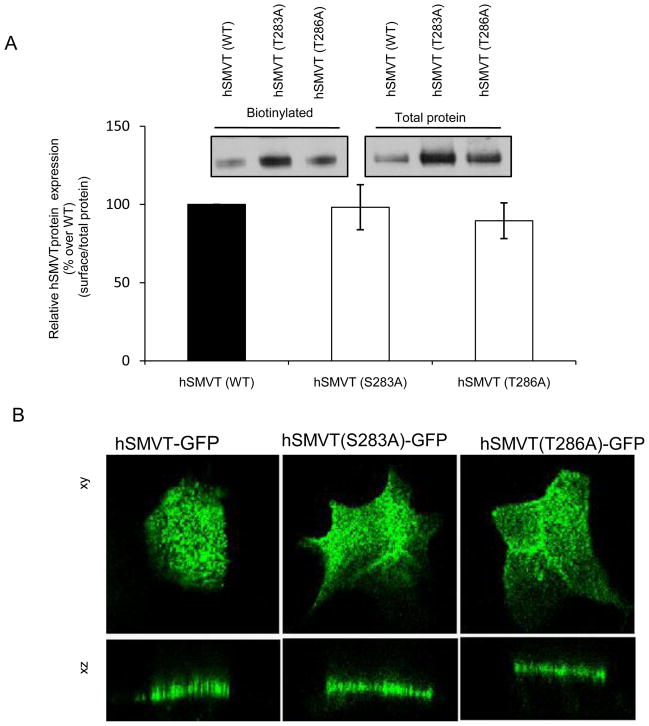
As mentioned earlier, the hSMVT polypeptide is predicted to have two potential PKC phosphorylation sites, one being Ser283 and the other Thr286 (Fig. 1A). To determine if these sites are important in mediating the PMA-induced inhibition in biotin uptake, we mutated these sites (to alanine) then examined the effect of this mutation on PMA-induced inhibition in the vitamin uptake. Since mutating a given amino acid of a transporter protein could lead to changes in the level of expression of its mRNA and/or protein as well as in its level of expression at the cell surface, we tested these possibilities first by means of quantitative PCR, Western blotting and membrane biotinylation assays/confocal imaging, respectively. Our quantitative PCR data showed no change in level of hSMVT mRNA expression as a result of mutating Ser283 and Thr286 (Fig. 6A). Similarly, Western blot analysis using total protein from ARPE19 cells expressing equal amounts of wild-type or mutated (Ser283 and Thr286) hSMVT showed no change in the level of expression of the protein (Fig. 6B). Finally, mutating Ser283 and Thr286 of the hSMVT protein did not affect its surface expression as indicated by results of biotinylation and by confocal imaging methods (Fig. 7A & B).
Fig. 6. Effect of mutating Ser283 and Thr286 on level of expression of hSMVT.
A: Effect on level of mRNA expression: Total RNA was isolated 48 h post transfection and real- time PCR was performed using specific primer against hSMVT. Data (normalized relative to β-actin) are mean ± SE of three independent experiments. B: Effect on level of protein expression: Western blot analysis was performed using 50 μg of total protein isolated from transiently transfected ARPE19 cells. The specific band for hSMVT was detected using anti-hSMVT polyclonal antibodies and the level of expression was normalized relative to β-actin. Data are mean ± SE of three experiments. Inset depicts representative Western blot image.
Fig. 7. Membrane expression of the Ser283 and Thr286 hSMVT mutants.
A: Biotinylation assay: Biotinylation was performed on ARPE19 cells transiently expressing the wild-type and mutant (S283A and T286A) hSMVT-GFP constructs 48 h post transfection. Western blotting was performed using anti-GFP monoclonal antibody and level of membranous expression was normalized relative to total amount of cellular hSMVT (see “Methods”). Data are mean ± SE of hSMVT membrane expression. Inset: representative Western blot image of biotinylated and total hSMVT protein. B: Confocal imaging: Cellular distribution of hSMVT-GFP along the lateral (xy) and axial (xz) sections of Caco-2 cells transiently expressing equal amount of wild-type or mutant hSMVT (4 μg). Imaging was performed 48 h post transfection.
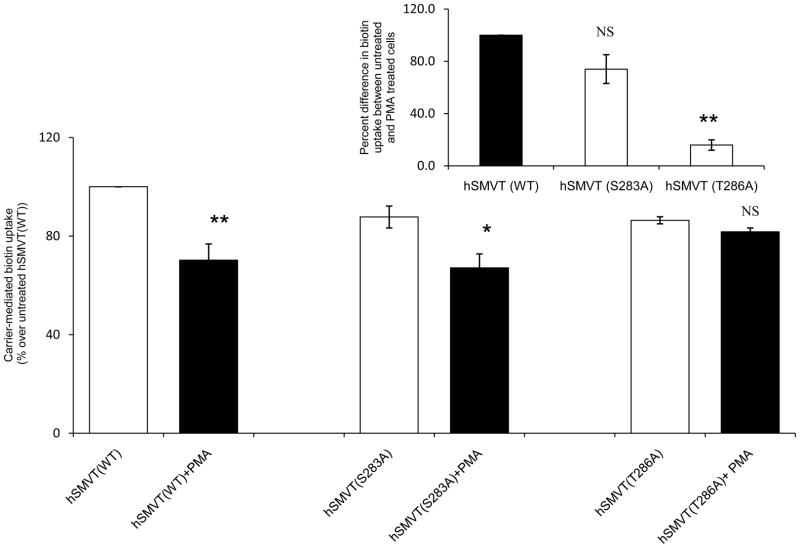
After establishing that mutating Ser283 and Thr286 of the hSMVT polypeptide does not affect the protein cellular and membrane expression, we examined the effect of these mutations on the PMA-induced inhibition in biotin uptake. For this, identical amounts (3 μg) of wild-type and mutated (at Ser283 and Thr286) hSMVT were transfected into ARPE19 cells followed by pre-treatment with PMA (10 μM for 1 h) and examination of biotin (9 nM) uptake; data was compared to untreated controls. The results showed that mutating Thr286 lead s to a disappearance in the PMA-induced inhibition in biotin uptake; while mutating Ser283 had no effect on PMA-induced inhibition in the vitamin uptake (Fig. 8). These findings suggest the involvement of Thr286 (but not Ser283) in the PMA (PKC)-induced inhibition in biotin uptake.
Fig. 8. Effect of mutating the potential PKC phosphorylation sites (Ser283 and Thr286) of hSMVT on the PMA-induced inhibition in biotin uptake.
ARPE19 cells were transiently transfected with equal amount (3μg) of wild- type or mutated (S283A and T286A) hSMVT. Carrier-mediated biotin uptake was then examined simultaneously in PMA pre-treated (10 μM for 1 h) and untreated cells. Data shown is the mean ± SE of the three independent experiments of biotin uptake by untreated and PMA treated cells, respectively. * p < 0.05, ** p < 0.01. The comparison was made relative to the appropriate untreated control construct as indicated in the figure. Inset: Uptake of the PMA treated cells was compared with respect to untreated set and relative percentage was plotted as percentage. **p < 0.01.
4. Discussion
The aim of this study was to investigate the role of the putative N-glycosylation and PKC-phosphorylation sites of the hSMVT polypeptide in the function and regulation of the carrier protein. The hSMVT system is the major biotin uptake system in intestinal epithelial cells [15]. The calculated molecular mass of the hSMVT protein (based on its primary amino acid sequence) was found to disagree with its observed molecular mass on Western blotting [14, 18, 21]. Whether this is due to glycosylation of the predicted N-glycosylation sites is not clear and was investigated in this study using different physiological and molecular approaches. Our investigations using tunicamycin (a nucleoside antibiotic that inhibits N-glycosylation) show a shift in the molecular weight of hSMVT on Western blot to a level that is in agreement with the theoretical prediction. Similar shifting in the molecular weight of hSMVT on Western blot was observed when treatment with PNGaseF was employed. These findings suggest that hSMVT is glycosylated and this glycosylation is the main post-translation modification affecting the mature hSMVT protein. The N-glycosylation of hSMVT appeared to be important for its function as pre-treatment of hSMVT-expressing cells with tunicamycin led to a significant inhibition in carrier-mediated biotin uptake.
We also determined which of the four N-glycosylation sites (Asn138, Asn489, Asn498, Asn534) of the hSMVT polypeptide is (are) important for its function. This was performed by examining the effect of mutating these sites individually on the functionality of hSMVT expressed in ARPE19 cells. The results showed that mutating Asn138 and Asn489 (but not Asn489 and Asn534) led to a significant reduction in biotin uptake, demonstrating the importance of these sites in hSMVT functionality. Analysis of the kinetic parameters of the biotin uptake process of cells expressing Asn138 and Asn489 hSMVT mutants showed a significant reduction in the Vmax, but not the apparent Km, of the biotin uptake process. This suggests reduction in the number and/or activity, but not affinity, of the carrier system.
The effect of mutating the Asn138 and Asn489 of the hSMVT protein on biotin uptake was not mediated via changes in the level of expression of the hSMVT mRNA as similar level of expression was found between the mutants and the wild-type hSMVT. However, mutating these sites, while led to shifting in protein size, also led to a significant decrease in the level of expression of the hSMVT protein. These findings suggest that these sites are important for glycosylation and for normal protein abundance.
We also investigated the role of the two putative PKC-phosphorylation sites of the hSMVT polypeptide in the previously observed PKC-mediated regulation of biotin uptake [16, 17, 24]. Using ARPE19 cells expressing hSMVT [where PMA (but not its negative control 4α PMA) inhibits biotin uptake], we found the PMA inhibition not to be mediated via an effect on the level of expression of the hSMVT mRNA or total cellular level of the transport protein. It was also not mediated by an effect on the level of expression of hSMVT at the cell membrane, thus excluding the possibility that the effect is mediated via removal of the protein from cell surface [22–24]. Based on these findings, it is reasonable to suggest that the PMA (PKC)-induced inhibition in biotin uptake is likely to be mediated by an effect on the activity of the hSMVT system itself. We next examined the role of the potential PKC phosphorylation sites (Ser283 and Thr286) of hSMVT in mediating the PMA-induced inhibition in biotin uptake. This was performed by examining the effect of mutating these sites individually on the PMA-induced inhibition on biotin uptake. Because mutating a given residue in a protein could affect that protein level of expression and/or stability, we addressed these issues first. This was achieved by mean of quantitative PCR, Western blotting and membrane biotinylation assays/confocal imaging studies, which showed that such mutations do not affect the expression or stability of hSMVT. Our studies on the effect of mutating Ser283 and Thr286 of hSMVT on the PMA-induced inhibition in biotin uptake showed that mutating the latter, but not the former, residue to lead to disappearance in the PMA-induced inhibition in the vitamin uptake. These findings suggest that Thr286 is involved in mediating the PMA (PKC)- induced inhibition in biotin uptake.
In conclusion, our studies demonstrate that the hSMVT protein is glycosylated and this glycosylation is important for function. Also, the predicted PKC phosphorylation site Thr286 (but not Ser283 ) appears to be involved in mediating the PKC- induced regulation of biotin uptake.
Highlights
The human sodium-dependent multivitamin transporter (hSMVT) is glycosylated
The glycosylation Asn138 and Asn489 are essential for hSMVT function.
The putative PKC-phosphorylation site Thr286 in the hSMVT polypeptide is important inthe PKC - mediated regulation of biotin uptake.
Acknowledgments
Supported by grants from the Department of Veterans Affairs and the National Institutes of Health (DK58057 and DK56061).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McMahon RJ. Biotin in metabolism and molecular biology. Annu Rev Nutr. 2002;22:221–239. doi: 10.1146/annurev.nutr.22.121101.112819. [DOI] [PubMed] [Google Scholar]
- 2.Wolf B. In: The metabolic and molecular basis of inherited disease. Scriver CR, Beaudet A, editors. McGraw-Hill; New York, NY: 2001. pp. 3935–3962. [Google Scholar]
- 3.Bowman BB, Rosenberg IH. Biotin absorption by distal rat intestine. J Nutr. 1987;117:2121–2126. doi: 10.1093/jn/117.12.2121. [DOI] [PubMed] [Google Scholar]
- 4.Wrong OM, Edmonds CJ, Chadwich VS. The Large Intenstine; Its Role In Mammalian Nutrition and Homeostasis. Wiley and Sons; New York: 1981. Vitamins; pp. 157–166. [Google Scholar]
- 5.Sorrell MF, Frank O, Thomson AD, Aquino A, Barker H. Absorption of vitamins from large intestine. Nutr Res Int. 1971;3:143–148. [Google Scholar]
- 6.Kasper H. Vitamin absorption in the colon. Am J Proctol. 1970;21:341–345. [PubMed] [Google Scholar]
- 7.Barth CA, Frigg M, Hogemeister H. Biotin absorption from the hindgut of the pig. J Anim Physiol Anim Nutr. 1986;55:128–134. [Google Scholar]
- 8.Said HM. Recent advances in carrier-mediated intestinal absorption of water soluble vitamins. Annu Rev Physiol. 2004;66:419–46. doi: 10.1146/annurev.physiol.66.032102.144611. [DOI] [PubMed] [Google Scholar]
- 9.Said HM, Seetharam B. In: Physiology of the gastrointestinal tract. Johnson LR, editor. Academic Press; San Diego: 2006. pp. 1791–1825. [Google Scholar]
- 10.Reidling JC, Subramanian VS, Dahhan T, Sadat M, Said HM. Mechanisms and regulation of vitamin C uptake: studies of the hSVCT systems in human liver epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1217–1227. doi: 10.1152/ajpgi.90399.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Huang W, Fei YJ, Xia H, Yang-Feng TL, Leibach FH, Devoe LD, Ganapathy V, Prasad PD. Human placentral Na+-dependent multivitamin transporter. Cloning, functional expression, gene structure, and chromosomal localization. J Biol Chem. 1999;274:14875–14883. doi: 10.1074/jbc.274.21.14875. [DOI] [PubMed] [Google Scholar]
- 12.Chatterjee NS, Kumar CK, Ortiz A, Rubin SA, Said HM. Molecular mechanism of the intestinal biotin transport process. Am J Physiol. 1999;277:C605–613. doi: 10.1152/ajpcell.1999.277.4.C605. [DOI] [PubMed] [Google Scholar]
- 13.Prasad PD, Wang H, Huang W, Fei YJ, Leibach FH, Devoe LD, Ganapathy V. Molecular and functional characterization of the intestinal Na+-dependent multivitamin transporter. Arch Biochem Biophys. 1999;366:95–106. doi: 10.1006/abbi.1999.1213. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian VS, Marchant JS, Boulware MJ, Ma TY, Said HM. Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells. Am J Physiol Cell Physiol. 2009;296:C663–671. doi: 10.1152/ajpcell.00396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balamurugan K, Ortiz A, Said HM. Biotin uptake by human intestinal and liver epithelial cells: role of the SMVT system. Am J Physiol Gastrointest Liver Physiol. 2003;285:G73–77. doi: 10.1152/ajpgi.00059.2003. [DOI] [PubMed] [Google Scholar]
- 16.Said HM. Cellular uptake of biotin: mechanisms and regulation. J Nutr. 1999;129:490S–493S. doi: 10.1093/jn/129.2.490S. [DOI] [PubMed] [Google Scholar]
- 17.Said HM, Ortiz A, McCloud E, Dyer D, Moyer MP, Rubin S. Biotin uptake by human colonic epithelial NCM460 cells: a carrier-mediated process shared with pantothenic acid. Am J Physiol. 1998;275:C1365–1371. doi: 10.1152/ajpcell.1998.275.5.C1365. [DOI] [PubMed] [Google Scholar]
- 18.Ghosal A, Said HM. Structure-function activity of the human sodium-dependent multivitamin transporter: role of His(115) and His(254) Am J Physiol Cell Physiol. 2010;300:C97–104. doi: 10.1152/ajpcell.00398.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reidling JC, Nabokina SM, Said HM. Molecular mechanisms involved in the adaptive regulation of human intestinal biotin uptake: A study of the hSMVt system. Am J Physiol Gastrointest Liver Physiol. 2007;292:G275–281. doi: 10.1152/ajpgi.00327.2006. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of the relative gene expression data using real-time quantative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Nabokina SM, Subramanian VS, Said HM. Association of PDZ-containing protein PDZ11 with human sodium dependent multivitamin transporter, hSMVT. Am J Physiol Gastrointest Liver Physiol. 2010 December 23; doi: 10.1152/ajpgi.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotmann A, Strand D, Martine U, Closs EI. Protein kinase C activation promotes the internalization of the human cationic amino acid transporter hCAT-1. A new regulatory mechanism for hCAT-1 activity. J Biol Chem. 2004;279:54185–54192. doi: 10.1074/jbc.M409556200. [DOI] [PubMed] [Google Scholar]
- 23.Padovano V, Massari S, Mazzucchelli S, Pietrini G. PKC induces internalization and retention of the EAAC1 glutamate transporter in recycling endosomes of MDCK cells. Am J Physiol Cell Physiol. 2009;297:C835–844. doi: 10.1152/ajpcell.00212.2009. [DOI] [PubMed] [Google Scholar]
- 24.Ma TY, Dyer DL, Said HM. Human intestinal cell line Caco-2: a useful model for studying cellular and molecular regulation of biotin uptake. Biochim Biophys Acta. 1994;1189:81–88. doi: 10.1016/0005-2736(94)90283-6. [DOI] [PubMed] [Google Scholar]