Abstract
The success of homogeneous catalysis can be attributed largely to the development of a diverse range of ligand frameworks that have been used to tune the behavior of various systems. Spectacular results in this area have been achieved using cyclic diaminocarbenes (NHCs) as a result of their strong σ-donor properties. Although it is possible to cursorily tune the structure of NHCs, any diversity is still far from matching their phosphorus-based counterparts, which is one of the great strengths of the latter. A variety of stable acyclic carbenes are known, but they are either reluctant to bind metals or they give rise to fragile metal complexes. During the last five years, new types of stable cyclic carbenes, as well as related carbon-based ligands (which are not NHCs), and which feature even stronger σ-donor properties have been developed. Their synthesis and characterization as well as the stability, electronic properties, coordination behavior, and catalytic activity of the ensuing complexes are discussed, and comparisons with their NHC cousins are made.
Keywords: carbenes, homogeneous catalysis, N-heterocyclic carbenes, phosphorus
1. Introduction
In 1988, three years before the seminal publication by Arduengo et al. on the synthesis of the crystalline cyclic diaminocarbene B1[1] we discovered that the (phosphino)-(silyl)carbene A1 was stable enough to be isolated by flash distillation[2] (Scheme 1). Later, we reported the single-crystal X-ray diffraction study and electron localization function (ELF) analysis of A2[3] that definitively confirmed the carbene nature of (phosphino)(silyl)carbenes, which had, for sometime, been debated.[4] In 2000,[5] apart from one carbene of type A, and some 50 N-heterocyclic carbenes (NHCs) of types B, C,[6] D,[7] and E,[8] only four other carbenes F,[9] G,[10] H,[11] and I,[12] had been structurally characterized, all of them bearing two heteroatom substituents. From 2000 to 2004, our research group expanded the variety of stable carbenes to (amino)(phosphino)carbenes such as J,[13] but also to mono-heteroatom-substituted carbenes K,[14] L,[15] and M,[16] for which the stabilization mode significantly differs throughout the series. These results were summarized in a previous review,[17] which gives a good indication of the electronic and steric requirements that make carbenes isolable.
Scheme 1.
Crystallographically characterized carbenes known before 2000 (A–I), and discovered between 2000 and 2004 (J–M), with the carbene bond angle given in parentheses. Dipp =2,6-iPr2C6H3, Dtbp =2,6-tBu2C6H3.
NHCs bind more strongly to metal centers than most classical ligands, such as phosphines (thus avoiding the necessity for the use of excess ligand). The NHC–transition-metal complexes are less sensitive to air and moisture, and have proven remarkably resistant to oxidation.[18] Moreover, they are strong σ-donor ligands, and their steric environment, which is best defined as fence- or fanlike,[19] differentiate them substantially from tertiary phosphines, which are usually regarded as a cone. Thanks to these features, the use of NHCs as ligands for transition metals have led to numerous breakthroughs in homogeneous catalysis, as exemplified by the second generation of Grubbs catalysts.[20,21] Moreover, they are excellent organic catalysts in their own right, as first shown by Enders and co-workers[22] in studies inspired by the work of Breslow on the thiazolium catalyst used in the benzoin condensation reaction.[23] This short analysis easily explains why so many variations of the NHC backbone have been reported, and a myriad of reviews devoted to NHC chemistry have been published.[24–27]
In marked contrast with NHCs, acyclic singlet carbenes A and G–M have found very limited applications.[28] Indeed, (phosphino)(silyl)- and (phosphino)(phosphonio)carbenes A and H are very reluctant to bind any transition metals, and complexes of carbenes I,[29–33] J,[34] K,[35] L,[36] and M[16] are much more fragile than their NHC counterparts. This is especially striking in the case of acyclic diaminocarbenes (ADCs), such as I, since these compounds feature, similar to the NHCs, two amino groups directly bonded to the carbene center. It was shown that, in contrast to the corresponding NHC analogues, [Mo(ADC)(CO)5] and [W(ADC)(CO)5] complexes are very unstable even at room temperature.[29] Similarly, reactions of ADCs with several PdII precursors resulted in reduction to palladium black,[30] and free ADCs failed to displace phosphine ligands in Grubbs-type ruthenium–alkylidene catalysts.[29b] Even a palladium–bis(ADC) complex was shown to undergo reversible opening of the chelate ring.[31] The reactions and coordination behavior of acyclic carbenes A and G–M, as well as ADCs and bis-(ADC)s, were reviewed recently by Bourissou and co-workers[37] and Slaughter,[32] respectively.
Herrmann et al. suggested that the poor coordination behavior of acyclic diaminocarbenes I, compared to NHCs B–E, might be due to the larger N-C-N angle (121° compared to 101–106°).[29b] This hypothesis was corroborated by theoretical studies by Schoeller et al.[38] on (phosphino)-(silyl)carbenes A. They concluded that the wide carbene bond angle (>150°) necessitates conformational changes to a bent carbene structure to allow complexation to a metal, a process that is energetically too costly. An extreme example was found experimentally: coordination to a {RhCl(nbd)} (nbd = norbornadiene) fragment resulted in the value of the carbene bond angle of (aryl)(phosphino)carbene K decreasing from 162° to 119° (Scheme 2).[35]
Scheme 2.
Contraction of the carbene bond angle of K upon complexation. nbd = norbornadiene.
This analysis suggests that there is little hope that acyclic carbenes could find applications as ligands for transition-metal catalysts. Moreover, it is generally admitted that acyclic carbenes are more thermally, air, and moisture sensitive than their cyclic counterparts, which clearly hampers the possibility of using them as organic catalysts. Therefore, beginning in 2005, we turned our attention to the design of novel types of cyclic carbenes, which are not diaminocarbenes; in other words, we started a project on the chemistry of cyclic non-NHCs. Since the efficiency of NHCs in transition-metal catalysis is mainly due to their strong σ-donor properties, electron-rich carbenes were targeted, namely cyclic diphosphinocarbenes (PHCs), (amino)(phosphino)carbenes (N-PHCs), (alkyl)(amino)carbenes (CAACs), and (amino)-(ylidic)carbenes (N-YHCs; Scheme 3). Since a rather acute carbene bond angle seems beneficial for coordination, an extreme example was investigated, namely cyclopropenylidenes (CPs). Moreover, the robustness of carbene complexes is partly due to the presence of a strong carbon–metal bond. Therefore, other types of carbon-based ligands, such as cyclic bent allenes (CBAs) and the related cyclic carbodiphosphoranes (CCDPs) and vinylidenephosphoranes (CVPs), as well as abnormal-NHCs (aNHCs) appeared to be highly desirable compounds.
Scheme 3.
Cyclic carbenes and related species discussed in this Review.
This Review summarizes the results obtained so far for all the species shown in Scheme 3. The rationale for the choice of the targeted compounds, their synthesis, characterization, stability, and coordination behavior, as well as the catalytic activity of the ensuing complexes will be discussed. Before the concluding remarks, the electronic properties of these species will be compared, which includes a description of the differences and similarities with NHCs.
2. Cyclic Diphosphinocarbenes
2.1. Background
One modification of the NHC backbone is to replace the two nitrogen atoms by their heavier analogues, namely phosphorus, to give cyclic diphoapninocarbenes (PHCs).[39] Examination of the literature pointed out some concerns regarding their stability and some difficulties in their synthesis, but it also indicated that if they are suitably designed, PHCs might act as strong σ-donor ligands for transition metals.
It was known that acyclic diphosphinocarbenes could not be characterized spectroscopically in solution, even at −78°C, mainly because of intramolecular processes, especially 1,2-migrations.[40] However, because of geometric constraints, these processes are much less favored in cyclic systems, and therefore it was reasonable to believe that PHCs should be more stable than their acyclic versions.
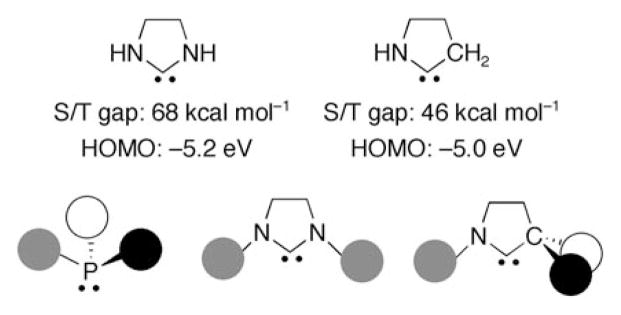
Calculations had shown that the nitrogen centers of the parent NHC are in a perfectly planar environment,[41] whereas the phosphorus centers of the parent PHC are strongly pyramidalized,[42] and therefore do not act as π donors. Consequently, the singlet/triplet gap drops from 79 kcal mol−1 [41a] for the parent unsaturated NHC of type B to 21 kcalmol−1 [42b] for the corresponding PHC; the latter species is predicted to be highly unstable with respect to dimerization.[42a] However, Schleyer et al.,[43] had stated that “in contrast to the still common misconception that 2p-3p overlap is ineffective, the inherent π-donor capabilities of the heavier elements (such as phosphorus) are as large as or even larger than their second row counterparts (such as nitrogen); the apparent inferior donor ability is due to the difficulty in achieving the optimum planar configuration”. In the same vein, Nyulaszi[44] had shown, examining several criteria, that the planar phosphole is more aromatic than pyrrole; for example the NICS value is −17.4 for planar phosphole and only −14.7 for pyrrole.
These results suggested that, if a planar environment could be imposed at the phosphorus centers, PHCs should be stable and also stronger σ-donor ligands than NHCs. Interestingly, one way to achieve planarity is to use bulky substituents and to incorporate the phosphorus center into rings.[44] To test this hypothesis, ab initio calculations were performed on PHC derivatives PHC1–3, which feature hydrogen, phenyl, and bulky 2,4,6-tri(tert-butyl)phenyl substituents at the phosphorus center.[39] Interestingly, the sum of the angles around the P center, the singlet-triplet energy gap, as well as the energy of the highest occupied molecular orbitals (HOMOs; Kohn–Sham (KS) orbital energies), increase significantly as the steric demands of the substituents increase (Scheme 4). Moreover the HOMO of the most bulky carbene PHC3 (−5.0 eV) is even higher in energy than that calculated at the same level of theory for triazolin-5-ylidene D (−5.1 eV);[7] this finding can be regarded as a good indication of the strongly basic character of PHC3.
Scheme 4.
Computational data for PHC1–3 and triazolin-5-ylidene D. S =singlet, T =triplet.
2.2. Synthesis, Characterization, and Stability
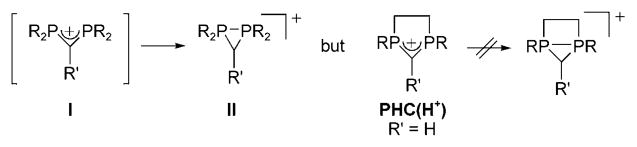
The classical precursors of NHCs are the corresponding well-known protonated species NHC(H+)s. However, the phosphorus analogues (PHC(H+)s) were unknown. Indeed, in contrast to the very stable amidinium salts, acyclic diphosphaallyl cations of type I are unstable towards rearrangements, especially ring closure, which surprisingly leads to the corresponding cyclic valence isomers II[45] (Scheme 5). This is again due to the reluctance of phosphorus to achieve a planar configuration, and also to its ready accommodation in three-membered rings. However, we reasoned that the rigid ring structure of PHC(H+)s could prevent such a rearrangement, and indeed one C-silylated four-[46] and one five-membered ring derivative (R′= SiMe3) were known.[47]
Scheme 5.
Spontaneous ring closure of phosphorus analogues of amidinium salts, which can be prevented by the ring structure of PHC(H+)s.
Another problem arose because none of the synthetic methods used for the preparation of NHC(H+)s can be extended to that of their heavier congeners. Thus, original synthetic approaches had to be designed. First, formal [3+2] cycloadditions of the transient diphosphaallylic cation 2 with a nitrile or a cyanamide were performed (Scheme 6).[39,48] The addition of silver trifluoromethanesulfonate to phosphaalkene 1,[49] which bears bulky 2,4,6-tri(tert-butyl)phenyl substituents, in the presence of a large excess of a nitrile or dimethylcyanamide, cleanly afforded the desired salts PHC3–5(H+). This synthetic route has some limitations. Alkynes, alkenes, ketones, and even imines cannot be used as dipolarophiles, and so far only C≡N species gave the desired cycloaddition reaction. Therefore, we attempted to develop a more broadly applicable route to PHC(H+)s starting from 1,3-dichloro-1,3-diphosphapropane 3.[50] The addition of GaCl3 leads to the transient phosphenium salt 4, which then reacts with excess nitrile or cyanamide to afford the five-membered heterocycles 5. Dehydrohalogenation with DBU gave rise to the desired gallium salts of PHC3–5(H+) in good yields. However, once again, only nitriles underwent cycloaddition.
Scheme 6.
Synthetic routes to PHC3–5(H+). Mes* =2,4,6-tBu3C6H2, Tf = trifluoromethanesulfonyl, DBU =1,8-diazabicyclo[5.4.0]undec-7-ene.
Then, we turned our attention to the deprotonation of PHC(H+)s, and found that the nature of the counteranion/base combination has a crucial influence on the fate of the reaction.[48] For example, the attempted deprotonation of PHC3(H+) (R =Me, X =GaCl4) with KH/potassium tert-butoxide did not afford the free carbene; instead its GaCl3 adduct 6 was obtained in 83% yield (Scheme 7). However, when the same PHC3(H+) with triflate as the anion was treated with lithium bis(trimethylsilyl)amide, the corresponding PHC3 (R =Me) was isolated as light yellow crystals in 72% yield after recrystallization. By using the same procedure, PHC4 (R =NMe2) was obtained in 66% yield.
Scheme 7.
Influence of the counteranion/base combination on the deprotonation of PHCs; preparation of PHC3 and PHC4. Ar =2,4,6-tBu3C6H2, HMDS = 1,1,1,3,3,3-hexamethyldisilazane.
The X-ray diffraction analysis of PHC3 (R =Me) (Figure 1) revealed the almost planar environment of the phosphorus centers (sum of the angles: 353 and 348°). However, the slight deviation from planarity (trans arrangement of the aryl substituents) makes PHC3 chiral in the solid state. In solution, even at −100°C, both 1H and 13C NMR spectroscopy show the equivalency of the diastereotopic tert-butyl groups. This finding suggests that the two enantiomers are in rapid equilibrium, which is in agreement with the expected low inversion barriers at the phosphorus centers. The strong donation of the phosphorus lone pairs of electrons to the electron-deficient carbene center is clearly apparent from the P–Ccarbene bond lengths (1.67 and 1.71 Å), which are significantly shorter than P–C single bonds (>1.80 Å). The other geometric parameters show that the interaction between the NC unit and the PCP fragment is weak. The same conclusions were drawn for the triazolin-5-ylidene D, which is the direct NHC analogue of PHC3. Interestingly, the carbene bond angle is very acute (98.2°); in fact it is very similar to the carbene bond angle recently reported for four-membered NHCs that feature a boron or a phosphorus atom in the ring skeleton (96.7° and 94.0°).[51]
Figure 1.

Solid-state structure of PHC3 showing the very weak pyramidalization of the phosphorus centers.
The signals for the carbene carbon atoms of PHC3,4 in the 13C NMR spectra (δ =184 and 187 ppm, respectively) are strongly deshielded compared to those of the PHC3,4(H+) precursors (δ =119 and 115 ppm); a similar trend was observed between NHC and NHC(H+)s. The 13C chemical shifts of the carbene are also at slightly higher field than those of NHCs (δ =205–244 ppm).[26b]
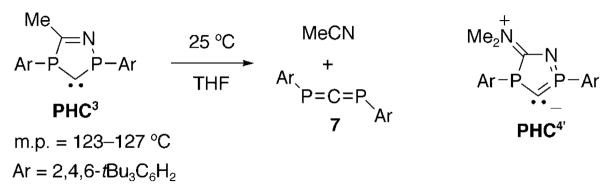
Although PHC3 is indefinitely stable at room temperature in the solid state (m.p. 123–127°C), it undergoes a [3+2] retro-cycloaddition in solution to afford the previously described 1,3-diphosphaallene 7[52] and acetonitrile (Scheme 8). This reaction shows first order kinetics, and a half-life for PHC3 in THF solution of about 5 h at 16°C. This behavior is very surprising since the nitrogen analogue, namely triazolin-5-ylidene D, is very stable under the same experimental conditions. However, there is no evidence of retro-cycloaddition of PHC4 after 24 h in benzene at 25°C. This is presumably due to the existence of a zwitterionic resonance form PHC4’, which significantly stabilizes the system thermodynamically.
Scheme 8.
Decomposition of PHC3 in solution, and resonance form PHC4.
From these results as a whole, it appears that a saturated backbone, or a six-membered ring skeleton, could be beneficial for the stability of PHCs, since the major issue faced so far is the retro-cycloaddition that leads to 1,3-diphosphaallenes such as 7. However, other synthetic routes have to be developed to test this hypothesis. Importantly, one could also conclude that PHCs can only be stable if a very bulky substituent is attached to both phosphorus centers. However, as shown in the following section, one can hope that one of the phosphorus nuclei could act as a spectator substituent (therefore, it would not have to bear a bulky group to impose planarity) without precluding isolation of the carbene. If this hypothesis is correct, many stable PHCs could be prepared.
2.3. Ligand Behavior
So far, only three complexes[39,48] have been prepared from free PHCs, namely [RhCl(cod)(PHC3)], [RhCl(CO)2-(PHC3)], and [RhCl(CO)2(PHC4)]. The rhodium carbonyl complexes will be discussed in Section 9, for comparison of the electronic properties of PHCs with the other cyclic non-NHCs and classical NHCs. [RhCl(cod)(PHC3)] was prepared by the simple addition of [{RhCl(cod)}2] to PHC3, and isolated as highly thermally stable single crystals (78% yield; m.p. 187–189°C). Notably, no significant decomposition was observed when a solution of the complex in dichloromethane was stirred for several hours under an atmosphere of air. As observed for the heteroatom–Ccarbene bond in NHCs, the complexation induces a very small lengthening of the P–C bonds, which however remain shorter than those in the corresponding cation PHC3(H+). Interestingly, the phosphorus centers are not strongly pyramidalized: the sum of the angles around the phosphorus center (350 and 351°) are essentially identical to those observed for the free PHC3. These data suggest a very weak π backdonation from the metal to the carbene ligand. This is confirmed by the Ccarbene–Rh bond length of 2.06 Å, which is at the upper limit of those observed for [RhCl(cod)(NHC)] complexes (2.00–2.06 Å), and significantly longer than that found for the analogous complex featuring NHC D as the ligand (2.00 Å).[53]
A zirconium complex with a PHC ligand was prepared by Le Floch and co-workers[54] by using a 1,3-diphospholene thioacetal as a carbene precursor (Scheme 9). This complex proved to be stable for at least a week in THF at room temperature, but unstable in the absence of solvent; moreover, it is highly water sensitive. So far, no catalytic data are available for PHC complexes.
Scheme 9.
Synthesis of a Zr-PHC complex by reduction of a thioacetal. Cp =cyclopentadienyl.
3. Cyclic (Amino)(phosphino)carbenes
3.1. Background
Acyclic (amino)(phosphino)carbenes such as J feature short N–C and long P–C bond lengths, as well as planar nitrogen and strongly pyramidalized phosphorus centers.[13] These geometric parameters indicate that only the nitrogen atom acts as a π donor towards the vacant orbital on the carbene, and therefore the phosphorus center is not involved in the stabilization of these species; electronically, P acts as a spectator substituent. Indeed, it is possible to functionalize the phosphorus, while retaining the carbene center, as shown with the reaction with sulfur (Scheme 10).[13a] Carbenes J thus behave as bidentate ligands, as demonstrated by the preparation of the palladium dichloride complex JPd,[34b] which readily promotes the aryl amination of electron-rich and electron-poor aryl bromides with morpholine, although low conversions were obtained with aryl chlorides.
Scheme 10.
Acyclic (amino)(phosphino)carbenes such as J feature an active lone pair of electrons on the P center. cod =1,5-cyclooctadiene.
Despite the presence of only one stabilizing substituent, carbenes of type J appeared to be quite stable. We reasoned that cyclic (amino)(phosphino)carbenes (N-PHCs) should be equally stable, and importantly behave as stronger donor ligands than NHCs, since phosphorus is more electropositive than nitrogen. Moreover, in contrast to the acyclic version in which P only acts through the inductive effect, in N-PHCs its ρ-donor ability can be favored by increasing the steric hindrance of the P substituent, as shown for PHCs (see Section 2).
3.2. Synthesis, Characterization, and Stability
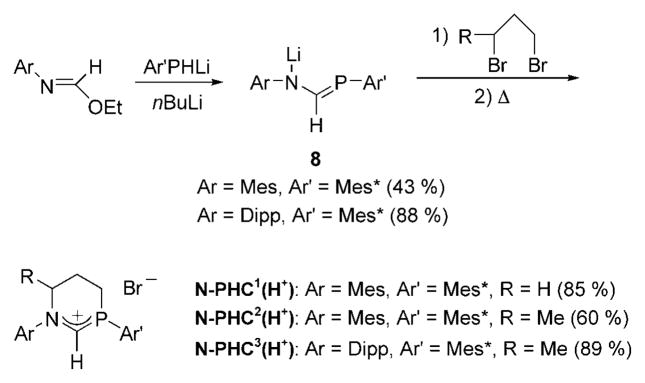
NHC(H+)s can be readily prepared by the addition of a compound with two leaving groups to 1,3-diazaallyl anions.[55] By analogy, phosphaformamidinates 8 appeared to be potential starting materials for the desired N-PHC precursors (Scheme 11). However, although a few examples of phosphaamidines and phosphaamidinates[56] had been reported, none of the known synthetic approaches could be used for the preparation of formyl derivatives. It was found that N-aryl formimidates with bulky aryl groups react with primary aryl phosphides, in the presence of one equivalent of n-butyl-lithium to afford phosphaformamidinates 8.[57] The addition of 1,3-dibromopropane or 1,3-dibromobutane in diethyl ether, followed by heating, then gives the desired N-PHC1-3(H+) in acceptable yields.[58]
Scheme 11.
Synthesis of phosphaformamidinates 8 and N-PHC1-3(H+). Mes =2,4,6-Me3C6H2, Mes* = 2,4,6-tBu3C6H2
Surprisingly, all attempts to deprotonate N-PHC1(H+) (R =H) with a variety of strong bases (LDA, LiHMDS, etc) led to the cyclic alkene 10a, which was isolated in 75% yield (Scheme 12). Monitoring the deprotonation reaction in THF at −78°C by multinuclear NMR spectroscopy showed the disappearance of N-PHC1(H+), and the clean formation of a new product, which was tentatively identified as the cyclic azomethine ylide 9a,[59] instead of the desired N-PHC. Warming the solution to room temperature again afforded the alkene 10a. The latter probably results from the intermolecular deprotonation of the carbon atom at the position β to the nitrogen atom by the negatively charged azomethine ylide carbon atom. These results, which are in marked contrast to those observed with NHCs, suggest that the electropositivity of phosphorus decreases the acidity of the iminium proton of N-PHC1(H+), and favors the deprotonation at the position α′ to the nitrogen atom. This is of course a good indication that, as expected, N-PHCs are more basic than NHCs. The position α′ to the nitrogen atom was then protected by a methyl substituent. However, deprotonation of N-PHC2(H+) again yielded the corresponding alkene 10b.
Scheme 12.
Deprotonation of N-PHC1,2(H+).
As explained for PHCs, bulky substituents decrease the inversion barrier at the phosphorus center, which allows for maximum donation of the lone pair of electrons. This phenomenon is also true for nitrogen centers,[43,44a] and consequently the presence of a bulky substituent at the N atom should increase the acidity of the iminium proton and also the stability of the ensuing N-PHC. Therefore, the deprotonation of N-PHC3(H+), which has a 2,6-diisopropyl-phenyl group (Dipp) instead of the mesityl group of N-PHC2(H+), was investigated. Depending on the experimental conditions and the nature of the base, heterocycles 10c and 11c, which result from the deprotonation in the position α′ to the nitrogen and phosphorus centers, respectively, were obtained and isolated in good yields (Scheme 13). However, the desired N-PHC3 was obtained when a solution of N-PHC3(H+) and LDA in THF was kept for only two minutes at −78°C and rapidly warmed to room temperature. The 31P NMR spectrum showed a signal at δ =−32.8 ppm (> 90%), while the 13C NMR spectrum showed a doublet at very low field (δ =314.5 ppm, JPC = 122 Hz). This carbon signal is shifted much further downfield than those observed for both NHCs (δ =205–245 ppm)[26b] and PHCs (δ = 184 ppm),[39] but is in the range observed for the acyclic (amino)(phosphino)carbenes J (δ =320–348 ppm, JPC = 22–101 Hz).[13] This finding implies that, despite the presence of the bulky 2,4,6-tri(tert-butyl)phenyl group, the phosphorus center plays the role of a spectator substituent, just as it does in the acyclic version J. Indeed, the X-ray crystal structure of N-PHC3(H+) reveals that the phosphorus center is in a strongly pyramidalized environment (sum of angles: 330°). All attempts to obtain single crystals of N-PHC3 failed.
Scheme 13.
Deprotonation of N-PHC3(H+), spectroscopic characterization, and rearrangement of N-PHC3. TMP =tetramethylpiperidide, LDA =lithium diisopropylamide.
After two days at −30°C, N-PHC3 rearranges quantitatively into 12c by carbene insertion into the C–H bond of a tert-butyl group (Scheme 13). This decomposition pathway is very surprising, since no C–H insertion has been observed for PHC3, which features the same 2,4,6-tri(tert-butyl)phenyl substituent, and this result is not yet understood. This observation does not, however, imply that N-PHCs cannot be isolated. One way to circumvent this difficulty would be to use a phosphorus substituent without reactive C–H bonds, and since the phosphorus lone pair of electrons does not seem to interact with the vacant orbital on the carbene, a simple phenyl group could be used. It is also necessary to protect the position α′ to the nitrogen atom, and maybe to the phosphorus atom, to avoid the competitive deprotonation; clearly, the preparation of unsaturated five-membered N-PHCs might be an excellent option. Interestingly, since the phosphorus lone pair of electrons probably remains active, it can potentially be used to change the coordination number of P, which offers an opportunity to tune the electronic properties of N-PHCs. So far, no complexes featuring a N-PHC as a ligand have been prepared.
4. Cyclic (Alkyl)(amino)carbenes
4.1. Background
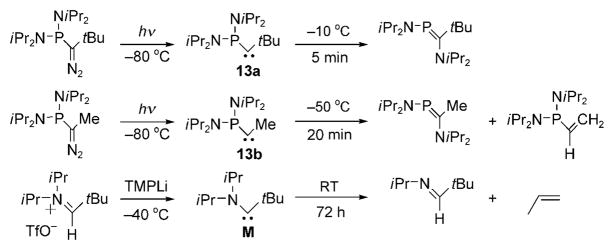
The direct observation of singlet alkyl carbenes usually requires matrix isolation conditions.[60] Indirect observation and kinetic measurements in solution can be performed by the pyridine ylide method developed by Platz and co-workers.[61] When the π-donating and σ-accepting methoxy substituent was present, Moss and co-workers[62] were able characterize the singlet (methoxy)(methyl)carbene by UV and IR spectroscopy, but only in a nitrogen matrix at 10 K, and in solution by a nanosecond time-resolved LFP technique (t1/2 < 2 ms at 20 °C). In 2002, we demonstrated that the [bis(diisopropylamino)phosphino](tert-butyl)carbene 13a has a lifetime of about three minutes at −10°C, whereas the corresponding [bis(diisopropylamino)phosphino](methyl)-carbene 13b could only be observed by 31P NMR apectroscopy up to −50°C (t1/2 ≈ 10 min at −50 °C; Scheme 14).[63] In both cases, 1,2-migration occurred. This is not surprising since the activation energies for 1,2-hydrogen shifts are essentially zero for simple alkyl carbenes, and were calculated to be between 11 and 25 kcalmol−1 for heteroatom-substituted alkylcarbenes.[64] In 2004 we were able to isolate the (tert-butyl)(diisopropylamino)carbene M, as light yellow crystals (m.p. <20°).[16] Carbene M can be stored indefinitely in the solid state at 0°C, but in solution at room temperature it transforms quantitatively within three days into the corresponding E imine and propene.
Scheme 14.
Evolution of persistent (alkyl)(phosphino)- and (alkyl)-(amino)carbenes in solution.
From these results, it can be concluded that 1) amino groups are more efficient than phosphino groups for stabilizing a carbene center, at least in the case of acyclic carbenes; and 2) the major obstacle for the isolation of a wide range of (amino and phosphino)(alkyl) carbenes are intramolecular processes, especially 1,2-migrations. These processes should be much less favored in cyclic systems, because of geometric constraints, and therefore we reasoned that cyclic (alkyl)-(amino)carbenes (CAACs), which have a quaternary carbon atom in the position α to the carbene center, should be very stable species.
Importantly, it was clear that the replacement of one of the electronegative amino substituents of NHCs by a σ-donor alkyl group would make CAAC ligands more electron rich than NHCs, and of course phosphines. Indeed, our calculations showed that the HOMO (−5.0 eV) of the parent CAAC (H at all positions) is slightly higher in energy than for the parent saturated NHC (−5.2 eV; Figure 2). We also found that the singlet–triplet gap for the parent CAAC (46 kcal mol−1) is significantly smaller than for the parent saturated NHC (68 kcalmol−1). Consequently, CAACs should be more nucleophilic (σ donating), but also more electrophilic (π accepting) than NHCs. Moreover, the presence of a quaternary carbon atom in a position α to the carbene center should provide steric environments that differentiate CAACs from all other ligands, and allows for the placement of a chiral center in a position α to the carbene.
Figure 2.
Calculated data for the parent saturated NHC and CAAC. Schematic representations of phosphines, NHCs, and CAACs, showing their very different steric environments.
4.2. Synthesis, Characterization, and Stability
The method used to prepare the precursor of (tert-butyl)(amino)carbene M, namely the alkylation of the corresponding enamine, cannot be extrapolated to cyclic versions. Therefore, a new synthetic approach had to be designed.[65] Aldimines with a secondary alkyl substituent at the carbon atom are readily available from aldehydes and amines. Deprotonation leads to the corresponding aza-allyl anion, which can react with a variety of compounds with two leaving groups, thereby giving rise to the desired carbene precursors, namely the cyclic aldiminium salts. This synthetic strategy was first tested with aldimine 14 prepared from 2,6-diisopropylaniline and the simplest aldehyde with a secondary alkyl substituent, namely 2-methylpropanal (Scheme 15). Deprotonation with LDA affords the aza-allyl anion, which readily induces the ring opening of 1,2-epoxy-2-methylpropane to afford the corresponding alkoxide 15. Subsequent treatment with triflic anhydride at −78°C gives rise to the triflate derivative, which upon warming to room temperature affords the aldiminium salt CAAC1(H+) in 58% yield (based on the imine). Deprotonation with LDA then gives carbene CAAC1 quantitatively as a pale yellow solid.
Scheme 15.
First synthetic route for the preparation of the small CAAC1.
Although the synthetic route described above has a broad scope of application, some of the reagents are quite expensive. Since we wish to make CAACs available in large quantities, we searched for a practical and economical synthesis of their direct precursors, namely their conjugate acids. Among the different methods we have developed, the “hydro-iminiumation” route appears to be the most suitable. The idea was based on one of the most appealing synthetic approaches for the preparation of nitrogen-containing heterocyclic systems, that is, the intramolecular hydroamination of alkenes.[66] Various sophisticated catalysts have been used to effect this transformation, but when an electron-withdrawing group (W) is present on the nitrogen atom, traces of acid promote the hydroamination reaction (Scheme 16).[67] The first step is protonation of the amine, followed by intra-molecular transfer of the proton to the double bond, and lastly trapping of the generated cation by the amino group. Accordingly, cyclization does not occur in the absence of electron-withdrawing groups at the nitrogen atom, because the excessive basicity of the amino group prevents the transfer of the proton to the olefin. Imines are certainly not overly basic, and therefore the feasibility of “hydro-iminiumation” reactions was quite likely, and indeed this chemical transformation is quite general.[68]
Scheme 16.
Hydro-amination (left) and hydro-iminiumation (right).
The preparation of the enantiomerically pure CAAC2 without time consuming enantio- or diastereoselective separation is given here as an illustration of this method (Scheme 17). There is a well-known propensity for relatively bulky reactants to approach a cyclohexane moiety from the equatorial direction (this effect being reinforced here by the presence of the isopropyl group); thus, 3-bromo-2-methyl-propene was added to the aza-allyl anion derived from (−)-menthone, and the corresponding enantiomerically pure alkenyl aldimine 16 was obtained in 94% yield. After addition of HCl to form the alkenyl aldiminium salt 17 (which can be isolated), the intramolecular hydro-iminiumation occurred, and was complete after 5 h at 50°C. The optically pure cyclic iminium salt CAAC2(H+) was isolated in 92% yield, and deprotonated with LDA to give CAAC2 in 95% yield.
Scheme 17.
Synthesis of enantiomerically pure CAAC2, which illustrates the hydro-iminiumation route.
It is apparent from the molecular structure of CAAC2 (Figure 3) that the steric environment is very different from that of phosphines and NHCs. The N–Ccarbene bond length (1.31 Å) is shorter than in NHCs (1.34–1.38 Å), which is not surprising since only one nitrogen atom interacts with the carbene center in CAACs. The Ccarbene–C bond length (1.52 Å) is in the range expected for a single bond, and the carbene bond angle (106.5°) is comparable to that observed for NHCs (102–107°). However, the signal for the carbene carbon atom of CAACs appears at much lower field (309–323 ppm) than for NHCs (205–244 ppm) in the 13C NMR spectrum.[26b]
Figure 3.

Solid-state structure of enantiomerically pure CAAC2.
CAAC1, which features a Dipp group at the nitrogen atom but only two methyl substituents on the carbon atom adjacent to the carbene center, is stable at room temperature in the solid state and in solution for at least two weeks. Therefore, it is clear that the choice of the substituents on the carbon atom is virtually unlimited (except that they cannot be H), without precluding isolation of CAACs. This offers the possibility of constructing stable carbenes with very different types of steric environment.
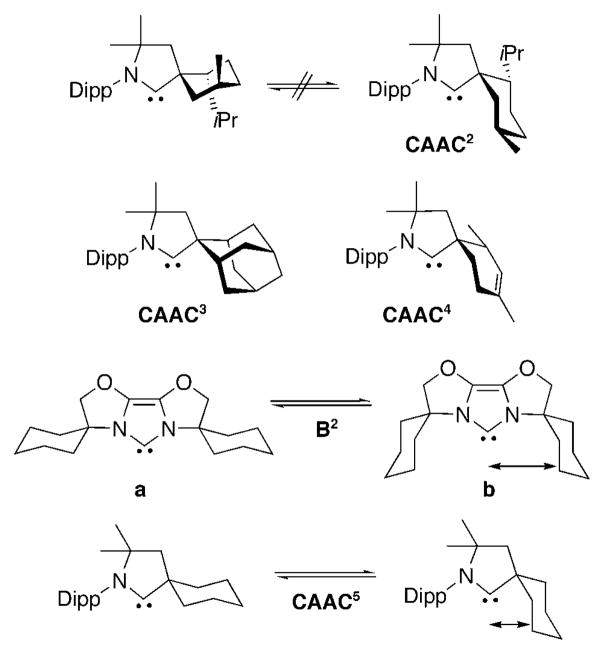
The enantiomerically pure CAAC2 is an example of what we named “rigid” CAACs. Indeed, the chair conformation of the cyclohexane is locked (Figure 3), since a ring flip would put both the isopropyl and methyl groups in unfavorable axial positions (Scheme 18); even a boat conformation would be highly adverse—in this conformation, the cyclohexane moiety constitutes a “wall of protection” not only for the carbene center, but also for any metal bound to the CAAC2. Other examples of rigid CAACs that we have also isolated are CAAC3[69] and CAAC4,[70] with the latter being by much cheaper to synthesize (Scheme 18). Indeed, CAAC2 and CAAC3 have to be prepared from the rather expensive (−)-menthone and 2-adamantanone, respectively, and an homologation step is required. CAAC4 is formed from a 95:5 mixture of cis- and trans-2,4-dimethyl-3-cyclohexenecarbox-aldehyde (trivertal), a common fragrance and flavor material produced in bulk quantities.
Scheme 18.
Rigid CAAC2–4, as well as NHC B2 and CAAC5 with flexible steric bulk.
CAAC5, with a nonsubstituted cyclohexane ring, illustrates the concept of “flexible steric bulk”. It was successfully developed for catalytic purposes by Glorius and co-workers,[25h,71] and can be incorporated into this ligand family. The idea is to have a ligand, which has a conformation that generates a small steric bulk (conformation a) to accept sterically hindered substrates, and another sufficiently bulky conformation (b) to support monoligation and promote reductive elimination (Scheme 18). In contrast to the NHCs B2 developed by Glorius and co-workers, there is only one cyclohexane ring in CAAC5, but it is much closer to the carbene and to the eventually coordinated metal center. Therefore, the effect of the “flexible wing” is amplified in CAAC5 compared to NHC B2.
Although a lot of substitution patterns are possible on the quaternary carbon atom, this is not the case for the substituent on the nitrogen atom. So far, only a Dipp group has allowed for the isolation of CAACs. However, it should be mentioned that with a 2,6-diethylphenyl group, the corresponding CAAC(H+)s can be deprotonated in situ in the presence of a metal fragment, thereby allowing the preparation of the corresponding CAAC–metal complex.[72] It seems quite likely that the instability of CAACs that do not bear a Dipp group on the nitrogen atom is due to the high basicity of the carbene center. The latter can deprotonate primary or secondary alkyl groups of the N-aryl substituents, which are more acidic than in NHCs because of strong donation of the single nitrogen atom to the carbene center.
4.3. Ligand Behavior
The coordination behavior of CAACs and the catalytic activity of the ensuing complexes are by far the most studied of all the species discussed in this Review. As can be seen below, CAACs ligands can give rise to unusual transition-metal complexes, and the catalytic activity of CAAC–palladium, –gold, and –ruthenium complexes is unique.
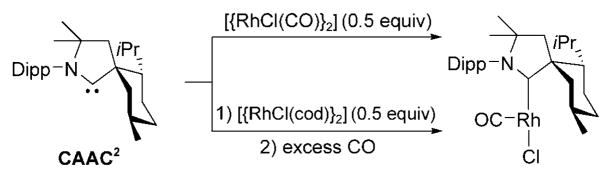
It has already been shown that sterically hindered NHCs allow for the isolation of low-coordinate unsaturated metal complexes,[73] as exemplified by the preparation of three-coordinate carbonyl nickel derivatives [Ni(CO)2(NHC)] (NHC =N,N′-di(tert-butyl)- or di(adamantyl)imidazol-2-yli-dene).[74] Along this line, a very unusual rhodium complex was prepared with the bulky, rigid CAAC2. The addition of [{RhCl(CO)2}2] to CAAC2, and even treatment of [RhCl-(cod)(CAAC2)] with excess CO, did not afford the expected dicarbonyl complex, but instead cleanly led to the 14-electron [RhCl(CO)(CAAC2)] complex (Scheme 19).[75]
Scheme 19.
Synthesis of 14-electron [RhCl(CO)(CAAC2)].
Related [RhCl(L)2] complexes, exemplified by the active species of Wilkinson3s catalyst [RhCl(PPh3)2], were known only as transient species.[76] They are only generated in situ by ligand dissociation[77] or by changes in the hapticity;[78] otherwise they readily form chloro-bridged dimers, even when two very bulky ligands L are present.[79] The surprising stability of [RhCl(CO)(CAAC2)], formally a 14-electron species, is partly due to the extreme hindrance provided by the menthyl ring, but also to the presence of metal–hydrogen interactions (Figure 4). Indeed, the X-ray crystal structure shows short Rh–H distances (2.18 and 2.23 Å), and in the 1H NMR spectrum there is a broad multiplet (1H) at d = 0.08 ppm. The complex [RhCl(CO)(CAAC2)] is indefinitely stable at room temperature in the open air. Although other neutral T-shaped formally 14-electron RhI complexes have been isolated, they are still very rare, and none of them have a halogen that can act as a bridging ligand.[80]
Figure 4.

Molecular structure of [RhCl(CO)(CAAC2)] showing the metal–hydrogen interactions.
CAAC2 has also been used to prepare the cationic 14-electron palladium complex [Pd(allyl)(CAAC2)]BF4 by simple treatment of the corresponding palladium chloride with AgBF4. As expected, this complex features a T-shaped geometry (Figure 5, left) with no interaction between the metal center and the tetrafluoroborate anion. However, similar to the previously mentioned [RhCl(CO)(CAAC2)] complex, at least one of the axial H atoms of the menthyl ring provides a stabilizing interaction (Pd–H: 2.05 and 2.51 Å), which is confirmed by the presence of a broad multiplet (1H) at δ =−0.17 ppm in the 1H NMR spectrum. This result is especially striking since all attempts failed to prepare similar complexes by using bulky NHCs and phosphines.[81] Indeed, even with the help of intramolecular stabilization by complexation with the alkene, complex 18 (Figure 5, right) appeared to be rather unstable, and was only characterized by 1H NMR spectroscopy.[82] [Pd(allyl)(CAAC2)]BF4 is the first example of a stable, formally 14-electron, PdII cation, although neutral, three-coordinate, T-shaped d8 palladium(II) complexes were isolated.[83]
Figure 5.
X-ray crystal structure of: left: [Pd(allyl)(CAAC2)]BF4 (anion omitted for clarity), and right: complex 18 characterized only by 1H NMR spectroscopy (right).
Similarly, bulky rigid CAACs can stabilize unusual cationic gold(I) species. The addition of the adamantyl-substituted CAAC3 to (Me2S)AuCl affords the [Au-(CAAC3)Cl] complex in excellent yield.[84] The chloride is then abstracted by reaction of a suspension of this complex in toluene with the silylium-like salt [(Tol)SiEt3]+ [B(C6F5)4] −[85] to afford CAAC3Au+. The X-ray diffraction study showed that it was not a naked [Au(L)]+ complex, since a toluene molecule was η2-coordinated to the metal center (Figure 6).[69] However, in CAAC3Au+ there is little perturbation of the aromatic toluene ring, which implies weak coordination. Interestingly, this complex appeared to be indefinitely stable in solution and in the solid state. Although this complex is not unique, only a few other similar π–arene complexes with very bulky phosphine ligands have been isolated.[86,87]
Figure 6.
X-ray crystal structure of [Au(CAAC3)(η2-toluene)]B(C6F5)4 (CAAC3Au+)
The isolation of low-coordinate Rh, Pd, and Au complexes clearly demonstrates that it is possible to design CAAC ligands that feature a wall of protection for the eventually coordinated metal center by manipulating the quaternary carbon atom adjacent to the carbene center. Moreover, since low-coordinate metals often play a key role in catalytic processes, these results show that catalysts based on CAAC ligands deserve extensive studies.
4.4. Catalysis
4.4.1. Palladium–CAAC Complexes
The steric and electronic properties of CAACs should benefit the numerous catalytic processes that require bulky electron-rich ligands at the metal center. As a first example, we studied the palladium-catalyzed α-arylation of carbonyl compounds, a process discovered simultaneously in 1997 by the research groups of Buchwald,[88] Hartwig,[89] and Miura.[90]
Three [PdCl(allyl)(CAAC)] complexes were prepared by the addition of [{Pd(allyl)(Cl)}2] to CAACs with very different steric environments at the quaternary carbon atom, which is next to the carbene center (Figure 7). They were isolated in high yields as air-stable colorless crystals. Table 1 summarizes the results obtained using these complexes for the α-arylation of propiophenone and isobutanal, the classical substrates for such a reaction.
Figure 7.
Solid-state structures of palladium complexes showing the different steric environments provided by small CAAC1 (left), bulky rigid CAAC2 (middle), and flexible CAAC5 (right).
Table 1.
Influence of the steric properties of CAAC ligands on the palladium-catalyzed α-arylation of propiophenone and isobutanal with aryl chlorides.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | R1 | R2 | Aryl chloride | Catalyst | Cat. [mol %] | T [°C] | t [h] | Yield [%] |
| 1 | Ph | H | PhCl | CAAC1Pd | 0.5 | 23 | 70 | 22 |
| 2 | CAAC2Pd | 0.5 | 23 | 1 | 100 | |||
| 3 | CAAC2Pd | 0.01 | 23 | 38 | 72 | |||
| 4 | CAAC5Pd | 0.5 | 23 | 70 | 29 | |||
| 5 | Ph | H | 2,6-Me2PhCl | CAAC1Pd | 0.5 | 23 | 70 | 0 |
| 6 | CAAC2Pd | 0.5 | 50 | 20 | 0 | |||
| 7 | CAAC5Pd | 0.5 | 23 | 36 | 32 | |||
| 8 | CAAC5Pd | 1 | 50 | 20 | 81 | |||
| 9 | H | Me | PhCl | CAAC2Pd | 1 | 23 | 16 | 98 |
With nonhindered aryl chlorides (entries 1–4), the most bulky CAAC2Pd complex is by far the best catalyst for the α-arylation of propiophenone. A turnover number (TON) of up to 7200 was obtained at room temperature (entry 3). This compares extremely favorably with the best TON reported so far of 4100 at 120°C by using an NHC ligand.[91] No catalytic activity was observed with CAAC2Pd nor with CAAC1Pd when a di-ortho-substituted aryl chloride was used (entries 5–8), but in marked contrast, CAAC5Pd was active, even at room temperature. Clearly, carbene CAAC1 is not sterically hindered enough to favor reductive elimination at room temperature with any aryl chloride. This step is easily promoted by the very rigid and bulky CAAC2 ligand. However, entry 6 shows that CAAC2 gives rise to a catalyst that is very sensitive to excessive steric hindrance, probably by preventing the oxidative addition step. This step becomes possible when the flexible carbene CAAC5 is used. In the solid state this carbene presents a steric environment around the metal center that is very similar to that of CAAC1 (Figure 7), however, in solution, the cyclohexane moiety of CAAC5 can easily undergo a ring flip. This second conformer certainly has a steric environment very similar to that of CAAC2, which consequently aids the reductive elimination step.
Although the α-arylation of carbonyl compounds has a broad scope of application,[92] very little success has been reported with aldehydes,[93] mostly because of the competing aldol condensation. By taking advantage of the mild conditions that can be used with Pd–CAAC complexes, it is possible to prevent this side reaction, as shown by the highly efficient coupling of chlorobenzene with isobutanal (entry 9). The α-arylation occurred at room temperature in 98% yield by using 1 mol% of CAAC2Pd, with no evidence of aldol condensation products.
Up to now, and despite enormous progress in the palladium-catalyzed α-arylation of ketones[94] and aldehydes,[95] CAACs are the only ligands that efficiently promote these reactions with aryl chlorides at room temperature. Similarly, no other α-arylations of carbonyl compounds with ortho-disubstituted aryl halides have been reported.
4.4.2. Gold–CAAC Complexes
A very surprising catalytic reaction was serendipitously discovered using [Au(CAAC3)(η2-toluene)]B(C6F5)4 (CAAC3Au+; Scheme 20).[69] Indeed, many transition-metal complexes, including gold complexes, are known to catalyze the addition of terminal alkynes to enamines, thereby affording propargyl amines.[96] In marked contrast, CAAC3Au+ efficiently mediates the catalytic coupling of enamines and terminal alkynes to yield allenes with the loss of imines. Mono-, di-, and trisubstituted enamines can be used, as well as aryl-, alkyl-, and trimethylsilyl-substituted terminal alkynes. The reaction tolerates sterically hindered substrates, and is diastereoselective. Importantly, when AuCl, AuCl/(Tol)SiEt3+B(C6F5)4−, [AuCl(PPh3)]/KB(C6F5)4, and even neutral [AuCl(CAAC)] complexes were used as catalysts, the propargyl amine was the major product (>95%), with traces of allene (<2%) detected only in the case of [AuCl(PPh3)]/KB(C6F5)4. From these results it is clear that the gold center must be coordinated by the CAAC ligand to efficiently catalyze formation of the allene, and it must also be rendered cationic by Cl abstraction. Mechanistic studies indicate that the reaction most probably proceeds through an unprecedented “carbene/vinylidene cross-coupling reaction”. This is the first general catalytic protocol to directly couple two unsaturated carbon centers to form the C3 allenic core.
Scheme 20.
Metal-catalyzed coupling of enamines and terminal alkynes.
Since NH3 is one of the largest volume and least expensive bulk chemicals, one of the greatest challenges of synthetic chemistry is to find atom-efficient processes that are capable of combining NH3 with simple organic molecules to create nitrogen–carbon bonds. The most appealing process is the addition of NH3 to C–C multiple bonds, a process that ideally occurs with 100% atom economy. Although various homogeneous catalysts have been used to effect the so-called hydroamination reaction,[66,97] none of them were reported to be effective when NH3 was used as the amine partner.
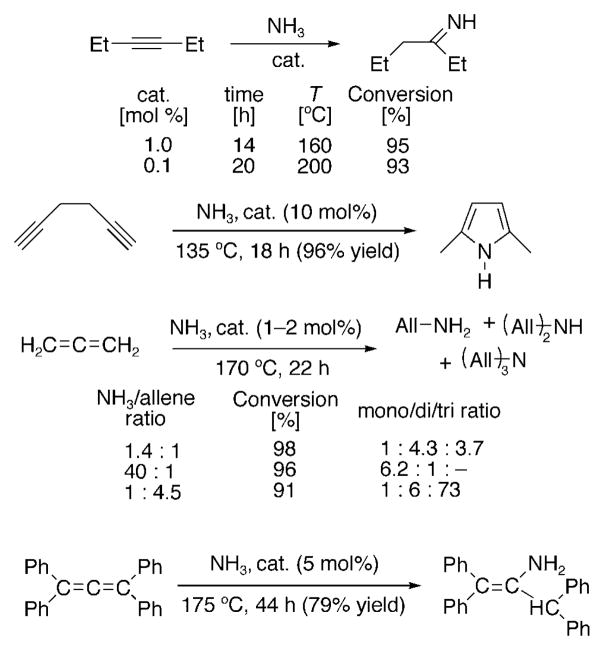
In 2008, it was found that cationic [Au(CAAC)]+ complexes, including the Werner-type complex [Au(CAAC3)-(NH3)]B(C6F5)4, readily catalyze the addition of ammonia to a variety of unactivated alkynes and allenes, thereby providing access to a diverse array of linear and cyclic nitrogen-containing compounds (Scheme 21).[98] As an illustration, the use of 3-hexyne as a substrateled to isolation of the corresponding imine in almost quantitative yield. Furthermore, the catalyst is very thermally robust, with no decomposition observed after heating it for 20 h at 200°C. Since nitrogen heterocycles are an important class of compounds that widely occur in natural products and often display potent biological activity, we attempted the direct synthesis of heterocycles from diynes and NH3. For example, the use of hexa-1,5-diyne led to the corresponding 2,5-disubstituted pyrrole in 96% yield. The scope of the NH3-hydroamination reaction was then expanded to allenes. A mixture of mono-, di-, and triallylamine was obtained in excellent yield by using 1,2-propadiene. Allyl amines are among the most versatile intermediates in synthesis and are of industrial importance. For example, the parent compound, which is produced commercially from ammonia and allyl chloride, is used in antifungal preparations and polymers. As can be seen, the selectivity of this reaction can be controlled by varying the NH3/allene ratio. It is particularly interesting that the parent allylamine and the triallylamine can be obtained with 86 and 91% selectivity, respectively (not optimized). The addition of NH3 to 1,2-dienes is not restricted to the parent allene: Even tetrasubstituted allenes undergo hydroamination with ammonia. However, a different regioselectivity is observed (probably because of steric factors), and only the mono hydroamination product is formed.
Scheme 21.
[Au(CAAC3)(NH3)]B(C6F5)4-catalyzed NH3 hydroamination of alkynes and allenes.
Catalytic systems that are able to promote the intermolecular hydroamination of alkynes and allenes with secondary amines are also quite rare. In the case of alkynes, it was reported that benzocyclic amines can be used, in the presence of cationic ruthenium hydride, in a hydroamination/C–H bond-activation process.[99] Similarly, lanthanide complexes were found to promote the addition of secondary amines to terminal alkynes, as a part of tandem hydroamination/C–C bond-forming processes.[100] In addition, several examples of the hydroamination of terminal alkynes with secondary (aryl)(alkyl)amines in the presence of mercury and thallium compounds are known,[101] but the high toxicity of the catalyst is a major drawback. The only hydroamination reaction of allenes with a secondary amine was reported by Nishina and Yamamoto,[102] who used morpholine, and mono- and disubstituted allenes at 80°C, with 10 mol% of a 1:1 mixture of [AuCl(Ar3P)] and AgOTf.
It was found that, in the presence of 5 mol% CAAC3Au+, diarylamine, arylalkylamine, and benzocyclic amine add to terminal alkynes as well as internal alkynes at 60–120°C, with reaction times of 7–24 h.[103] More strikingly, this catalytic process is also efficient for simple dialkylamines such as diethylamine (Scheme 22). The only noticeable difficulties were found with phenylacetylene, because of competitive oligomerization processes. The expected mixture of Markovnikov and anti-Markovnikov products was obtained with methylphenylacetylene, but more surprising were the results observed with diethylacetylene. Indeed, a mixture of the expected hydroamination adduct and an isomer, in which the unsaturation had been shifted, was observed. So far, there is no explanation for this isomerization.
Scheme 22.
Hydroamination of alkynes with diethylamine by using 5 mol% CAAC3Au+.
Similarly, CAAC3Au+ promotes the hydroamination of allenes with a variety of amines.[104] Morpholine, as well as benzylic and benzocyclic amines, react smoothly at 70–90°C to afford the hydroamination products in yields of 93–99% after only 8–12 h. More importantly, although drastic conditions are required with diethylamine (130–165°C, 24–36 h), the addition occurs to yield the Markovnikov adduct in 61–98% yield (Scheme 23). These results emphasize the robustness of the CAAC catalyst.
Scheme 23.
Hydroamination of allenes with diethylamine by using 5 mol% CAAC3Au+.
The availability of catalysts able to perform the hydroamination reaction of alkynes with secondary amines (Scheme 22) opens the way for cascade reactions. By combining the reactions showed in Schemes 20 and 22, the one-pot preparation of allenes by coupling two alkynes was first investigated by using a sacrificial secondary amine. As an example, the homocoupling of tert-butylacetylene in the presence of one equivalent of 1,2,3,4-tetrahydroisoquinoline (THQ) was complete after 16 h at 120°C by using 5 mol% CAAC3Au+, and the expected allene was formed in 89% yield (Scheme 24).[103] The scope of this tandem reaction was expanded to the cross-coupling reaction of alkynes. Solutions of an internal alkyne and 0.9 equivalent of THQ in benzene were first heated at 120°C in the presence of 5 mol% CAAC3Au+. The reactions were monitored by NMR spectroscopy, and after complete conversion of the amine, 0.9 equivalent of a terminal alkyne was added to the reaction mixtures. The expected allenes were formed in good to excellent yields after heating the solution for 16 h at 130°C. This reaction appeared to be quite general, although with some regioselectivity issues, and is of course limited to the use of terminal alkynes for the second step.
Scheme 24.
One-pot synthesis of allenes from two alkynes and a sacrificial amine by using 5 mol% CAAC3Au+. THQ = 1,2,3,4-tetrahydroisoquinoline.
Inspired by the recent works of Yi et al.[99,105] and Che and co-workers,[106] we then studied the one-pot three-component synthesis of 1,2-dihydroquinoline derivatives by a tandem hydroamination-hydroarylation reaction (Scheme 25).[70] Both homo- and cross-coupling reactions are possible, the only serious limitation is, as for the tandem reaction shown in Scheme 23, the use of a terminal alkyne for the second step. Consequently, the dihydroquinoline skeleton can be readily decorated with three different (R1, R2, and R3) substituents. For the cross-coupling process, the hydroamination of the first alkyne has to be monitored by spectroscopy, and only after complete conversion can the terminal alkyne be added.
Scheme 25.
Tandem hydroamination-hydroarylation reaction, promoted by CAAC3Au+.
Tanaka and co-workers[107] reported that a cationic gold(I) complex, similar to CAAC3Au+ but bearing triphenylphosphine as an ancillary ligand, promoted the intermolecular hydroamination of terminal as well as internal alkynes with a variety of primary aryl amines, but the protocol did not tolerate alkyl amines and secondary amines.[108] Similarly, NHC analogues of CAACAu+ did not allow the use of internal alkynes for the three-component synthesis of 1,2-dihydroquinoline derivatives.[99,105] The comparison of these results with our findings clearly demonstrates the specific properties of the CAAC ancillary ligand.
On the basis of preliminary mechanistic studies we postulated that the key step in the catalytic cycle for the hydroamination processes described above was the formation of a tricoordinate gold complex, which was followed by an inner-sphere C–N bond formation, as first postulated by Tanaka et al.,107 as well as by Nishina and Yamamoto.[109] We attempted to isolate such a tricoordinate gold(I) complex to confirm this hypothesis. For entropic reasons, intramolecular hydroamination reactions occur under much milder conditions than the intermolecular version, and are therefore better suited for characterizing reaction intermediates. We chose 2-alkynyl-N,N-dimethylbenzenamine 19, since the rigidity of the phenyl spacer places both the amino group and the alkyne in perfect positions to coordinate the metal center. Moreover, the absence of an N–H bond would prevent the hydroamination process going to completion. A stoichiometric amount of CAAC3Au+ was added at room temperature, and we observed the instantaneous disappearance of 19 and the concomitant formation of complex 20, which was isolated in 98% yield. The single-crystal X-ray diffraction study demonstrated that 20 was not the desired tricoordinate gold(I) complex, but a gold(I)–vinyl complex resulting from the addition of the tertiary amino group to the coordinated alkyne (Scheme 26).[110] Complex 20 is reminiscent of complexes recently isolated by Hammond and co-workers[111] as well as by Gagné and co-workers[112] in the gold-promoted cyclization of allenoates and intramolecular hydroarylation of allenes, respectively.[87c,113] The formation of 20 argues against the hypothesis of an inner-sphere mechanism for the hydroamination, and is in favor of an outer-sphere nucleophilic attack on the alkyne π complex. Such a mechanism had been postulated for several gold-catalyzed reactions.[108a,b,114]
Scheme 26.
Isolation of gold(I)–(η1-alkene) complex 20, as well as examples of catalytic hydroammoniumation and methylamination reactions to give 21 and 22, respectively.
Not surprisingly, treatment of complex 20 with one equivalent of trifluoromethanesulfonic acid instantaneously induced the protodeauration to afford the corresponding gold-free cyclic ammonium salt 21. The stoichiometric two-step transformation of 19 into 21 via 20 led to the question of whether this process could be catalytic in gold, or if the presence of triflic acid would induce the protonation of the basic tertiary amine and prevent the cyclization process. Protonation of 19 but no cyclization occurred in the absence of a gold catalyst, but in the presence of triflic acid, even under heating in a sealed tube at 120°C for three days. In contrast, heterocycle 21 was obtained in 98% yield after only 3 h at 70°C in the presence of 5 mol% of a 1:1 mixture of [AuCl(CAAC3)]/AgOTf and one equivalent of triflic acid, (Scheme 26). The scope of this hydroammoniumation reaction was briefly explored. It was found that aryl and alkyl groups are tolerated on the alkyne, and that the cyclization process occurs under milder conditions when a weaker basic amine is used.
Examples of the direct carboamination of alkynes (the addition of a carbon–nitrogen bond to a carbon–carbon triple bond) are very rare. Yamamoto and co-workers[115] reported the platinum- and palladium-catalyzed intramolecular C–N bond addition of amides and N,O-acetals, and Cacchi et al.[116] the palladium-catalyzed cyclization of 2-alkynyl-N-allyl-N′-trifluoroacetylbenzenamine. Although, in both cases, the cleavage of a relatively weak carbon–nitrogen bond was involved, these results prompted us to investigate the related methylamination reaction. 2-Alkynyl-N,N′-dimethylbenzenamines, such as 19, were transformed into 2,3-disubstituted indoles 22 in good to excellent yields after 20 h at 160°C in the presence of 10 mol% of a 1:1 mixture of [AuCl(CAAC3)]/KB(C6F5)4 (Scheme 26).
It is noteworthy that some of the catalytic hydroamination reactions discussed in this section were performed at very high temperatures, which demonstrates the robustness of cationic gold(I)–CAAC complexes. In fact, no decomposition was observed on heating [Au(CAAC3)(NH3)]B(C6F5)4 at 200°C for 2 days.
4.4.3. Ruthenium–CAAC Complexes
The most dramatic advance in ruthenium catalysts for olefin metathesis was observed after exchanging a single PCy3 ligand of Gr1[20] and HG1[117] with an NHC of type C (Scheme 27). The higher activity of Gr2 and HG2 has been rationalized by the superior σ-donating ability of the NHC over PCy3, which increases the affinity of the metal center for olefinic substrates.[118] Therefore, CAACs appeared to be excellent candidates as ligands for ruthenium catalysts for olefin metathesis, a project that we are developing with the the Grubbs research group.
Scheme 27.
Classical ruthenium olefin metathesis catalysts Gr1,2 and HG1,2. CAAC1,5,6 were used for preparing 24a,b, and CAAC1,5,6Ru.
We first attempted to prepare ruthenium–CAAC complexes by substituting the pyridine ligands of 23.[72a] However, in contrast to NHCs, which replace both pyridine ligands and retains the phosphine to give Gr2,[119] the addition of CAACs led to pyridine adducts 24a,b. These results are not yet understood. We then moved to the Hoveyda–Grubbs catalyst HG1, and were able to exchange the phosphine ligand and isolate complexes CAAC1,5,6Ru in good to excellent yields (Scheme 27). X-ray diffraction studies on CAAC1,5,6Ru show that the Ru–Ccarbene bond lengths are about 0.04–0.05 Å shorter and the Ru–O bond lengths 0.04–0.09 & longer than the corresponding NHC complex HG2. These findings are consistent with the CAACs acting as stronger σ donors than their NHC counterparts.
The catalytic activity of the air-stable CAAC complexes CAAC1,5,6Ru in ring-closing metathesis reactions was explored with three prototypical substrates that targeted di-, tri-, and tetrasubstituted olefins (Scheme 28). The best results were obtained with CAAC6Ru, but none of these CAAC complexes were active for the formation of the tetrasubstituted olefins.[72a] The dramatic increase in activity observed after slightly decreasing the steric bulk of the N-aryl group [Dipp (2,6-diisopropylphenyl) to Dep (2,6-diethylphenyl)] is attributed to catalyst initiation. We postulate that this step requires dissociation of the ether moiety and rotation of the benzylidene ring into a plane parallel to the N-aryl group to open a coordination site for the incoming olefin; for complexes CAAC5Ru and CAAC5Ru (with the Dipp group) this process may be disfavored for steric reasons.
Scheme 28.
Application of CAAC1,5,6Ru in ring-closing metathesis reactions.
We then turned our attention to the catalytic activity of complexes CAAC1,5,6Ru toward olefin cross-metathesis reactions, an area of significant interest. Indeed, highly active NHC based catalysts such as Gr2 and HG2 generally produce mixtures in which the thermodynamic products predominate: more E than Z isomers, and more internal olefins than terminal olefins (ethenolysis).[24w,120] We found[72b] that relative to the commercially available catalysts Gr1,2 and HG1,2, CAAC-substituted complexes enhanced the Z/E stereoselectivity in favor of the desired Z olefin (3:1 at 70% conversion) in the cross-metathesis of cis-1,4-diacetoxy-2-butene with allylbenzene. Similar Z/E ratios were only observed by Blechert and co-workers[121] when utilizing a ruthenium complex bearing an unsymmetrically substituted NHC.
More striking are the results obtained with complexes CAAC1,5,6Ru for the ethenolysis of methyl oleate.[72b] This is an important process that transforms internal olefins derived from seed oils to terminal olefin feedstocks,[122] and for which a highly efficient and selective catalyst has yet to be developed. Previous detailed studies showed that Gr1 and HG1[24w,120] are highly selective for the production of the desired terminal olefins c and d (over self-metathesis products e and f; Scheme 29). However, catalyst decomposition, as a consequence of the instability of the propagating methylidene species, and catalyst inhibition by the ethenolysis products occurred. Conversely, Gr2 and HG2 demonstrated relatively low selectivity for the synthesis of the desired terminal olefins. Under the same conditions, at a loading of 100 ppm, catalysts CAAC1,5,6Ru exhibited good selectivity (73–94%) for terminal olefins c and d, and achieved TONs ranging from 4200 to 5600. By lowering the catalyst loading of CAAC6Ru to 10 ppm, TONs of 35000 were achieved. Prior to this study, the highest TON reported was 14000, which was obtained when using a bis(9-cyclohexyl-9-phospha-9H-bicyclononane)ruthenium complex.[123]
Scheme 29.
Comparative catalytic activity of various ruthenium catalysts for the ethenolysis of methyl oleate.
Catalyst CAAC6Ru, the smallest of the series, appeared to be the most active in all the metathesis reactions studied so far. These results provide a clear direction for the design of ruthenium catalysts for olefin metathesis.
5. Cyclic (Amino)(ylidic)carbenes
5.1. Background
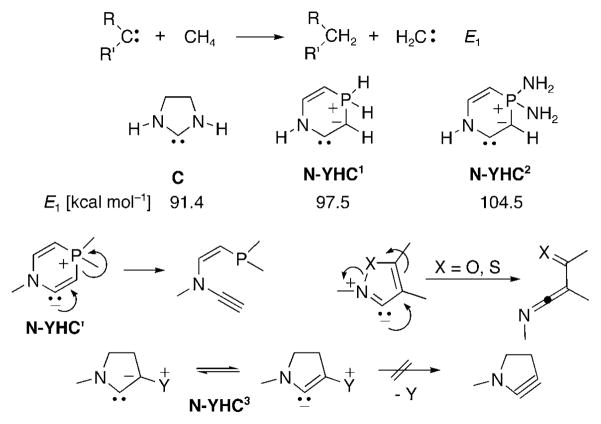
Replacing one nitrogen center of NHCs by an sp3-hybridized carbon atom leads to CAACs, which are already more nucleophilic (σ donating). One way to increase the electron density at the carbene carbon atom even further is to replace the sp3-carbon atom of CAACs by a carbanion. Of course, a carbene has to be neutral, which implies that the carbanion has to be part of an ylide, and as a consequence some of its electron density will be shifted towards the cationic component. This rather intuitive approach was confirmed by calculations. Nuylasziet al.[124] showed that there is an excellent linear correlation between the nucleophilicity of carbenes and the value of the energy E1 obtained in the isodesmic reaction shown in Scheme 30. For the parent saturated NHC C, an E1 value of 91.4 kcalmol−1[124,42a] was calculated, while for carbenes N-YHC2 and N-YHC2, which feature a phosphorus ylide moiety, the E1 values were 97.5 and 104.5 kcalmol−1, respectively.[42a] These results not only show the superior donor ability of a carbanion compared to an amino group in the α position of the carbene, but also show that the substituents on the phosphorus atom could allow for a fine-tuning of the carbene’s electronic properties. Clearly, compared to H, the NH2 substituents stabilize the P+ moiety of N-YHC2, thereby preventing some back donation of the carbanion lone pair of electrons to the P center. Experimentally, carbenes N-YHC1,2 are not interesting targets since, considering the resonance structure N-YHC′, it is quite likely that such carbenes would be unstable towards ring opening, as found for isoxazole[125] and isothiazole carbenes.[126] To prevent such a process, the positive part (Y) of the ylide has to be exocyclic, as in N-YHC3, since the formation of an endocyclic triple bond is highly unlikely.
Scheme 30.
The E1 value correlates with the nucleophilicity of the carbenes. The cationic part of the ylide has to be exocyclic to prevent ring-opening processes, as observed for other cyclic carbenes.
5.2. Synthesis, Characterization, and Stability
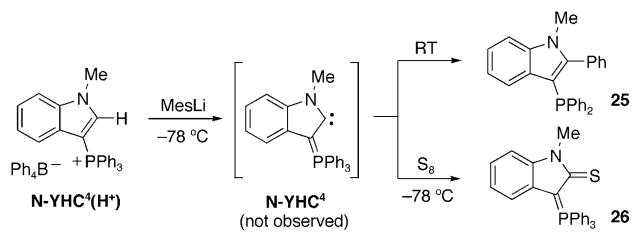
All the reports on N-YHC were published in 2008.[127–130] The first attempt to prepare a free N-YHC was reported by Kawashima and co-workers.[127] Treatment of phosphonium tetraphenylborate salt N-YHC4(H+) with mesityllithium at −78°C, and then warming the mixture to room temperature afforded heterocycle 25 as the major product (Scheme 31). As postulated by the authors, it is quite likely that 25 results from the transient formation of the desired N-YHC4 followed by a formal 1,3-phenyl shift. Although, the half-life of N-YHC4 did not permit its characterization by NMR spectroscopy, its transient formation was demonstrated by the formation of thioamide 26 when the deprotonation was carried out at −78°C in the presence of elemental sulfur. Furthermore, the transient N-YHC4 was also trapped with transition-metal species. Importantly, calculations showed that although N-YHC4 is not a poorer π acceptor than NHCs, it should behave as a very strong σ-donor ligand since its HOMO (−4.4 eV) is significantly higher than those of NHCs (−5.2 eV) and even CAACs (−5.0 eV).
Scheme 31.
Generation, rearrangement, and trapping of N-YHC4.
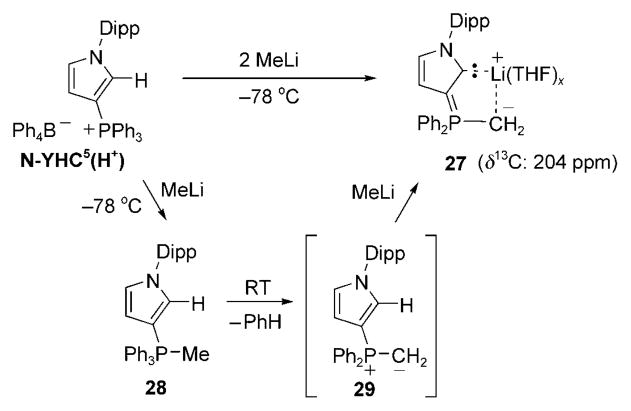
Our research group[128] attempted the deprotonation of another potential precursor of N-YHC by also using a triphenylphosphorus ylide as well as a ring system based on the skeleton of imidazolin-2-ylidenes (unsaturated NHCs B), instead of the benzimidazolin-2-ylidene E used by Kawashima and co-workers.[127] Treatment of the tetraphenyl borate salt of N-YHC5(H+) with a variety of bases (LDA, TMPLi, tBuLi, KHMDS) led to a complex mixture of products, but with no evidence for the desired carbene. However, the use of two equivalents of methyllithium resulted in a clean reaction taking place and isolation of the lithium complex 27 (Scheme 32). Monitoring the reaction of N-YHC5(H+) with one equivalent of methyllithium by variable-temperature multinuclear NMR spectroscopy showed the formation of the phosphorane intermediate 28. Based on literature precedents,[131] it is reasonable to postulate that the next step is the elimination of benzene with concomitant formation of ylide 29. The second equivalent of methyllithium can then deprotonate the heterocycle to afford the observed lithium complex 27. Although 27 is not the expected carbene, it can be viewed as a lithium adduct of an N-YHC, with the metal cation being coordinated further by an ylidic carbon atom and THF molecules.[132] Therefore, one can expect that the observed chemical shift of the carbene carbon atom of 27 (δ =204 ppm, JPC = 54 Hz) in the 13C NMR spectrum gives a good indication of the chemical shift of free N-YHCs (see below).
Scheme 32.
Deprotonation of N-YHC5(H+) affords stable lithium adduct 27, which has an N-YHC as part of a bidentate ligand.
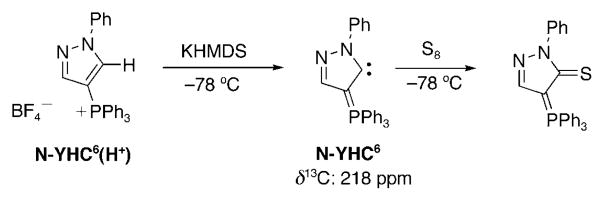
Very interestingly, Fürstner et al.,[129] using a triazolin-5-ylidene-like skeleton (D) with a phenyl group at the nitrogen atom instead of a benzimidazolin-2-ylidene as Kawashima and co-workers[127] or an imidazolin-2-ylidene as our research group,[128] was able to generate the free N-YHC6 (Scheme 33). This compound was sufficiently stable for spectroscopic characterization. A signal was observed at δ =218 ppm (JPC = 51.2 Hz) in the 13C NMR spectrum, which leaves no doubt to the formation of N-YHC6. Furthermore, the addition of sulfur gave the corresponding thioamide.
Scheme 33.
Synthesis, spectroscopic characterization, and trapping of N-YHC6.
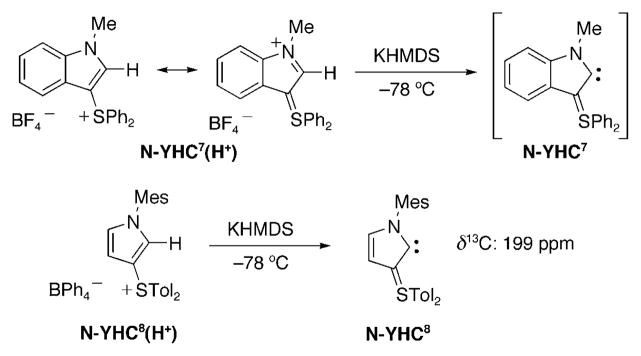
In the same publication, Fürstner et al.[129] reported the attempted preparation of N-YHC7 with a sulfur ylide moiety instead of a phosphorus ylide, and using a benzimidazolin-2-ylidene-like skeleton (Scheme 34). They noted that the solid-state structure of the precursor (N-YHC7(H+) revealed a significant degree of charge delocalization from the sulfonium group into the ring, and concluded that this transmission of charge predisposed the salt for deprotonation. However, although they could trap the putative N-YHC7 with metal species, they were not able to characterize it spectroscopically. In marked contrast, Kawashima and co-workers[130] used an imidazolin-2-ylidene type ring system and mesityllithium as a base, and observed a signal at δ =199 ppm in the 13C NMR spectrum recorded at −40°C. This is an unambiguous identification of N-YHC8, although they mentioned that N-YHC8 was part of a complex mixture of unidentified products.
Scheme 34.
Preparation of the transient N-YHC7 and persistent N-YHC8, showing the importance of the ring skeleton.
By comparing Schemes 26 and 29, one could conclude that benzimidazolin-2-ylidenes are not the correct skeleton to build stable N-YHCs. However, it should be noted that a methyl group has always been used as the substituent on the nitrogen atom; therefore, the instability of the corresponding N-YHCs might be due to a lack of steric bulk. From the results of the deprotonation of N-YHC5(H+) and N-YHC6(H+), it is clear that the triazolin-5-ylidene is better than the imidazolin-2-ylidene-like skeleton, which is in line with the weaker basicity of the former. Lastly, the fate of the deprotonation reaction of N-YHC5(H+) versus N-YHC8(H+) tends to indicate that sulfur ylides are more appropriate than phosphorus ylides for stabilizing N-YHCs.
As shown by Fürstner et al.,[129] other types of ylides can be envisaged. It is quite likely that the right combination of ylides and ring skeleton will allow for the preparation of isolable N-YHCs, which are desirable ligands for transition-metal-based catalysts. Simply by combining the observations described above, one can predict that a sulfur ylide analogue of N-YHC6 should be isolable.
5.3. Ligand Behavior and Catalysis
Very few studies have been carried out on the coordination properties of N-YHCs. [RhCl(CO)2(N-YHC)] complexes were prepared, essentially to compare the electronic properties of N-YHCs with the other cyclic non-NHCs and classical NHCs (see Section 9). [RhCl(cod)(N-YHC)] complexes of most of the carbenes discussed in this section were also synthesized. The Rh–Ccarbene bond length and other parameters around the metal center are within the range of those reported for the analogous NHC complexes. However, it was noted that the Ccarbene–N bond lengths (ca. 1.38 Å) are slightly longer than those observed for NHCs, thus suggesting that, as expected, π donation of the amino group to the carbene center is weaker.
The two palladium complexes N-YHC4Pd [127] and YHC5Pd[128] were also characterized crystallographically. Preliminary experiments showed that 5 mol% of YHC5Pd promotes the amination of p-tolyl bromide and morpholine at 80°C in 2 h.[127a] Although these results are not spectacular, they suggest that N-YHCs deserve further study.

6. Cyclopropenylidenes
6.1. Background
Up until 2006, four-membered NHCs[51] were the smallest ring systems featuring a carbene center. Moreover, it was generally believed that singlet carbenes could be isolated only if their electron deficiency was reduced by the presence of at least one π-donor heteroatom, preferably nitrogen or phosphorus, directly bonded to the carbene center. Clearly, three-membered ring carbenes with two (or one) nitrogen atoms such as 30 (Scheme 35) could not be stable; they would readily isomerize into carbodiimides (or ketene-imines). Since some kind of electronic stabilization is necessary for a carbene to be isolated, we targeted cyclopropenylidenes. Indeed, they are the conjugate bases of cyclopropenium ions, the prototypical 2π-Huckel aromatic compounds discovered by Breslow over 50 years ago,[133] and therefore could benefit from aromaticity.
Scheme 35.

Three-membered N-heterocyclic carbenes would rearrange into cumulenes.
The parent cyclopropenylidene CP1 (Scheme 36) is the most abundant cyclic hydrocarbon observed in interstellar space,[134] but is recognized to be highly unstable in condensed phases. Reisenauer et al.[135] were able to detect the molecule in a solid argon matrix by infrared spectroscopy, but it survives for only several hours at 35 to 40 K before polymerizing. The quest for free cyclopropenylidenes in the laboratory had not been restricted to the parent compound CP1. As amino groups are known to stabilize the corresponding cyclopropenium salts,[136] bis(dialkylamino)cyclopropenylidenes, such as CP2, have been among the most frequently targeted derivatives. The research groups of Weiss and Yoshida attempted lithium–halogen exchange from CP2-(Cl+)X− (X = ClO4),[137] and deprotonation of CP2(H+) X−(X = ClO4),[138] respectively, with nBuLi. They were not able to isolate the resulting product. Initially, Yoshida et al. claimed the successful synthesis of the free cyclopropenylidene CP2,[138] but several years later he[139] and Weiss[137] concluded concurrently that the compound in question was more likely to be the carbene-LiClO4 adduct. However, the only spectroscopic data that exist for this adduct are the single report of a 7Li NMR chemical shift.[139b] In the 1990s, Tamm et al.[140] repeated the lithium–halogen exchange reaction of CP2(Cl+)X− (X =ClO4 and CF3SO3) with n-butylllithium, and described the product as stable only at low temperatures. It has also been shown that this compound, generated in situ, effectively transfers the cyclopropenylidene moiety CP2 to a number of substrates, including transition metals and main-group species.[137–141]
Scheme 36.
CP1 and CP2, as well as the potential precursors CP2(H+) and CP2(Cl+).
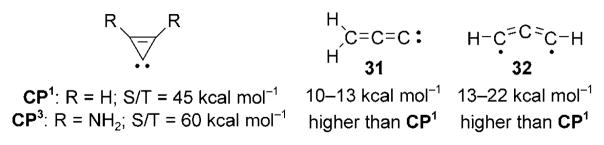
Despite these previous rather discouraging results, calculations prompted us to tackle the synthesis of a stable, free cyclopropenylidene. Indeed, it was predicted that the rearrangement of CP1 into the other C3H2 isomers, propadienylidene (31) and propynylidene (32), was quite unlikely, because the latter were predicted to be higher in energy by 10–13 and 13–22 kcalmol−1, respectively, depending on the level of calculations (Scheme 37).[142] Our own calculations predicted a relatively small singlet–triplet energy gap (45 kcal mol−1) for the parent cyclopropenylidene CP1, which might explain its tendency to polymerize. In contrast, we found a singlet–triplet energy gap of 60 kcalmol−1 for the simplest amino-substituted derivative CP3 (R =NH2), which should definitely prevent the dimerization and subsequent polymerization.[143] Indeed, this value is comparable to that found for triazolin-5-ylidene D (58 kcalmol−1).[144]
Scheme 37.
Singlet–triplet energy gap for CP1 and CP3, as well as the relative energy of CP1 isomers 31 and 32.
6.2. Synthesis, Characterization, and Stability
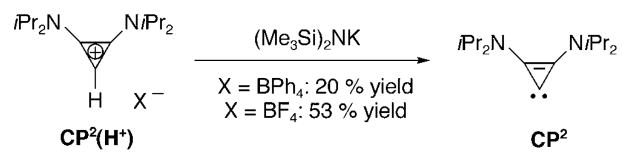
First, we reproduced the Weiss–Yoshida–Tamm lithium–halogen exchange reaction with n-butyllithium, but using the tetrafluoroborate salt of CP2(Cl+) as a precursor to avoid any potential explosive hazards arising from the perchlorate anion.[145] Luckily, a very clean reaction occurred. A single-crystal X-ray diffraction study revealed that the resulting product 33 was a polymeric chain, with an overall stoichiometry of five LiBF4 units for four carbene ligands, and with each cyclopropenylidene moiety bonded to a lithium cation (Figure 8, left). To test the lability of the C–Li bond and attempt to isolate the free cyclopropenylidene CP2, we tried to sequester the metal ion into strong complexing agents. The addition of an excess of [12]crown-4 to a solution of 33 in diethyl ether led to isolation of the tertiary complex 34 in 60% yield (Figure 8, right). In contrast to all known carbene–lithium complexes characterized crystallographically at that time,[146] 34 is a monomeric carbene–lithium complex. These results are in contrast to the observation of Alder et al.[33a] that the addition of [12]crown-4 to the N,N-diisopropyltetra-hydropyrimid-2-ylidene lithium BF4 complex induces the liberation of the free carbene. This result suggests that cyclopropenylidene CP2 coordinates lithium cations very strongly compared to diaminocarbenes. Indeed, the carbene–lithium bond length in 34 (2.093 Å) is significantly shorter than those observed in the few other reported carbene–lithium ion adducts (2.135–2.155 Å).[146] The polymeric carbene–lithium complex 33 can also be cleanly obtained by deprotonation of the tetrafluoroborate salt of CP2(H+) with n-butyllithium.
Figure 8.
Solid-state structures of CP2-LiBF4 polymer 33 (left) and tertiary complex 34 with the BF4 anion omitted for clarity (right).
Alder et al. have shown that sodium or potassium bases must be used, instead of lithium bases, if the salt-free derivative of a highly basic compound is desired.[147] After several unsuccessful attempts, it was found that potassium bis(trimethylsilyl)amide reacted with the tetraphenylborate salt of cyclopropenium CP2(H+) to afford the desired free cyclopropenylidene CP2 in 20% yield.[148] Later on, CP2 was isolated in 53% yield by using the same base but the terafluoroborate salt[145] (Scheme 38). Importantly, it was shown that only certain combinations of counteranion and base allowed for the isolation of free cyclopropenylidene CP2, as well as its lithium complexes 33 and 34. This explains why the early attempts to isolate CP2, and even its lithium complexes, failed.
Scheme 38.
Only certain combinations of anion and base allow for the preparation of CP2.
The X-ray diffraction study of bis(diisopropylamino)cyclopropenylidene CP2 (Figure 9) indicates, as expected, significant π donation from the amino groups to the electron-deficient ring; however, this donation is weaker than in the starting conjugate acid CP2(H+). Not surprisingly, the carbene bond angle in CP2 (57.2°) is extremely acute, and it is smaller than that in its conjugate acid precursor (62.6°). This trend is observed for all known stable singlet carbenes. Similarly, the signal for the carbene carbon atom of CP2 in the 13C NMR spectrum (δ =189 ppm) is strongly deshielded compared to that of CP2(H+) (δ =100 ppm).
Figure 9.
Solid-state structure of CP2.
Although CP2 is sensitive to air, it is thermally very stable (m.p. 107–109°C); heating a toluene solution at 80°C for two hours resulted in only approximately 10% decomposition.
Tamm and co-workers[149] have shown that the amino substituents can easily be varied, and of special interest is that this allowed the introduction of chirality. By using the deprotonation route, they prepared cyclopropenylidene CP4, which features two (R)-1-phenylethylamino groups (60% yield). The spectroscopic data, especially the 13C NMR chemical shift (δ = 188 and 161 ppm), are very similar to those observed for CP2.

Diphenylcyclopropenylidene CP5 has recently been characterized by McMahon and co-workers[150] by infrared spectroscopy, but only in an argon matrix at 10 K. It was obtained by photochemical isomerization of the triplet diphenylpropynylidene (35), a process that is photochemically reversible at λ =232 nm (Scheme 39).
Scheme 39.
Preparation of CP5, which is in photochemical equilibrium with 35.
Attempts to prepare cyclopropenylidene CP6 with extremely bulky aryl substituents failed.[151] When a solution of dichlorocyclopropene 36 in THF was treated with an excess of magnesium at room temperature, triafulvalenes 38 were obtained in a total yield of 94% as a mixture of E and Z isomers (Scheme 40). The E/Z ratio (60:40) reflects the relative thermodynamic stability of 38a and 38b arising from steric repulsion between the bulky substituents. Derivatives 38 are the first examples of triafulvalenes[152] that could be isolated in pure form. These compounds are the smallest members of a class of hydrocarbons named fulvalenes,[153] which are formed by formally cross-conjugating two rings through a common exocyclic double bond.
Scheme 40.
Preparation of triafulvalenes 38, and the possible reaction intermediates. Trip =2,4,6-iPr3C6H2.
It is important to note that there is no evidence for the mechanism of the reaction that leads to 38. Breslow et al. have shown that zinc cleanly promotes the coupling of cyclopropenium salts to afford bis(chlorocyclopropene) of type 37.[154] Thus, the formation of triafulvalenes 38 does not imply that CP6 is involved as an intermediate, and consequently does not prove that diarylcyclopropenylidenes cannot be isolated. Indeed, there are several reports that demonstrate that, in some cases, both a carbene and its dimer can exist.[9,155] Moreover, a singlet–triplet energy gap of 43 kcal mol−1 was calculated for diphenylcyclopropenylidene CP5.[150] Although this value is smaller than that of the bis-(amino)cyclopropenylidene CP3 (60 kcalmol−1),[148] it is much larger than that of the already isolated (phosphino)-(silyl)carbene A (27 kcalmol−1). In addition, the noncatalyzed dimerization[9,124,147,156,157] of singlet carbenes is believed to follow a nonleast motion pathway.[158] The reaction involves the attack of the occupied in-plane σ lone pair of electrons of one singlet carbene center on the out-of-plane vacant pπ orbital of a second carbene, with the latter orbital being reasonably high in energy because of the 2π-electron system of the ring. In other words, there is an energy barrier for the dimerization that is due to electronic factors, and bulky substituents might be able to enhance this barrier sufficiently by kinetic stabilization to make diarylcyclopropenylidenes isolable.
6.3. Ligand Behavior and Catalysis
A few transition-metal complexes have been prepared from stable, free cyclopropenylidene CP2. However, it should be mentioned that at the end of the 1960s Öfele had already reported chromium pentacarbonyl[159] and palladium dichloride[160] complexes bearing a diarylcyclopropenylidene ligand. The metal complexes of the so-called carbocyclic carbenes were recently reviewed by the pioneers of the field.[161] Therefore, we will focus in this section on the coordination behavior of the free CP2,[162] but will summarize all the catalytic data available for cyclopropenylidene complexes, independently of the method used to prepare them.
CP2 is able to cleave [{Rh(CO)2Cl}2] to afford the [RhCl(CO)2(CP2)] complex, which will be used to assess the donor capabilities of cyclopropenylidenes (see Section 9). More surprisingly, it reacts with [{Rh(cod)Cl}2] to afford [Rh(cod)(CP2)2]+[RhCl2(cod)]− (Scheme 41), a type of complex rarely obtained with NHCs.[163] CP2 can also displace standard ligands, and even neutral bidentate ligands, as shown by the clean formation of [RhCl(PPh3)2(CP2)] and [PdMe2-(CP2)2] from Wilkinson’s catalyst and [PdMe2(tmeda)], respectively. This ligand-exchange reaction is also efficient for metal(0) complexes, as evidence by the formation of [Ni(cod)(CP2)2] from [Ni(cod)2] at room temperature. This type of complex has not been isolated with NHC ligands, where either a bridging cod ligand connects two {Ni(NHC)2} fragments[164] or alternatively a homoleptic [Ni(NHC)2] complex is formed.[165]
Scheme 41.
Complexes prepared from free CP2. tmeda = N,N,N′,N′-tetramethylethylenediamine.
We believe that the minimal steric bulk of CP2 is responsible for the formation of some of the rather unusual complexes mentioned above. Importantly, it is quite likely that the availability of stable cyclopropenylidenes paves the way for the synthesis of complexes with a variety of metals in the zero oxidation state, since such complexes are more difficult to prepare without free carbenes.
With the exception of a short note by Tamm and coworkers,[149] which stated that free chiral CP5 promotes the benzoin condensation of benzaldehyde with enantioselectivities of only up to 18% ee, no catalytic reactions have been reported when using free cyclopropenylidene as an organo-catalyst or as a precursor for transition-metal catalysts. However, the first catalyzed reaction involving a cyclopropenylidene metal complex was described as early as 1988 by Yoshida and co-workers.[166] They found that a series of air-stable cyclopropenylidene palladium complexes efficiently catalyze the exothermic isomerization of quadricyclane to norbornadiene. Interestingly, they found that the most active catalyst was [{PdCl2(CP2)}2] (Scheme 42).
Scheme 42.
Isomerization of quadricyclane to norbornadiene promoted by [PdCl2(CP)] dimers.
Several mixed palladium(II) complexes bearing a 2,3-diarylcyclopropenylidene and a phosphine were tested by Wass et al.[167,168] and Herrmann et al.[169,170] as catalysts in Suzuki–Miyaura and Heck coupling reactions, as well as in the Hartwig–Buchwald aromatic amination. Wass et al. found high activities, especially for the Heck reaction. Herrmann et al. concluded after a detailed study that these complexes were less active than the most effective NHC–phosphine systems, but, in contrast to the latter,[171] they did not exhibit an induction period.[172] The best results were obtained with the most bulky CP that bears mesityl groups.
The attempts to use rhodium(III) complexes with a diphenylcyclopropenylidene ligand and two phosphines in hydroformylation catalysis were rather disappointing.[173] Indeed, no hydroformylation of 1-hexene was observed after 3 h at 90°C under 20 bar of CO and H2. High conversion was seen when Zn was used as a reducing agent, but the authors suggested that the cyclopropenylidene fragment was lost during the process. In marked contrast, rhodium complexes bearing classical NHCs led to very promising results.[25c]
It is important to mention that all the coupling reactions described above have been performed with diaryl-substituted cyclopropenylidenes. Since all these processes are known to benefit from strong σ donors, it is quite likely that much higher catalytic activities could be obtained by using bis(amino)cyclopropenylidenes such as CP2. As a matter of fact, the successful isomerization of quadricyclane to norbornadiene was performed with palladium catalysts bearing CP2.
7. Cyclic Bent Allenes, Carbodiphosphoranes, and Vinylidenephosphoranes
7.1. Background
All the carbenes described so far are stable because of the donation of a lone pair of electrons from a heteroatom (in the α or β position) into the vacant p orbital of the carbene. In other words, the carbene carbon atom has a lone pair of electrons (σ orbital) and a pseudo-filled p orbital. Consequently, they are strong σ-donor and weak π-acceptor ligands. We reasoned that one way to retain the strong σ-donor properties and, at the same time, to further diminish the π-acceptor character of the carbon atom would be to design a compound featuring a carbon atom with two lone pairs of electrons.
On playing with the resonance forms of diaminocyclo-propenylidenes, such as CP2 (Scheme 43), we realized that one of them (g) is an allene, while another (h) features two lone pairs of elctrons on the carbon atom. However, in line with the hybridization theory, allenes have a linear C-C-C skeleton with orthogonal pairs of substituents.[174] The allene framework is so rigid that even minor deviations from linearity are of note. In a publication from 1995 entitled “A remarkably bent allene. X-ray crystal structure and ab initio calculations”, Weber et al.[175] described 39 with a C-C-C bond angle of 170.1°. Therefore, resonance form g of diaminocyclopropenylidenes seems unrealistic, since the carbene bond angle is close to 57° and furthermore the amino substituents are coplanar with the ring. Nevertheless, by analogy, we wondered if strong π-donating substituents, such as amino groups, at the two termini of an acyclic allene framework, as shown in 40, could polarize the C–C bonds up to the breaking point of the π system, thereby leading to a resonance form similar to h. If this hypothesis proved to be correct, the C-C-C backbone of 40 should not be linear, and the central carbon atom would formally have two lone pairs of electrons. We also recognized that this type of compounds, which we named bent allenes, resembles carbodiphosphoranes 41. This is a well-known family of stable phosphorus compounds, which are also nonlinear.[176,177]
Scheme 43.
Resonance forms of diaminocyclopropenylidenes; 39, the most severely bent acyclic allene known up to 2008; resonance forms of tetrakis(amino)allene 40 and carbodiphosphorane 41; calculated carbodicarbene 42 and the concept of carbon(0); synthesis of acyclic bent-allene 44.
Before we published our experimental studies, Tonner and Frenking[178] reported a detailed computational investigation of derivative 42, which is directly related to bent-allenes 40 and also carbodiphoshoranes 41 (Scheme 43). They described 42 as a “carbodicarbene”, a compound with “a divalent carbon(0)”[179] and two “NHC ligands”.[180] They predicted an equilibrium geometry for 42 with a C-C-C bond angle of 131.8°. Experimentally, we were pleased to find that bis(deprotonation) of 43 with potassium hexamethyldisilazane afforded 44.[181] Although the allene bond lengths are only slightly longer (C–C 1.34 &) than the standard C=C bond length of an allene (1.31 Å),[182] the two NCN planes are not perpendicular, but twisted by 69°. More strikingly, the allene framework is severely bent with a C-C-C bond angle of 134.8°, a value very similar to that calculated by Tonner and Frenking for 42. Clearly the central carbon atom is not sp hybridized as in a typical allene, but likely approaches a configuration with two lone pairs of electrons. Although extremely water sensitive, allene 44 is indefinitely stable at room temperature both in solution and in the solid state (m.p. 150–152°C).
Bent-allene 44, as well as other carbon(0) derivatives (L:→C←:L), are predicted to be extremely basic (strong σ-donor ligands). Computational studies showed that bent-allene 42 not only has a very high first proton affinity (294 kcalmol−1, versus 262 kcalmol−1 for imidazolin-2-yli-denes B), but also a large second proton affinity (168 kcal mol−1 versus 72 kcalmol−1 for B).[183] Consequently, bent allenes are prone to double protonation, and should also be capable of bonding two transition metals at the same carbon site. This hypothesis was supported by the existence of dimetalated carbodiphosphoranes,[184] and confirmed by recent results by Fürstner et al.,[185,186] who were able to isolate a dinuclear gold complex of a carbon(0) derivative, with the carbon atom bound to a phosphine and a dialkoxy-carbene as “ligands” (Scheme 44).
Scheme 44.
Synthesis a dinuclear gold complex of a carbon(0) derivative.
These computational and experimental results clearly showed that cyclic bent allenes (CBAs) were desirable ligands. Examination of the literature showed that even the low-temperature NMR spectroscopic characterization of cyclic allenes was limited to those containing more than seven carbon atoms;[187] the only exception was the 1,2,4,6-cycloheptatetraene (45), which was elegantly incarcerated in a molecular container by Warmuth and Marvel[188] (Scheme 45). The kinetically protected 1,2-cyclooctadiene 46 (calculated C-C-C angle: 158°),[189] the trisilicon-[190] and diphosphorus-containing[191] six-membered rings 47 and 48, respectively (crystallographically observed C-C-C angles: 166, 161, and 156°, respectively), and the hafnium-containing five-membered ring 49[192] were the smallest cyclic allenes isolated. The presence of a heavier main-group element or a transition metal does not result in the allene fragments of 46–49 being significantly more distorted than in the eight-membered ring 45. Smaller ring allenes were known as reaction intermediates,[193] which is in line with the predictions that strain should approximately double with each successive removal of a carbon atom from the ring.[194]
Scheme 45.
Smallest ring allenes isolated before 2008, with the C-C-C bond angles.
The lack of stability of cyclic “regular” allenes is of course associated with the energy required for bending the allene skeleton. However, from consideration of the bent geometry of acyclic allene 44, it seemed likely that push-push allenes could be incorporated into rings that were typically too strained to accommodate a C=C=C framework. This hypothesis was reinforced by calculations by Tonner and Frenking, who found that widening the bond angle of 42 from the equilibrium geometry (131.8°) up to the “classical” linear structure requires only 3.7 kcalmol−1.[178] Push-push allenes are clearly not rigid but highly flexible.
7.2. Synthesis, Characterization, and Stability
By analogy with the ring size of the most studied NHCs, we chose to attempt the synthesis of a five-membered ring allene. The thermally and air-stable 3,5-diaminopyrazolium salt CBA2(H+),[195] which is readily prepared from dichloro derivative CBA1(H+),[196] was a logical precursor. Indeed, the C-C-C fragment of the ensuing CBA would be substituted by four π-donor amino groups, similar to the isolated acyclic bent-allene 44. Deprotonation with n-butyllithium did not lead to the free cyclic bent-allene CBA2, but to its monomeric lithium adduct 50, which was isolated as thermally stable orange crystals in 26% yield[197] (Scheme 46).
Scheme 46.
Synthesis of CBA2-lithium adduct 50, and stable free cyclic bent-allene CBA3. Ar =2,6-Me2C6H3.
Just as for the lithium adduct of diaminocyclopropenylidene CP2, all attempts to sequester the metal ion of 50 into a strong complexing agent failed. Moreover, although the free CP2 could be obtained by using a potassium base, no clean reaction occurred on treating CBA2(H+) with KN(SiMe3)2 or KN(iPr2)2. This is an indication that bent allenes are highly basic and strongly coordinate metals. We therefore decided to modify the pyrazolium scaffold to make the central carbon atom less basic. The exocyclic amino groups of CBA2(H+) were replaced by weaker π donors and more electronegative aryloxy groups. Indeed deprotonation of CBA3(H+) with KHMDS led to the desired free, cyclic bent-allene CBA3, which was isolated as pale yellow crystals in 47% yield (Scheme 46).
A single-crystal X-ray diffraction study showed that CBA3 features an extremely acute C-C-C bond angle of 97.5° (Figure 10). In contrast to the perpendicular arrangement of the substituents found in classical allenes, and the 69° twist angle observed for the acyclic bent-allene 44, the two nitrogen and two oxygen atoms of CBA3 are coplanar with the allene fragment. The C–C bond lengths (1.37 Å) are significantly longer than the standard allene value (1.31 Å).[182] All these peculiar features are in agreement with a strong polarization of the allenic π bonds towards the central carbon atom. Surprisingly, CBA3 exists in the solid state as a racemic mixture as a result of the pyramidalization of the nitrogen atoms, with a trans arrangement of the Ph groups (sum of the angles at N: 347 and 351°). Therefore, although the endocyclic C–N bond lengths (1.38 Å) are shorter than single bonds, the nitrogen lone pairs of electrons do not significantly interact with the allene π system. Consequently, the exocyclic phenoxy groups are responsible for the polarization of the C-C-C framework. Indeed, the oxygen centers are sp2 hybridized (CO-C: 120 and 117°); they are arranged so that the lone pair of electrons can conjugate with the allene π system (C-O-C-C torsional angles: 2.6 and 6.3°), and the O–C bonds are short (1.35 Å).
Figure 10.

Molecular structure of CBA3 in the solid state.
The chemical shift of the signals for the central and terminal allenic carbon nuclei of CBA3 in the 13C NMR spectrum (δ =115 and 175 ppm, respectively) is similar to that observed for the acyclic bent-allene 44 (δ =110 and 145 ppm, respectively), but is the reverse of that observed for non-polarized allenes (δ =185–215 and 60–130 ppm, respectively),[198] thus arguing again in favor of the presence of an electron-rich central carbon atom.
Cyclic bent-allene CBA3 is stable for weeks at room temperature, both in solution and in the solid state (m.p.: 95°C, decomp). Since CBA3 has been prepared by deprotonation of the pyrazolium ion CBA3(H+), it has been suggested that its stability comes from its aromatic character.[199,200] On the other hand, Tuononen and co-workers[201] concluded their computational investigation by stating that CBA3 is best described as a carbenoid. Calculations,[202] including HOMA and NICS aromaticity indices, showed that allenes derived from 3,5-bis(π-donor)-substituted pyrazolium salts are weakly aromatic to non-aromatic. More surprisingly, we found experimentally that 1) CBA3(H+) with exocyclic aryloxy substituents features planar endocyclic nitrogen atoms (Figure 11, left), as expected for an aromatic system; and 2) CBA4(H+) with stronger exocyclic π-donor amino substituents exhibits highly pyramidalized endocyclic nitrogen centers, but planarized exocyclic ones (Figure 11, right). Therefore, it seems that exocyclic delocalization is preferred at the expense of aromaticity in CBA3, and even in CBA4(H+).
Figure 11.

Molecular structures of CBA3(H+) (left; the Dipp groups on the oxygen atoms have been removed for clarity), and CBA4(H+) (right) in the solid state.
To assess the limit of the stability of cyclic bent allenes we attempted to synthesize an all-carbon four-membered ring allene, which cannot gain any stabilization other than from the two exocyclic π-donor substituents. Treatment of 51[203] with triethyloxonium tetrafluoroborate, followed by the addition of excess piperidine gave rise to salt CBA5(H+) in 72% yield (Scheme 47). The addition of LDA at room temperature to a solution of CBA5(H+) in THF at −20°C cleanly led to CBA5 (or possibly its lithium adduct).[204] The signals for the central and terminal carbon atoms of the allene moiety appear at δ =151 and 185 ppm in the 13C NMR spectrum; these resonances are shifted downfield by δ =58 and 13 ppm, respectively, compared to those of the conjugate acid precursor.
Scheme 47.
Synthesis of CBA5(H+) and persistent cyclic allene CBA5.
Importantly, we found that the addition of an excess of the tetrafluoroboric acid/diethyl ether complex to CBA5 [or CBA5(H+)] led to the corresponding dication 52, which was isolated in 90% yield as white crystals, which were subjected to a diffraction study. This double protonation of cyclic allene CBA5 (Scheme 48) provides definitive evidence for the possibility of using two negative charges at the central carbon atom of cyclic bent allenes.
Scheme 48.
Double protonation of CBA5.
CBA5 is only stable for several hours at −20°C, and readily decomposes above −5°C to give a complex mixture of unidentified products. However, the relative stability of this very small carbocycle clearly indicates that a wide variety of push-push substituted cyclic allenes can be isolated.
As mentioned at the beginning of this section, there are strong similarities between bent allenes and carbodiphosphoranes. Schmidbaur et al.[205] described the synthesis of cyclic carbodiphosphoranes CCDP1–3 in 1980, and more recently, Baceiredo and co-workers[206] prepared CCDP4 (Scheme 49).
Scheme 49.
Syntheses of cyclic carbodiphosphoranes CCDP1–4.
According to X-ray diffraction studies, six- and five-membered carbodiphosphoranes CCDP2 and CCDP4 (Figure 12) feature a PCP angle of 117 and 105°, respectively. The signal corresponding to the central carbon atom is noteworthy, as it appears at extremely high field (CCDP2: δ = −3.1; CCDP4: δ =+21.5 ppm) in the 13C NMR spectrum—as expected for a strongly negatively charged carbon atom.
Figure 12.

Solid-state structure of CCDP4.
The major problem associated with cyclic carbodiphosphoranes is their thermal instability. Schmidbaur et al.[205] reported that five-membered CCDP1 decomposed within a few hours at 20°C, and even the six-membered CCDP2 was unstable above 35°C; however, they did not discuss the decomposition pathways. Baceiredo and co-workers[207] found that heating a solution of CCDP4 in benzene for 60 h at 80°C induces its rearrangement into 1,2λ5-azaphosphete 54 (Scheme 50). This rearrangement is analogous to that observed for cyclic diphosphinocarbene PHC3. Indeed, a [3+2] retro-cycloaddition occurs in both cases to afford benzonitrile and diphosphinocarbene 53, and acetonitrile and 1,3-diphosphaallene 7,[39] respectively. Phosphinocarbene 53 then reacts in a formal [2+2] cycloaddition with benzonitrile to afford the isolated azaphosphete 54; the latter reaction has precedents with other phosphinocarbenes.[28a,208]
Scheme 50.
Rearrangement of CCDP4; analogy with PHC3.
From these results as a whole, it appears that cyclic carbodiphosphoranes are less stable than cyclic bent allenes, and this is a real concern for their potential use. On the other hand, as indicated by the 13C NMR spectroscopic data (see Section 9), the carbon center of CCDPs is more electron-rich than that of CBAs, which is of course a very interesting feature. This analysis led us to consider a mixed system with a phosphorus–carbon ylidic bond and a carbon–carbon double bond; such compounds are known as vinylidenephosphoranes (Scheme 51). In contrast to the numerous examples of acyclic derivatives,[176,209] only one cyclic vinylidenephosphorane CVP1 had been reported. Interestingly, this compound could be isolated in a crystalline form, and was stable “for a limited period with cooling”, but no 13C NMR spectroscopic data, crystallographic data, or coordination chemistry were reported.[210]
Scheme 51.
Cyclic vinylidenephosphoranes (CVPs), which are hybrid compounds between CBAs and CCDPs; CVP1, the only derivative reported[210] before 2008.
Since no dimerization pathway and skeleton rearrangements, or even fragmentation processes seem possible, we believed that the reported limited stability of CVP1 was due to its air sensitivity. Therefore, as a proof of concept, we investigated the synthesis of a simple CVP derivative, not bearing a heteroatom at the carbon terminus (Scheme 52).[211] The conjugate acid CVP2(H+) was prepared in three steps from the readily available diene 55[212] by using slightly modified known procedures. A formal [4+1] cycloaddition with bis(diisopropylamino)phosphenium triflate[213] gave rise to phospholenium salt 56. Deprotonation with NaNH2 in ammonia, and subsequent treatment with one equivalent of CCl4 at room temperature led directly to the desired phospholium chloride CVP2(H+).[214] Deprotonation with KHMDS finally afforded CVP2, which was isolated as brownish red crystals in 32% yield.
Scheme 52.
Synthesis of CVP2.
According to a single-crystal X-ray diffraction study (Figure 13), CVP2 has a planar five-membered ring. The most surprising feature is the P–C bond distance of 1.783 Å, which is essentially the same as that of the phosphonium precursor CVP2(H+) (1.786 Å). This bond is exceptionally long compared to those observed in nonstabilized phosphorus ylides (1.66 Å),[215] the five-membered cyclic carbodiphosphorane CCDP4 (1.64–1.66 Å),[206] and, even more strikingly, in acyclic vinylidenephosphoranes (1.68 Å).[216] This observation clearly indicates that there is almost no back donation from the lone pair of electrons on the carbon atom to the phosphonium center. The only significant change in geometry between CVP2(H+) and CVP2 is the P-C-C bond angle, which decreases significantly from 107 to 100°. This phenomenon is always observed for carbenes and bent allenes when compared with their conjugate acid precursors. The signal of the central carbon atom in the 13C NMR spectrum was observed at δ =184 ppm, which is significantly low-field shifted relative to the starting material (δ =109 ppm), but also to bent allenes (δ =110–151 ppm) and cyclic carbodiphosphoranes (δ =−3–+ 2 ppm).
Figure 13.

Molecular structure of CVP2 in the solid state.
Derivative CVP2 appeared to be quite stable in solution and in the solid state (m.p.: 81–82°C), but was, as expected, very sensitive to moisture. Future initiatives will include the preparation of cyclic vinylidenephosphoranes with a π-donor group at the carbon terminus to increase even further the electron density at the central carbon atom.
From the results discussed in this section it can be concluded that carbodiphosphoranes are potentially the most electron-rich ligands of all of the compounds described so far in this Review. However, since their thermal stability is a serious concern, cyclic bent allenes and vinylidenephosphoranes constitute better alternatives, and certainly deserve further study.
7.3. Ligand Behavior and Catalysis
As for cyclopropenylidenes, this section will focus on the coordination behavior of the free cyclic bent allenes, carbodiphosphoranes, and vinylidenephosphoranes, but will summarize all the catalytic data available, independently of the method used to prepare the complexes bearing these ligands.
It is first worth mentioning that “regular allenes” react with transition-metal species to give η2 complexes involving one of the C–C π bonds.[217] In marked contrast, an η1-coordination mode to rhodium and iridium centers was observed for CBAs[197,204] (but also for the acyclic bent-allene 44),[181] as shown for [RhCl(CO)2(CBA3)] (Figure 14, left), and [RhCl(cod)(CBA5)] (Figure 14, right). This is a striking demonstration of the very peculiar electronic structure of bent allenes.
Figure 14.
X-ray crystal structures of [RhCl(CO)2(CBA3)] and [RhCl-(cod)(CBA5)].
Similarly, free cyclic carbodiphophorane CCDP4 and vinylidenephosphorane CVP2 react with different metal fragments to afford the corresponding η1 complexes in high yields (Scheme 53). Baceiredo and co-workers[206] noted that the P–C bond lengths in the CCDP4 complexes lay between those of single and double bonds, thus indicating that the remaining lone pair of electrons on the carbon atom interacts with the two phosphonio groups. Interestingly, the metal–carbon bonds (Pd–C: 2.12 Å; Rh–C: 2.11 Å) are significantly longer than those reported for the corresponding NHC complexes (Pd–C and Rh–C: 2.00–2.06 Å), which suggests very weak π donation from the metal to the carbodiphosphorane.
Scheme 53.
Complexes synthesized from free CCDP4 and CVP2. all =allyl.
In 2009, the first applications of CCDP4 for catalysis were disclosed in which CCDP4Cu and CCDP4Au complexes prepared by depronation of CCDP4(H+) with potassium tert-butoxide were used, followed by the addition of CuCl and AuCl(SMe2), respectively[218] (Scheme 54). Although the Cu complex is air- and moisture-sensitive, the gold complex was described as stable in the solid state in the presence of air. Both complexes were active in the addition of amines and alcohols to acrylonitrile. Higher conversions were obtained with copper, and the hydroxylation was slower than hydroamination. A 72% conversion was obtained at room temperature after 40 h when phenol was used. The authors noted that this catalytic performance was superior to that reported for analogous Cu–NHC complexes (64%, 80°C, 40 h),[219] but also that decomposition of the catalyst occurred, which prevented the achievement of better conversions.
Scheme 54.
First catalytic applications of complexes bearing a CCDP as a ligand.
Lastly, Huynh and co-workers[220,221] reported the catalytic activity of palladium complexes featuring cyclic bent allenes based on the pyrazole skeleton as ligands (Scheme 55). These complexes were prepared by oxidative addition of 4-iodopyr-azolium salts to [Pd2(dba)3], followed by addition of PPh3 or pyridine. They were found to promote Suzuki–Miyaura coupling reactions of activated substrates at ambient temperature in water, under aerobic conditions. However, low yields ranging from 14 to 52% were obtained with more difficult substrates such as 4-chlorobenzaldehyde. The same conclusions apply to the Heck cross-coupling reaction.
Scheme 55.
Synthesis of complexes used for Suzuki–Miyaura coupling reactions. dba =trans,trans-dibenzylideneacetone.
The conclusions we drew following the disappointing catalytic activity observed so far for cyclopropenylidene complexes, also apply here. Indeed, Huynh and co-workers have shown that the best results are obtained with the bulkier pyrazolin-4-ylidene, and this ligand has simple phenyl groups in position 3 and 5. It is quite likely that much higher catalytic activities should be attainable with more sterically hindered cyclic bent allenes such as CBA3.
8. Abnormal NHCs
8.1. Background
For many years, our research group has been interested in the synthesis of stable, unusual isomers of well-known compounds.[222] Cyclic bent allenes derived from pyrazolium salts are constitutional isomers of imidazol-2-ylidenes, the well-known unsaturated NHCs B (Scheme 56). C5-Depro-tonated imidazolium salts (aNHCs) appeared as good candidates as alternative stable isomers of CBA and NHC B. Transition-metal complexes with aNHCs as ligands were known. In 2001 Crabtree and co-workers[223] discovered that imidazolium salts, which usually add oxidatively to metals through the C2–H bond to afford a typical NHC complex, can also bind “in the wrong way” at C5,[223] as shown in 57. Since no reasonable canonical resonance forms for the free ligand aNHC can be drawn that show a carbene center without introducing additional charges, the C5-bound compounds are often referred to as “abnormal carbene” complexes or aNHC complexes.
Scheme 56.
Different resonance forms of CBA (derived from pyrazolium salts), NHC B, and aNHC. Synthesis of the abnormal NHC complex 57.
Since 2001, many other complexes featuring aNHC ligands have been prepared, and the results have been summarized in several reviews.[224] Most studies have concentrated on developing synthetic protocols for preparing metal complexes of aNHCs (including metalation by C5–H activation, oxidative addition of a C5–halogen bond, and transmetalation), and on assessing the electronic and structural properties imposed by this ligand. In the abstract of one of the most recent publications[225] on aNHC complexes, Albrecht and co-workers[226] wrote: “Analytical investigations using X-ray diffraction and X-ray photoelectron spectroscopy indicate that the C5 bonding mode increases the electron density at the metal center substantially, classifying C5-bound carbene ligands amongst the most basic neutral donors known thus far”. Therefore, the synthesis of free aNHCs was an exciting challenge.
Before our studies, no free aNHCs had been isolated or even characterized in solution, but a report by Lassaletta and co-workers[163c] is noteworthy. They showed that deprotonation of imidazo[1,5-a]pyridinium salts NHC1(H+) leads to free NHC1 that can be isolated (Scheme 57). In contrast, they did not observe the corresponding free aNHC1 when they used C2-substituted precursors such as aNHC1(H+). However, they were able to isolate the corresponding aNHC1(Rhcod) complex by performing the deprotonation reaction in the presence of [{RhCl(cod)}2]. The latter result clearly indicates that free aNHC1 has a reasonable lifetime, and this encouraged us to attempt the preparation and isolation of an aNHC.
Scheme 57.
Synthesis of NHC1, and evidence for the transient formation of aNHC1 through the formation of aNHC1(Rhcod).[163c]
Calculations predict that the parent aNHC (H atoms at C and N) is about 17 kcalmol−1 higher in energy than its NHC isomer.[227] However, in contrast to classical singlet carbenes, no dimerization pathway is possible for aNHCs, and skeleton rearrangements or fragmentation processes seem quite unlikely.
8.2. Synthesis, Characterization, and Stability
Calculations of the acidity constants for the C2-bound proton (leading to NHCs) and C5-H (leading to aNHCs) revealed pKa values of 24.9[228] and 33.0, respectively.[229] The latter value is comparable to that calculated for the CAAC precursors. Thus, bases used to prepare CAACs, such as n-butyllithium, LDA, KHMDS, etc., could certainly deprotonate the imidazolium salts at the C5 carbon atom. Since the C2-bound proton is more acidic than the C5-H proton, a phenyl group was placed at C2. A bulky 2,6-diisopropyl-phenyl (Dipp) substituent was appended to both nitrogen atoms and a second phenyl group was attached at C4 to offer kinetic protection to the C5 position. Imidazolium salts aNHC2(H+) with various counterions were prepared in high yields after slight modifications to known synthetic procedures (Scheme 58).[230] All attempts to deprotonate the tetrafluoroborate salt of imidazolium aNHC2(H+) failed. However, small anions are known to accelerate heterolytic C–H bond cleavage through hydrogen bonding, and interestingly this effect has been used with C2- and C5-unsubstituted imidazolium salts to favor metalation at C2 (with the more acidic proton) over C5.[231] Since the C2 position of aNHC2(H+) is protected, small anions should promote the desired deprotonation reaction at C5. Indeed, deprotonation occurred when aNHC2(H+) with HCl2− as the anion was treated with two equivalents of a lithium base such as n-butyllithium or LDA, but, as already observed with diamino-cyclopropenylidene CP2 and cyclic bent-allene CBA2, the aNHC2(Li+) adduct was formed. Potassium bases, especially KHMDS, have also proven here to be more appropriate for generating the desired free aNHC2, which was isolated after workup as a green powder in 68% yield.[232]
Scheme 58.
Synthesis of free aNHC2 and of its lithium adduct aNHC2(Li+).
In the solid state, free aNHC2 features a fully planar ring, with the five ring atoms also being in a planar environment (maximum deviation, 1.9 pm; Figure 15). The endocyclic C–N (1.33–1.40 Å) and C–C bond lengths (1.35 Å) are halfway between those of single and double bonds. These geometric parameters suggest a delocalization of the π system. Interestingly, the carbene bond angle for aNHC2 (101.0°) is more acute than the corresponding angle in its cationic precursor aNHC2(H+) (108.0°), which is consistent with an increased s character of the σ lone-pair orbital on the carbene atom compared to the C–H+ bonding orbital of the precursor. As already mentioned, this is the general trend for all the species discussed in this Review.
Figure 15.

Molecular structure of aNHC2 in the solid state.
In the 13C NMR spectrum the C5 atom of aNHC2 gives rise to a resonance at δ =202 ppm, which is significantly downfield shifted compared with the corresponding resonance for aNHC2(H+) (δ =124 ppm), and coincidently in the range observed for classical NHCs.[26b]
Although aNHC2 is stable at room temperature for a few days, both in the solid state (m.p. 65°C, decomp) and in solution, it rearranges quantitatively to 58 upon heating in benzene at 50°C for 48 h (Scheme 59). This fused heterocycle most likely results from the deprotonation of an isopropyl substituent of the Dipp group by the carbene center, followed by nucleophilic addition of the resulting benzyl anion to C2.
Scheme 59.
Rearrangement of aNHC2 upon heating in solution.
The results reported above, including the latter rearrangement, gives a good indication of the substituent patterns that will allow for the isolation of other aNHCs. Taking into account that aNHC2 is stable despite the presence of a simple phenyl group on the carbon atom adjacent to the carbene center, steric bulk does not seem to be a requirement. This is in line with the absence of a possible dimerization pathway. However, accessible C–H bonds spatially close to the carbene center should be avoided to prevent the intramolecular deprotonation as observed in the rearrangement leading to 58. Notably, the substituent at C4 undergoes conjugation with the carbene center, which opens the possibility of substantially modulating the electronic character of the ring system.
8.3. Ligand Behavior and Catalysis
As for cyclopropenylidenes and cyclic bent allenes, this section is limited to the coordination behavior of the free aNHC2, but all the catalytic data available for complexes bearing an aNHC ligand are summarized.
In fact, [AuCl(aNHC2)] is the only complex reported so far with the stable aNHC2. It appears to be very stable, and its catalytic activity is under investigation. Its preparation serves as a proof of concept to demonstrate that, not surprisingly, the availability of the free species will allow for the synthesis of a variety of metal–aNHC complexes which are not always readily available by other routes.
As far as the catalytic activity of aNHC complexes is concerned, just a few reports are available. The first was the discovery in 2004 by Lebel et al.,[233] who showed that the mixed [PdCl2(NHC)(aNHC)] complex 59 promoted Suzuki–Miyaura and Heck reactions (Scheme 60). Although, the catalytic activity of 59 was found to be inferior to that observed for palladium complexes bearing a single normal NHC, they noted that the corresponding bis(NHC) complex 60 was inactive for both coupling reactions.
Scheme 60.
Palladium complexes for Suzuki–Miyaura and Heck reactions (59 and 60), as well as for copper- and amine-free Sonogashira coupling reactions (61).
A series of PEPPSI-based palladium complexes 61 (Scheme 60) have been reported to be efficient precatalysts for the Sonogashira coupling of aryl iodides and bromides with terminal acetylenes.[234] These reactions were carried out in air in a mixed aqueous medium under copper-free and amine-free conditions. Significantly better performances were obtained with 61 than with PdCl2 and [PdCl2(NHC)-(pyridine)].
Albrecht and co-workers[226,235] have shown that palladium complex 62aNHC (Scheme 61), with a cis-chelating di(aNHC) ligand, promotes the hydrogenation of cyclooctene at room temperature and 1 atm hydrogen. Comparative experiments allowed them to conclude that, irrespective of the solvent, the conversions are substantially higher than with the corresponding complex 62NHC, which bears the analogous bidentate ligand with a normal NHC. Later, the same research group showed that similar bidentate aNHC rhodium(III) complexes are active in hydrogen-transfer catalysis.[236] With 63aNHC, the most active catalyst, the reduction of benzophenone to diphenylmethanol is essentially complete in 1 h, with isopropanol acting as a hydrogen donor. Several other ketones are efficiently hydrogenated, but no reaction was observed with imines as substrates. Although, the catalytic activity is one or two orders of magnitude lower than the most active systems known today,[237] it is interesting to note that, here also, the corresponding NHC complex 63NHC is essentially inactive.
Scheme 61.
aNHC complexes 62aNHC and 63aNHC are active in hydrogenation and hydrogen-transfer catalysis, respectively, while NHC complexes 62NHC and 63NHC are not.
In a series of studies, Peris and co-workers have compared the catalytic activity of analogous complexes containing an NHC, an aNHC, and a pyrazol-3-ylidene ligand. They tested the alkylation of benzyl alcohol with n-butanol, tert-butyl-amine with benzyl alcohol, and aniline with n-hexylamine,[238] as well as the benzylation of toluene with benzyl alcohol by using iridium complexes 64, 65, and 66 (Scheme 62).[239] All the complexes showed good activities, although 64 is slightly more efficient. A remarkable feature is that these processes were carried out in the absence of base or any other additive.
Scheme 62.
Comparison of iridium complexes bearing NHC (64), aNHC (65), and pyrazol-3-ylidene (66) ligands for alkylation reactions.
Peris and co-workers used ruthenium–p-cymene complexes 67–69 to investigate the catalytic dimerization of phenylacetylene, as well as the β-alkylation of secondary alcohols with primary alcohols (Scheme 63).[240] For the first process, high conversions were achieved in all cases, although the dimerization competes with the formation of trisubstituted benzenes. For the second process, the normal NHC 67 shows the lowest activity, with only moderate yields obtained after long reaction times. Both 68 and 69 show good activity in terms of conversion, although longer reaction times are needed for 68 than for 69. These results lie among the best reported to date.[241]
Scheme 63.
Comparative activity of ruthenium complexes bearing NHC (67), aNHC (68), and pyrazol-3-ylidene (69) ligands for the β-alkylation of secondary alcohols with primary alcohols. M =major product, m =minor product.
9. Comparative Electronic Properties of Cyclic Non-NHCs and NHCs
For a given metal, the chemical properties of a complex are determined by the electronic and steric effects imposed by the ligands. Several methods have, therefore, been developed to assess the electronic properties of ligands, most of them being the subject of debate in recent publications. Tolman’s electronic parameter (TEP) is arguably the most well-known method for phosphines and other classical ligands.[242,243] However, A method derived from the TEP, but based on the CO vibrational frequencies of rhodium and iridium complexes of type cis-[M(CO)2Cl(NHC)], is commonly used for NHCs. Crabtree and co-workers[244] have shown that a good linear correlation is found between the average νCO value for [Ir(CO)2Cl(L)] complexes versus the Tolman electronic parameter. It has recently been confirmed that estimating the donor strength by using [Ni(CO)3(L)], [RhCl(CO)2(L)], and [IrCl(CO)2(L)] as reference systems gives very similar trends.[243,245] Gusev[246] argued that although a single parameter such as TEP is valuable for ranking ligands in the same series (phosphines, carbenes, amines …), this is not the case for comparing ligands of different types (for example, phosphines versus carbenes). The donor properties are sometimes compared by using the trans-CO stretching frequency, because it is presumed to be least influenced by steric effects. This approach is misleading, since two absorptions arise in the IR spectrum from symmetric and asymmetric stretching vibrations, and they cannot be assigned to the cis- and trans-CO modes.[247]
These introductory remarks explain why cis-[RhCl(CO)2(L)] complexes (sometimes, also the iridium analogues) of cyclic non-NHCs have been prepared. The symmetrical, asymmetrical, and average CO stretching frequencies are given in Scheme 64.[248–251] The corresponding values for complexes bearing NHCs B–D have been added for comparison.
Scheme 64.
Symmetric, asymmetric, and average CO stretching frequencies for cis-[RhCl(CO)2(L)] (top) and cis-[IrCl(CO)2(L)] complexes (bottom).
From the average νavCO frequencies of cis-[RhCl(CO)2(L)] it clearly appears that the donor strength of the ligands follows the order: CCDPs (2001 cm−1) > N-YHCs ≈ CBAs ≈ CVPs (2009–2017 cm−1) > PHCs ≈ aNHCs (2022–2025 cm−1) > CPs ≈ CAACs (2031–2036 cm−1) > NHCs (2039–2049 cm−1). The same relative donor strength is also apparent using the few examples of cis-[IrCl(CO)2(L)] complexes. The most striking feature is certainly the wide range of donating ability, which can be achieved using different scaffolds; the νavCO frequencies span from 2001 up to 2049 cm−1. Even by varying the nature of the atoms of the skeleton and the size of the ring, cyclic diaminocarbenes (classical NHCs) can barely reach the donor strength of CPs and CAACs. The only exceptions are the five-membered 70,[247] and six-membered NHCs 71,[252] 72,[253] and 73,[254] with the latter reaching the donor strength of CBAs and N-YHCs (Scheme 65).
Scheme 65.
Members of the NHC family reaching the donor strength of CBAs and N-YHCs.
Importantly, in contrast to the general statement that NHCs are weak π acceptors, it has recently been shown experimentally[255] and computationally[256] that the metal–NHC bond consists of components originating from donation and back-donation, with both being of comparable importance. In that regard, it is generally admitted that the ν(CO) frequencies of cis-[MCl(CO)2(L)] complexes reflect the overall donor ability of the ligand L, but this is also under debate.[257] Thus, it is of interest to consider other methods to access the electronic properties of ligands. The research groups of Yates[228] and Frenking et al.[183] have shown that there is significant correlation between the calculated proton affinities (PAs) and the pKa value and the energy of the HOMO. Of course, to compare the basicity of different ligands, it is only possible to use PA values, which have been calculated at the same level of theory, although these values are not yet available for all the species discussed in this Review. However, as can be seen in Scheme 66, the first results suggest that the order of basicity is CBAs >aNHCs >CCDP >NHC. The biggest difference between this and the order of donor strength derived from IR data of cis-[MCl(CO)2(L)] complexes is the relatively low calculated donor strength of CCDP, and this is not yet rationalized. It is also important to note that the second proton affinity is especially high for CBAs and CCDPs, which is in line with the experimentally observed diprotonation of CBA5.[204]
Scheme 66.
Calculated first and second proton affinity [kcalmol−1].
A way to distinguish the σ-donor and π-acceptor properties of a ligand is to locate its HOMO and LUMO, although the energy of the latter can not usually be accurately determined. Alternatively, it is possible to calculate the singlet–triplet energy gap. Unfortunately, these values are not available at the same level of theory for the whole series of ligands discussed in this Review. However, the energies of the HOMO and LUMO of N-YHCs and NHCs B–D,[127] as well as of CAACs and NHC B and C[258] were determined by using the same method, as well as the singlet–triplet gap and HOMO of CAACs and NHC C[259] (Scheme 67). These studies confirmed that both N-YHCs and CAACs are stronger σ donors than NHCs, but more importantly, they show that they are better π acceptors by far, which is not apparent from the ν (CO) frequencies of cis-[MCl(CO)2(L)] complexes. This is of considerable importance for the chemical reactivity of free carbenes, and indeed we have shown that free CAACs can activate CO,[260] H2, and NH3,[259] whereas free NHCs are unreactive.[261] Similarly, it is safe to predict that complexes bearing CAACs and N-YHCs will behave very differently from the NHC analogues.
Scheme 67.
Calculated energy of the HOMO and LUMO (top),[127,258] and of the singlet–triplet energy gap and HOMO (bottom) for selected carbenes.[259]
10. Summary and Outlook
The chemistry of cyclic non-NHCs is in its infancy and many issues have still to be solved, but the preliminary results are very encouraging, and point to clear directions for the design of useful species.
New synthetic routes to PHCs and N-PHCs that allow the preparation of different ring skeletons have to be discovered. Interestingly, it is not necessary for the lone pair of electrons on one (or the) phosphorus center to interact with the carbene vacant orbital to make these species stable. Consequently, the lone pair of electrons can potentially be used to change the coordination number of the P atom, which offers an opportunity to tune the electronic properties of PHCs and N-PHCs.
CAACs are by far the most studied of the non-NHCs, which is in line with their earlier discovery in 2005. The replacement of one of the electronegative amino substituents of NHCs by a strong σ-donor alkyl group makes CAAC ligands not only more electron rich than NHCs, but also more electrophilic. The presence of a quaternary carbon atom in position α to the carbene center provides steric environments that differentiate them dramatically from all other ligands, and allows for the placement of a chiral center next to the carbene. Bulky, rigid, flexible, small, and enantiomerically pure CAACs are readily available. The larger CAACs allow for the preparation of stable transition-metal complexes with unusually low coordination numbers, which confer an extreme robustness to classical complexes. These properties have been used to perform catalytic reactions that require high temperatures, as exemplified by the first examples of hydroamination reactions of alkynes and allenes with NH3. The possibility of tuning the steric properties of CAACs has been shown in the palladium-catalyzed α-arylation of ketones and aldehydes. The first examples of such catalytic processes occurring at room temperature with non-activated aryl chlorides, including di-ortho-substituted derivatives, have been reported. Moreover, small CAACs give rise to active ruthenium catalysts for olefin metathesis. In this catalytic area, it is noteworthy that the unsymmetrical nature of CAACs favors the formation of the kinetic products in olefin cross-metathesis reactions. Turnover numbers of 35000 have been achieved in the ethenolysis of methyl oleate, which is so far unparalleled.
Although no N-YHCs have yet been isolated, preliminary results indicate that the right combination of the type of ylide and ring skeleton will allow for the preparation of very stable N-YHCs. The variety of ylides available makes this class of carbenes highly tunable electronically.
So far, only diamino-substituted cyclopropenylidenes have been isolated. However, the calculated singlet–triplet energy gap of aryl-substituted CPs is much larger than those of already isolated carbenes, and therefore bulky substituents might be able to enhance the energy barrier for dimerization to such extent that diarylcyclopropenylidenes could be isolated. The extremely acute carbene bond angle makes this class of compounds very peculiar and attractive ligands for transition-metal complexes. It is important to note that most of the catalytic applications of CP complexes have been performed by using small diaryl-substituted cyclopropenylidenes. It is quite likely that much higher catalytic activities will be obtained by using bis(amino)cyclopropenylidenes, which are much stronger donors, or alternatively with bulkier CPs. As a matter of fact, the excellent catalytic results reported for the isomerization of quadricyclane to norbornadiene have been observed with palladium catalysts bearing bis(diisopropylamino)cyclopropenylidene.
Although only discovered in 2008, it already appears that a variety of stable cyclic bent allenes are readily available. CBAs resulting from the deprotonation of pyrazolium salts that are 3,5-disubstituted by π-donor groups can be prepared in large quantities from cheap precursors. They are much stronger σ donors than NHCs or even CAACs, and in contrast to regular allenes, which react with transition-metal fragments to give η2 complexes, an η1-coordination mode to metals was observed. The electronic and steric properties of CBAs can easily be tuned by changing the carbon substituents, and preliminary observations indicate that bulky substituents are not required for their isolation. The only catalytic results reported to date have been obtained by using CBAs that bear a very small alkyl or aryl group at both carbon atoms, and it is quite likely that much higher catalytic activities should be attainable with more sterically hindered and electron-rich CBAs.
As for PHCs and N-PHCs, novel synthetic routes have to be designed to make cyclic carbodiphosphoranes and vinyl-idenephopshoranes attractive compounds, since so far they have only been prepared by tedious multiple-step processes. CCDPs are the strongest σ donors of all the non-NHCs studied so far, which make them very attractive ligands. However, it seems that the corresponding complexes are rather fragile, and therefore CVPs constitute interesting alternatives, especially since their electronic properties should be easily tunable through the carbon substituent.
In contrast to classical carbenes, including NHCs, no dimerization pathway is possible for aNHCs, and skeleton rearrangements or fragmentation processes are quite unlikely. Therefore, a variety of these compounds should be available. Indeed, the only aNHC isolated today bears a simple phenyl group on the carbon atom adjacent to the carbene center, which proves that steric bulk is not a requirement. Importantly, the substituent at C4 undergoes conjugation with the carbene center, which opens the possibility of substantially modulating the electronic character of the ring system. The few catalytic experiments that have been reported are encouraging. For example, the results obtained for the β-alkylation of secondary alcohols with primary alcohols by using ruthenium–p-cymene complexes bearing an aNHC lie among the best reported to date. Abnormal NHCs can be considered as a novel class of stable meso-ionic compounds, and we believe that those resulting from C5 deprotonation of imidazolium salts are just the first type of a large series of aNHCs.[262] Indeed, there is no reason that other nitrogen-, phosphorus-, or even sulfur-containing cationic heterocycles cannot be deprotonated to afford stable derivatives.
In 2002,[263] Herrmann wrote: “from the work in numerous academic laboratories and in industry, a revolutionary turning point in organometallic catalysis is emerging thanks to NHCs”. However, it is arguable that phosphorus ligands are still the ligands of choice for most industrial processes. This is certainly due to their long history but also to their enormous structural diversity. Although it is possible to cursorily tune the structure of NHCs, any diversity is still far from matching their phosphorus-based counterparts, and we believe that the new carbon-based ligands discussed in this Review narrow the gap. Section 9 demonstrates the wide range of donating ability which can be achieved with the various non-NHCs. Interestingly, cyclic diaminocarbenes (classical NHCs) can barely reach the donor strength of most of the non-NHCs, even when the nature of the atoms of the skeleton and the size of the ring is varied.
So far, only CAACs have been proven to afford complexes with really exciting catalytic activities, but one has to keep in mind that although transition-metal–NHC complexes have been known since the 1960s,[264] their first application in catalysis appeared only in 1995,[265] and clearly this has been facilitated by the availability of storable NHCs.
Acknowledgments
Our work was supported by the NSF (CHE-0808825 and -0924410), NIH (R01 GM 68825), DOE (DE-FG02-09ER16069), and RHODIA Inc. G.B. is grateful to his dedicated co-workers, who are co-authors of the papers cited in this Review.
Biographies

Mohand Melaimi was born in Charenton Le Pont (near Paris) in 1976. He studied chemistry at the Université Pierre et Marie Curie in Paris, and completed his PhD at the Ecole Polytechnique (2003) under the supervision of Pascal Le Floch and François Mathey. After postdoctoral research (2004–2006) at Texas A&M in the group of François Gabbaï, he moved to the UCR/CNRS Joint Research Laboratory at the University of California at Riverside (USA), where he is currently working as a “Chargé de Recherche CNRS”. His research focuses on carbenes and bent allenes, and their coordination with transition metals.

Michèle Soleilhavoup studied chemistry at the University Paul Sabatier in Toulouse and received her PhD in 1993 under the supervision of Guy Bertrand. She then worked for BASF AG at Ludwigshafen (1993–1995), before moving to the University Paris VI as a “Chargée de Recherche CNRS”. From 2000 to 2001, she worked with Remi Chauvin at the Laboratoire de Chimie de Coordination in Toulouse, before joining the UCR/CNRS Joint Research Laboratory at the University of California at Riverside (USA). Her research focuses on carbenes and their application as tunable ligands for transition-metal catalysts.

Guy Bertrand studied Chemistry at Montpellier, and obtained his PhD from the University of Toulouse. 1988–1998 he was a “Director of Research” at the Laboratoire de Chimie de Coordination du CNRS, and 1998–2005 the Director of the Laboratoire d’Hétérochimie Fondamentale et Appliquée at the University Paul Sabatier (Toulouse). Since 2001 he has been Distinguished Professor and Director of the UCR/CNRS Joint Research Chemistry Laboratory at the University of California at Riverside. His research concerns the interface between organic and inorganic chemistry. He is a member of the French Academy of Sciences.
References
- 1.Arduengo AJ, III, Harlow RL, Kline M. J Am Chem Soc. 1991;113:361–363. [Google Scholar]
- 2.a) Igau A, Grützmacher H, Baceiredo A, Bertrand G. J Am Chem Soc. 1988;110:6463–6466. [Google Scholar]; b) Igau A, Baceiredo A, Trinquier G, Bertrand G. Angew Chem. 1989;101:617–618. [Google Scholar]; Angew Chem Int Ed Engl. 1989;28:621–622. [Google Scholar]
- 3.The first X-ray diffraction study of a (phosphino)silylcarbene was only reported in 2000: Kato T, Gornitzka H, Baceiredo A, Savin A, Bertrand G. J Am Chem Soc. 2000;122:998–999.
- 4.Arduengo AJ, III, Harlow RL, Kline M. J Am Chem Soc. 1991;113:2801–2801. [Google Scholar]
- 5.Bourissou D, Guerret O, Gabbaï FP, Bertrand G. Chem Rev. 2000;100:39–92. doi: 10.1021/cr940472u. [DOI] [PubMed] [Google Scholar]
- 6.Arduengo AJ, III, Goerlich JR, Marshall WJ. J Am Chem Soc. 1995;117:11027–11028. [Google Scholar]
- 7.Enders D, Breuer K, Raabe G, Runsink J, Teles JH, Melder JP, Ebel K, Brode S. Angew Chem. 1995;107:1119–1122. [Google Scholar]; Angew Chem Int Ed Engl. 1995;34:1021–1023. [Google Scholar]
- 8.Hahn FE, Wittenbecher L, Boese R, Bläser D. Chem Eur J. 1999;5:1931–1935. [Google Scholar]
- 9.Arduengo AJ, III, Goerlich JR, Marshall WJ. Liebigs Ann. 1997;365:374. [Google Scholar]
- 10.Alder RW, Butts CP, Murray M, Orpen G. J Am Chem Soc. 1998;120:11526–11527. [Google Scholar]
- 11.Soleilhavoup M, Baceiredo A, Treutler O, Ahlrichs R, Nieger M, Bertrand G. J Am Chem Soc. 1992;114:10959–10961. [Google Scholar]
- 12.Alder RW, Allen PR, Murray M, Orpen AG. Angew Chem. 1996;108:1211–1213. [Google Scholar]; Angew Chem Int Ed Engl. 1996;35:1121–1123. [Google Scholar]
- 13.a) Merceron N, Miqueu K, Baceiredo A, Bertrand G. J Am Chem Soc. 2002;124:6806–6807. doi: 10.1021/ja026556z. [DOI] [PubMed] [Google Scholar]; b) Merceron-Saffon N, Baceiredo A, Gornitzka H, Bertrand G. Science. 2003;301:1223–1225. doi: 10.1126/science.1086860. [DOI] [PubMed] [Google Scholar]
- 14.Buron C, Gornitzka H, Romanenko V, Bertrand G. Science. 2000;288:834–836. doi: 10.1126/science.288.5467.834. [DOI] [PubMed] [Google Scholar]
- 15.Sole S, Gornitzka H, Schoeller WW, Bourissou D, Bertrand G. Science. 2001;292:1901–1903. doi: 10.1126/science.292.5523.1901. [DOI] [PubMed] [Google Scholar]
- 16.Lavallo V, Mafhouz J, Canac Y, Donnadieu B, Schoeller WW, Bertrand G. J Am Chem Soc. 2004;126:8670–8671. doi: 10.1021/ja047503f. [DOI] [PubMed] [Google Scholar]
- 17.Canac Y, Soleilhavoup M, Conejero S, Bertrand G. J Organomet Chem. 2004;689:3857–3865. [Google Scholar]
- 18.Peris E, Crabtree RH. C R Acad Sci Ser IIc. 2003;6:33–37. [Google Scholar]
- 19.a) Hillier AC, Sommer WJ, Yong BS, Petersen JL, Cavallo L, Nolan SP. Organometallics. 2003;22:4322–4326. [Google Scholar]; b) Viciu MS, Navarro O, Germaneau RF, Kelly RA, Sommer WJ, Marion N, Stevens ED, Cavallo L, Nolan SP. Organometallics. 2004;23:1629–1635. [Google Scholar]
- 20.a) Scholl M, Ding S, Lee CW, Grubbs RH. Org Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]; b) Trnka TM, Grubbs RH. Acc Chem Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]
- 21.See also: Huang JK, Stevens ED, Nolan SP, Petersen JL. J Am Chem Soc. 1999;121:2674–2678.
- 22.Teles JH, Melder JP, Ebel K, Schneider R, Gehrer E, Harder W, Brode S, Enders D, Breuer K, Raabe G. Helv Chim Acta. 1996;79:61–83. [Google Scholar]
- 23.Breslow R. J Am Chem Soc. 1958;80:3719–3726. [Google Scholar]
- 24.Reviews on NHCs published in 2007: Enders D, Niemeier O, Henseler A. Chem Rev. 2007;107:5606–5655. doi: 10.1021/cr068372z.Kamber NE, Jeong W, Waymouth RM, Pratt RC, Lohmeijer BGG, Hedrick JL. Chem Rev. 2007;107:5813–5840. doi: 10.1021/cr068415b.Diez-Gonzalez S, Nolan SP. Synlett. 2007:158–2167.Kantchev EAB, O’Brien CJ, Organ MG. Angew Chem. 2007;119:2824–2870.Angew Chem Int Ed. 2007;46:2768–2813. doi: 10.1002/anie.200601663.Marion N, Díez-González S, Nolan SP. Angew Chem. 2007;119:3046–3058. doi: 10.1002/anie.200603380.Angew Chem Int Ed. 2007;46:2988–3000. doi: 10.1002/anie.200603380.Lee HM, Chun-Chin L, Pi-Yun C. Curr Org Chem. 2007;11:1491–1524.Pugh D, Danopoulos AA. Coord Chem Rev. 2007;251:610–641.Lin IJB, Vasam CS. Coord Chem Rev. 2007;251:642–670.Diver ST. Coord Chem Rev. 2007;251:671–701. doi: 10.1016/j.ccr.2006.09.008.Douth-waite RE. Coord Chem Rev. 2007;251:702–717.Gade LH, Bellemin-Laponnaz S. Coord Chem Rev. 2007;251:718–725.Colacino E, Martinez J, Lamaty F. Coord Chem Rev. 2007;251:726–764.Dragutan V, Dragutan I, Delaude L, Demonceau A. Coord Chem Rev. 2007;251:765–794.Esteruelas MA, López AM, Oliván M. Coord Chem Rev. 2007;251:795–840.Mata JA, Poyatos M, Peris E. Coord Chem Rev. 2007;251:841–859.Sommer WJ, Weck M. Coord Chem Rev. 2007;251:860–873.Diez-Gonzalez S, Nolan SP. Coord Chem Rev. 2007;251:874–883.Kascatan-Nebioglu A, Panzner MJ, Tessier CA, Cannon CL, Youngs WJ. Coord Chem Rev. 2007;251:884–895.Liddle ST, Edworthy IS, Arnold PL. Chem Soc Rev. 2007;36:1732–1744. doi: 10.1039/b611548a.Barnard PJ, Berners-Price SJ. Coord Chem Rev. 2007;251:1889–1902.Bielawski CW, Grubbs RH. Prog Polym Sci. 2007;32:1–29.Bourissou D, Moebs-Sanchez S, Martín-Vaca B. C R Chim. 2007;10:775–794.Deshmukh PH, Blechert S. Dalton Trans. 2007:2479–2491. doi: 10.1039/b703164p.
- 25.Reviews on NHCs published in 2008: Buchmeiser MR. Chem Rev. 2008;109:303–321. doi: 10.1021/cr800207n.Hahn FE, Jahnke MC. Angew Chem. 2008;120:3166–3216. doi: 10.1002/anie.200703883.Angew Chem Int Ed. 2008;47:3122–3172. doi: 10.1002/anie.200703883.Praetorius JM, Crudden CM. Dalton Trans. 2008:4079–4094. doi: 10.1039/b802114g.Cavell K. Dalton Trans. 2008:6676–6685. doi: 10.1039/b811449h.Clement ND, Routaboul L, Grote-vendt A, Jackstell R, Beller M. Chem Eur J. 2008;14:7408–7420. doi: 10.1002/chem.200800858.Díez-González S, Nolan SP. Acc Chem Res. 2008;41:349–358. doi: 10.1021/ar7001655.Marion N, Nolan SP. Acc Chem Res. 2008;41:1440–1449. doi: 10.1021/ar800020y.Würtz S, Glorius F. Acc Chem Res. 2008;41:1523–1533. doi: 10.1021/ar8000876.Normand AT, Cavell KJ. Eur J Inorg Chem. 2008:2781–2800.Veige AS. Polyhedron. 2008;27:3177–3189.Pastre JC, Correia CRD. Quim Nova. 2008;31:872–884.
- 26.Reviews on NHCs published in 2009: Kühl O. Coord Chem Rev. 2009;253:2481–2492.Tapu D, Dixon DA, Roe C. Chem Rev. 2009;109:3385–3407. doi: 10.1021/cr800521g.Lin JCY, Huang RTW, Lee CS, Bhattacharyya A, Hwang WS, Lin IJB. Chem Rev. 2009;109:3561–3598. doi: 10.1021/cr8005153.Arnold PL, Casely IJ. Chem Rev. 2009;109:3599–3611. doi: 10.1021/cr8005203.Díez-González S, Marion N, Nolan SP. Chem Rev. 2009;109:3612–3676. doi: 10.1021/cr900074m.Poyatos M, Mata JA, Peris E. Chem Rev. 2009;109:3677–3707. doi: 10.1021/cr800501s.Samojłowicz C, Bieniek M, Grela K. Chem Rev. 2009;109:3708–3742. doi: 10.1021/cr800524f.van Otterlo WAL, de Koning CB. Chem Rev. 2009;109:3743–3782. doi: 10.1021/cr900178p.Monfette S, Fogg DE. Chem Rev. 2009;109:3783–3816. doi: 10.1021/cr800541y.Alcaide B, Almendros P, Luna A. Chem Rev. 2009;109:3817–3858. doi: 10.1021/cr9001512.Hindi KM, Panzner MJ, Tessier CA, Cannon CL, Youngs WJ. Chem Rev. 2009;109:3859–3884. doi: 10.1021/cr800500u.Corberán R, Mas-Marzá E, Peris E. Eur J Inorg Chem. 2009:1700–1716.de Frémont P, Marion N, Nolan SP. Coord Chem Rev. 2009;253:862–892.Herndon JW. Coord Chem Rev. 2009;253:1517–1595.Jacobsen H, Correa A, Poater A, Costabile C, Cavallo L. Coord Chem Rev. 2009;253:687–703.Radius U, Bickelhaupt FM. Coord Chem Rev. 2009;253:678–686.Delaude L. Eur J Inorg Chem. 2009:1681–1699.Arduengo AJ, III, Iconaru LI. Dalton Trans. 2009:6903–6914. doi: 10.1039/b907211j.McGuinness D. Dalton Trans. 2009:6915–6923. doi: 10.1039/b906479f.Teyssot ML, Jarrousse AS, Manin M, Chevry A, Roche S, Norre F, Beaudoin C, Morel L, Boyer D, Mahiou R, Gautier A. Dalton Trans. 2009:6894–6902. doi: 10.1039/b906308k.Cazin CSJ. C R Chim. 2009;12:1173–1180.
- 27.Books on NHCs: Glorius F. N-Heterocyclic Carbenes in Transition Metal Catalysis. Vol. 21. Springer; Heidelberg: 2007. (Topics in Organometallic Chemistry)Nolan SP. N- Heterocyclic Carbenes in Synthesis. Wiley-VCH; Weinheim: 2006.
- 28.a) Alcaraz G, Wecker U, Baceiredo A, Dahan F, Bertrand G. Angew Chem. 1995;107:1358–1359. [Google Scholar]; Angew Chem Int Ed Engl. 1995;34:1246–1248. [Google Scholar]; b) Piquet V, Baceiredo A, Gornitzka H, Dahan F, Bertrand G. Chem Eur J. 1997;3:1757–1764. [Google Scholar]; c) Goumri-Magnet S, Kato T, Gornitzka H, Baceiredo A, Bertrand G. J Am Chem Soc. 2000;122:4464–4470. [Google Scholar]; d) Krysiak J, Kato T, Gornitzka H, Baceiredo A, Mikolajczyk M, Bertrand G. J Org Chem. 2001;66:8240–8242. doi: 10.1021/jo010586n. [DOI] [PubMed] [Google Scholar]; e) Illa O, Gornitzka H, Branchadell V, Baceiredo A, Bertrand G, Ortuño RM. Eur J Org Chem. 2003:3147–3152. doi: 10.1021/jo0350460. [DOI] [PubMed] [Google Scholar]; f) Krysiak J, Lyon C, Baceiredo A, Gornitzka H, Mikolajczyk M, Bertrand G. Chem Eur J. 2004;10:1982–1986. doi: 10.1002/chem.200305722. [DOI] [PubMed] [Google Scholar]
- 29.a) Denk K, Sirsch P, Herrmann WA. J Organomet Chem. 2002;649:219–224. [Google Scholar]; b) Herrmann WA, Ofele K, von Preysing D, Herdtweck E. J Organomet Chem. 2003;684:235–248. [Google Scholar]
- 30.Frey GD, Herrmann WA. J Organomet Chem. 2005;690:5876–5880. [Google Scholar]
- 31.Wanniarachchi YA, Slaughter LM. Organometallics. 2008;27:1055–1062. [Google Scholar]
- 32.Slaughter LM. Comments Inorg Chem. 2008;29:46–72. [Google Scholar]
- 33.For recent papers on ADC, see Alder RW, Blake ME, Chaker L, Harvey JN, Paolini F, Schütz J. Angew Chem. 2004;116:6020–6036. doi: 10.1002/anie.200400654.Angew Chem Int Ed. 2004;43:5896–5911. doi: 10.1002/anie.200400654.Kremzow D, Seidel G, Lehmann CW, Fürstner A. Chem Eur J. 2005;11:1833–1853. doi: 10.1002/chem.200400928.Moncada AI, Manne S, Tanski JM, Slaughter LM. Organometallics. 2005;25:491–505.Dhudshia B, Thadani AN. Chem Commun. 2006:668–670. doi: 10.1039/b516398f.Frey GD, Herdtweck E, Herrmann WA. J Organomet Chem. 2006;691:2465–2478.Rosen EL, Sanderson MD, Saravanakumar S, Bielawski CW. Organometallics. 2007;26:5774–5777.Wanniarachchi YA, Kogiso Y, Slaughter LM. Organometallics. 2007;27:21–24.Wanniarachchi YA, Slaughter LM. Chem Commun. 2007:3294–3296. doi: 10.1039/b703769d.Hirsch-Weil D, Snead DR, Inagaki S, Seo H, Abboud KA, Hong S. Chem Commun. 2009:2475–2477. doi: 10.1039/b821169h.Luzyanin KV, Tskhovrebov AG, Carias MC, Guedes da Silva MFC, Pombeiro AJL, Kukushkin VY. 2009;28:6559–6566.Organometallics Ruiz J, Perandones BF. Organometallics. 2009;28:830–836.Snead DR, Ghiviriga I, Abboud KA, Hong S. Org Lett. 2009;11:3274–3277. doi: 10.1021/ol9013156.Wanniarachchi YA, Subramanium SS, Slaughter LM. J Organomet Chem. 2009;694:3297–3305.
- 34.a) Merceron-Saffon N, Gornitzka H, Baceiredo A, Bertrand G. J Organomet Chem. 2004;689:1431–1435. [Google Scholar]; b) Teuma E, Lyon-Saunier C, Gornitzka H, Mignani G, Baceiredo A, Bertrand G. J Organomet Chem. 2005;690:5541–5545. doi: 10.1016/j.jorganchem.2005.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Despagnet E, Miqueu K, Gornitzka H, Dyer PW, Bourissou D, Bertrand G. J Am Chem Soc. 2002;124:11834–11835. doi: 10.1021/ja027201i. [DOI] [PubMed] [Google Scholar]
- 36.Cattoën X, Gornitzka H, Bourissou D, Bertrand G. J Am Chem Soc. 2004;126:1342–1343. doi: 10.1021/ja0396854. [DOI] [PubMed] [Google Scholar]
- 37.Vignolle J, Cattoën X, Bourissou D. Chem Rev. 2009;109:3333–3384. doi: 10.1021/cr800549j. [DOI] [PubMed] [Google Scholar]
- 38.a) Schoeller WW, Eisner D, Grigoleit S, Rozhenko AR, Alijah A. J Am Chem Soc. 2000;122:10115–10120. [Google Scholar]; b) Schoeller WW, Rozhenko AR, Alijah A. J Organomet Chem. 2001;617–618:435–443. [Google Scholar]
- 39.Martin D, Baceiredo A, Gornitzka H, Schoeller WW, Bertrand G. Angew Chem. 2005;117:1728–1731. doi: 10.1002/anie.200462239. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:1700–1703. doi: 10.1002/anie.200462239. [DOI] [PubMed] [Google Scholar]
- 40.Baceiredo A, Igau A, Bertrand G, Menu MJ, Dartiguenave Y, Bonnet JJ. J Am Chem Soc. 1986;108:7868–7869. doi: 10.1021/ja00284a081. [DOI] [PubMed] [Google Scholar]
- 41.a) Dixon DA, Arduengo AJ., III J Phys Chem. 1991;95:4180–4182. [Google Scholar]; b) Boehme C, Frenking G. J Am Chem Soc. 1996;118:2039–2046. [Google Scholar]
- 42.a) Fekete A, Nyulászi L. J Organomet Chem. 2002;643–644:278–284. [Google Scholar]; b) Schoeller WW, Eisner D. Inorg Chem. 2004;43:2585–2589. doi: 10.1021/ic030234u. [DOI] [PubMed] [Google Scholar]
- 43.Kapp J, Schade C, El-Nahasa AM, von P, Schleyer R. Angew Chem. 1996;108:2373–2376. [Google Scholar]; Angew Chem Int Ed Engl. 1996;35:2236–2238. [Google Scholar]
- 44.a) Nyulászi L. Tetrahedron. 2000;56:79–84. [Google Scholar]; b) Nyulaszi L. Chem Rev. 2001;101:1229–1246. doi: 10.1021/cr990321x. [DOI] [PubMed] [Google Scholar]
- 45.a) Kato T, Gornitzka H, Baceiredo A, Schoeller WW, Bertrand G. Science. 2000;289:754–756. doi: 10.1126/science.289.5480.754. [DOI] [PubMed] [Google Scholar]; b) Kato T, Gornitzka H, Baceiredo A, Schoeller WW, Bertrand G. J Am Chem Soc. 2002;124:2506–2512. doi: 10.1021/ja010347h. [DOI] [PubMed] [Google Scholar]; c) Martin D, Baceiredo A, Bertrand G. Eur J Inorg Chem. 2004:3533–3537. [Google Scholar]
- 46.Sebastian M, Schmidt O, Fuchs A, Nieger M, Szieberth D, Nyulaszi L, Niecke E. Phosphorus Sulfur Silicon Relat Elem. 2004;179:779–783. [Google Scholar]
- 47.Fuchs A, Nieger M, Niecke E, Nyulaszi L, Schmidt O, Schoeller W, Sebastian M. Phosphorus Sulfur Silicon Relat Elem. 2002;177:1605–1608. [Google Scholar]
- 48.Masuda JD, Martin D, Lyon-Saunier C, Baceiredo A, Gornitzka H, Donnadieu B, Bertrand G. Chem Asian J. 2007;2:178–187. doi: 10.1002/asia.200600300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karsch HH, Reisacher HU, Müller G. Angew Chem. 1986;98:467–468. [Google Scholar]; Angew Chem Int Ed Engl. 1986;25:454–455. [Google Scholar]
- 50.Karsch HH, Kühler FH, Reisacher H. Tetrahedron Lett. 1984;25:3687–3690. [Google Scholar]
- 51.a) Despagnet-Ayoub E, Grubbs RH. J Am Chem Soc. 2004;126:10198–10199. doi: 10.1021/ja047892d. [DOI] [PubMed] [Google Scholar]; b) Ishida Y, Donnadieu B, Bertrand G. Proc Natl Acad Sci USA. 2006;103:13585–13588. doi: 10.1073/pnas.0604761103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.a) Appel R, Fölling P, Josten B, Siray M, Winkhaus V, Knoch F. Angew Chem. 1984;96:620–621. [Google Scholar]; Angew Chem Int Ed Engl. 1984;23:619–620. [Google Scholar]; b) Karsch HH, Reisacher HU, Müller G. Angew Chem. 1984;96:619–620. [Google Scholar]; Angew Chem Int Ed Engl. 1984;23:618–619. [Google Scholar]
- 53.Herrmann WA, Elison M, Fischer J, Kocher C, Artus GRJ. Chem Eur J. 1996;2:772–780. [Google Scholar]
- 54.a) Cantat T, Mezailles N, Maigrot N, Ricard L, Le Floch P. Chem Commun. 2004:1274–1275. doi: 10.1039/b403436h. [DOI] [PubMed] [Google Scholar]; b) Cantat T, Mezailles N, Auffrant A, Le Floch P. Dalton Trans. 2008:1957–1972. doi: 10.1039/b717600g. [DOI] [PubMed] [Google Scholar]
- 55.Jazzar R, Liang H, Donnadieu B, Bertrand G. J Organomet Chem. 2006;691:3201–3205. doi: 10.1016/j.jorganchem.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.a) Issleib K, Schmidt H, Meyer H. J Organomet Chem. 1978;160:47–57. [Google Scholar]; b) Becker VG, Uhl W, Wessely HJ. Z Anorg Allg Chem. 1981;479:41–56. [Google Scholar]; c) Issleib K, Schmidt H, Leissring E. Synth React Inorg Met-Org Chem. 1988;18:215–223. [Google Scholar]; d) Paasch K, Nieger M, Niecke E. Angew Chem. 1995;107:2600–2602. [Google Scholar]; Angew Chem Int Ed Engl. 1995;34:2369–2371. [Google Scholar]; e) Westerhausen M, Digeser MH. Z Anorg Allg Chem. 1997;623:1237–1242. [Google Scholar]; f) Westerhausen M, Digeser MH. Inorg Chem. 1997;36:521–527. [Google Scholar]; g) Becker G, Heck JR, Hübler U, Schwarz W, Würthwein EU. Z Anorg Allg Chem. 1999;625:2008–2024. [Google Scholar]; h) Wang ZX, Wang DQ, Dou JM. J Organomet Chem. 2003;665:205–213. [Google Scholar]; i) Boeré RT, Cole ML, Junk PC, Masuda JD, Wolmershäuser G. Chem Commun. 2004:2564–2565. doi: 10.1039/b408994d. [DOI] [PubMed] [Google Scholar]
- 57.Song M, Donnadieu B, Soleilhavoup M, Bertrand G. Chem Asian J. 2007;2:904–908. doi: 10.1002/asia.200700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frey GD, Song M, Bourg JB, Donnadieu B, Soleilhavoup M, Bertrand G. Chem Commun. 2008;4711:4713. doi: 10.1039/b812333k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.a) Tsuge O, Kanemasa S. In: Advances in Heterocyclic Chemistry. Katritzky AL, editor. Vol. 5. Academic Press; New York: 1989. pp. 231–349. [Google Scholar]; b) Coskun N, Tuncman S. Tetrahedron. 2006;62:1345–1350. [Google Scholar]; c) Lopez-Calle E, Keller M, Eberbach W. Eur J Org Chem. 2003:1438–1453. [Google Scholar]
- 60.a) Moss RA, Platz MS, Jones M Jr, editors. Reactive Intermediate Chemistry. Wiley-Interscience; Hoboken: 2004. [Google Scholar]; b) Brinker UH, editor. Advances in Carbene Chemistry. Jai; Greenwich: 1994 and 1998. [Google Scholar]
- 61.a) Scaiano JC. In: Reactive Intermediate Chemistry. Moss RA, Platz MS, Jones M Jr, editors. Wiley-Interscience; Hoboken: 2004. pp. 847–872. [Google Scholar]; b) Tippmann EM, Platz MS, Svir IB, Klymenko OV. J Am Chem Soc. 2004;126:5750–5762. doi: 10.1021/ja039693k. [DOI] [PubMed] [Google Scholar]; c) Jackson JE, Platz MS. In: Advances in Carbene Chemistry. Brinker UH, editor. Vol. 1. Jai; Greenwich: 1994. p. 89. [Google Scholar]
- 62.Sheridan RS, Moss RA, Wilk BK, Shen S, Wlostowski M, Kesselmayer MA, Subramanian R, Kmiecik-Lawrynowicz G, Krogh-Jespersen K. J Am Chem Soc. 1988;110:7563–7564. [Google Scholar]
- 63.Despagnet E, Gornitzka H, Rozhenko AB, Schoeller WW, Bourissou D, Bertrand G. Angew Chem. 2002;114:2959–2961. doi: 10.1002/1521-3773(20020802)41:15<2835::AID-ANIE2835>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2002;41:2835–2837. doi: 10.1002/1521-3773(20020802)41:15<2835::AID-ANIE2835>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 64.Evanseck JD, Houk KN. J Phys Chem. 1990;94:5518–5523. [Google Scholar]
- 65.Lavallo V, Canac Y, Prasang C, Donnadieu B, Bertrand G. Angew Chem. 2005;117:5851–5855. doi: 10.1002/anie.200501841. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:5705–5709. doi: 10.1002/anie.200501841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.For reviews on hydroamination reactions, see Müller TE, Hultzsch KC, Yus M, Foubelo F, Tada M. Chem Rev. 2008;108:3795–3892. doi: 10.1021/cr0306788.Severin R, Doye S. Chem Soc Rev. 2007;36:1407–1420. doi: 10.1039/b600981f.Aillaud I, Collin J, Hannedouche J, Schulz E. Dalton Trans. 2007:5105–5118. doi: 10.1039/b711126f.Matsunaga S. J Synth Org Chem Jpn. 2006;64:778–779.Roesky PW. Z Anorg Allg Chem. 2006;632:1918–1926.Odom AL. Dalton Trans. 2005:225–233. doi: 10.1039/b415701j.Hazari N, Mountford P. Acc Chem Res. 2005;38:839–849. doi: 10.1021/ar030244z.Hartwig JF. Pure Appl Chem. 2004;76:507–516.Hong S, Marks TJ. Acc Chem Res. 2004;37:673–686. doi: 10.1021/ar040051r.Beller M, Seayad J, Tillack A, Jiao H. Angew Chem. 2004;116:3448–3479. doi: 10.1002/anie.200300616.Angew Chem Int Ed. 2004;43:3368–3398. doi: 10.1002/anie.200300616.
- 67.a) Schlummer B, Hartwig JF. Org Lett. 2002;4:1471–1474. doi: 10.1021/ol025659j. [DOI] [PubMed] [Google Scholar]; b) Rosenfeld DC, Shekhar S, Takemiya A, Utsunomiya M, Hartwig JF. Org Lett. 2006;8:4179–4182. doi: 10.1021/ol061174+. [DOI] [PubMed] [Google Scholar]; c) Anderson LL, Arnold J, Bergman RG. J Am Chem Soc. 2005;127:14542–14543. doi: 10.1021/ja053700i. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lapis AAM, DaSilveira Neto BA, Scholten JD, Nachtigall FA, Eberlin MN, Dupont J. Tetrahedron Lett. 2006;47:6775–6779. [Google Scholar]; e) Li ZG, Zhang JL, Brouwer C, Yang CG, Reich NW, He C. Org Lett. 2006;8:4175–4178. doi: 10.1021/ol0610035. [DOI] [PubMed] [Google Scholar]; f) Seshu Babu N, Reddy KM, Prasad PSS, Suryanarayana I, Lingaiah N. Tetrahedron Lett. 2007;48:7642–7645. [Google Scholar]
- 68.a) Jazzar R, Dewhurst RD, Bourg JB, Donnadieu B, Canac Y, Bertrand G. Angew Chem. 2007;119:2957–2960. doi: 10.1002/anie.200605083. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:2899–2902. doi: 10.1002/anie.200605083. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Jazzar R, Bourg JB, Dewhurst RD, Donnadieu B, Bertrand G. J Org Chem. 2007;72:3492–3499. doi: 10.1021/jo0703909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lavallo V, Frey GD, Kousar S, Donnadieu B, Bertrand G. Proc Natl Acad Sci USA. 2007;104:13569–13573. doi: 10.1073/pnas.0705809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng X, Frey GD, Kinjo R, Donnadieu B, Bertrand G. J Am Chem Soc. 2009;131:8690–8696. doi: 10.1021/ja902051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.a) Altenhoff G, Goddard R, Lehmann CW, Glorius F. Angew Chem. 2003;115:3818–3821. doi: 10.1002/anie.200351325. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:3690–3693. doi: 10.1002/anie.200351325. [DOI] [PubMed] [Google Scholar]; b) Altenhoff G, Goddard R, Lehmann CW, Glorius F. J Am Chem Soc. 2004;126:15195–15201. doi: 10.1021/ja045349r. [DOI] [PubMed] [Google Scholar]
- 72.a) Anderson DR, Lavallo V, O’leary DJ, Bertrand G, Grubbs RH. Angew Chem. 2007;119:7400–7403. doi: 10.1002/anie.200702085. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:7262–7265. doi: 10.1002/anie.200702085. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Anderson DR, Ung T, Mkrtumyan G, Bertrand G, Grubbs RH, Schrodi Y. Organometallics. 2008;27:563–566. doi: 10.1021/om7008028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.For a recent review, see Scott NM, Nolan SP. Eur J Inorg Chem. 2005;1815:1828.
- 74.Dorta R, Stevens ED, Hoff CD, Nolan SP. J Am Chem Soc. 2003;125:10490–10491. doi: 10.1021/ja0362151. [DOI] [PubMed] [Google Scholar]
- 75.a) Lavallo V, Canac Y, Dehope A, Donnadieu B, Bertrand G. Angew Chem. 2005;117:7402–7405. doi: 10.1002/anie.200502566. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:7236–7239. doi: 10.1002/anie.200502566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Collman JP, Hegedus LS, Norton JR, Finke RG, editors. Principles and Applications of Organotransition Metal Chemistry. University Science Books; Mill Valley: 1987. [Google Scholar]
- 77.a) Ford PC, Netzel TL, Spillett CT, Pourreau DB. Pure Appl Chem. 1990;62:1091–1094. [Google Scholar]; b) Maguire JA, Boese WT, Goldman ME, Goldman AS. Coord Chem Rev. 1990;97:179–192. [Google Scholar]; c) Vigalok A, Ben-David Y, Milstein D. Organometallics. 1996;15:1839–1844. [Google Scholar]
- 78.a) Hofmann P, Meier C, Hiller W, Heckel M, Riede J, Schmidt MU. J Organomet Chem. 1995;490:51–70. [Google Scholar]; b) Werner H, Schäfer M, Nürnberg O, Wolf J. Chem Ber. 1994;127:27–38. [Google Scholar]; c) Schäfer M, Mahr N, Wolf J, Werner H. Angew Chem. 1993;105:1377–1379. [Google Scholar]; Angew Chem Int Ed Engl. 1993;32:1315–1318. [Google Scholar]; d) Price RT, Anderson RA, Muetterties EL. J Organomet Chem. 1989;376:407–417. [Google Scholar]; e) Fryzuk MD, McConville DH, Rettig SJ. J Organomet Chem. 1993;445:245–256. [Google Scholar]
- 79.a) Canepa G, Brandt CD, Werner H. Organometallics. 2004;23:1140–1152. [Google Scholar]; b) Canepa G, Brandt CD, Werner H. J Am Chem Soc. 2002;124:9666–9667. doi: 10.1021/ja020560t. [DOI] [PubMed] [Google Scholar]
- 80.a) Urtel H, Meier C, Eisenträger F, Rominger F, Joschek JP, Hofmann P. Angew Chem. 2001;113:803–806. doi: 10.1002/1521-3773(20010216)40:4<781::aid-anie7810>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2001;40:781–784. doi: 10.1002/1521-3773(20010216)40:4<781::aid-anie7810>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]; b) Budzelaar PHM, Moonen NNP, de Gelder R, Smith JMM, Gal AW. Eur J Inorg Chem. 2000:753–769. [Google Scholar]; c) Yared YW, Miles SL, Bau R, Reed CA. J Am Chem Soc. 1977;99:7076–7078. [Google Scholar]
- 81.a) Viciu MS, Zinn FK, Stevens ED, Nolan SP. Organometallics. 2003;22:3175–3177. [Google Scholar]; b) Ding Y, Goddard R, Pörschke KR. Organometallics. 2005;24:439–445. [Google Scholar]
- 82.Burstein C, Lehmann CW, Glorius F. Tetrahedron. 2005;61:6207–6217. [Google Scholar]
- 83.Yamashita M, Hartwig JF. J Am Chem Soc. 2004;126:5344–5345. doi: 10.1021/ja0315107. [DOI] [PubMed] [Google Scholar]
- 84.Frey GD, Dewhurst RD, Kousar S, Donnadieu B, Bertrand G. J Organomet Chem. 2008;693:1674–1682. doi: 10.1016/j.jorganchem.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lambert JB, Zhang S, Stern CL, Huffman JC. Science. 1993;260:1917–1918. doi: 10.1126/science.260.5116.1917. [DOI] [PubMed] [Google Scholar]
- 86.Herrero-Gómez E, Nieto-Oberhuber C, López S, Benet-Buchholz J, Echavarren AM. Angew Chem. 2006;118:5581–5585. doi: 10.1002/anie.200601688. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:5455–5459. doi: 10.1002/anie.200601688. [DOI] [PubMed] [Google Scholar]
- 87.A few well-defined examples of cationic two-coordinate gold(I) π-alkene and π-alkyne complexes have also been reported: Zuccaccia D, Belpassi L, Tarantelli F, Macchioni A. J Am Chem Soc. 2009;131:3170–3171. doi: 10.1021/ja809998y.de Fremont P, Marion N, Nolan SP. J Organomet Chem. 2009;694:551–560.Akana JA, Bhattacharyya KX, Muller P, Sadighi JP. J Am Chem Soc. 2007;129:7736–7737. doi: 10.1021/ja0723784.Shapiro ND, Toste FD. Proc Natl Acad Sci USA. 2008;105:2779–2782.Hooper TN, Green M, McGrady JE, Patel JR, Russell CA. Chem Commun. 2009:3877–3879. doi: 10.1039/b908109g.Brown TJ, Dickens MG, Widenhoefer RA. Chem Commun. 2009:6451–6453. doi: 10.1039/b914632f.
- 88.Palucki M, Buchwald SL. J Am Chem Soc. 1997;119:11108–11109. [Google Scholar]
- 89.Hamann BC, Hartwig JF. J Am Chem Soc. 1997;119:12382–12383. [Google Scholar]
- 90.Satoh T, Kawamura Y, Miura M, Nomura M. Angew Chem. 1997;109:1820–1822. [Google Scholar]; Angew Chem Int Ed Engl. 1997;36:1740–1742. [Google Scholar]
- 91.Ehrentraut A, Zapf A, Beller M. Adv Synth Catal. 2002;344:209–217. [Google Scholar]
- 92.For a review, see Culkin DA, Hartwig JF. Acc Chem Res. 2003;36:234–245. doi: 10.1021/ar0201106.
- 93.For the first example of α-arylation of aldehydes, see Terao Y, Fukuoka Y, Satoh T, Miura M, Nomura M. Tetrahedron Lett. 2002;43:101–104.
- 94.For recent examples of palladium-catalyzed α-arylation of ketones with aryl chloride, see Biscoe MR, Buchwald SL. Org Lett. 2009;11:1773–1775. doi: 10.1021/ol900295u.Winkelmann OH, Riekstins A, Nolan SP, Navarro O. Organometallics. 2009;28:5809–5813.Grasa GA, Colacot TJ. Org Process Res Dev. 2008;12:522–529.Ackermann L, Vicente R, Hofmann N. Org Lett. 2009;11:4274–4276. doi: 10.1021/ol901597d.
- 95.a) Martín R, Buchwald SL. Angew Chem. 2007;119:7374–7377. [Google Scholar]; Angew Chem Int Ed. 2007;46:7236–7239. doi: 10.1002/anie.200703009. [DOI] [PubMed] [Google Scholar]; b) Martín R, Buchwald SL. Org Lett. 2008;10:4561–4564. doi: 10.1021/ol8017775. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Vo GD, Hartwig JF. Angew Chem. 2008;120:2157–2160. doi: 10.1002/anie.200705357. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:2127–2130. doi: 10.1002/anie.200705357. [DOI] [PubMed] [Google Scholar]
- 96.Blay G, Monleon A, Pedro JR. Curr Org Chem. 2009;13:1498–1539. [Google Scholar]
- 97.For recent reviews on gold-catalyzed hydroamination, see Li Z, Brouwer C, He C. Chem Rev. 2008;108:3239–3265. doi: 10.1021/cr068434l.Arcadi A. Chem Rev. 2008;108:3266–3325. doi: 10.1021/cr068435d.Gorin DJ, Sherry BD, Toste FD. Chem Rev. 2008;108:3351–3378. doi: 10.1021/cr068430g.Patil NT, Yamamoto Y. Chem Rev. 2008;108:3395–3442. doi: 10.1021/cr050041j.Widenhoefer RA. Chem Eur J. 2008;14:5382–5391. doi: 10.1002/chem.200800219.Krause N, Belting V, Deutsch C, Erdsack J, Fan HT, Gockel B, Hoffmann-Röder A, Morita N, Volz F. Pure Appl Chem. 2008;80:1063–1069.Fürstner A, Davies PW. Angew Chem. 2007;119:3478–3519.Angew Chem Int Ed. 2007;46:3410–3449. doi: 10.1002/anie.200604335.Widenhoefer RA, Han X. Eur J Org Chem. 2006:4555–4563.
- 98.Lavallo V, Frey GD, Donnadieu B, Soleilhavoup M, Bertrand G. Angew Chem. 2008;120:5302–5306. doi: 10.1002/anie.200801136. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:5224–5228. doi: 10.1002/anie.200801136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yi CS, Yun SY, Guzei IA. J Am Chem Soc. 2005;127:5782–5783. doi: 10.1021/ja042318n. [DOI] [PubMed] [Google Scholar]
- 100.Li Y, Marks TJ. J Am Chem Soc. 1998;120:1757–1771. [Google Scholar]
- 101.a) Barluenga J, Aznar F, Liz R, Rodes R. J Chem Soc Perkin Trans 1. 1980:2732–2737. [Google Scholar]; b) Barluenga J, Aznar F. Synthesis. 1977:195–196. [Google Scholar]
- 102.Nishina N, Yamamoto Y. Synlett. 2007;1767:1770. [Google Scholar]
- 103.Zeng X, Frey GD, Kousar S, Bertrand G. Chem Eur J. 2009;15:3056–3060. doi: 10.1002/chem.200802626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zeng X, Soleilhavoup M, Bertrand G. Org Lett. 2009;11:3166–3169. doi: 10.1021/ol901418c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yi C, Yun SY. J Am Chem Soc. 2005;127:17000–17006. doi: 10.1021/ja055608s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.a) Liu XY, Ding P, Huang JS, Che CM. Org Lett. 2007;9:2645–2648. doi: 10.1021/ol070814l. [DOI] [PubMed] [Google Scholar]; b) Liu XY, Che CM. Angew Chem. 2008;120:3865–3870. [Google Scholar]; Angew Chem Int Ed. 2008;47:3805–3810. doi: 10.1002/anie.200800160. [DOI] [PubMed] [Google Scholar]
- 107.Mizushima E, Hayashi T, Tanaka M. Org Lett. 2003;5:3349–3352. doi: 10.1021/ol0353159. [DOI] [PubMed] [Google Scholar]
- 108.Gold(III)-catalyzed intermolecular hydroamination of terminal alkynes with arylamines has also been reported: Zhou CY, Chan PWH, Che CM. Org Lett. 2006;8:325–328. doi: 10.1021/ol052696c.Zhang Y, Donahue JP, Li CJ. Org Lett. 2007;9:627–630. doi: 10.1021/ol062918m.
- 109.a) Nishina N, Yamamoto Y. Angew Chem. 2006;118:3392–3395. [Google Scholar]; Angew Chem Int Ed. 2006;45:3314–3317. doi: 10.1002/anie.200600331. [DOI] [PubMed] [Google Scholar]; b) Nishina N, Yamamoto Y. Tetrahedron. 2009;65:1799–1808. [Google Scholar]
- 110.Zeng X, Kinjo R, Donnadieu B, Bertrand G. Angew Chem. 2010;122:954–957. doi: 10.1002/anie.200905341. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:942–945. doi: 10.1002/anie.200905341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu L, Xu B, Mashuta MS, Hammond GB. J Am Chem Soc. 2008;130:17642–17643. doi: 10.1021/ja806685j. [DOI] [PubMed] [Google Scholar]
- 112.Weber D, Tarselli MA, Gagne MR. Angew Chem. 2009;121:5843–5846. doi: 10.1002/anie.200902049. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:5733–5736. doi: 10.1002/anie.200902049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.See also: Seidel G, Mynott R, Fürstner A. Angew Chem. 2009;121:2548–2551. doi: 10.1002/anie.200806059.Angew Chem Int Ed. 2009;48:2510–2513. doi: 10.1002/anie.200806059.
- 114.a) Kennedy-Smith JJ, Staben ST, Toste FD. J Am Chem Soc. 2004;126:4526–4527. doi: 10.1021/ja049487s. [DOI] [PubMed] [Google Scholar]; b) Hashmi ASK, Weyrauch JP, Frey W, Bats JW. Org Lett. 2004;6:4391–4394. doi: 10.1021/ol0480067. [DOI] [PubMed] [Google Scholar]
- 115.a) Nakamura I, Mizushima Y, Yamagishi U, Yamamoto Y. Tetrahedron. 2007;63:8670–8676. [Google Scholar]; b) Shimada T, Nakamura I, Yamamoto Y. J Am Chem Soc. 2004;126:10546–10547. doi: 10.1021/ja047542r. [DOI] [PubMed] [Google Scholar]
- 116.a) Cacchi S. J Organomet Chem. 1999;576:42–64. [Google Scholar]; b) Cacchi S, Fabrizi G, Pace P. J Org Chem. 1998;63:1001–1011. [Google Scholar]
- 117.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J Am Chem Soc. 2000;122:8168–8179. [Google Scholar]
- 118.Sanford MS, Love JA, Grubbs RH. J Am Chem Soc. 2001;123:6543–6554. doi: 10.1021/ja010624k. [DOI] [PubMed] [Google Scholar]
- 119.Sanford MS, Love JA, Grubbs RH. Organometallics. 2001;20:5314–5318. [Google Scholar]
- 120.Burdett KA, Harris LD, Margl P, Maughon BR, Mokthar-Zadeh T, Saucier PC, Wasserman EP. Organometallics. 2004;23:2027–2047. [Google Scholar]
- 121.Vehlow K, Maechling S, Blechert S. Organometallics. 2006;25:25–28. [Google Scholar]
- 122.Corma A, Iborra S, Velty A. Chem Rev. 2007;107:2411–2502. doi: 10.1021/cr050989d. [DOI] [PubMed] [Google Scholar]
- 123.Forman GS, McConnell AE, Hanton MJ, Slawin AMZ, Tooze RP, van Rensburg WJ, Meyer WH, Dwyer C, Kirk MM, Serfontein DW. Organometallics. 2004;23:4824–4827. [Google Scholar]
- 124.Nyulászi L, Veszpremi T, Forro A. Phys Chem Chem Phys. 2000;2:3127–3129. [Google Scholar]
- 125.a) Woodward RB, Woodman DJ. J Am Chem Soc. 1966;88:3169–3170. [Google Scholar]; b) Woodman DJ, Davidson AI. J Org Chem. 1973;38:4288–4295. doi: 10.1021/jo00964a018. [DOI] [PubMed] [Google Scholar]
- 126.Dehope A, Lavallo V, Donnadieu B, Schoeller WW, Bertrand G. Angew Chem. 2007;119:7047–7050. doi: 10.1002/anie.200702272. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:6922–6925. doi: 10.1002/anie.200702272. [DOI] [PubMed] [Google Scholar]
- 127.a) Nakafuji SY, Kobayashi J, Kawashima T. Angew Chem. 2008;120:1157–1160. doi: 10.1002/anie.200704746. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:1141–1144. doi: 10.1002/anie.200704746. [DOI] [PubMed] [Google Scholar]; b) Nakafuji SY, Kobayashi J, Kawashima T. Phosphorus Sulfur Silicon Relat Elem. 2008;183:642–643. [Google Scholar]
- 128.Asay M, Donnadieu B, Baceiredo A, Soleilhavoup M, Bertrand G. Inorg Chem. 2008;47:3949–3951. doi: 10.1021/ic800459p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fürstner A, Alcarazo M, Radkowski K, Lehmann CW. Angew Chem. 2008;120:8426–8430. doi: 10.1002/anie.200803200. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:8302–8306. doi: 10.1002/anie.200803200. [DOI] [PubMed] [Google Scholar]
- 130.Kobayashi J, Nakafuji SY, Yatabe A, Kawashima T. Chem Commun. 2008;6233:6235. doi: 10.1039/b813454e. [DOI] [PubMed] [Google Scholar]
- 131.a) Seyferth D, Hughes WB, Heeren JK. J Am Chem Soc. 1965;87:2847–2854. [Google Scholar]; b) Seyferth D, Hughes WB, Heeren JK. J Am Chem Soc. 1965;87:3467–3474. [Google Scholar]
- 132.Several transition-metal complexes featuring a bidentate NHC-ylide ligand have recently been reported: Canac Y, Duhayon C, Chauvin R. Angew Chem. 2007;119:6429–6431. doi: 10.1002/anie.200701490.Angew Chem Int Ed. 2007;46:6313–6315. doi: 10.1002/anie.200701490.Canac Y, Lepetit C, Abdalilah M, Duhayon C, Chauvin R. J Am Chem Soc. 2008;130:8406–8413. doi: 10.1021/ja801159v.Abdellah I, Debono N, Canac Y, Duhayon C, Chauvin R. Dalton Trans. 2009:7196–7220. doi: 10.1039/b907019b.
- 133.a) Breslow R. J Am Chem Soc. 1957;79:5318–5318. [Google Scholar]; b) Breslow R, Yuan C. J Am Chem Soc. 1958;80:5991–5994. [Google Scholar]; c) Breslow R, Groves JT, Ryang G. J Am Chem Soc. 1967;89:5048–5048. [Google Scholar]
- 134.Kaiser RI. Chem Rev. 2002;102:1309–1358. doi: 10.1021/cr970004v. [DOI] [PubMed] [Google Scholar]
- 135.Reisenauer HP, Maier G, Riemann A, Hoffmann RW. Angew Chem. 1984;96:596. [Google Scholar]; Angew Chem Int Ed Engl. 1984;23:641. [Google Scholar]
- 136.Komatsu K, Kitagawa K. Chem Rev. 2003;103:1371–1427. doi: 10.1021/cr010011q. [DOI] [PubMed] [Google Scholar]
- 137.Weiss R, Priesner C, Wolf H. Angew Chem. 1978;90:486–487. [Google Scholar]; Angew Chem Int Ed Engl. 1978;17:446–447. [Google Scholar]
- 138.Yoshida Z, Konishi H, Sawada S, Ogoshi H. J Chem Soc Chem Commun. 1977;850:851. [Google Scholar]
- 139.a) Konishi H, Matsumoto S, Kamitori Y, Ogoshi H, Yoshida Z. Chem Lett. 1978:241–244. [Google Scholar]; b) Yoshida Z. Pure Appl Chem. 1982;54:1059–1074. [Google Scholar]; c) Miki S, Ohno T, Iwasaki H, Yoshida Z. J Phys Org Chem. 1988;1:333–349. [Google Scholar]
- 140.a) Tamm M, Grzegorzewski A, Hahn FE. J Organomet Chem. 1995;501:309–313. [Google Scholar]; b) Schumann H, Glanz M, Girgsdies F, Hahn FE, Tamm M, Grzegorzewski A. Angew Chem. 1997;109:2328–2330. [Google Scholar]; Angew Chem Int Ed Engl. 1997;36:2232–2234. [Google Scholar]
- 141.a) Gompper R, Bartmann E. Angew Chem. 1978;90:490–491. [Google Scholar]; Angew Chem Int Ed Engl. 1978;17:456–457. [Google Scholar]; b) Gompper R, Wolf U. Liebigs Ann Chem. 1979:1406–1425. [Google Scholar]; c) Weiss R, Wolf H. Chem Ber. 1980;113:1746–1753. [Google Scholar]
- 142.a) Taatjes CA, Klippenstein SJ, Hansen N, Miller JA, Cool TA, Wang J, Lawd ME, Westmoreland PR. Phys Chem Chem Phys. 2005;7:806–813. doi: 10.1039/b417160h. [DOI] [PubMed] [Google Scholar]; b) Patterson EV, Stanton JF, McMahon RJ. J Am Chem Soc. 1997;119:5847–5856. [Google Scholar]; c) Maier G, Preiss T, Reisenauer HP, Hess BA, Jr, Schaad LJ. J Am Chem Soc. 1994;116:2014–2018. [Google Scholar]
- 143.For recent computational studies concerning the stability of cyclopropenes, see Johnson LE, Dupre DB. J Phys Chem A. 2007;111:11066–11073. doi: 10.1021/jp0717894.Scherer W, Tafipolsky M, Ofele K. Inorg Chim Acta. 2008;361:513–520.Schoeller WW, Frey GD, Bertrand G. Chem Eur J. 2008;14:4711–4718. doi: 10.1002/chem.200701926.Johnson LE, Dupre DB. J Phys Chem A. 2007;112:7448–7454. doi: 10.1021/jp802214u.
- 144.Heinemann C, Thiel W. Chem Phys Lett. 1994;217:11–16. [Google Scholar]
- 145.Lavallo V, Ishida Y, Donnadieu B, Bertrand G. Angew Chem. 2006;118:6804–6807. doi: 10.1002/anie.200602701. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:6652–6655. doi: 10.1002/anie.200602701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.a) Boche G, Hilfe C, Harms K, Marsch M, Lohrenz JCW. Angew Chem. 1995;107:509–511. [Google Scholar]; Angew Chem Int Ed Engl. 1995;34:487–489. [Google Scholar]; b) Hilf C, Bosold F, Harms K, Lohrenz JCW, Marsch M, Schimeczek M, Boche G. Chem Ber. 1997;130:1202–1212. [Google Scholar]; c) Hilf C, Bosold F, Harms K, Marsch M, Boche G. Chem Ber. 1997;130:1213–1221. [Google Scholar]; d) Arduengo AJ, III, Tamm M, Calabrese JC, Davidson F, Marshall WJ. Chem Lett. 1999:1021–1022. [Google Scholar]; e) Fränkel R, Birg C, Kernbach U, Habereder T, Nöth H, Fehlhammer WP. Angew Chem. 2001;113:1961–1964. doi: 10.1002/1521-3773(20010518)40:10<1907::aid-anie1907>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2001;40:1907–1910. doi: 10.1002/1521-3773(20010518)40:10<1907::aid-anie1907>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]; f) Arnold PL, Mungur SA, Blake AJ, Wilson C. Angew Chem. 2003;115:6163–6166. doi: 10.1002/anie.200352710. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:5981–5984. doi: 10.1002/anie.200352710. [DOI] [PubMed] [Google Scholar]; g) Arnold PL, Rodden M, Davis KM, Scarisbrick AC, Blake AJ, Wilson C. Chem Commun. 2004:1612–1613. doi: 10.1039/b404614e. [DOI] [PubMed] [Google Scholar]
- 147.Ref. [33a].
- 148.Lavallo V, Canac Y, Donnadieu B, Schoeller WW, Bertrand G. Science. 2006;312:722–724. doi: 10.1126/science.1126675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Holschumacher D, Hrib CG, Jones PG, Tamm M. Chem Commun. 2007;3661:3663. doi: 10.1039/b706708a. [DOI] [PubMed] [Google Scholar]
- 150.DePinto JT, Deprophetis WA, Menke JL, McMahon RJ. J Am Chem Soc. 2007;129:2308–2315. doi: 10.1021/ja066300j. [DOI] [PubMed] [Google Scholar]
- 151.Kinjo R, Ishida Y, Donnadieu B, Bertrand G. Angew Chem. 2009;121:525–528. doi: 10.1002/anie.200804372. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:517–520. doi: 10.1002/anie.200804372. [DOI] [PubMed] [Google Scholar]
- 152.Even triafulvalenes, which feature thermodynamically highly stabilizing benzo substituents, were only characterized by 1H NMR spectroscopy and mass spectrometry: Neidlein R, Poignée V, Kramer W, Glück C. Angew Chem. 1986;98:735–736.Angew Chem Int Ed Engl. 1986;25:731–732.Halton B, Cooney MJ, Jones CS, Boese R, Bläser D. Org Lett. 2004;6:4017–4020. doi: 10.1021/ol048307g.
- 153.For recent reviews on fulvalenes, see Halton B. Eur J Org Chem. 2005:3391–3414.Hopf H. Classics in Hydrocarbon Chemistry. Wiley-VCH; Weinheim: 2000.
- 154.a) Breslow R, Gal P. J Am Chem Soc. 1959;81:4747–4748. [Google Scholar]; b) Breslow R, Gal P, Chang HW, Altman LJ. J Am Chem Soc. 1965;87:5139–5144. [Google Scholar]
- 155.a) Alder RW, Blake ME. Chem Commun. 1997:1513–1514. [Google Scholar]; b) Hahn FE, Wittenbecher L, Le Van D, Fröhlich R. Angew Chem. 2000;112:551–554. doi: 10.1002/(sici)1521-3773(20000204)39:3<541::aid-anie541>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2000;39:541–544. doi: 10.1002/(sici)1521-3773(20000204)39:3<541::aid-anie541>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]; c) Liu Y, Lindler PE, Lemal DM. J Am Chem Soc. 1999;121:10626–10627. [Google Scholar]
- 156.For studies of noncatalyzed dimerization of carbenes, see for example: Denk MK, Hezarkhani A, Zheng FL. Eur J Inorg Chem. 2007:3527–3534.Böhm VPW, Herrmann WA. Angew Chem. 2000;112:4200–4202.Angew Chem Int Ed. 2000;39:4036–4038. doi: 10.1002/1521-3773(20001117)39:22<4036::aid-anie4036>3.0.co;2-l.Warkentin J. In: Advances in Carbene Chemistry. Brinker UH, editor. Vol. 2. Jai; London: 1998. p. 245.
- 157.For other studies on the dimerization of carbenes, see for example: Graham DC, Cavell KJ, Yates BF. J Phys Org Chem. 2005;18:298–309.Liu Y, Lemal DM. Tetrahedron Lett. 2000;41:599–602.Lemal DM, Lovald RA, Kawano KI. J Am Chem Soc. 1964;86:2518–2519.Alder RW, Chaker L, Paolini FPV. Chem Commun. 2004:2172–2173. doi: 10.1039/b409112d.
- 158.a) Trinquier G, Malrieu JP. J Am Chem Soc. 1987;109:5303–5315. [Google Scholar]; b) Malrieu JP, Trinquier G. J Am Chem Soc. 1989;111:5916–5921. [Google Scholar]; c) Jacobsen H, Ziegler T. J Am Chem Soc. 1994;116:3667–3679. [Google Scholar]
- 159.Öfele K. Angew Chem. 1968;80:1032–1033. [Google Scholar]; Angew Chem Int Ed Engl. 1968;7:950–950. [Google Scholar]
- 160.Öfele K. J Organomet Chem. 1970;22:C9–C11. [Google Scholar]
- 161.Öfele K, Tosh E, Taubmann C, Herrmann WA. Chem Rev. 2009;109:3408–3444. doi: 10.1021/cr800516g. [DOI] [PubMed] [Google Scholar]
- 162.Kuchenbeiser G, Donnadieu B, Bertrand G. J Organomet Chem. 2008;693:899–904. doi: 10.1016/j.jorganchem.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.For examples, see Herrmann WA, Fischer J, Öfele K, Artus GRJ. J Organomet Chem. 1997;530:259–262.Mata JA, Peris E, Incarvito C, Crabtree RH. Chem Commun. 2003:184–185. doi: 10.1039/b210726k.Alcarazo M, Roseblade SJ, Cowley AR, Fernandez R, Brown JM, Lassaletta JM. J Am Chem Soc. 2005;127:3290–3291. doi: 10.1021/ja0423769.Stylianides N, Danopoulos AA, Tsoureas N. J Organomet Chem. 2005;690:5948–5958.
- 164.a) Schaub T, Radius U. Chem Eur J. 2005;11:5024–5030. doi: 10.1002/chem.200500231. [DOI] [PubMed] [Google Scholar]; b) Schaub T, Backes M, Radius U. Organometallics. 2006;25:4196–4209. [Google Scholar]
- 165.a) Arduengo AJ, Gamper SF, Calabrese JC, Davidson F. J Am Chem Soc. 1994;116:4391–4394. [Google Scholar]; b) Arnold PL, Cloke GN, Geldbach T, Hitchcock PB. Organometallics. 1999;18:3228–3233. [Google Scholar]; c) Clement ND, Cavell KJ, Jones C, Elsevier CJ. Angew Chem. 2004;116:1297–1299. doi: 10.1002/anie.200353409. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:1277–1279. doi: 10.1002/anie.200353409. [DOI] [PubMed] [Google Scholar]; d) Caddick S, Cloke FGN, Hitchcock PB, de AK, Lewis K. Angew Chem. 2004;116:5948–5951. [Google Scholar]; Angew Chem Int Ed. 2004;43:5824–5827. doi: 10.1002/anie.200460955. [DOI] [PubMed] [Google Scholar]
- 166.Miki S, Ohno T, Iwasaki H, Maeda Y, Yoshida Z. Tetrahedron Lett. 1988;44:55–60. [Google Scholar]
- 167.Wass DF, Haddow MF, Hey TW, Orpen AG, Russell CA, Wingad RL, Green M. Chem Commun. 2007;2704:2706. doi: 10.1039/b702827j. [DOI] [PubMed] [Google Scholar]
- 168.Wass DF, Hey TW, Rodriguez-Castro J, Russell CA, Shishkov IV, Wingad RL, Green M. Organometallics. 2007;26:4702–4703. [Google Scholar]
- 169.Herrmann WA, Öfele K, Taubmann C, Herdweck E, Hoffmann SD. J Organomet Chem. 2007;692:3846–3854. [Google Scholar]
- 170.Taubmann C, Tosh E, Öfele K, Herdtweck E, Herrmann WA. J Organomet Chem. 2008;693:2231–2236. [Google Scholar]
- 171.Herrmann WA, Böhm VPW, Gstöttmayr CWK, Grosche M, Reisinger CP, Weskamp T. J Organomet Chem. 2001:617–618. 616–628. [Google Scholar]
- 172.Similarly, analogous complexes bearing another carbocyclic carbene, namely cycloheptatrienylidene, does not exhibit an induction period: Herrmann WA, Öfele K, Schneider SK, Herdtweck E, Hoffmann SD. Angew Chem. 2006;118:3943–3947. doi: 10.1002/anie.200503589.Angew Chem Int Ed. 2006;45:3859–3862. doi: 10.1002/anie.200503589.
- 173.Green M, McMullin CL, Morton GJP, Orpen AG, Waas DF, Wingad RL. Organometallics. 2009;28:1476–1479. [Google Scholar]
- 174.Krause N, Hashmi ASK, editors. Modern Allene Chemistry. Wiley-VCH; Weinheim: 2004. [Google Scholar]
- 175.Weber E, Seichter W, Hess B, Will G, Dasting HJ. J Phys Org Chem. 1995;8:94–96. [Google Scholar]
- 176.For reviews on ylides and carbodiphosphoranes, see Kolodiazhnyi OI. Phosphorus Ylides: Chemistry and Application in Organic Synthesis. Wiley-VCH; Weinheim: 1999. Schmidbaur H. Angew Chem. 1983;95:980–1000.Angew Chem Int Ed Engl. 1983;22:907–927.
- 177.For a discussion on other compounds featuring a carbon(0) species, see Tonner R, Öxler F, Neumüller B, Petz W, Frenking G. Angew Chem. 2006;118:8206–8211. doi: 10.1002/anie.200602552.Angew Chem Int Ed. 2006;45:8038–8042. doi: 10.1002/anie.200602552.Schmidbaur H. Angew Chem. 2007;119:3042–3043.Angew Chem Int Ed. 2007;46:2984–2985. doi: 10.1002/anie.200700139.Frenking G, Neumüller B, Petz W, Tonner R, Öxler F. Angew Chem. 2007;119:3044–3045. doi: 10.1002/anie.200602552.Angew Chem Int Ed. 2007;46:2986–2987.
- 178.Tonner R, Frenking G. Angew Chem. 2007;119:8850–8853. [Google Scholar]; Angew Chem Int Ed. 2007;46:8695–8698. doi: 10.1002/anie.200701632. [DOI] [PubMed] [Google Scholar]
- 179.For references on atomic carbon, see Shevlin PB. In: Reactive Intermediate Chemistry. Moss RA, Platz MS Jr, Jones M, editors. Wiley; New York: 2004. pp. 463–500.Joo H, Shevlin PB, Mckee ML. J Am Chem Soc. 2006;128:6220–6230. doi: 10.1021/ja060216m.
- 180.For recent computational developments of divalent element(0) compounds, see Takagi N, Shimizu T, Frenking G. Chem Eur J. 2009;15:8593–8604. doi: 10.1002/chem.200901401.Frenking G, Tonner R. Pure Appl Chem. 2009;81:597–614.Takagi N, Shimizu T, Frenking G. Chem Eur J. 2009;15:3448–3456. doi: 10.1002/chem.200802739.Tonner R, Frenking G. Chem Eur J. 2008;14:3260–3272. doi: 10.1002/chem.200701390.Tonner R, Frenking G. Chem Eur J. 2008;14:3273–3289. doi: 10.1002/chem.200701392.Deshmukh MM, Gadre SR, Tonner R, Frenking G. Phys Chem Chem Phys. 2008;10:2298–2301. doi: 10.1039/b803068e.
- 181.Dyker CA, Lavallo V, Donnadieu B, Bertrand G. Angew Chem. 2008;120:3250–3253. doi: 10.1002/anie.200801176. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:3206–3209. doi: 10.1002/anie.200705620. [DOI] [PubMed] [Google Scholar]
- 182.Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R. J Chem Soc Perkin Trans. 1987;2:S1–S19. [Google Scholar]
- 183.Tonner R, Heydenrych G, Frenking G. ChemPhysChem. 2008;9:1474–1481. doi: 10.1002/cphc.200800208. [DOI] [PubMed] [Google Scholar]
- 184.a) Schmidbaur H, Sherbaum F, Huber B, Müller G. Angew Chem. 1988;100:441–443. [Google Scholar]; Angew Chem Int Ed Engl. 1988;27:419–421. [Google Scholar]; b) Schmidbaur H, Gasser O. Angew Chem. 1976;88:542–543. [Google Scholar]; Angew Chem Int Ed Engl. 1976;15:502–503. [Google Scholar]; c) Vicente J, Singhal AR, Jones PG. Organometallics. 2002;21:5887–5890. [Google Scholar]; d) Romeo I, Bardaji M, Gimeno MC, Laguna M. Polyhedron. 2000;19:1837–1841. [Google Scholar]
- 185.a) Fürstner A, Alcarazo M, Goddard R, Lehmann CW. Angew Chem. 2008;120:3254–3258. doi: 10.1002/anie.200705798. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:3210–3214. doi: 10.1002/anie.200705798. [DOI] [PubMed] [Google Scholar]; b) Alcarazo M, Lehmann CW, Anoop A, Thiel W, Fürstner A. Nat Chem. 2009;1:295–301. doi: 10.1038/nchem.248. [DOI] [PubMed] [Google Scholar]
- 186.See also: Dyker CA, Bertrand G. Nat Chem. 2009;1:265–266. doi: 10.1038/nchem.265.
- 187.a) Christl M. In: Modern Allene Chemistry. Krause N, Hashmi ASK, editors. Vol. 1. Wiley-VCH; Weinheim: 2004. pp. 243–358. [Google Scholar]; b) Johnson RP. Chem Rev. 1989;89:1111–1124. [Google Scholar]
- 188.a) Warmuth R, Marvel MA. Angew Chem. 2000;112:1168–1171. doi: 10.1002/(sici)1521-3773(20000317)39:6<1117::aid-anie1117>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2000;39:1117–1119. doi: 10.1002/(sici)1521-3773(20000317)39:6<1117::aid-anie1117>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]; b) Warmuth R, Marvel MA. Chem Eur J. 2001;7:1209–1220. doi: 10.1002/1521-3765(20010316)7:6<1209::aid-chem1209>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 189.Price JD, Johnson RP. Tetrahedron Lett. 1986;27:4679–4682. [Google Scholar]
- 190.a) Pang Y, Petrich SA, Young VG, Jr, Gordon MS, Barton TJ. J Am Chem Soc. 1993;115:2534–2536. [Google Scholar]; b) Lin JB, Pang Y, Young VG, Barton TJ. J Am Chem Soc. 1993;115:3794–3795. [Google Scholar]; c) Shimizu T, Hojo F, Ando W. J Am Chem Soc. 1993;115:3111–3115. [Google Scholar]
- 191.Hofmann MA, Bergstrasser U, Reiss GJ, Nyulaszi L, Regitz M. Angew Chem. 2000;112:1318–1320. doi: 10.1002/(sici)1521-3773(20000403)39:7<1261::aid-anie1261>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2000;39:1261–1263. [Google Scholar]
- 192.a) Ugolotti J, Dierker G, Kehr G, Fröhlich R, Grimme S, Erker G. Angew Chem. 2008;120:2662–2665. doi: 10.1002/anie.200705615. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:2622–2625. doi: 10.1002/anie.200705615. [DOI] [PubMed] [Google Scholar]; b) Rosenthal U. Angew Chem. 2008;120:5196–5199. [Google Scholar]; Angew Chem Int Ed. 2008;47:5118–5121. doi: 10.1002/anie.200801571. [DOI] [PubMed] [Google Scholar]
- 193.For examples, see Wang J, Sheridan RS. Org Lett. 2007;9:3177–3180. doi: 10.1021/ol071290s.Azizoglu A, Demirkol O, Kilic T, Yildiz YK. Tetrahedron. 2007;63:2409–2413.Christl M, Braun M, Fischer H, Groetsch S, Müller G, Leusser D, Deuerlein S, Stalke D, Arnone M, Engels B. Eur J Org Chem. 2006:5045–5058.Nikitina AF, Sheridan RS. Org Lett. 2005;7:4467–4470. doi: 10.1021/ol051733x.Ceylan M, Yalcin S, Secen H, Sutbeyaz Y, Balci M. J Chem Res. 2003:21–23.
- 194.Daoust KJ, Hernandez SM, Konrad KM, Mackie ID, Winstanley J, Johnson RP. J Org Chem. 2006;71:5708–5714. doi: 10.1021/jo060698k. [DOI] [PubMed] [Google Scholar]
- 195.Eid AI, Kira MA, Fahmy HH. J Pharm Belg. 1978;33:303–311. [PubMed] [Google Scholar]
- 196.a) Gilbert AM, Failli A, Shumsky J, Yang Y, Severin A, Singh G, Hu W, Keeney D, Petersen PJ, Katz AH. J Med Chem. 2006;49:6027–6036. doi: 10.1021/jm060499t. [DOI] [PubMed] [Google Scholar]; b) Shoeb HA, Ibrahim M, Kamel OK. Egypt J Chem. 1972;15:603–608. [Google Scholar]; c) Kira MA, Osman AI, Fahmy HH. Egypt J Chem. 1978;21:341–348. [Google Scholar]
- 197.Lavallo V, Dyker CA, Donnadieu B, Bertrand G. Angew Chem. 2008;120:5491–5494. doi: 10.1002/anie.200801176. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:5411–5414. doi: 10.1002/anie.200801176. [DOI] [PubMed] [Google Scholar]
- 198.a) Saalfrank RW, Maid H. Chem Commun. 2005:5953–5967. doi: 10.1039/b510062n. [DOI] [PubMed] [Google Scholar]; b) Saalfrank RW. Isr J Chem. 1985;26:181–190. [Google Scholar]
- 199.a) Christl M, Engels B. Angew Chem. 2009;121:1566–1567. [Google Scholar]; Angew Chem Int Ed. 2009;48:1538–1539. doi: 10.1002/anie.200803476. [DOI] [PubMed] [Google Scholar]
- 200.Lavallo V, Dyker CA, Donnadieu B, Bertrand G. Angew Chem. 2009;121:1568–1570. [Google Scholar]; Angew Chem Int Ed. 2009;48:1540–1542. [Google Scholar]
- 201.Hänninen MM, Peuronen A, Tuononen HM. Chem Eur J. 2009;15:7287–7291. doi: 10.1002/chem.200900928. [DOI] [PubMed] [Google Scholar]
- 202.Fernández I, Dyker CA, DeHope A, Donnadieu B, Frenking G, Bertrand G. J Am Chem Soc. 2009;131:11875–11881. doi: 10.1021/ja903396e. [DOI] [PubMed] [Google Scholar]
- 203.Brand JS, de Candole BC, Brown JA. Org Lett. 2003;5:2343–2346. doi: 10.1021/ol034701n. [DOI] [PubMed] [Google Scholar]
- 204.Melaimi M, Parameswaran P, Donnadieu B, Frenking G, Bertrand G. Angew Chem. 2009;121:4886–4889. doi: 10.1002/anie.200901117. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:4792–4795. doi: 10.1002/anie.200901117. [DOI] [PubMed] [Google Scholar]
- 205.Schmidbaur H, Costa T, Milewski-Mahrla B, Schubert U. Angew Chem. 1980;92:557–558. [Google Scholar]; Angew Chem Int Ed Engl. 1980;19:555–556. [Google Scholar]
- 206.Marrot S, Kato T, Gornitzka H, Baceiredo A. Angew Chem. 2006;118:2660–2663. doi: 10.1002/anie.200504396. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:2598–2601. doi: 10.1002/anie.200504396. [DOI] [PubMed] [Google Scholar]
- 207.Marrot S, Kato T, Cossio FP, Gornitzka H, Baceiredo A. Angew Chem. 2006;118:7607–7610. doi: 10.1002/anie.200603151. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:7447–7450. [Google Scholar]
- 208.Tejeda J, Reau R, Dahan F, Bertrand G. J Am Chem Soc. 1993;115:7880–7881. [Google Scholar]
- 209.Cristau HM. Chem Rev. 1994;94:1299–1313. [Google Scholar]
- 210.Bestmann HJ, Roth K, Saalfrank RW. Angew Chem. 1977;89:915–916. [Google Scholar]; Angew Chem Int Ed Engl. 1977;16:877–878. [Google Scholar]
- 211.Asay M, Kato T, Saffon-Merceron N, Cossío FP, Baceiredo A, Bertrand G. Angew Chem. 2008;120:7640–7643. doi: 10.1002/anie.200802741. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:7530–7533. doi: 10.1002/anie.200802741. [DOI] [PubMed] [Google Scholar]
- 212.a) Birney D, Lim TK, Koh JHP, Pool BR, White JM. J Am Chem Soc. 2002;124:5091–5099. doi: 10.1021/ja025634f. [DOI] [PubMed] [Google Scholar]; b) Avenati M, Pilet O, Carrupt PA, Vogel P. Helv Chim Acta. 1982;65:178–187. [Google Scholar]
- 213.a) Cowley AH, Kemp RA, Lasch JG, Norman NC, Stewart CA. J Am Chem Soc. 1983;105:7444–7445. [Google Scholar]; b) Cowley AH, Kemp RA, Lasch JG, Norman NC, Stewart CA, Whittlesey BR, Wright TC. Inorg Chem. 1986;25:740–749. [Google Scholar]
- 214.a) Cristau HJ, Grenier J, Lexouret P, Torreilles E. Heteroat Chem. 1996;7:471–480. [Google Scholar]; b) Grützmacher H, Pritzkow H. Angew Chem. 1991;103:721–723. [Google Scholar]; Angew Chem Int Ed Engl. 1991;30:709–710. [Google Scholar]; c) Heim U, Pritzkow H, Schoenberg H, Grützmacher H. J Chem Soc Chem Commun. 1993:673–674. [Google Scholar]
- 215.Bart JCJ. J Chem Soc B. 1969;350:365. [Google Scholar]
- 216.Burzlaff H, Voll U, Bestmann HJ. Chem Ber. 1974;107:1949–1956. [Google Scholar]
- 217.For reviews on transition-metal–allene complexes, see Jacobs TL. In: The Chemistry of the Allenes. Landor SR, editor. Vol. 2. Academic Press; New York: 1982. pp. 277–347.Shaw BL, Stringer AJ. Inorg Chim Acta Rev. 1973;7:1–10.
- 218.Corberan R, Marrot S, Dellus N, Merceron-Saffon N, Kato T, Peris E, Baceiredo A. Organometallics. 2009;28:326–330. [Google Scholar]
- 219.Munro-Leighton C, Blue ED, Gunnoe TB. J Am Chem Soc. 2006;128:1446–1447. doi: 10.1021/ja057622a. [DOI] [PubMed] [Google Scholar]
- 220.Han Y, Huynh HV, Tan GK. Organometallics. 2007;26:6581–6585. [Google Scholar]
- 221.Han Y, Lee LJ, Huynh HV. Organometallics. 2009;28:2778–2786. [Google Scholar]
- 222.See, for example: Canac Y, Bourissou D, Baceiredo A, Gornitzka H, Schoeller WW, Bertrand G. Science. 1998;279:2080–2082. doi: 10.1126/science.279.5359.2080.Scheschkewitz D, Amii H, Schoeller WW, Bourissou D, Bertrand G. Science. 2002;295:1880–1881. doi: 10.1126/science.1068167.Scheschkewitz D, Amii H, Gornitzka H, Schoeller WW, Bourissou D, Bertrand G. Angew Chem. 2004;116:595–597. doi: 10.1002/anie.200352944.Angew Chem Int Ed. 2004;43:585–587. doi: 10.1002/anie.200352944.Rodriguez A, Olsen RA, Ghaderi N, Scheschkewitz D, Tham FS, Mueller LJ, Bertrand G. Angew Chem. 2004;116:4988–4991. doi: 10.1002/anie.200460475.Angew Chem Int Ed. 2004;43:4880–4883. doi: 10.1002/anie.200460475.Gandon V, Bourg JB, Tham FS, Schoeller WW, Bertrand G. Angew Chem. 2008;120:161–165. doi: 10.1002/anie.200704008.Angew Chem Int Ed. 2008;47:155–159.
- 223.a) Gründemann S, Kovacevic A, Albrecht M, Faller JW, Crabtree RH. Chem Commun. 2001:2274–2275. doi: 10.1039/b107881j. [DOI] [PubMed] [Google Scholar]; b) Gründemann S, Kovacevic A, Albrecht M, Faller JW, Crabtree RH. J Am Chem Soc. 2002;124:10473–10481. doi: 10.1021/ja026735g. [DOI] [PubMed] [Google Scholar]
- 224.For reviews, see Arnold PL, Pearson S. Coord Chem Rev. 2007;251:596–609.Albrecht M. Chem Commun. 2008:3601–3610. doi: 10.1039/b806924g.Schuster O, Yang L, Raubenheimer HG, Albrecht M. Chem Rev. 2009;109:3445–3478. doi: 10.1021/cr8005087.Albrecht M. Chimia. 2009;63:105–110.
- 225.Recent articles dealing with aNHCs: Viciano M, Feliz M, Corberán R, Mata JA, Clot E, Peris E. Organometallics. 2007;26:5304–5314.Baya M, Eguillor B, Esteruelas MA, Oliván M, Oñate E. Organometallics. 2007;26:6556–6563.Eguillor B, Esteruelas MA, Oliván M, Puerta M. Organometallics. 2008;27:445–450.Wolf J, Labande A, Daran JC, Poli R. Eur J Inorg Chem. 2008:3024–3030.Appelhans LN, Incarvito CD, Crabtree RH. J Organomet Chem. 2008;693:2761–2766.Crittall MR, Ellul CE, Mahon MF, Saker O, Whittlesey MK. Dalton Trans. 2008:4209–4211. doi: 10.1039/b807923d.Holschumacher D, Bannenberg T, Hrib CG, Jones PG, Tamm M. Angew Chem. 2008;120:7538–7542. doi: 10.1002/anie.200802705.Angew Chem Int Ed. 2008;47:7428–7432. doi: 10.1002/anie.200802705.Heckenroth M, Kluser E, Neels A, Albrecht M. Dalton Trans. 2008:6242–6249. doi: 10.1039/b812405a.Tang CY, Smith W, Vidovic D, Thompson AL, Chaplin AB, Aldridge S. Organometallics. 2009;28:3059–3066.Maron L, Bourissou D. Organometallics. 2009;28:3686–3690.Albrecht M. Science. 2009;289:532–533. doi: 10.1126/science.1181553.
- 226.Heckenroth M, Neels A, Garnier MG, Aebi P, Ehlers AW, Albrecht M. Chem Eur J. 2009;15:9375–9386. doi: 10.1002/chem.200900249. [DOI] [PubMed] [Google Scholar]
- 227.Tonner R, Heydenrych G, Frenking G. Chem Asian J. 2007;2:1555–1567. doi: 10.1002/asia.200700235. [DOI] [PubMed] [Google Scholar]
- 228.Magill AM, Cavell KJ, Yates BF. J Am Chem Soc. 2004;126:8717–8724. doi: 10.1021/ja038973x. [DOI] [PubMed] [Google Scholar]
- 229.Magill AM, Yates BF. Aust J Chem. 2004;57:1205–1210. [Google Scholar]
- 230.a) Bambirra S, Leusen DV, Meetsma A, Hessen B, Teuben JH. Chem Commun. 2003:522–523. doi: 10.1039/b208502j. [DOI] [PubMed] [Google Scholar]; b) Korotkikh MI, Kiselov AV, Pekhtereva TM, Shvaika OP. Ukr Khim Zh. 2001;67:97–103. [Google Scholar]
- 231.a) Appelhans LN, Zuccaccia D, Kovacevic A, Chianese AR, Miecznikowski JR, Macchioni A, Clot E, Eisenstein O, Crabtree RH. J Am Chem Soc. 2005;127:16299–16311. doi: 10.1021/ja055317j. [DOI] [PubMed] [Google Scholar]; b) Kovacevic A, Gründemann S, Miecznikowski JR, Clot E, Eisenstein O, Crabtree RH. Chem Commun. 2002:2580–2581. [Google Scholar]
- 232.Aldeco-Perez E, Rosenthal AJ, Donnadieu B, Parameswaran P, Frenking G, Bertrand G. Science. 2009;326:556–559. doi: 10.1126/science.1178206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lebel H, Janes MK, Charette AB, Nolan SP. J Am Chem Soc. 2004;126:5046–5047. doi: 10.1021/ja049759r. [DOI] [PubMed] [Google Scholar]
- 234.John A, Shaikh MM, Ghosh P. Dalton Trans. 2009;10581:10591. doi: 10.1039/b913068c. [DOI] [PubMed] [Google Scholar]
- 235.Heckenroth M, Kluser E, Neels A, Albrecht M. Angew Chem. 2007;119:6409–6412. doi: 10.1002/anie.200702199. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2007;46:6293–6296. doi: 10.1002/anie.200702199. [DOI] [PubMed] [Google Scholar]
- 236.Yang L, Krüger A, Neels A, Albrecht M. Organometallics. 2008;27:3161–3171. [Google Scholar]
- 237.For reviews, see Samec JSM, Backvall JE, Andersson PG, Brandt P. Chem Soc Rev. 2006;35:237–248. doi: 10.1039/b515269k.Morris RH. Chem Soc Rev. 2009;38:2282–2291. doi: 10.1039/b806837m.Wang C, Wu XF, Xiao JL. Chem Asian J. 2008;3:1750–1770. doi: 10.1002/asia.200800196.Gladiali S, Alberico E. Chem Soc Rev. 2006;35:226–236. doi: 10.1039/b513396c.
- 238.Prades A, Corberan R, Poyatos M, Peris E. Chem Eur J. 2008;14:11474–11479. doi: 10.1002/chem.200801580. [DOI] [PubMed] [Google Scholar]
- 239.Prades A, Corberan R, Poyatos M, Peris E. Chem Eur J. 2009;15:4610–4613. doi: 10.1002/chem.200802740. [DOI] [PubMed] [Google Scholar]
- 240.Prades A, Viciano M, Sanau M, Peris E. Organometallics. 2008;27:4254–4259. [Google Scholar]
- 241.da Costa AP, Viciano M, Sanau M, Merino S, Tejeda J, Peris E, Royo B. Organometallics. 2008;27:1305–1309. [Google Scholar]
- 242.Tolman CA. Chem Rev. 1977;77:313–348. [Google Scholar]
- 243.Cyclic voltametry of [Rh(Cl)L(cod)] complexes is an alternative method that allows the ranking of the donor strength of a family of ligands, but so far this method has only been used with a restricted number of L ligands: Wolf S, Plenio H. J Organomet Chem. 2009;694:1487–1492.
- 244.Chianese AR, Li XW, Janzen MC, Faller JW, Crabtree RH. Organometallics. 2003;22:1663–1667. [Google Scholar]
- 245.Tonner R, Frenking G. Organometallics. 2009;28:3901–3905. [Google Scholar]
- 246.Gusev DG. Organometallics. 2009;28:763–770. [Google Scholar]
- 247.Fürstner A, Alcarazo M, Krause H, Lehmann CW. J Am Chem Soc. 2007;129:12676–12677. doi: 10.1021/ja076028t. [DOI] [PubMed] [Google Scholar]
- 248.Dyker CAV, Lavallo G. Bertrand unpublished results [Google Scholar]
- 249.Chianese AR, Kovacevic A, Zeglis BM, Faller JW, Crabtree RH. Organometallics. 2004;23:2461–2468. [Google Scholar]
- 250.Song G, Zhang Y, Li X. Organometallics. 2008;27:1936–1943. [Google Scholar]
- 251.a) Leuthäuβer S, Schwarz D, Plenio H. Chem Eur J. 2007;13:7195–7203. doi: 10.1002/chem.200700228. [DOI] [PubMed] [Google Scholar]; b) Kelly RA, III, Clavier H, Giudice S, Scott NM, Stevens ED, Bordner J, Samardjiev I, Hoff CD, Cavallo L, Nolan SP. Organometallics. 2008;27:202–210. [Google Scholar]
- 252.Bazinet P, Ong TG, O’Brien JS, Lavoie N, Bell E, Yap GPA, Korobkov I, Richeson DS. Organometallics. 2007;26:2885–2895. [Google Scholar]
- 253.Präsang C, Donnadieu B, Bertrand G. J Am Chem Soc. 2005;127:10182–10183. doi: 10.1021/ja052987g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mayr M, Wurst K, Ongania KH, Buchmeiser MR. Chem Eur J. 2004;10:1256–1266. doi: 10.1002/chem.200305437. [DOI] [PubMed] [Google Scholar]
- 255.a) Sanderson MD, Kamplain JW, Bielawski CW. J Am Chem Soc. 2006;128:16514–16515. doi: 10.1021/ja067475w. [DOI] [PubMed] [Google Scholar]; b) Khramov DM, Lynch VM, Bielawski CW. Organometallics. 2007;26:6042–6049. [Google Scholar]
- 256.Srebro M, Michalak A. Inorg Chem. 2009;48:5361–5369. doi: 10.1021/ic900336r. [DOI] [PubMed] [Google Scholar]
- 257.Huynh HV, Han Y, Jothibasu R, Yang JA. Organometallics. 2009;28:5395–5404. [Google Scholar]
- 258.Tukov AA, Normand AT, Nechaev MS. Dalton Trans. 2009;7015:7028. doi: 10.1039/b906969k. [DOI] [PubMed] [Google Scholar]
- 259.Frey GD, Lavallo V, Donnadieu B, Schoeller WW, Bertrand G. Science. 2007;316:439–441. doi: 10.1126/science.1141474. [DOI] [PubMed] [Google Scholar]
- 260.Lavallo V, Canac Y, Donnadieu B, Schoeller WW, Bertrand G. Angew Chem. 2006;118:3568–3571. doi: 10.1002/anie.200600987. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:3488–3491. doi: 10.1002/anie.200600987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.CAACs and NHCs also react very differently with elemental phosphorus: Masuda JD, Schoeller WW, Donnadieu B, Bertrand G. Angew Chem. 2007;119:7182–7185. doi: 10.1002/anie.200703055.Angew Chem Int Ed. 2007;46:7052–7055. doi: 10.1002/anie.200703055.Masuda JD, Schoeller WW, Donnadieu B, Bertrand G. J Am Chem Soc. 2007;129:14180–14181. doi: 10.1021/ja077296u.Back O, Kuchenbeiser G, Donnadieu B, Bertrand G. Angew Chem. 2009;121:5638–5641. doi: 10.1002/anie.200902344.Angew Chem Int Ed. 2009;48:5530–5533. doi: 10.1002/anie.200902344.
- 262.Guisado-Barrios G, Bouffard J, Donnadieu B, Bertrand G. Angew Chem. 2010;122:4869–4872. doi: 10.1002/anie.201001864. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:4759–4762. doi: 10.1002/anie.201001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Herrmann WA. Angew Chem. 2002;114:1342–1363. doi: 10.1002/1521-3773(20020415)41:8<1363::aid-anie1363>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2002;41:1290–1309. doi: 10.1002/1521-3773(20020415)41:8<1290::aid-anie1290>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 264.a) Öfele K. J Organomet Chem. 1968;12:42–43. [Google Scholar]; b) Cardin DJ, Cetinkaya B, Lappert MF. Chem Rev. 1972;72:545–574. [Google Scholar]
- 265.Herrmann WA, Elison M, Fischer J, Köcher C, Artus GRJ. Angew Chem. 1995;107:2602–2605. [Google Scholar]; Angew Chem Int Ed Engl. 1995;34:2371–2374. [Google Scholar]