Abstract
Yeast can proliferate in environments containing very high Ca2+ primarily due to the activity of vacuolar Ca2+ transporters Pmc1 and Vcx1. Yeast mutants lacking these transporters fail to grow in high Ca2+ environments, but growth can be restored by small increases in environmental Mg2+. Low extracellular Mg2+ appeared to competitively inhibit novel Ca2+ influx pathways and to diminish the concentration of free Ca2+ in the cytoplasm, as judged from the luminescence of the photoprotein aequorin. These Mg2+-sensitive Ca2+ influx pathways persisted in yvc1 cch1 double mutants. Based on mathematical models of the aequorin luminescence traces, we propose the existence in yeast of at least two Ca2+ transporters that undergo rapid feedback inhibition in response to elevated cytosolic free Ca2+ concentration. Finally, we show that Vcx1 helps return cytosolic Ca2+ toward resting levels after shock with high extracellular Ca2+ much more effectively than Pmc1 and that calcineurin, a protein phosphatase regulator of Vcx1 and Pmc1, had no detectable effects on these factors within the first few minutes of its activation. Therefore, computational modeling of Ca2+ transport and signaling in yeast can provide important insights into the dynamics of this complex system.
Keywords: Calcium homeostasis, Calcium signaling, Response curve, Competitive inhibition
1. Introduction
In eukaryotic cells, Ca2+ functions as a highly versatile intracellular signal molecule regulating many different biological processes such as proliferation, muscle contraction, neurotransmitter release, programmed cell death and gene expression, etc. [2,22,41,42]. In budding yeast (Saccharomyces cerevisiae), calcium signals are used to effect important adaptations in response to mating pheromones [24], membrane damaging compounds [8,9], and a variety of environmental stresses such as high salt, high pH, high osmolarity, and others [37].
Like mammalian cells, budding yeast maintain cytosolic Ca2+ concentration at very low levels in growing yeast cells (50–200 nM) [31] through the actions of Ca2+ pumps and exchangers (see Fig. 1A) [7,12]. A vacuolar calcium ATPase Pmc1 [13,15] and a vacuolar Ca2+/H+ exchanger Vcx1 [14,32] independently transport cytosolic Ca2+ into large vacuoles, the major Ca2+ store and sink. Cytosolic Ca2+ is also pumped into ER and Golgi through a secretory pathway calcium ATPase termed Pmr1 [44,47], which may also promote Ca2+ extrusion from yeast cells [52]. Unlike in the case of plant and animal cells, Ca2+ pumps and exchangers have not been detected on the plasma membrane of yeast cells [47,52]. When yeast cells experience a severe hypertonic shock, vacuolar Ca2+ can be released into the cytosol through the mechanosensitive Ca2+ channel Yvc1, a distant homolog of mammalian TRPC-type Ca2+ channels [36], which results in transient elevation of cytosolic Ca2+ concentration. A plasma membrane voltage-gated Ca2+ channel (VGCC) composed of Cch1 and Mid1 becomes activated in response to depolarization [10], depletion of secretory Ca2+ [27], pheromone stimulation [34], hypotonic shock [4], In unstimulated yeast cells growing in standard laboratory conditions, small amounts of extracellular Ca2+ enter the cell through unidentified transporters on the plasma membrane [27].
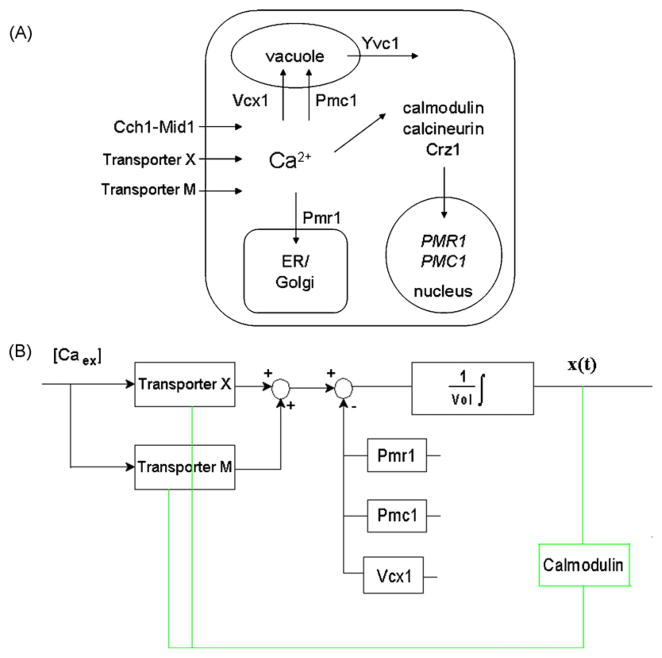
Fig. 1.
Schematic graph of the system and control block diagram. Panel A, A schematic graph of Ca2+ homeostasis/signaling system in yeast cells (for details, please see the text in Section 1). Transporter M is newly detected in this work and is assumed to open under extremely high extracellular Ca2+ concentration. Panel B, Control block diagram of our model. In yvc1 cch1 yeast cells, the cytosolic Ca2+ influx is through Transporter X and an assumed Transporter M, the cytosolic Ca2+ efflux is through Pmc1, Pmr1 and Vcx1. For yeast cells with fixed volume, the cytosolic Ca2+ concentration (i.e., x(t)) is the integral of the flux rate difference (i.e., influx rate subtracting efflux rate) divided by the cytosolic volume. The green lines describe the feedback loop: cytosolic Ca2+ concentration is sensed by the calmodulin and Ca2+-bound calmodulin is assumed to inhibit the activity of both transporters M and X. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
In response to sudden increases in extracellular Ca2+, cytosolic free Ca2+ levels rapidly rise and fall with complex dynamics, as judged by luminescence measurements of yeast cells expressing aequorin [31]. In response to these changes, the universal Ca2+ sensor protein calmodulin can bind and activate the protein phosphatase calcineurin, which inhibits the function of Vcx1 and induces the expression of Pmc1 and Pmr1 via activation of the Crz1 transcription factor [13,14,16,25,46]. These calcineurin-sensitive adaptations appear to be crucial for proliferation of yeast cells in high calcium environments. Mathematical modeling of these feedback networks successfully recapitulated the observed changes in aequorin luminescence [12]. However, that initial model relied on extremely rapid effects of calcineurin on Pmc1 and Pmr1 expression that were not physiologically realistic.
Here we show that the calcineurin-dependent feedback networks described above have little or no effect on aequorin luminescence traces within the first few minutes of Ca2+ shock. Therefore, the initially complex dynamics of cytosolic Ca2+ homeostasis must arise through other mechanisms. The observed dynamics are well described by a new mathematical model that omits calcineurin-dependent feedback and instead includes rapid Ca2+-dependent feedback inhibition of Ca2+ influx. Though the feedback mechanism and the Ca2+ influx transporters all remain to be identified, experimental evidence suggests that the primary Ca2+ influx transporters are competitively inhibited by extracellular Mg2+. Indeed, the impaired growth of yeast cells in high calcium environments was ameliorated by inclusion of Mg2+ salts in the medium and the best fitting mathematical model incorporates two Mg2+-sensitive Ca2+ influx pathways with different cation affinities. These findings extend a recent report of Ca2+ influx pathways in yeast that become (re)activated upon withdrawal of extracellular Mg2+ [49] and demonstrate the power of combining experimental and computational methodologies.
2. Methods
2.1. Experimental methods
The ability of extracellular Mg2+ to prevent toxicity of extra-cellular Ca2+ to yeast was measured as in [13]. Briefly, stationary phase cultures of each yeast strain were diluted 1000-fold into fresh YPD pH 5.5 medium containing varying concentrations of CaCl2 and MgCl2, incubated at 30 °C for 24 h, and then optical density was measured at 650 nm using a 96-well platereader (Molecular Devices). The concentration of CaCl2 causing a 50% inhibition of growth (the IC50) was obtained by fitting the data to the simple sigmoid equation.
Cytosolic free Ca2+ concentrations were monitored in populations of yeast cells expressing aequorin essentially as described [34]. Briefly, yeast cells were transformed with plasmid pEVP11 and grown to mid-log phase in synthetic medium lacking leucine to select for plasmid maintenance. Cells were harvested by centrifugation, washed, and resuspended at OD600 = 20 in medium containing 10% ethanol and 25 μg/mL coelenterazine (Molecular Probes, Inc.). After 45 min incubation at room temperature in the dark, cells were washed twice with YPD pH 5.5 medium and shaken at 30 °C for an additional 90 min. Aliquots of the cell suspension (450 μL) were pipetted into tubes containing appropriate volumes of 1 M MgCl2, mixed, and placed into a Sirius tube luminometer (Bertholdfinc) Inc.). Luminescence was recorded at 0.2 s intervals for 0.5 min prior to and 3.0 min post injection of 2 M CaCl2 (300 μL). Output is plotted as relative luminescence units per second (RLU) over time using similar numbers of cells per sample.
2.2. Mathematical modeling
2.2.1. Control block diagram
In the control block diagram as shown in Fig. 1B, we can see that for yeast cells with fixed volume, we can calculate the concentration of cytosolic Ca2+ (i.e., x(t)) by taking an integral of the flux rate difference (i.e., the influx rate through Transporter M and Transporter X into the cytosol subtracting the sequestering rates of Pmc1, Pmr1 and Vcx1) divided by the cytosolic volume. Since the slow gene expression feedback pathway through calcineurin has now been shown not to be accounting for the observed response spikes, there should be some other quick feedback mechanisms the details of which we are currently quite ignorant to. Since calmodulin has been reported to have the ability of directly regulating the opening probability of Ca2+ influx pathways [51], here for simplicity, we just assume that the activity of Transporter M and Transporter X are both directly inhibited by Ca2+-bound calmodulin (see the green lines in Fig. 1B). If the volume of yeast cells changes due to growth or hypertonic shock, etc., then we need to further consider the effects caused by the cellular volume change.
The mathematical modeling for simulating calcium response curves in yvc1 cch1 yeast cells can now be divided into three parts: feedback part modeling, protein modeling (including Transporter M, Transporter X, Pmc1, Pmr1 and Vcx1) and volume evolution modeling.
2.2.2. Feedback part modeling
Sensing cytosolic Ca2+
Due to the usual defect of one of the two C-terminal EF-hands (site IV), yeast calmodulin can only bind to a maximum of three molecules of Ca2+ [16]. By law of mass action, we can use the following equation to describe the cytosolic Ca2+ sensing process under the assumption of strong cooperativity existing among the three active sites [12,23]:
| (1) |
where m(t) denotes the concentration of Ca2+-bound calmodulin and m′ (t) denotes its change rate, denotes the forward rate contant, denotes the backward rate constant, x(t) denotes cytosolic calcium ion concentration and [CaMtotal] denotes the total concentration of calmodulin (including Ca2+-free and Ca2+-bound form).
Once we know the concentration of Ca2+-bound calmodulin, we use a putative algebraic expression to model its assumed inhibitory regulation on Transporter M and Transporter X as follows:
| (2) |
| (3) |
where ka, kb denote the feedback control constants. JM and JX denote the calcium ion flux through Transporter M and Transporter X, respectively. JMO and JXO denote the calcium ion flux through Transporter M and Transporter X in the case of without calmodulin inhibition. Obviously with such simple algebraic expressions the inhibitory relation (i.e., when m(t) rises, the activity of Transporter M and Transporter X will both drop) can be represented.
2.2.3. Volume evolution modeling (under hypertonic shock)
As we will see later, our aequorin response curves are all obtained under condition of applying hypertonic shocks (e.g., by the sudden injection of 800 mM CaCl2 into the extracellular medium) to the yeast cells. Under such great osmotic perturbation, yeast cells will quickly shrink due to the existence of water channels in the plasma membrane [20,29,45]. The evolution of the cell volume is governed by the following equation [29]:
| (4) |
where V(t) and A(t) denote the volume and the surface area of yeast cells at time t, respectively, V′ (t) denotes the change rate of yeast cell volume, Lp denotes the hydraulic membrane permeability, σ denotes the reflection coefficient, R denotes the ideal gas constant, T denotes the room temperature (294.15 K), ns denotes the apparent number of osmotically active molecules in the cell, b denotes the non-osmotic volume and Πex denotes the osmotic pressure of the external medium which can be calculated as Πex = Π0 + 3([Caex] + [Mgex])RT where Π0 denotes the initial osmotic pressure of the external medium, [Caex] and [Mgex] denote extracellular Ca2+ concentration and extracellular Mg2+ concentration, respectively (please note that we can calculate A(t) = 4π (3V(t)/4/π)2/3 because yeast cells are somewhat spherical).
The initial intracellular osmotic pressure (Πi(0)) for yeast cells in standard medium is around 0.636 Osm [20], according to Boyle van’t Hoff’s law, Πi(t) = RT(n0 + x(t))/(V(t) − b) where x(t) denotes cytosolic calcium ion concentration. This means that the initial apparent number of osmotically active molecules n0 in yeast cell is around 0.636 × 0.4 mol/L × V0 = 3.82 × 10−14 mol (please note that b is 0.4V0 and V0 = 100 μm3 is the initial volume of the yeast cell [20]). As a common knowledge, x(t) is always <1 mM (even under extremely high hypertonic shock) which is small compared with n0. So we can assume ns = n0 + x(t) as a constant.
2.2.4. Protein modeling
Experimental results show that the uptake behaviors of the four involved proteins (Transporter X [27], Pmc1 [48], Pmr1 [44] and Vcx1 [35]) conform to the Michaelis–Menten equation, which is a general equation for describing the single site ion uptake behavior of many kinds of transport proteins [2,11,12]. If we assume that the uptake behavior of Transporter M can also be modeled using this equation and that Mg2+ is a competitive inhibitor of this transporter, according to the classical enzyme kinetics theory of competitive inhibition [17,38], the uptake rate of Transporter M can be expressed as:
| (5) |
where JMO denotes the uptake rate of Transporter M (without calmodulin inhibition), [Caex] and [Mgex] denote extracellular Ca2+ concentration and extracellular Mg2+ concentration, respectively, Vmax is the maximum uptake rate of Transporter M, Km is the binding constant and KIM is the inhibition constant. And we can build a similar mathematical model for Transporter X which has been shown to be Mg2+-sensitive [27].
2.2.5. A concise model
Since now we have built the uptake models for all the five proteins involved in Ca2+ transport and models for the relevant feedback regulation, according to the control block diagram (Fig. 1B) and by further taking consideration of the effect of cell volume shrinkage, we can derive the main equation of our calcium homeostasis problem as follows [19]:
| (6) |
where x′(t) denotes the change rate of cytosolic free Ca2+ concentration, JM, JX, JPmc1, JVcx1 and JPmr1 denote the calcium ion flux through Transporter M, Transporter X, Pmc1, Vcx1 and Pmr1, respectively, Vol(t) denotes the volume of the cytosol at time t which can be roughly calculated as 10%V(t) and Vol′(t) denotes its change rate, f denotes the calcium buffer effect constant [19]. As stated in page 105 of Ref. [19], “fi (i.e., f) can be interpreted as the fraction of (i.e., total cytosolic calcium) which is free. Typical measured values for fi are 0.01–0.05.” In yeast cells, the calcium buffer effect is especially severe (we believe that >99% of total cytosolic calcium is bound).
We can further write the above main equation in fully detailed mathematical form:
| (7) |
where x(t) denotes cytosolic calcium ion concentration, m(t) denotes the concentration of Ca2+-bound calmoculin, Km, Kx, K1, K2 and K3 are the Michaelis binding constants of Transporter M, Transporter X, Pmc1, Vcx1 and Pmr1, respectively, Vm, Vx, V1, V2 and V3 are the corresponding rate parameters.
Previously published data [27] show that after 2.5 h of incubation, the maximum Ca2+ accumulation due to Transporter X is around 390 nmol/109 cells. We can roughly calculate parameter Vx in the main equation (Eq. (7)) as follows (for details, please see Eq. (18) of Ref. [12]):
| (8) |
where α denotes the growth rate constant the value of which is 0.006 min−1 [18].
The main equation (Eq. (7)) together with the calcium sensing equation (Eq. (1)) and volume evolution equation (Eq. (4)) constitute a concise mathematical model for simulating response curves in yvc1 cch1 yeast cells under hypertonic shocks. The whole set of parameters (except the control parameters [Caex] and [Mgex]) in our model which will be used in the subsequent simulations are listed in Table 1.
Table 1.
Model parameters for which all results (except Fig. 4C) are calculated unless otherwise stated
| Parameter | Value | Description | |
|---|---|---|---|
| Km | 505.43 mM | The binding constant of Transporter M | |
| Kx | 500 μM | The binding constant of Transporter X [27] | |
| K1 | 4.3 μM | The binding constant of Pmc1 [48] | |
| K2 | 0.1 μM | The binding constant of Pmr1 [44] | |
| K3 | 25 μM | The binding constant of Vcx1 [35] | |
| KIM | 149.18 mM | The Mg2+ inhibition constant of Transporter M | |
| KIX | 3.51 × 10−6 μM | The Mg2+ inhibition constant of Transporter X | |
| Vm | 1.239 × 10−16 mol min−1 | The rate parameter of Transporter M | |
| Vx | 3.2 × 10−17 mol min−1 | The rate parameter of Transporter X (calculated from [27]) | |
| V1 | 4.459 × 10−17 mol min−1 | The rate parameter of Pmc1 | |
| V2 | 4.394 × 10−17 mol min−1 | The rate parameter of Pmr1 | |
| V3 | 1.682 × 10−15 mol min−1 | The rate parameter of Vcx1 | |
| ka | 0.0448 | The feedback control constant of Transporter M | |
| kb | 4.319 | The feedback control constant of Transporter X | |
|
|
3.751 (μM)−3 min−1 | The forward rate constant of Eq. (1) | |
|
|
0.02445 min−1 | The backward rate constant of Eq. (1) | |
| [CaMtotal ] | 25 μM | The total concentration of calmodulin [12] | |
| Lmax | 1.9996 × 109 RLUs | The maximal aequorin luminescence | |
| f | 0.007626 | The calcium buffer effect constant | |
| Lp | 6 × 10−12 m s−1 Pa−1 | The hydraulic membrane permeability [29] | |
| σ | 0.035 | The reflection coefficient | |
| b | 0.4V0 | The non-osmotic volume of yeast cell [20] | |
| ns | 3.82 × 10−14 mol | The apparent number of osmotically active molecules in the cell (calculated from [20]) | |
| Π0 | 0.24 Osm | The initial osmotic pressure of the external medium [20] | |
| V0 | 100 μm3 | The initial volume of the yeast cell [20] |
2.2.6. Conversion to aequorin luminescence unit (RLUs)
Here we use the following experimentally reported function [3,21] to convert calcium ion concentration from μM to aequorin luminescence unit (i.e., RLU: relative luminescence unit) so that we can make intuitive comparison between our numerical solutions (in μM) with experimentally obtained aequorin response curves (in RLU):
| (9) |
where x denotes Ca2+ concentration value in unit μM and Lmax denotes the maximal aequorin luminescence.
2.3. Parameter estimation method
The above described two transporters model (Eqs. (1), (4) and (7). We name this model as two transporter model because in this model, it is assumed that there are two influx pathways: transporters M and X) has 25 parameters, 13 of which (Km, KIM, KIX, Vm, V1, V2, V3, ka, kb, , Lmax and f) are free parameters which need to be estimated. Here we use a hybrid optimization algorithm to estimate the model parameters by minimizing the difference between the experimental data and the model prediction. The hybrid optimization algorithm consists of a combination of a stochastic evolutionary algorithm [6,50] for the global search to find a good initial guess followed by a local search performed using Levenberg–Marquardt algorithm to refine the quality of the fit.
To describe a bit more specifically, the stochastic evolutionary algorithm is a stochastic process that modifies an original population of individuals from iteration to iteration with the aim of minimizing an objective function. In this study, we use a modified (μ, λ)-Evolutionary Strategy (ES), based on stochastic fitness ranking [6,50]. This method is simple and has proven to be more efficient than most other classical evolutionary algorithms for large parameter estimation problems [33,39,40]. The local search was performed using the Matlab (The Mathworks, Inc.) function “LSQCURVEFIT” which employs Levenberg–Marquardt algorithm to do the curve fitting for non-linear data.
We simultaneously fit the model to both the wild-type data (see Fig. 2B) and the mutant data (see Fig. 2C) by minimizing the least square error (LSE). As usual, we proportionally weight the wild-type data based on the maximum amplitude of each data set and take mutant data weight to be 1/3 of the wild-type data because the mutant data is less important than the wild-type data. This leads us to minimize the weighted least square error given as:
| (10) |
where a denotes the type of data (wild-type or mutant). Let S = {0, 0.3 mM, 3 mM, 30 mM, 90 mM}, then m denotes the case in which extracellular MgCI2 is the mth element of S. n denotes the time point and Tn denotes the number of data points in the dataset for a single experimental response curve. Thus denotes the concentration for the data type a with extracellular Mg2+ = S[m] at time t = n × td where td denotes the time difference between the consecutive data points (in our case, td = 0.2 s and Tn = 300), F(a, m, n) is the simulated data and wa,m,n is the associated weight.
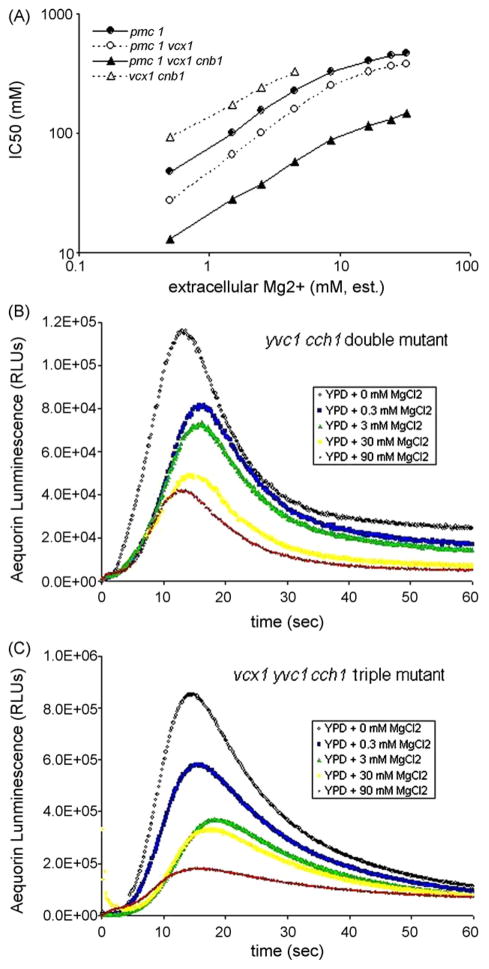
Fig. 2.
Experimental results. Panel A, the concentrations of CaCl2 that caused a 50% inhibition of growth (i.e., the IC50 for CaCl2 ) were shown for pmc1 (filled circles), pmc1 vcx1 (open circles), pmc1 vcx1 cnb1 (filled triangles) and vcx1 cnb1 (open triangles) mutants after 24 h of growth standard YPD culture medium supplemented with 0–32 mM MgCl2 . Panel B, A yvc1 cch1 double mutant expressing apo-aequorin from a plasmid was incubated with coelenterazine co-factor to reconstitute aequorin in situ. The cells bearing reconstituted aequorin were returned to growth medium for an additional 90 min, divided into equal aliquots, treated with varying amounts (0 mM, 0.3 mM, 3 mM, 30 mM, 90 mM) of MgCl2 , placed into a tube luminometer, and monitored for luminescence before and after injection of 800 mM CaCl2 . Panel C, the corresponding aequorin luminescence curves for vcx1 yvc1 cch1 mutant.
In addition to fitting the two transporters model to the experimental data, we also performed fitting for the one transporter model. In this case, Transporter X is assumed to be the only influx pathway (i.e., under the condition of Vm = 0, Eqs. (1), (4) and (7) constitute the one transporter model) and only 9 parameters (KIX, V1, V2, V3, kb, , Lmax and f) are free parameters which need to be estimated. Because of the stochastic nature of the optimization strategy used here, lots of fitting were made for both the one transporter and two transporters model. In order to be able to compare both models, the same parameter boundary was given for each parameter and the optimization was run under the same condition (500 iterations of global search and 10,000 iterations of local search). The estimated parameters values together with the remaining parameter values for the best fit of two transporters model are shown in Table 1. The simulation results of the best fit for two transporters model and for one transporter model are shown in Fig. 4A–C.
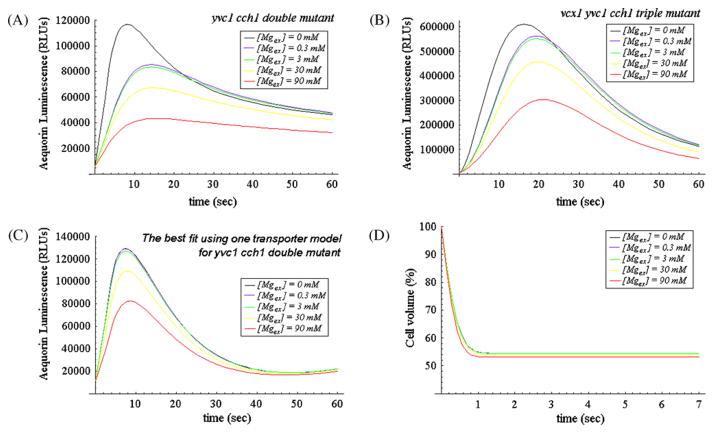
Fig. 4.
Simulated response curves and volume evolution curves. Panel A, the simulated response curves of the best fit using two transporters model for yvc1 cch1 mutant. The black, blue, green, yellow and red curves depict the simulated x(t) curves for parameter [Mgex ] suddenly increasing from 0 mM to various concentrations (0 mM, 0.3 mM, 3 mM, 30 mM, 90 mM) at t = 0, respectively (please note that at the same time t = 0 parameter [Caex ] suddenly increases from 0 mM to 800 mM in these simulations). Panel B, the corresponding simulated response curves using two transporters model for vcx1 yvc1 cch1 mutant. Panel C, the simulated response curves of the best fit using one Mg2+-sensitive transporter model (i.e., in this case we assume that Transporter X is the sole influx pathway) for yvc1 cch1 mutant. The black, blue, green, yellow and red curves depict the simulated x(t) curves for parameter [Mgex ] suddenly increasing from 0 mM to various concentrations (0 mM, 0.3 mM, 3 mM, 30 mM, 90 mM) at t = 0, respectively (please note that at the same time t = 0 parameter [Caex ] suddenly increases from 0 mM to 800 mM in these simulations). Panel D, the simulated volume evolution curves using the two transporters model for yvc1 cch1 mutant under hypertonic shock (step increase of [Caex ] from 0 mM to 800 mM with simultaneous step increase of [Mgex ] to various concentrations). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3. Results
3.1. Mg2+ blocks Ca2+ toxicity and Ca2+ influx in yeast
Yeast mutants lacking the vacuolar Ca2+ ATPase Pmc1p grow as well as wild-type yeast strains in standard culture media but they grow much poorer than wild-type when environmental Ca2+ is elevated [13], suggesting that elevated cytosolic free Ca2+ can be toxic to yeast. However, we noticed that Ca2+ toxicity was blocked by a contaminant present in certain batches of agar (data not shown). The contaminant that blocked Ca2+ toxicity was traced to Mg2+ because (1) the toxicity blocking activity was abolished by addition of the Mg2+ chelator EDTA, (2) crude preparations of agar are known to contain millimolar Mg2+, and (3) pure MgCl2 but not NaCl could block Ca2+ toxicity in pmc1 mutants in standard culture media.
To investigate the mechanism of Mg2+ suppression of Ca2+ toxicity, the concentration of CaCl2 that caused a 50% inhibition of growth (i.e., the IC50 for CaCl2) was determined for pmc1 mutants after 24 h of growth standard YPD culture medium supplemented with 0–32 mM MgCl2. Remarkably, for pmc1 knockout mutants the IC50 for CaCl2 increased with increasing MgCl2 up to ~8 mM after which the effectiveness of MgCl2 began to decrease (Fig. 2A, filled circles). Double mutants lacking both Pmc1 and the vacuolar Ca2+/H+ exchanger Vcx1 behaved similarly except the IC50 values were shifted downward by ~1.6-fold (Fig. 2A, open circles). The sole remaining Ca2+ transporter Pmr1, a Ca2+/Mn2+ ATPase of the Golgi complex, is essential for growth of pmc1 vcx1 double mutants and is strongly up-regulated by calcineurin [14]. A pmc1 vcx1 cnb1 triple mutant that also lacks calcineurin exhibited ~2.3-fold lower tolerance to CaCl2 than the pmc1 vcx1 double mutant and MgCl2 suppressed CaCl2 toxicity over a similar range of concentrations (Fig. 2A, filled triangles). A vcx1 cnb1 double mutant in which Pmc1 and Pmr1 are expressed only at basal levels exhibited ~6.6-fold increase of IC50 for CaCl2 as expected and also exhibited MgCl2 suppression of CaCl2 toxicity (Fig. 2A, open triangles). These findings demonstrate that MgCl2 suppresses CaCl2 toxicity independent of all known Ca2+ transporters, which is consistent with the possibility that Mg2+ competitively inhibits one or more Ca2+ influx pathways.
Two Ca2+ channels have been characterized in yeast, the vacuolar Ca2+ release channel Yvc1 and the plasma membrane Ca2+ influx channel Cch1-Mid1. The yvc1 cch1 pmc1 vcx1 quadruple mutant exhibited similar levels of MgCl2 suppression of CaCl2 toxicity as the pmc1 vcx1 double mutants (data not shown), suggesting that Mg2+ blocks some other Ca2+ influx pathways. To test this possibility directly, cytosolic free Ca2+ concentrations were monitored directly after a sudden increase in extracellular CaCl2 by following luminescence of the Ca2+-sensitive photoprotein aequorin. The yvc1 cch1 double mutant expressing apo-aequorin from a plasmid was incubated with coelenterazine co-factor to reconstitute aequorin in situ. The cells bearing reconstituted aequorin were returned to growth medium for an additional 90 min, divided into equal aliquots, treated with varying amounts of MgCl2, placed into a tube luminometer, and monitored for luminescence before and after injection of 800 mM CaCl2. In the absence of added MgCl2, aequorin luminescence rose quickly after CaCl2 injection, peaked after ~13.3 s, and declined to a level that was well above the starting level (Fig. 2B). Increasing concentrations of MgCl2 progressively lowered the rates of luminescence rise, the maximum achievable luminescence, and the new baseline levels following decline (Fig. 2B). Remarkably, the loss of Vcx1 resulted in a dramatic increase in the rate and peak height of aequorin luminescence (Fig. 2C). Additionally, the loss of Vcx1 resulted in ~60% slower rate of luminescence decline after the peak regardless of MgCl2 concentration. Though calcineurin inhibits Vcx1 function in long-term growth assays [14], the activity of Vcx1 in these short-term luminescence experiments was not detectably affected by addition of the calcineurin inhibitor FK506 (data not shown). The further loss of Pmc1 also had little effect on aequorin responses (data not shown). These findings identify Vcx1 as the major Ca2+-sequestering transporter in short-term responses to high Ca2+ environments and confirm the hypothesis that Mg2+ interferes with one or more novel Ca2+ influx pathways. A similar hypothesis was proposed recently to explain the increased Ca2+ influx and elevated cytosolic Ca2+ concentration observed in yeast upon Mg2+ sudden withdrawal [49]. The proteins responsible for Mg2+-sensitive Ca2+ influx have not been identified but the Alr, Alr2, and Mnr2 proteins have been identified as hetero-oligomeric proteins required for Mg2+ uptake that also promote sensitivity to high environmental Ca2+ [28].
3.2. Computational modeling of Ca2+ influx and sequestration
The multiphasic nature and calcineurin-independence of the aequorin luminescence curves suggested complex dynamics of the novel Ca2+ influx pathway(s). To help understand these dynamics, a mathematical model was constructed in which the Ca2+ transporters Pmr1, Pmc1, and Vcx1 were assumed to function without calcineurin feedback in vivo according to standard Michaelis–Menten kinetics. The optimal fitting to the experimental data using a hybrid optimization algorithm (see Section 2.3) shows that simulations assuming two Mg2+-sensitive Ca2+ influx transporters (termed transporters M and X) each with distinct properties (as discussed below in more detail) can closely fit the experimental data (see Fig. 4A and B and compare them with Fig. 2B and C) whereas simulations using just one Mg2+-sensitive Ca2+ influx transporter poorly fit the experimental data (see Fig. 4C and compare it with Fig. 2B).
3.2.1. Steady state properties
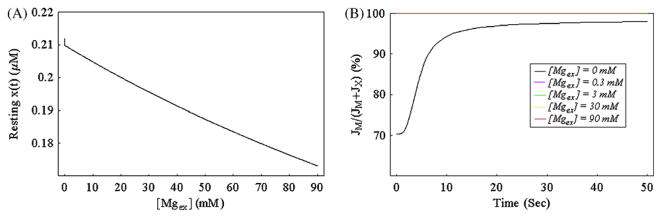
The model for yvc1 cch1 mutant under hypertonic shock consists of three equations (Eqs. (1), (4) and (7), please see Section 2.2) with three unknowns: x(t), m(t) and V(t). By performing steady state analysis of our model with fixed parameter [Caex] = 800 mM, we can first depict the steady state value of x(t) as a function of parameter [Mgex] as shown in Fig. 3A. In general, the resting level of x(t) decreases almost linearly as [Mgex] increases. Only in the case of very low [Mgex] (≤1 μM), the curves shows a strange bending.
Fig. 3.
Steady state analysis and flux analysis of the system. Panel A, the steady state value of x(t) as a function of parameter [Mgex ] for simulated yvc1 cch1 mutant in extracellular medium with high calcium concentration (parameter [Caex ] = 800 mM). The simulated cytosolic calcium level of our model yvc1 cch1 mutant rests within 0.173–0.212 μM (regardless of the initial conditions) as the media Mg2+ level (parameter [Mgex ]) ranges from 0 mM to 90 mM. Panel B, the simulated flux proportion of Transporter M in the total cytosolic Ca2+ influx as a function of t for yvc1 cch1 mutant under hypertonic shock (at t = 0, parameter [Caex ] suddenly increases from 0 mM to 800 mM and at the same time parameter [Mgex ] suddenly increases from 0 mM to various concentrations (0 mM, 0.3 mM, 3 mM, 30 mM, 90 mM)). Please note that the blue, green and yellow curves coincide with the red curve in this graph. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.2.2. Transients and mutant behavior
By setting reasonable initial conditions (x(0) = 100 nM, m(0) = 0 M and V(0) = 100 μM3) and then solving the three equations (Eqs. (1), (4) and (7), using the parameters listed in Table 1) numerically, we can depict the x(t) curves for parameter [Mgex] suddenly increasing from 0 mM to various concentrations (0 mM, 0.3 mM, 3 mM, 30 mM, 90 mM) at t = 0 as shown in Fig. 4A (please note that at the same time, parameter [Caex] suddenly increases from 0 mM to 800 mM in these simulations). In general, the simulated x(t) rises due to the hypertonic shock, forms a peak and then declines to a resting value; for higher value of parameter [Mgex], the peak value of the curve is lower and appears later. For example, by observing the top black curve for parameter [Mgex] = 0 mM, we can see that due to the hypertonic shock, the simulated x(t) quickly rises (in around 9 s) from an initial value of 5700RLUs (~100 nM) to a high peak of around 116600RLUs (~0.54 μM), then gradually decreases to a value of 46110RLUs (~0.35 μM) when t = 1 min. Further investigation shows that it will further decreases to steady state value of 17500RLUs (~0.21 μM). By observing the blue curve for parameter [Mgex] rising from 0 mM to 0.3 mM, we can see that the simulated cytosolic x(t) rises slower than the black curve and its peak value (around 85200RLUs (~0.47 μM)) which appears when t = 14 s is much lower than that of the black curve. However, the green curve for parameter [Mgex] rising from 0 mM to 3 mM seems just a little bit lower and slower than the blue curve.
In Fig. 4B, we depict the simulated x(t) curves for vcx1 yvc1 cch1 triple mutant under hypertonic shock (step increase of [Caex] from 0 mM to 800 mM with simultaneous step increase of [Mgex] to various concentrations at t = 0, please note that V3 is set to be 0 in these simulations, for the rest parameters please see Table 1). By comparison of these curves with their corresponding curves for simulated yvc1 cch1 double mutant in Fig. 4A, the peak values of these simulated response curves seem much higher. For example, the peak value of the top black curve for [Mgex] = 0 mM in Fig. 4B is around 609000RLUs (~1.07 μM) which is certainly much higher than the peak value 116600RLUs (~0.54 μM) of its corresponding black curve in Fig. 4A.
In Fig. 4C, the simulated x(t) curves of the best fit using one Mg2+-sensitive transporter model (i.e., Transporter X is assumed to be the only influx pathway) for yvc1 cch1 mutant under hyper-tonic shock (step increase of [Caex] from 0 mM to 800 mM with simultaneous step increase of [Mgex] from 0 mM to various concentrations at t = 0) are shown. In this figure, the top two curves (i.e., the black curve and the blue curve) coincide with each other. The peak values of the blue, green, yellow, red curves are 129000RLUs (~0.413 μM), 127000RLUs (~0.41 μM), 109000RLUs (~0.382 μM), 82340RLUs (~0.334 μM), respectively. The peaks of both blue and green curves appear when t = 7.5 s whereas the peaks of the yellow and red curves appear at t = 7.9 s and t = 8.6 s, respectively.
3.2.3. Flux analysis and cell volume evolution
To discriminate the different influx contributions from two Ca2+ influx pathways, in Fig. 3B we depict the simulated flux proportion of Transporter M in the total cytosolic Ca2+ influx as a function of t for yvc1 cch1 mutant under hypertonic shock (step increase of [Caex] from 0 mM to 800 mM with simultaneous step increase of [Mgex] from 0 mM to various concentrations at t = 0). By observing the bottom black curve for [Mgex] = 0 mM, we can see that at the beginning, 70.4% of the cytosolic Ca2+ influx is contributed by Transporter M and this proportion quickly rises to 98%. The other curves seem to almost coincide and are flat with a constant value of 100%.
In Fig. 4D, the simulated volume evolution curves using to transporters model for yvc1 cch1 mutant under hypertonic shock (step increase of [Caex] from 0 mM to 800 mM with simultaneous step increase of [Mgex] from 0 mM to various concentrations at t = 0) are shown. As we can see in this figure, in general, the cell volume quickly decreases (in about 1 s) to a steady state value of 53–55% of the initial volume and the difference among different color curves are quite subtle.
3.2.4. Extracellular Mg2+ depletion and Ca2+ challenge
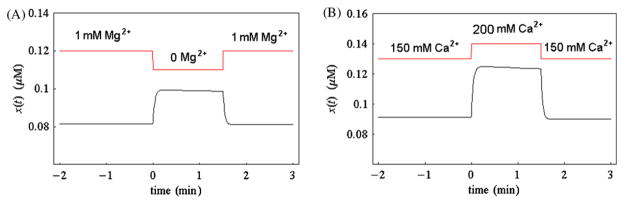
To check the behavior of cytosolic Ca2+ level (i.e., x(t)) of our model yvc1 cch1 mutant cell upon extracellular Mg2+ depletion, in Fig. 5A we depict the simulated x(t) response curve for parameter [Mgex] suddenly decreasing from 1 mM to 0 mM at t = 0 and being reset to 1 mM after 90 s (parameter [Caex] = 150 mM in this simulation). From this figure, we can see that when t = 0, the simulated cytosolic Ca2+ level rises quickly (in 15 s) from its original resting level of 0.081 μM to a value of 0.099 μM, almost keeps at that value and recovers quickly to its original resting level of 0.081 μM when t = 1.5 min.
Fig. 5.
Simulated cytosolic Ca2+ level (i.e., x(t)) for yvc1 cch1 mutant increases upon extracellular Mg2+ depletion or extracellular Ca2+ challenge. Panel A, the simulated x(t) response curve (the black curve) for parameter [Mgex ] (the red curve) suddenly decreasing from 1 mM to 0 mM at t = 0 and being reset to 1 mM after 90 s (please note that in this simulation, parameter [Caex ] = 150 mM). Panel B, the simulated x(t) response curve (the black curve) for parameter [Caex ] (the red curve) suddenly increasing from 150 mM to 200 mM at t = 0 and being reset to 150 mM after 90 s (please note that in this simulation, parameter [Mgex ] = 0 mM). The basal level of [Caex ] in these two simulations is set to be 150 mM instead of 2 mM as in Wiesenberger’s paper [49] because our model is only valid for hypertonic shock (extracellular Ca2+ >132 mM). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
To further check the behavior of cytosolic Ca2+ level of our model yvc1 cch1 mutant cell upon extracellular Ca2+ challenge, in Fig. 5B we depict the simulated x(t) response curve for parameter [Caex] suddenly increasing from 150 mM to 200 mM at t = 0 and being reset to 150 mM after 90 s (parameter [Mgex] = 0 mM in this simulation). From this figure, we can see that the simulated cytosolic Ca2+ level rises quickly (in 15 s) from its original resting level of 0.091 μM to a value of 0.125 μM and decreases very gradually to a value of 0.123 μM (at t = 1.5 min), then it decreases quickly to its original resting value.
4. Discussion
We present experimental data that are consistent with the existence of Mg2+-sensitive Ca2+ influx pathways in yeast. Increasing concentrations of extracellular Mg2+ increased the concentration of extracellular Ca2+ necessary for inhibition of yeast cell growth over a 24 h period. By extrapolation of log-transformed plots of Mg2+ concentration versus IC50, we estimate that standard YPD medium effectively contains 0.5 mM Mg2+, in good agreement with the assayed value of 0.7 mM [1]. Increasing concentrations of extra-cellular Mg2+ also inhibited Ca2+ influx as judged by decreasing rates of aequorin luminescence following a sudden increase of extracellular Ca2+. While this work was in progress, a complementary study also showed that sudden withdrawal of all extracellular Mg2+ from synthetic growth medium caused a rapid and reversible influx of Ca2+ into yeast cells [49]. An earlier study of 45Ca2+ influx demonstrated Mg2+ inhibition of a constitutive Ca2+ influx pathway [27]. Finally, overexpression of the yeast Alr1 or Alr2 Mg2+ transporters – homologs of the bacterial CorA Mg2+ transporters –resulted in increased sensitivity to high environmental Ca2+ [28]. All these results are consistent with a model where Mg2+ competitively inhibits one or more Ca2+ influx pathways.
As just mentioned, previously published data demonstrate the existence of a constitutive Mg2+-sensitive Ca2+ influx pathway, which we named as Transporter X [27]. From Fig. 2B, we can see that a subtle difference of extracellular Mg2+ concentration results in the great difference between the profiles of the black (for [Mgex] = 0) and blue curve (for [Mgex] = 0.3 mM). In the case of one transporter model, this can only happen when Transporter X has a very small inhibition constant KIX so that a subtle increase of extracellular Mg2+ concentration will have great inhibitory effect on the calcium influx (see Eq. (7), please note that Vm = 0 for one transporter model. The only influx is through Transporter X which is expressed as
Given fixed Vx, Kx and [Caex], smaller KIX can make this influx term more sensitive to the change of [Mgex]). However, a very small KIX will lead to even greater inhibitory effect when [Mgex] = 3 mM or higher concentration. Thus for one transporter model, it either cannot reproduce the great difference between the profiles of the black and the blue experimental curves in Fig. 2B or cannot reproduce the relatively subtle difference between the profiles of the blue and green curves in Fig. 2B. However, it is possible for two transporters model to reproduce both differences when in addition to Transporter X with an extremely low KIX which functions only in the case of extremely low extracellular Mg2+, there is another transporter with a high magnesium inhibition constant whose influx is much less sensitive to the change of extracellular Mg2+ than that of Transporter X.
As shown in Fig. 4C, simulations using just one Mg2+-sensitive Ca2+ influx transporter poorly fit the experimental data because the best fit of one Mg2+-sensitive transporter model (see Fig. 4C) using hybrid optimization algorithm cannot reproduce the great difference between the black and blue experimental curves shown in Fig. 2B whereas simulations assuming two Mg2+-sensitive Ca2+ influx transporters (termed transporters X and M with very distinct inhibition constants: KIX = 3.51 × 10−6 μM and KIM = 149.18 mM) can closely fit the experimental data (see Fig. 4A and B). All these results confirm the theoretical analysis in the previous paragraph and our simulation results strongly suggest the existence of a new transporter named Channel M on the plasma membrane of yvc1 cch1 mutant. As shown in Fig. 3B, for [Mgex] = 0 (see the black curve in Fig. 3B), both transporters M and X make considerable contribution to the total Ca2+ influx whereas for relatively high [Mgex] (see the rest curves in Fig. 3B) the flux contribution of Transporter X becomes negligible. The strange bending shown in Fig. 3A appears because of the opening of Transporter X in the case of extremely low [Mgex].
A mathematical model of Transporter M and other components of the system was constructed here. By comparison of simulated response curves in Fig. 4A with corresponding experimental curve in Fig. 2B for yvc1 cch1 mutant, we can see that the numerical simulations of our model can quantitatively reproduce the main characteristics of the experimental results such as low levels of extracellular Mg2+ can slow Ca2+ influx and diminish cytosolic free Ca2+ elevation. The model assumed that Transporter M has very low affinity for Ca2+ (Km = 505.43 mM), competitive inhibition by extracellular Mg2+ (KIM = 149.18 mM), and rapid feedback inhibition by intracellular Ca2+. The mechanism of feedback was found to be independent of calcineurin but otherwise its components remain wholly unknown. The relatively low affinity for Ca2+ relative to Mg2+ also suggests that Transporter M may function primarily as a Mg2+ transporter in physiological conditions. If so, the cellular response to high extracellular Ca2+ may include Mg2+ starvation in addition to Ca2+ influx, as suggested recently [49]. It is very likely that both transporters M and X are homo/hetero-oligomers of Alr1, Alr2 and Mnr2—uniporters primarily involved in Mg2+ uptake (note that uniporter is an integral membrane protein that is involved in facilitated diffusion whose uptake behavior can be well described with Michaelis–Menton kinetics [2]). A more realistic understanding of Transporter M and Transporter X should be possible after their genes and regulators are identified and characterized.
The computer simulations (see Fig. 4A and B) also reproduced the experimentally determined effects of the vacuolar H+/Ca2+ exchanger Vcx1 on cytosolic free Ca2+ dynamics. The presence or absence of calcineurin inhibitor had no effect on the aequorin traces in the presence or absence of Vcx1 (data not shown), suggesting no significant inhibition of Vcx1 by calcineurin within 3 min following Ca2+ shock. In long-term growth experiments, calcineurin appears to strongly inhibit Vcx1 function in addition to strongly inducing Pmc1 function [14]. Perhaps these effects of calcineurin can be observed in aequorin experiments performed over longer time scales and used to computationally model the long-term effects of Ca2+ on yeast growth (Fig. 2A).
From Fig. 4D, we can see that the shrinkage of our simulated yvc1 cch1 model cell is quickly accomplished (in less than 1 s). More simulations show that the volume shrinkage rate of our model cell is mainly determined by the reflection coefficient σ, the value of which used here (i.e., 0.035) is actually a value for sorbitol obtained by fitting the experimental curve [29]. Although the reflection coefficient σ for the current solute is not exactly known, further investigations show that the value of σ (in the investigations, we let this value range from 1000 to 0.001) seems to have insignificant influence on our main simulation results shown in Figs. 3 and 4A–C.
Wiesenberger et al. reported in their experimental paper [49] that removal of Mg2+ in extracellular medium (with the presence of extracellular Ca2+) resulted in an immediate increase in free cytoplasmic Ca2+ and this signal was reversible. As we can see from Fig. 5A, the sudden step decrease of [Mgex] from 1 mM to 0 mM at t = 0 incurs an immediate quick rise of simulated cytosolic Ca2+ level (i.e., x(t)) and after parameter [Mgex] is reset to 1 mM at t = 1.5 min, simulated cytosolic Ca2+ drops and recovers to its original resting level. Moreover, by comparison of two simulated response curves shown in Fig. 5A and B, we can see that the manner of the behavior of the simulated cytosolic Ca2+ level under Mg2+ depletion is quite similar to that under Ca2+ challenge. All these simulation results show that our model can reproduce (although roughly) the relevant experimental results reported by Wiesenberger et al. [49].
Finally there are still two issues worthy of discussion here. The first issue is about the reversibility of Ca2+ sequestration through Vcx1 and Pmc1. Intuitively there should exist a Ca2+ efflux from the vacuole into the cytosol. However, this is not the case for yeast cells. Dunn et al. [18] ever measured the rate of Ca2+ efflux from yeast vacuoles both in vitro and in vivo. Their experiments indicated that in vivo vacuolar Ca2+ efflux is very low (essentially zero). We think that it is more appropriate to use standard Michaelis–Menten kinetics (as we did here) rather than reversible Michaelis–Menten kinetics to describe the uptake behavior of Vcx1 and Pmc1 because the later kinetics will introduce an unreal vacuolar Ca2+ efflux. The second issue is about the validity of using Michaelis–Menten kinetics for modeling the uptake behavior of the transporters M and X. It is well-known that Michaelis–Menten kinetics assumes a rapid equilibrium between the enzyme and substrate to form an intermediate complex [17]. So we need to check if the uptake of Ca2+ and Mg2+ through the transporters is fast enough that it could be considered as steady-state at all times. Ion channels enable rapid (~107 ion s−1) movement of selected ions through pores in biological membranes [5]. Ca2+ channel can recognize its substrate and let it permeate within 10–100 ns [30]. Carriers (i.e., transporters) are characterized by turnover numbers that are typically 1000-fold lower than ion channels [26]. This means the average time needed for a Ca2+ to pass through the plasma membrane via the help of an ion transporter is within 10–100 μs, which is several order smaller than the time frame (in seconds) of the Ca2+ peaks shown in Fig. 2B and C. Moreover, Michaelis–Menten kinetics has been used in a mathematical model for describing the iron uptake behavior of plasma membrane iron transporter in the iron homeostasis system of Escherichia coli (see the first term in Eq. (1) in Ref. [43]). So here we think that it is appropriate to use Michaelis–Menten kinetics for modeling the uptake behavior of the transporters M and X.
As mentioned before, the mathematical model presented here is only valid for short-term (about 3 min) following hypertonic Ca2+ shock. This model needs to be modified for low extracellular Ca2+ (<132 mM) because Eq. (4) is only valid for hypertonic shock and it does not include factors such as turgor pressure which will arise in the case of low extracellular Ca2+. Moreover, for simplicity, this model assumes direct inhibition of Ca2+-bound calmodulin on both two transporters M and X, which is not backed by any experimental data. In the real cells, the relevant feedback regulation pathways may be more complicated. And the relative coarseness of the model accounts for the differences (e.g., the noticeable bias in the downward phase of the peak) shown by the comparison of Fig. 2B with Fig. 4A (also by comparison of Fig. 2C with Fig. 4B). On the other hand, the novelty of the present work lies in that by combining computational and experimental methodology, we detect the existence of a new Mg2+-sensitive Ca2+ transporter named as Transporter M on the yeast plasma membrane of yvc1 cch1 mutant cell working together with previously found Transporter X under hypertonic Ca2+ shock. The eventual accomplishment of a complete and accurate mathematical model of dynamic Ca2+ signaling networks in yeast will facilitate similar endeavors in all cell types and organisms, but doing so still requires additional mechanistic insights obtained from the fusion of experimental data and mathematical models.
Acknowledgments
J. Cui sincerely thanks his group leader Prof. P.M.A. Sloot for sustaining support for his research. We would like to thank Dr. Gertien J. Smits (University of Amsterdam) for her critical comments on our work. We are indebted to Prof. Patrick Gervais (Laboratoire de Génie des Procédés Alimentaires et Biotechnologiques) for his advice on volume evolution modeling under hypertonic shock. We thank Dr. Ariel Caride and an anonymous referee for their detailed and valuable comments on the previous version of this paper. J. Cui was funded by the Dutch Science Foundation on his project “Mesoscale simulation paradigms in the silicon cell” and later funded by EU on MORPHEX project. This work was partially supported by grant GM053082 from the National Institute of Health, USA (to KWC).
Footnotes
Conflict of interest statement
None.
References
- 1.Abelovska L, Bujdos M, Kubova J, Petrezselyova S, Nosek J, Tomaska L. Comparison of element levels in minimal and complex yeast media. Can J Microbiol. 2007;53:533–535. doi: 10.1139/W07-012. [DOI] [PubMed] [Google Scholar]
- 2.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. Garland Publishing, Inc; New York: 2002. [Google Scholar]
- 3.Allen DG, Blinks JR, Prendergast FG. Aequorin luminescence: relation of light emission to calcium concentration—a calcium-independent component. Science. 1977;195:996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
- 4.Batiza AF, Schulz T, Masson PH. Yeast respond to hypotonic shock with a calcium pulse. J Biol Chem. 1996;271:23357–23362. doi: 10.1074/jbc.271.38.23357. [DOI] [PubMed] [Google Scholar]
- 5.Beckstein O, Biggin PC, Bond P, Bright JN, Domene C, Grottesi A, et al. Ion channel gating: insights via molecular simulations. FEBS Lett. 2003;555:85–90. doi: 10.1016/s0014-5793(03)01151-7. [DOI] [PubMed] [Google Scholar]
- 6.Beyer H-G. Toward a theory of evolution strategies: self-adaptation. Evol Comput. 1996;3:311–347. [Google Scholar]
- 7.Bonilla M, Cunningham KW. Calcium release and influx in yeast: TRPC and VGCC rule another kingdom. Sci STKE. 2002;127:pe17. doi: 10.1126/stke.2002.127.pe17. [DOI] [PubMed] [Google Scholar]
- 8.Bonilla M, Cunningham KW. MAP kinase stimulation of Ca2+ signaling is required for survival of endoplasmic reticulum stress in yeast. Mol Biol Cell. 2003;14:4296–4305. doi: 10.1091/mbc.E03-02-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonilla M, Nastase KK, Cunningham KW. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 2002;21:2343–2353. doi: 10.1093/emboj/21.10.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 11.Chao Y, Fu D. Kinetic study of the antiport mechanism of an Escherichia coli zinc transporter. ZitB, J Biol Chem. 2004;279:12043–12050. doi: 10.1074/jbc.M313510200. [DOI] [PubMed] [Google Scholar]
- 12.Cui J, Kaandorp JA. Mathematical modeling of calcium homeostasis in yeast cells. Cell Calcium. 2006;39:337–348. doi: 10.1016/j.ceca.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham KW, Fink GR. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol. 1994;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham KW, Fink GR. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham KW, Fink GR. Ca2+ transport in Sacchromyces cerevisiae. J Exp Biol. 1994;196:157–166. doi: 10.1242/jeb.196.1.157. [DOI] [PubMed] [Google Scholar]
- 16.Cyert MS. Genetic analysis of calmodulin and its targets in Sacchromyces cerevisiae. Annu Rev Genet. 2001;35:647–672. doi: 10.1146/annurev.genet.35.102401.091302. [DOI] [PubMed] [Google Scholar]
- 17.Dixon M. Enzymes. 3. Longman Group Limited; London: 1979. [Google Scholar]
- 18.Dunn T, Gable K, Beeler T. Regulation of cellular Ca2+ by yeast vacuoles. J Biol Chem. 1994;269:7273–7278. [PubMed] [Google Scholar]
- 19.Fall CP, Marland ES, Wagner JM, Tyson JJ. Computational Cell Biology. Springer-Verlag; New York, Inc: 2002. [Google Scholar]
- 20.Gennemark P, Nordlander B, Hohmann S, Wedelin D. A simple mathematical model of adaptation to high osmolarity in yeast. In Silico Biol. 2006;6:193–214. [PubMed] [Google Scholar]
- 21.Gupta SS, Ton V-K, Beaudry V, Rulli S, Cunningham KW, Rao R. Antifungal activity of amiodarone is mediated by disruption of calcium homeostasis. J Biol Chem. 2003;278:28831–28839. doi: 10.1074/jbc.M303300200. [DOI] [PubMed] [Google Scholar]
- 22.Halachmi D, Eilam Y. Calcium homeostasis in yeast cells exposed to high concentrations of calcium: roles of vacuolar H+-ATPase and cellular ATP. FEBS Lett. 1993;316:73–78. doi: 10.1016/0014-5793(93)81739-m. [DOI] [PubMed] [Google Scholar]
- 23.Hardie DG. Biochemical Messengers. Chapman and Hall; London: 1991. [Google Scholar]
- 24.Iida H, Yagawa Y, Anraku Y. Essential role for induced Ca2+ influx followed by [Ca2+]i rise in maintaining viability of yeast cells late in the mating pheromone response pathway. J Biol Chem. 1990;265:13391–13399. [PubMed] [Google Scholar]
- 25.Kingsbury TK, Cunningham KW. A conserved family of calcineurin regulators. Genes Dev. 2000;14:1595–1604. [PMC free article] [PubMed] [Google Scholar]
- 26.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 27.Locke EG, Liang L, Bonilla M, Takita Y, Cunningham KW. A homolog of voltage-gated channels stimulated by depletion of Ca2+ secretory pools in yeast. Mol Cell Biol. 2000;20:6686–6694. doi: 10.1128/mcb.20.18.6686-6694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdiarmid CW, Gardner RC. Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J Biol Chem. 1998;273:1727–1732. doi: 10.1074/jbc.273.3.1727. [DOI] [PubMed] [Google Scholar]
- 29.de Marañón IM, Gervais P. Determination of cells’ water membrane permeability: unexpected high osmotic permeability of Saccharomyces cerevisiae. Biotechnol Bioeng. 1997;56:62–70. doi: 10.1002/(SICI)1097-0290(19971005)56:1<62::AID-BIT7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 30.Miller C. Ion channels: doing hard chemistry with hard ions. Curr Opin Chem Biol. 2000;4:148–151. doi: 10.1016/s1367-5931(99)00068-x. [DOI] [PubMed] [Google Scholar]
- 31.Miseta A, Fu L, Kellermayer R, Buckley J, Bedwell DM. The Golgi Apparatus plays a significant role in the maintenance of Ca2+ homeostasis in the vps33Δ vacuolar biogenesis mutant of Saccharomyces cerevisiae. J Biol Chem. 1999;274:5939–5947. doi: 10.1074/jbc.274.9.5939. [DOI] [PubMed] [Google Scholar]
- 32.Miseta A, Kellermayer R, Aiello DP, Fu L, Bedwell DM. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 1999;451:132–136. doi: 10.1016/s0014-5793(99)00519-0. [DOI] [PubMed] [Google Scholar]
- 33.Moles CG, Mendes P, Banga JR. Parameter estimation in biochemical pathways: a comparison of global optimization methods. Genome Res. 2003;13:2467–2474. doi: 10.1101/gr.1262503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller E, Locke EG, Cunningham KW. Differential regulation of two Ca2+ influx systems by pheromone signaling in Saccharomyces cerevisiae. Genetics. 2001;159:1527–1538. doi: 10.1093/genetics/159.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohsumi Y, Ankaru Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1983;258:5614–5617. [PubMed] [Google Scholar]
- 36.Palmer CP, Zhou X, Lin J, Loukin SH, Kung C, Saimi Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proc Natl Acad Sci USA. 2001;98:7801–7805. doi: 10.1073/pnas.141036198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Putney JW, editor. Calcium Signaling. 2. CRC Press; 2005. [Google Scholar]
- 38.Rubinow SI. Introduction to Mathematical Biology. John Wiley & Sons, Inc; 1975. [Google Scholar]
- 39.Runarsson TP, Yao X. Stochastic ranking for constrained evolutionary optimization. IEEE Trans Evol Comput. 2000;4:284–294. [Google Scholar]
- 40.Runarsson TP, Yao X. Search biases in constrained evolutionary optimisation. IEEE Trans Syst Man Cybern, Part C. 2005;35:233–243. [Google Scholar]
- 41.Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signalling. Plant Cell. 2002;14(Suppl):401–417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semsey S, Anderson AMC, Krishna S, Jensen MH, Massé E, Sneppen K. Genetic regulation of fluxes: iron homeostasis of Escherichia coli. Nucleic Acids Res. 2006;34:4960–4967. doi: 10.1093/nar/gkl627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sorin A, Rosas G, Rao R. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from Sarco/endoplasmic reticulum and plasma membrane calcium pumps. J Biol Chem. 1997;272:9895–9901. doi: 10.1074/jbc.272.15.9895. [DOI] [PubMed] [Google Scholar]
- 45.Soveral G, Veiga A, Loureiro-Dias MC, Tanghe A, Van Dijck P, Moura TF. Water channels are important for osmotic adjustments of yeast cells at low temperature. Microbiology. 2006;152:1515–1521. doi: 10.1099/mic.0.28679-0. [DOI] [PubMed] [Google Scholar]
- 46.Stathopoulos-Gerontides A, Guo JJ, Cyert MS. Yeast calcineurin regulates nuclear localization of the Crz1 transcription factor through dephosphorylation. Genes Dev. 1999;13:798–803. doi: 10.1101/gad.13.7.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strayle J, Pozzan T, Rudolph HK. Steady-state free Ca2+ in the yeast endoplasmic reticulum reaches only 10 μM and is mainly controlled by the secretory pathway pump Pmr1. EMBO J. 1999;18:4733–4743. doi: 10.1093/emboj/18.17.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takita Y, Engstrom L, Ungermann C, Cunningham KW. Inhibition of the Ca2+-ATPase Pmc1 by the v-SNARE protein Nyv1. J Biol Chem. 2001;276:6200–6206. doi: 10.1074/jbc.M009191200. [DOI] [PubMed] [Google Scholar]
- 49.Wiesenberger G, Steinleitner K, Malli R, Graier WF, Vormann J, Schweyen RJ, Stadler JA. Mg2+ deprivation elicits rapid Ca2+ uptake and activates Ca2+/calcineurin signaling in Saccharomyces cerevisiae. Euk Cell. 2007;6:592–599. doi: 10.1128/EC.00382-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nanfack YF, Kaandorp JA, Blom JG. Efficient parameter estimation for spatio-temporal models of pattern formation: case study of Drosophila melanogaster. Bioinformatics. 2007;23:3356–3363. doi: 10.1093/bioinformatics/btm433. [DOI] [PubMed] [Google Scholar]
- 51.Zamponi GW. The L-type calcium channe C-terminus: sparking interest beyond its role in calcium-dependent inactivation. J Physiol. 2003;552:333. doi: 10.1113/jphysiol.2003.052852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zappe A. Unterschiedliche funktionen der lonenpumpe Pmr1. http://elib.uni-stuttgart.de/opus/volltexte/2003/1425.