Abstract
Sleep inertia is the impaired cognitive performance immediately upon awakening, which decays over tens of minutes. This phenomenon has relevance to people who need to make important decisions soon after awakening, such as on-call emergency workers. Such awakenings can occur at varied times of day or night, so the objective of the study was to determine whether or not the magnitude of sleep inertia varies according to the phase of the endogenous circadian cycle. Twelve adults (mean, 24 years; 7 men) with no medical disorders other than mild asthma were studied. Following 2 baseline days and nights, subjects underwent a forced desynchrony protocol composed of seven 28-h sleep/wake cycles, while maintaining a sleep/wakefulness ratio of 1:2 throughout. Subjects were awakened by a standardized auditory stimulus 3 times each sleep period for sleep inertia assessments. The magnitude of sleep inertia was quantified as the change in cognitive performance (number of correct additions in a 2-min serial addition test) across the first 20 min of wakefulness. Circadian phase was estimated from core body temperature (fitted temperature minimum assigned 0°). Data were segregated according to: (1) circadian phase (60° bins); (2) sleep stage; and (3) 3rd of the night after which awakenings occurred (i.e., tertiary 1, 2, or 3). To control for any effect of sleep stage, the circadian rhythm of sleep inertia was initially assessed following awakenings from Stage 2 (62% of awakening occurred from this stage; n = 110). This revealed a significant circadian rhythm in the sleep inertia of cognitive performance (p = 0.007), which was 3.6 times larger during the biological night (circadian bin 300°, ~2300–0300 h in these subjects) than during the biological day (bin 180°, ~1500–1900 h). The circadian rhythm in sleep inertia was still present when awakenings from all sleep stages were included (p = 0.004), and this rhythm could not be explained by changes in underlying sleep drive prior to awakening (changes in sleep efficiency across circadian phase or across the tertiaries), or by the proportion of the varied sleep stages prior to awakenings. This robust endogenous circadian rhythm in sleep inertia may have important implications for people who need to be alert soon after awakening.
Keywords: circadian rhythm, cognitive performance, grogginess, jet lag, shift work, sleep, sleep inertia
Upon awakening cognitive performance is transiently impaired. This state of grogginess is known as “sleep inertia” (Tassi and Muzet, 2000). The magnitude of sleep inertia can be large. For example, the deficit in cognitive performance, as assessed with a 2-min serial addition test, immediately upon awakening after a full night of sleep is far greater than the deficit caused by staying awake for an entire 24 h (Wertz et al., 2006). Studies have shown the impairment caused by sleep inertia to be comparable to the effects of alcoholic intoxication (Roehrs et al., 2003; Arnedt et al., 2005). The detrimental effects of sleep inertia are likely to be most important in those individuals who have to make important decisions, perform potentially dangerous tasks, or respond to an emergency immediately after awakening (e.g., military personnel, emergency workers, airline pilots, truck drivers, shift workers, parents of infants). Immediate decision making following awakening can occur at any time of day or night. Thus, it is important to assess whether or not the circadian pacemaker affects the magnitude of sleep inertia. Previous studies have suggested the existence of a circadian rhythm in sleep inertia (Naitoh, 1981; Dinges et al., 1985), although results were confounded by interference from sleep deprivation and/or lack of control of sleep stage upon awakening, and this rhythm could not be confirmed by later studies (Naitoh et al., 1993; Achermann et al., 1995). It was concluded that there is a need for direct evidence that sleep inertia exhibits a circadian rhythm (Tassi and Muzet, 2000). Since there is an endogenous circadian rhythm in sleep propensity with a peak during the biological night (Dijk and Czeisler, 1994) and in alertness with a trough during the biological night (Johnson et al., 1992), we tested the hypothesis that there exists an endogenous circadian rhythm in sleep inertia of cognitive performance, with maximal inertia during the biological night.
MATERIALS AND METHODS
The protocol was approved by the institutional Human Research Committee, and written informed consent was obtained from all participants.
Participants
This study was part of a larger study designed to assess the influence of circadian and sleep/wake influences upon cardiopulmonary function (Hu et al., 2004a, 2004b; Shea et al., 2007). Of 16 consecutive volunteers studied, 12 met the inclusion criterion of having sleep inertia data during awakenings from stage 2 sleep in at least 4 of 6 circadian phase bins. These 12 volunteers had a mean age of 23.7 years (SD, 4.9 years) and included 7 men. All subjects were healthy apart from mild asthma (n = 8), had stable circadian rhythms, and had no habits or conditions that affected the variables of interest. To ensure this, the following exclusion criteria were used: any acute or chronic psychiatric or medical condition (other than asthma) as detected by history, physical, 12-lead electrocardiogram (ECG), chest x-ray, complete blood count, blood and urine comprehensive metabolic panel analysis, and overnight polysomnography (apnea/hypopnea index, >10/h; periodic leg movement index, >15/h); obesity (BMI ≥ 30 kg/m2); age < 18 and > 45 years; current smoking (verified by urine cotinine assay) or >10 pack-year history of smoking; >20 units of alcohol per week or >500 mg of caffeine per day (no alcohol or caffeine for 3 weeks prior to in-laboratory stay); unstable circadian rhythms (recent shift work, and >2-h time zone change in prior 3 months); extreme morning types (Horne-Ostberg Owl and Lark Questionnaire score, > 69) and extreme evening types (Horne-Ostberg Owl and Lark Questionnaire score, < 31); current medication use (other than oral contraceptives in females and β2-adrenergic receptor agonist inhaler in subjects with asthma).
Experimental Protocol and Measurements
After 3 weeks of a regular sleep/wake schedule at home, including 8-h sleep opportunities at each subject’s average habitual times, subjects underwent an 11-day laboratory protocol. This protocol consisted of 2 baseline days and nights, with 8-h sleep opportunities at their respective habitual times, followed by 7 recurring 28-h sleep/wake cycles under dim light conditions (<8 lux) to prevent the influence of light on the circadian system and on cognitive performance (Fig. 1). This “forced desynchrony” (FD) protocol desynchronizes the sleep/wake cycle from the circadian cycle by distributing sleep and wakefulness across all phases of the circadian cycle (Czeisler et al., 1999), allowing assessment of the effect of the independent endogenous circadian system on cognitive performance separate from the behavioral sleep/wake cycle (Johnson et al., 1992). Core body temperature was measured continuously by rectal thermistor for assessment of circadian phase and period using a nonorthogonal spectral analysis (NOSA) technique (Czeisler et al., 1999). The fitted composite minimum of core body temperature was assigned circadian phase 0°. In this FD protocol, the scheduled sleep/wakefulness ratio was maintained at 1:2 per 28-h cycle, identical to that in the ambulatory and baseline days. Throughout each sleep opportunity, standard polysomnography (PSG) was recorded, with 2 electroencephalography (EEG) channels (C3–A2; C4–A1), 2 electrooculography (EOG) channels, and 1 submental electromyography (EMG) channel (Vitaport, Netherlands). Sleep opportunities were 10 h 20 min including 3 evenly spaced awakenings induced by a standardized auditory stimulus (an alarm clock that escalated in loudness) for assessment of sleep inertia (Fig. 1). Immediately upon awakening, subjects remained in bed and an investigator entered the suite to raise the head of the bed to 45 degrees and move a computer console in front of the subject. This took ~1 to 2 min. Immediately thereafter, subjects performed a 2-min computerized serial 2-number addition test (e.g., 51 + 38 = ?, etc.), the same cognitive performance test used by other workers studying sleep inertia (Jewett et al., 1999; Wertz et al., 2006). Subjects were instructed to perform as quickly and as accurately as possible. The addition task (“ADD test”) was repeated 20 min after awakening. The magnitude of sleep inertia was quantified as the increase in the number of correct additions from the 1st to the 2nd ADD tests, that is, across 20 min of wakefulness. Following the 2 ADD tests, subjects went back to sleep, unless it was the 3rd awakening, whereupon their “day” began. To reduce any learning effect on the ADD test, individuals practiced the test 8 times over the 2 baseline days. Subjects also performed the ADD test 5 times across the remainder of each scheduled wake episode for estimation of any residual learning effect across the FD protocol.
Figure 1.
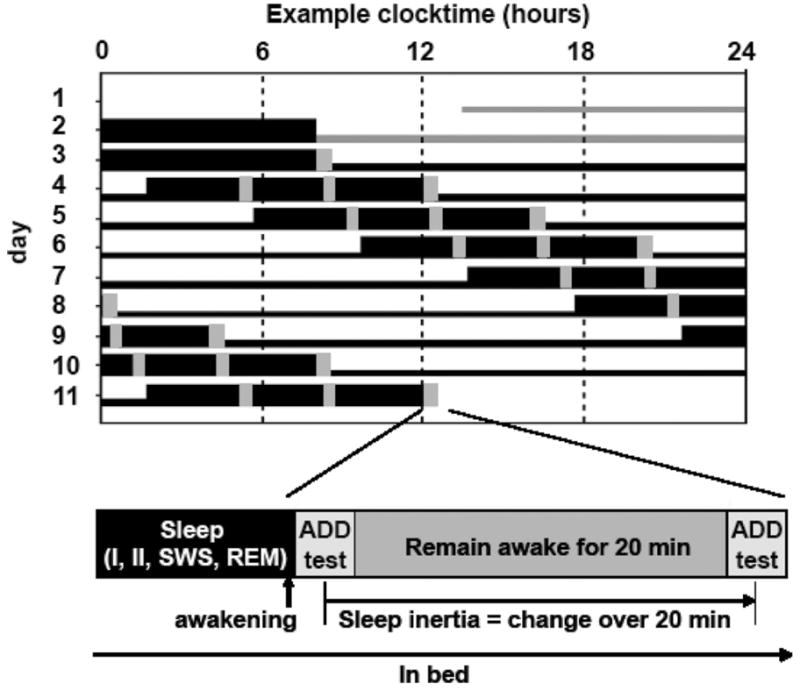
Study protocol. (Top) Schematic example of the forced desynchrony (FD) protocol for a subject with a habitual wake time at 0800 h. Thick horizontal black bars depict scheduled sleep opportunities; vertical gray bars indicate times of sleep inertia testing. Days 1 and 2 are the baseline days, after which seven 28-h “days” occur. The scheduled sleep periods are evenly distributed across all circadian phases. (Bottom) Sequence of tests immediately following awakenings from sleep. Subjects were awakened by a standardized auditory stimulus and performed a serial addition test 1 to 2 min following awakening and again 20 min following awakening, while remaining awake in a semirecumbent posture. The impairment during the test immediately upon awakening relative to 20 min following awakening was quantified as the measure of the severity of sleep inertia.
Note, between the 1st and 2nd ADD test following awakenings, subjects performed standard pulmonary function testing, reported elsewhere (Shea et al., 2007). Subjects with asthma (n = 8) were, based on symptoms of respiratory discomfort, permitted at any time to take the locally acting bronchodilator inhaler (albuterol; see Statistical Analysis, below).
Statistical Analysis
Any sleep inertia test data were excluded from analysis if: (1) the subject was already awake at the time of the scheduled awakening; (2) a test was not presented at the scheduled time (e.g., due to computer malfunction or if a subject requested a bedpan); (3) the PSG recording could not be scored for sleep stage (e.g., due to movement artifact, loose leads, or halted recording); (4) the datum represented an outlier (>±3 SD from the individual’s mean; assumed to be caused by aberrant factors, such as the subject falling asleep during the ADD task or technical problems); or (5) a subject with asthma had taken inhaler medication within 6 h of scheduled awakening. These steps resulted in 29% data loss. Thus, of 252 sleep inertia tests (12 subjects × 7 sleep episodes × 3 tests per sleep period), 178 tests were included in the analysis.
Next, to account for any systematic residual learning effects across the protocol (i.e., beyond the rapid learning effect on the baseline days), the number of correct additions in each sleep inertia ADD test was adjusted using the slope of the linear regression for each individual’s ADD test results collected during scheduled wakefulness periods across the FD protocol. The average improvement in the ADD test was 0.012 correct additions per minute per hour into the FD protocol (range among subjects was −0.006 to 0.028).
Next, to account for interindividual differences in ADD test performance, data were normalized by expressing data as percentage differences from each subject’s mean. Then, all individuals’ data were assigned circadian phases, from 0 to 359 degrees, with minimum core body temperature assigned as 0°, as described above, and separated into six 60° bins. Then, data were grouped according to sleep stage at awakening (stage 1, 2, 3/4 combined [slow wave sleep, SWS], or REM sleep; Rechtschaffen and Kales 1968), according to the most frequently occurring sleep stage in the ten 30-sec epochs before the awakening. Then data were segregated according to the specific 3rd of the night from which awakening occurred (i.e., 1st, 2nd, and 3rd tertiary).
Sleep stage at awakening varied out of the investigators’ control, so an exact balance of data from all sleep stages, in each tertiary, and for each circadian bin could not be achieved. Of the 178 valid assessments of sleep inertia, 110 were made following awakenings from stage 2 sleep (62% of valid observations), 16 (9%) from stage 1 sleep, 15 (8%) from SWS, and 35 (20%) from REM sleep.
As our primary analysis, to determine if sleep inertia was affected by circadian phase, we first compared data following awakenings from the same sleep stage (to prevent potential confounding by sleep stage) using type III mixed-model analyses of variance (ANOVA; Statistica software; StatSoft, Inc., Tulsa, OK). Since the majority of valid observations occurred after awakenings from stage 2 sleep, only those stage 2 data were used in this primary analysis.
We performed a number of secondary analyses to address potential confounding factors and generalizability of results. (1) To determine if sleep inertia was affected by sleep stage upon awakening it was necessary to compare data between sleep stages matched for circadian phase bin. Thus, sleep inertia data were computed for each individual following awakenings from any pair of different sleep stages matched to within ± 30 circadian degrees. To ensure that individuals contributed evenly, the within-subject averages for each sleep stage combination were calculated first and the group effect tested by Wilcoxon matched-pairs signed-ranks test. (2) Data were also analyzed, including awakening from all sleep stages combined, to test generalizability of the main finding (type III mixed-model ANOVA). (3) To determine whether the circadian rhythm in sleep inertia was confounded by variations in underlying homeostatic sleep pressure, the circadian profile of sleep inertia was compared with that of sleep efficiency across each of the tertiaries. (4) We classified sleep stage upon awakening as the most frequently occurring stage across the 5-min preceding awakening. To test whether the variability in sleep inertia may be due to sleep properties across the whole tertiary, we performed linear regression analyses between the magnitude of sleep inertia versus the average sleep efficiency and versus the average sleep stage percentages within each tertiary preceding the awakening for each individual separately (using 18 combinations of circadian phase and tertiaries).
RESULTS
For our primary analysis focusing on awakenings from stage 2 sleep, subjects performed an average (±SEM) of 10.3 ± 1.2 correct additions per minute immediately upon awakening, which improved to 12.4 ± 1.2 after 20 min (i.e., sleep inertia = −17%; Fig. 2). There was a large amplitude significant circadian variation in cognitive performance immediately upon awakening from stage 2 sleep (Fig. 2, solid black circles; F5,93 = 5.54; p = 0.0002). The peak to trough circadian variation was 2.6 correct additions per minute, with worst performance at circadian bin 300°. There was a significant circadian variation in sleep inertia of cognitive performance (Fig. 2, vertical difference between solid black circles and open black circles; F5,93 = 3.46; p = 0.007). Thus, the greatest degree of sleep inertia occurred during the biological night (i.e., circadian bin 300°; −28 ± 4.6%, equivalent to 3.5 correct additions per minute), which was almost 4 times larger than occurred during the biological day (i.e., circadian bin 180°; −8 ± 5.8%; equivalent to 1.0 correct additions per minute), and nearly twice as large as occurred during the biological morning (i.e., circadian bin 60°; 7minus;17 ± 5.3%). In addition, there was a smaller amplitude significant circadian variation in cognitive performance when measurements were taken during the scheduled prolonged wake episodes (Fig. 2, open gray squares; F5,388 = 2.39; p = 0.037). The peak to trough circadian variation was 0.8 correct additions per minute (less than one-third of that immediately upon awakening), with worst performance at circadian bin 0°. Comparison of the open circles and open squares in Figure 2 shows that the majority of sleep inertia on this test had dissipated in 20 min after awakening at most circadian phases.
Figure 2.
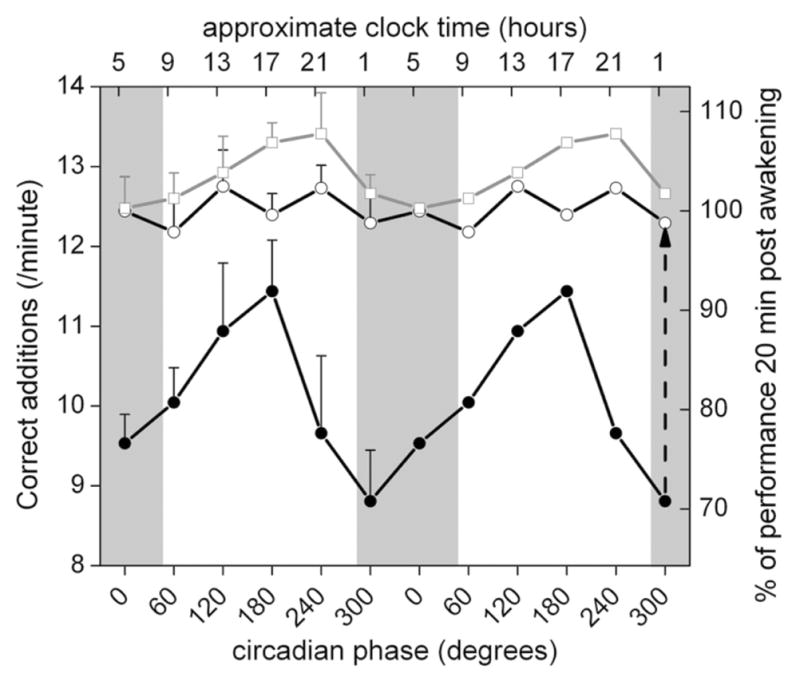
Circadian rhythm in sleep inertia of cognitive performance. Cognitive performance immediately upon awakening from stage 2 non-REM sleep exhibited a large amplitude circadian variation (solid black circles). Furthermore, the vertical difference between solid black circles (immediately upon awakening) and open black circles (20 min after awakening)—representing the magnitude of sleep inertia and exemplified by the height of the vertical dashed arrow for 1 circadian phase—also exhibited a significant circadian variation. Measurements taken during the scheduled prolonged wake episodes are shown as open gray squares. On the abscissa, data are aligned according to circadian phase from 0° to 359° (6 “bins,” with 0° = core body temperature minimum). Performance is expressed in absolute units based on the group average (left ordinate) and as a percentage of each individual’s average performance 20 min following awakening (right ordinate). The data represent mean data that are double plotted to aid visualization of rhythmicity. Gray shaded areas indicate the group average time of habitual sleep episodes. Error bars indicate SEM.
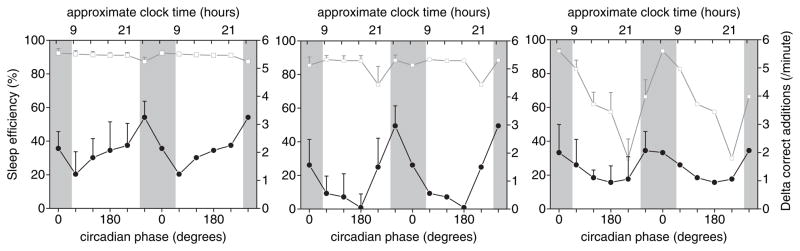
Secondary analyses revealed: (1) There was no detectable effect of sleep stage at awakening on the degree of sleep inertia of cognitive performance when matched for circadian phase bin. Specifically, in the 8 individuals with data at equivalent circadian phases, the average sleep inertia was 2.4 ± 0.3 correct additions per minute following stage 2 sleep and 1.6 ± 0.5 correct additions per minute following REM sleep (Wilcoxon test, p = 0.48; n = 8 pairs). Similarly, the average sleep inertia was 3.0 ± 0.7 correct additions per minute following stage 2 sleep and 1.7 ± 0.6 correct additions per minute following SWS (Wilcoxon test, p = 0.13; n = 7 pairs). A comparison between SWS and REM sleep was not performed because there were only 3 subjects with such data at matched circadian phases. Stage 1 sleep data were excluded because this was considered a transitory sleep stage. (2) Including awakening from all sleep stages, there was a significant circadian rhythm in sleep inertia (F5,159 = 3.68; p = 0.004). Including awakening from all sleep stages and testing for a circadian and tertiary effect, there was: (a) a significant effect of circadian phase (F5,97 = 2.49; p = 0.036); (b) no significant effect of tertiary (F2,36 = 2.05; p = 0.14); and (c) no significant interaction between circadian phase and tertiary (F10,73 = 0.68; p = 0.74) on sleep inertia (Figure 3).
Figure 3.
Circadian rhythm in sleep inertia and sleep efficiency according to time into sleep episode. Sleep inertia after awakening from combined sleep stages exhibits a similar circadian rhythm amplitude and phase for each of the 3 tertiaries, with a peak at 300°. In contrast, sleep efficiency shows no circadian rhythm during the first tertiary and a very large rhythm during the last tertiary. Filled symbols indicate the increase in number of correct additions across the first 20 min following awakening as a measure of dissipation of sleep inertia. Open symbols indicate sleep efficiency (total sleep time as a percentage of scheduled sleep episode). Error bars indicate SEM; n = 9 subjects (3 subjects were excluded due to incomplete data, e.g., due to inhaler use).
(3) Figure 3 shows clearly that the circadian rhythm in sleep inertia is present in all 3 tertiaries, while a circadian rhythm in sleep efficiency emerged only in the 3rd tertiary. Thus, the circadian rhythm in sleep inertia was unlikely to be mediated via changes in sleep efficiency. (4) Correlation analyses for each individual between sleep efficiency, REM, SWS, stage 2 sleep (as independent variables), and sleep inertia magnitude (as the dependent variable) show that the individual R2 values are very small, in the range of 0.06–0.10 (average R2 for individuals: sleep efficiency% = 0.06; REM% = 0.09; SWS% = 0.07; stage2% = 0.10).
In further analyses: (5) we found that in 83% of the cases, the sleep stage in the last epoch (30 sec, as used in some prior studies) was identical to the most frequently occurring sleep stage across the last 5 min for stage 2. (6) We found no cumulative buildup of homeostatic sleep pressure across the FD protocol when assessed at a similar circadian phase (i.e., sleep efficiency did not increase from sleep episode #3 to sleep episode #9 (89% vs. 85%, respectively; p = 0.31; n = 8, excluding 4 subjects who used inhaler medication within 6 h of any portion of the sleep episode). (7) For every subject the number of attempted additions (as used in some prior studies) was highly correlated with the number of correct additions (mean R2 = 0.93; range, 0.85–0.99). (8) There was no significant effect of asthma on the circadian rhythm in sleep inertia upon awakening from stage II sleep (type I mixed-model ANOVA; interaction between asthma vs. non-asthma as a covariate and circadian bin as fixed factor; F42,51 = 0.96; p = 0.55).
DISCUSSION
These data demonstrate an endogenous circadian rhythm in sleep inertia of cognitive performance, with worst impairment upon awakening during the biological night (300° bin; equivalent to ~2300–0300 h) and little impairment during the biological day (180°; ~1500–1900 h). Prior work has not been conclusive as to whether or not the sleep inertia of cognitive performance depends on the circadian phase at awakening (Naitoh, 1981; Dinges et al., 1985; Naitoh et al., 1993; Achermann et al., 1995; Tassi and Muzet, 2000). Dinges and colleagues (1985) studied cognitive performance upon awakening following 2-h naps at 2 circadian phases occurring across a 54-h period of sleep deprivation. These authors found similar sleep inertia of cognitive performance at the trough of body temperature as compared with 12 h later at the peak of body temperature, despite 12 h more sleep deprivation. This result was suggestive of a circadian influence on performance counteracting the effect of prolonged sleep deprivation, but these effects could not be distinguished. Moreover, it is possible that the improvement over time despite further sleep deprivation may have been partly caused by a learning effect. Two studies by Naitoh and colleagues resulted in conflicting conclusions regarding the presence or absence of a circadian rhythm in sleep inertia (Naitoh, 1981; Naitoh et al., 1993). Achermann and colleagues (1995) failed to find a difference in the mean level of sleep inertia, or the time constant of dissipation of sleep inertia when assessed at 2 circadian phases, albeit following prolonged sleep in the morning or a short nap in the evening. Thus, the lack of prior direct evidence for an independent circadian rhythm in sleep inertia was likely due to a number of study limitations, including (1) assessment of sleep inertia at only 2 times of day; (2) assessment of sleep inertia on a varying background of long-term sleep deprivation, or different amounts of sleep; (3) lack of compensation of any learning effect in the performance measure; (4) minimal or no control over body posture or lighting conditions; and/or (5) circadian analysis based on between-subject rather than within-subject comparisons. Our current study design addresses all of these limitations.
Three-Process Model
The neural mechanisms underlying sleep inertia are unknown, but may involve adenosine buildup in the basal forebrain (Van Dongen et al., 2001) and reduced cerebral blood flow, particularly in the prefrontal cortex (Balkin et al., 2002), a primary site responsible for planning and decision making (Tanji and Hoshi, 2001). The mammalian internal circadian pacemaker has numerous projections to sleep and arousal-related nuclei throughout the brain (Aston-Jones et al., 2001), but the specific pathways that mediate the circadian modulation of sleep inertia are currently unknown.
The current data add toward a quantitative understanding of a 3-process model of daytime performance composed of a homeostatic process S, a circadian process C, and a sleep/wake transition dependent inertia process W (Folkard and Åkerstedt, 1992). Here we provide statistical evidence that there is an interaction between process C and process W, such that the circadian modulation of cognitive performance is strong when the level of sleep inertia is high (immediately after awakening from sleep) and relatively weak when sleep inertia is dissipated (compare solid black circles with open squares on Fig. 2). This is reminiscent of the interaction demonstrated between process C and process S (Dijk and Czeisler, 1994), where the effects of the 2 processes cannot simply be summated to predict an outcome. Thus, the circadian wake drive may be most noticeable if there is concurrently a strong sleep drive—either caused by homeostatic buildup of sleep pressure during wakefulness or by sleep inertia. Based on the current observations, the existing mathematical models could be refined by implementing this interaction between process C and sleep inertia process W.
Factors Influencing the Buildup of Sleep Inertia
Sleep stage is often assumed to have an important influence on the magnitude of sleep inertia. For instance, Dinges and colleagues (1985), when comparing sleep stages, found the highest correlation between performance on a 3-min math test and the amount of SWS in the preceding nap period. This result is also a common perception; for instance, a number of recently developed commercial devices purport to wake people up more quickly by targeting an alarm to coincide with lighter stages of sleep. In contrast, Jewett and colleagues (1999) found no significant effect of sleep stage on sleep inertia magnitude when using the same 2-min ADD test as used in the current study. Notably, prior to our work, no study had effectively dissociated the independent effect of sleep stage from that of circadian phase on sleep inertia. When matching for circadian phase and applying a within-subject comparison, we found no significant difference in sleep inertia magnitude between awakening from stage 2 sleep and REM sleep, or between stage 2 sleep and SWS. However, larger data sets are desirable to confirm or refute this negative result. It is likely that the degree of sleep inertia is not simply related to the sleep stage in the last epoch before awakening (as used in some studies). That is why the most frequently occurring sleep stage in the last 10 epochs was used in the present study. This was arbitrary and it is possible that a different integrated measure of sleep depth over a different preceding period is more predictive of the degree of sleep inertia. However, when we used a longer integrative measure of the prevailing homeostatic sleep drive (as assessed by sleep efficiency, SWS% or REM% spanning a whole 3rd of the scheduled sleep episode), we again found no correlation with the degree of sleep inertia. We cannot rule out the possibility that other measures of sleep depth, such as EEG coherence prior to awakening, may be more related to the degree of sleep inertia than sleep stage (Tassi et al., 2006).
Time Course of Dissipation of Sleep Inertia
In our study, we did not assess the time course of sleep inertia. Instead, we assessed the change in cognitive performance over a fixed time period of 20 min. It is conceivable that any changes in our data across circadian phase could be influenced by changes in the magnitude of sleep inertia and/or changes in the time constant of sleep inertia. As noted above, before our study there was no conclusive evidence of a circadian influence on sleep inertia magnitude. Similarly, there is no consensus on the time course of dissipation of sleep inertia, with estimates ranging from 1 min (Webb and Agnew 1964) to 2–4 h (Jewett et al., 1999). These different estimates likely emerge due to differences in the type of task, the specific outcome variables and experimental conditions, as well as confounding influences from learning effects, accruing wakefulness, and/or notable changes in circadian phases, which would particularly affect time constants estimated over several hours. However, most evidence suggests that the effects of sleep inertia on cognitive performance rarely exceed 30 min (Tassi and Muzet, 2000). For instance, the time constant of dissipation of sleep inertia for a reaction time-dependent memory task was 18 min (and unchanged by measurements at different circadian phases, albeit following different amounts of sleep; Achermann et al., 1995). The negative effect of sleep inertia on decision-making performance lasted for 30 min, although it was almost completely dissipated after 20 min (Bruck and Pisani, 1999). In addition, applying the same cognitive task (2-min serial addition test with pairs of 2-digit numbers) and dependent variable (number of correct additions per time unit) as used in the current study, Wertz and colleagues (2006) demonstrated that sleep inertia of cognitive performance following awakening from an 8-h sleep episode on this test was dissipated after 20 min. The data from the current study suggest that the majority of the sleep inertia of cognitive performance on this test is dissipated after 20 min at any circadian phase, with little further improvement thereafter (Fig. 2, compare open circles and open squares).
There was a lack of a circadian variation in cognitive performance 20 min after awakening (Fig. 2, open circles), while there was a circadian variation present in the average across the wake episode (Fig. 2, open squares). This discrepancy could result from: (1) an interaction between circadian and homeostatic influences on performance, with circadian rhythmicity only becoming apparent after a certain duration of wakefulness as discussed above; and/or (2) a different rate of dissipation of sleep inertia across the circadian cycle. This second explanation would seem unlikely because this would imply fastest dissipation of sleep inertia at the temperature minimum (i.e., where performance 20 min after awakening was equal to the average of all other measurements spread across the wake episode). To conclusively determine whether the time constant of the dissipation of sleep inertia is affected by circadian phase will require studies such as the current FD protocol, but with repeated cognitive performance assessments for a few hours after each awakening.
Discussion of Potential Limitations
Although our main finding of the circadian rhythm of sleep inertia is robust, there are several potential limitations to the secondary end points. First, we found no significant effect of time into the sleep episode on sleep inertia, but we cannot exclude the possibility that an increase in cumulative prior sleep duration and concomitant decrease in homeostatic sleep pressure across the sleep episode have opposing effects and cancel each other out. Second, auditory arousal threshold may have varied across circadian phase. Nonetheless, we expect that any circadian variation in auditory arousal threshold would have resulted in a sleep inertia profile opposite to that observed (because arousal threshold is likely highest during the biological night, requiring a stronger arousing stimulus, and—if anything—reducing the magnitude of sleep inertia). Third, there may be more precise circadian phase markers than core body temperature (Klerman et al., 2002); but our time resolution was ~4-h circadian bins, thus any added precision is unlikely to have affected our conclusions. Fourth, following practice with the ADD test in the baseline days, there was a small residual learning effect across the FD protocol. However, we doubt this affected our interpretations because we removed the remaining linear learning effect and the main outcome variable was the change in cognitive performance across 20 min following awakening. Fifth, between the ADD tests performed upon awakening and 20 min later, subjects performed pulmonary function testing, which may have alerted the subjects and caused sleep inertia to dissipate more rapidly than if the subjects were left undisturbed. However, a previous study indicated no noticeable effect of behavioral activity, body posture, and lighting conditions on the time constant of sleep inertia of cognitive throughput (Jewett et al., 1999). Moreover, any possible alerting effect would have been present at all circadian phases, and may present a more true-to-life situation when both physical and mental tasks are performed upon awakening. Finally, our data were collected in unique laboratory circumstances and using a specific test, so future studies will be required to verify the results in real-life situations.
Implications
Our main finding of a strong circadian modulation of sleep inertia of cognitive performance demonstrates an interaction between the circadian timing system and the processes regulating sleep inertia, where the separate effects are not simply additive. If this finding holds true in an operational setting, this would have important implications for professions requiring decision making immediately upon awakening, including firefighters, airline pilots, and on-call medical professionals, especially because long-standing shift work and habitual sleep deprivation may worsen the detrimental effects of sleep inertia on cognitive performance. This finding may help develop strategies such as: (i) schedule naps during extended work hours, which allow for recuperation (Arora et al., 2006) while minimizing the adverse side effects of impaired cognitive performance immediately upon awakening, and (ii) timed blockade of the effect of sleep inertia, for example, using adenosine receptor antagonists such as caffeine (Van Dongen et al., 2001) while minimizing the adverse side effects during the scheduled sleep episodes.
Acknowledgments
We thank the volunteers, research staff, and recruiters who participated in this study. This work was supported by National Institutes of Health grants HL76446, AT002713, and RR002635.
References
- Achermann P, Werth E, Dijk DJ, Borbély AA. Time course of sleep inertia after nighttime and daytime sleep episodes. Arch Ital Biol. 1995;134:109–119. [PubMed] [Google Scholar]
- Arnedt JT, Owens J, Crouch M, Stahl J, Carskadon MA. Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion. JAMA. 2005;294:1025–1033. doi: 10.1001/jama.294.9.1025. [DOI] [PubMed] [Google Scholar]
- Arora V, Dunphy C, Chang VY, Ahmad F, Humphrey HJ, Meltzer D. The effects of on-duty napping on intern sleep time and fatigue. Ann Intern Med. 2006;144:792–798. doi: 10.7326/0003-4819-144-11-200606060-00005. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- Balkin TJ, Braun AR, Wesensten NJ, Jeffries K, Varga M, Baldwin P, Belenky G, Herscovitch P. The process of awakening: A PET study of regional brain activity patterns mediating the re-establishment of alertness and consciousness. Brain. 2002;125:2308–2319. doi: 10.1093/brain/awf228. [DOI] [PubMed] [Google Scholar]
- Bruck D, Pisani DL. The effects of sleep inertia on decision-making performance. J Sleep Res. 1999;8:95–103. doi: 10.1046/j.1365-2869.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker [see comments] Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Orne MT, Orne EC. Assessing performance upon abrupt awakening from naps during quasi-continuous operations. Behavior Research Methods, Instruments & Computers. 1985;17:37–45. [Google Scholar]
- Folkard S, Åkerstedt T. A three-process model of the regulation of alertness-sleepiness. In: Broughton RJ, Ogilvie R, editors. Sleep, Arousal and Performance: Problems and Promises. Boston: Birkhluser; 1992. pp. 11–26. [Google Scholar]
- Hu K, Ivanov P, Chen Z, Hilton MF, Stanley HE, Shea SA. Non-random fluctuations and multi-scale dynamics regulation of human activity. Physica A. 2004a;337:307–318. doi: 10.1016/j.physa.2004.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Ivanov P, Hilton MF, Chen Z, Ayers RT, Stanley HE, Shea SA. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Acad Sci U S A. 2004b;101:18223–18227. doi: 10.1073/pnas.0408243101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett ME, Wyatt JK, Ritz-De Cecco A, Khalsa SB, Dijk DJ, Czeisler CA. Time course of sleep inertia dissipation in human performance and alertness. J Sleep Res. 1999;8:1–8. doi: 10.1111/j.1365-2869.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, Czeisler CA. Short-term memory, alertness and performance: A reappraisal of their relationship to body temperature. J Sleep Res. 1992;1:24–29. doi: 10.1111/j.1365-2869.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:281–293. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- Naitoh P. Circadian cycles and restorative power of naps. In: Johnson LC, Tepas DI, Colquhoun P, editors. Biological Rhythms, Sleep and Shiftwork. New York: Spectrum; 1981. pp. 553–580. [Google Scholar]
- Naitoh P, Kelly T, Babkoff H. Sleep inertia: Best time not to wake up? Chronobiol Int. 1993;10:109–118. doi: 10.1080/07420529309059699. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. NIH Publication No. 204. Washington, DC: US Government Printing Office; 1968. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. [Google Scholar]
- Roehrs T, Burduvali E, Bonahoom A, Drake C, Roth T. Ethanol and sleep loss: A “dose” comparison of impairing effects. Sleep. 2003;26:981–985. doi: 10.1093/sleep/26.8.981. [DOI] [PubMed] [Google Scholar]
- Shea SA, Scheer FA, Hilton MF. Predicting the daily pattern of asthma severity based on relative contributions of the circadian timing system, the sleep-wake cycle and the environment. Sleep. 2007;30:A65. [Google Scholar]
- Tanji J, Hoshi E. Behavioral planning in the pre-frontal cortex. Curr Opin Neurobiol. 2001;11:164–170. doi: 10.1016/s0959-4388(00)00192-6. [DOI] [PubMed] [Google Scholar]
- Tassi P, Bonnefond A, Engasser O, Hoeft A, Eschenlauer R, Muzet A. EEG spectral power and cognitive performance during sleep inertia: The effect of normal sleep duration and partial sleep deprivation. Physiol Behav. 2006;87:177–184. doi: 10.1016/j.physbeh.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Tassi P, Muzet A. Sleep inertia. Sleep Med Rev. 2000;4:341–353. doi: 10.1053/smrv.2000.0098. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Price NJ, Mullington JM, Szuba MP, Kapoor SC, Dinges DF. Caffeine eliminates psychomotor vigilance deficits from sleep inertia. Sleep. 2001;24:813–819. doi: 10.1093/sleep/24.7.813. [DOI] [PubMed] [Google Scholar]
- Webb WB, Agnew H. Reaction time and serial response efficiency on arousal from sleep. Percept Mot Skills. 1964;18:783–784. doi: 10.2466/pms.1964.18.3.783. [DOI] [PubMed] [Google Scholar]
- Wertz AT, Ronda JM, Czeisler CA, Wright KP., Jr Effects of sleep inertia on cognition. JAMA. 2006;295:163–164. doi: 10.1001/jama.295.2.163. [DOI] [PubMed] [Google Scholar]