Abstract
Objective
Women in the postpartum period are at high-risk for complications from influenza. Pharmacokinetic data of oseltamivir phosphate in postpartum women, however, are lacking.
Study Design
Seven healthy patients within 48 hours of delivery were recruited. Each woman received 75 mg of oseltamivir phosphate. Plasma and breast milk samples were obtained at times 0, 0.5, 1, 2, 4, 8, 12, and 24 hours after the first dose. The samples were analyzed for oseltamivir and oseltamivir carboxylate levels. Using a noncompartmental model, area-under-the-curve (AUC), maximum concentration (Cmax), time-to-maximum concentration (Tmax), and half-life (T1/2) were estimated.
Results
Oseltamivir phosphate and oseltamivir carboxylate were found in breast milk, though later and in lower levels than found in plasma. The Cmax and AUC 0–24 was higher for the active metabolite than for the prodrug in both plasma and breast milk.
Conclusion
Oseltamivir carboxylate was present in breast milk but in concentrations significantly lower than considered therapeutic in infants.
Keywords: Oseltamivir, breast milk pharmacokinetics, Oseltamivir excretion
INTRODUCTION
Increased morbidity and mortality from influenza in pregnancy have been reported for seasonal and pandemic influenza. The 2009 H1N1 pandemic demonstrated that pregnant women are a high risk group with increased rates of hospital admission, intensive care admission and death. (1–10) This high-risk status extends to women in the postpartum period. While it is difficult to say at what time period postpartum a woman's immune system returns to non-pregnant status, the Centers for Disease Control and Prevention (CDC) identified women up to two weeks postpartum as being at increased risk of complications of H1N1 and recommended prompt initiation of antiviral treatment with oseltamivir. (10, 11)
Oseltamivir is a neuraminidase inhibitor that is effective against both influenza A and influenza B (12, 13) that works by blocking progeny virion release (14–16). It has been shown to reduce the duration of viral replication, the severity and duration of influenza symptoms, the levels of biochemical markers of host inflammatory response, and the incidence of secondary infections. There are no published studies addressing dosing during the postpartum period. Moreover, the only human data regarding transfer of oseltamivir or its active metabolite into breast milk is a published journal correspondence. (17) To provide some guidance on these issues, we studied the pharmacokinetics of first dose oseltamivir in women in the immediate postpartum time period.
MATERIALS & METHODS
This study was approved by the Internal Review Board at the University of Texas Southwestern Medical Center, Dallas, Texas. The study was performed at Parkland Memorial Hospital from March 2010 to May 2010 by the Departments of Obstetrics and Gynecology and Pediatrics at UT Southwestern and in conjunction with the Pediatric Pharmacology Research Unit (PPRU) Network.
Patients age 18 and older without other medical problems following an uncomplicated delivery of a singleton gestation and who had elected to exclusively bottle feed their infants were eligible for the study. Patients were excluded if their delivery was complicated by chorioamnionitis, metritis, or postpartum hemorrhage. Patients were also excluded if they had an allergy or prior adverse reaction to oseltamivir or known kidney or liver disease.
Patients who consented to participate in the study received a single 75 mg capsule of oseltamivir phosphate (Tamiflu). They had blood and breast milk samples collected the time of the first dose of medication and at 0.5, 1, 2, 4, 8, 12, and 24 hours after the single dose of oseltamivir. Blood samples were processed to separate plasma, and plasma and breast milk samples were stored at −80° Celsius for batch analysis.
Plasma and breast milk samples were shipped to BASI Laboratory (United Kingdom) and analyzed for oseltamivir phosphate and oseltamivir carboxylate content using a validated tandem mass spectrometric method (method on file). The method was accurate and sensitive ranging from 1 to 10 ng/mL (RSD < 5%) for oseltamivir phosphate and oseltamivir carboxylate, respectively.
Oseltamivir and oseltamivir carboxylate concentrations (ng/mL) in plasma and breast milk were plotted versus time (hours) for each of the respective subjects. Using a noncompartmental model, area-under-the-curve (AUC), maximum concentration (Cmax), time-to-maximum concentration (Tmax), and half-life (T1/2) were estimated using Thermo Kinetica 5.0 (Thermo Fischer Scientific, Waltham, MA). The pharmacokinetic estimates were summarized. An Analyses of Variance and Ryan-Einot-Gabriel-Welsh Multiple Range Test were used to determine significance between study variables (SAS 9.2, Cary NC).
RESULTS
Seven women were enrolled who were exclusively bottle feeding. Of the seven patients enrolled, they ranged in age from 19–24 years of age. Six of the seven women enrolled were multiparous, and their BMI ranged from 19–36. Three patients were African American, two were Hispanic, and two were Caucasian. These women were enrolled when the authors were available to perform the multiple sample collections.
We observed both the oseltamivir phosphate prodrug and the oseltamivir carboxylate active metabolite in breast milk (Table 1). As shown in Figures 1 and 2, both oseltamivir phosphate and oseltamivir carboxylate were found in detectible amounts in breast milk later than in plasma, and the breast milk levels of both the prodrug and active metabolite were lower than those in plasma. Individual data are available on request.
Table 1.
Pharmacokinetic parameters for oseltamivir phosphate prodrug and oseltamivir carboxylate in plasma and breast milk.
| OSELTAMIVIR PHOSPHATE | OSELTAMIVIR CARBOXYLATE | |||
|---|---|---|---|---|
| Plasma | Breast milk | Plasma | Breast milk | |
| Cmax (ng/mL) | 70.1 ± 72.6 | 26.9 ± 14.0 | 224.3 ± 40.5 | 41.9 ± 21.0 |
| Tmax (hour) | 2.6 ± 2.4 | 3.4 ± 2.2 | 4.6 ± 1.5 | 18.9 ± 6.4 |
| T1/2 (hour) | 4.2 ± 2.4 | 4.2 ± 1.2 | 16.1 ± 8.0 | n/a |
| AUC0–24 h (ng·h/mL) | 155.6 ± 57.9 | 143.4 ± 73.4 | 2611.7 ± 349.0 | 569 ± 405.8 |
Data reported as mean ± SD
Figure 1.
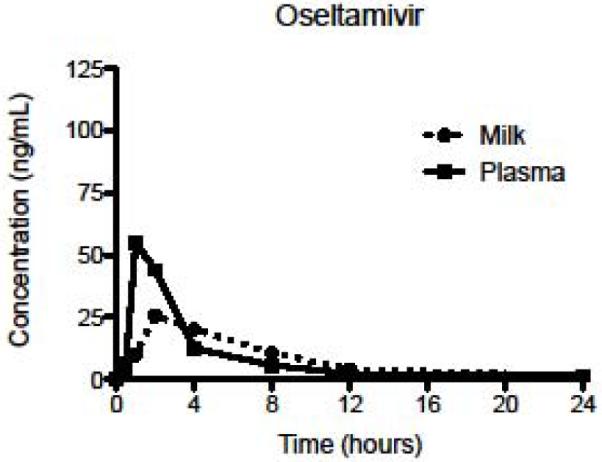
Mean concentrations of oseltamivir phosphate in plasma and breast milk after first dose.
Figure 2.
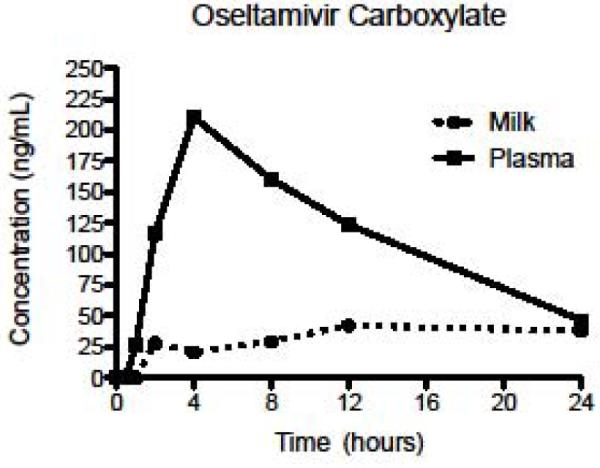
Mean concentrations of oseltamivir carboxylate in plasma and breast milk after first dose.
Table 1 details the first dose pharmacokinetics indices for oseltamivir phosphate and oseltamivir carboxylate in plasma and in breast milk. The Cmax (ng/mL) and AUC0–24 (ng·hr/mL) for the active metabolite were higher than for the prodrug in both plasma and breast milk. Similarly, the time to reach maximum concentration was higher for the active metabolite than for the prodrug in both plasma and breast milk. The half-life of the active metabolite in breast milk could not be calculated with the collection time points used.
COMMENT
Our data are consistent with the findings in animal models that both the prodrug oseltamivir phosphate and the active metabolite oseltamivir carboxylate are excreted in breast milk. The only prior human data available is from a case report by Wenges-Van Holthe and colleagues who reported a lactating patient treated with oseltamivir for influenza who consented to provide breast milk samples for analysis (17). They found oseltamivir and oseltamivir carboxylase were present in breast milk at low levels corresponding to 0.012 mg/kg to the infant daily, well below pediatric dosing (17).
Our data show following a single dose (75 mg) of oseltamivir, breast milk concentrations of oseltamivir reached a steady state at 12 to 24 hours (Figure 2). We averaged concentrations obtained at 12 and 24 hours to calculate infant exposure; the resulting mean concentration was 2.8 ng/mL. Assuming a 2.75 kg infant ingests 750 mL of breast milk daily, the approximated exposure to oseltamivir is 2.1 micrograms/day or 0.76 microgram/kg/day. When compared with oseltamivir dosing for infants less than 12 months of age (1–7 mg/kg/day) (18), infants exposed to oseltamivir from breast will receive a significantly lower amount of drug (e.g., 1 vs 0.00076 mg/kg/day).
Considering the pharmacokinetics of oseltamivir in plasma in the puerperum, we found values consistent with concentrations achieved in prior reported studies of non-pregnant adults but with some differences in timing. (19) Specifically, the time to achieve maximum oseltamivir concentration was 2.6 ± 2.4 hours in our post partum patients which is longer than the reported 0.9–2.3 hours. For the active metabolite, however, our finding of 4.6 ± 1.5 hours was consistent with prior reports in non-pregnant adults of 3.4–4.6 hours. Similarly, our values for T1/2 for both oseltamivir (4.2 ± 2.4) and oseltamivir carboxylate (16.0 ± 8.) were longer than the half-lives reported in non pregnant adults of 1–3 hours (12, 15, 18) and 6–10 hours (12, 14, 18) for oseltamivir and oseltamivir carboxylate, respectively. The observed maximum concentrations in plasma for oseltamivir phosphate and oseltamivir carboxylate following first-dose oseltamivir phosphate were lower than those reported by the manufacturer for non-pregnant patients. (19) We observed values of 70.1 ± 72.6 ng/mL for oseltamivir phosphate and 224.3 ± 40.5 ng/mL of oseltamivir carboxylate. Brewster et al reported similar values in non-pregnant individuals of 75.1 ng/mL for oseltamivir phosphate and 276 ng/mL for oseltamivir carboxylate. (12)
Our study has several limitations. First, the study was designed to assess the pharmacokinetics after the first dose of medication; we do not have steady state data. Second, our patients were in the immediate postpartum period and had not begun breast feeding; oseltamivir levels may differ in women with established breastfeeding. Colostrums, found initially after delivery, has a higher amino acid and protein concentration but less sugar and fat. This is important as individual drug binding to lipids and proteins differs, affecting drug excretion into breast milk. Higher drug levels may be found in colostrums than in established breast milk if the drug is bound to proteins. Conversely, lipid-soluble drugs may be found in lower concentrations in colostrums. However, this study clearly shows that oseltamivir phosphate and oseltamivir carboxylate are excreted into breast milk though in significantly lower concentrations than those in plasma. The active metabolite, oseltamivir carboxylate, remains detectable in breast milk at 24 hours after initial dosing though at significantly lower valves than equates to therapeutic dosing for infants.
Acknowledgments
Sources of funding: University of Texas Southwestern Medical Center Department of Obstetrics and Gynecology and by Grant # 5-U10-HD046000-05 from National Institutes of Health supporting the Pediatric Pharmacology Research Unit (PPRU) Network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reprints will not be available
REFERENCES
- 1.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 Influenza virus infection during pregnancy in the USA. The Lancet. 2009;374:451–58. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen SA, Jamieson DJ, MacFarlane K, Cragan JD, Williams J, Henderson Z. Pandemic Influenza and Pregnant Women: Summary of a Meeting of Experts. Am J Public Health. 2009;99(Supp 2):248–53. doi: 10.2105/AJPH.2008.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hewagama S, Walker SP, Stuart RL, et al. 2009 H1N1 Influenza A and Pregnancy Outcomes in Victoria, Australia. Clin Infect Dis. 2010;50:686–90. doi: 10.1086/650460. [DOI] [PubMed] [Google Scholar]
- 4.Louie JK, Acosta M, Jamieson DJ, Honein M. Severe 2009 H1N1 Influenza in Pregnant and Postpartum Women in California. New Eng J Med. 2010;362(1):27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 5.Archer BN, Cohen C, Naidoo D, et al. Interim Report on Pandemic H1N1 influenza virus infections in South Africa, April to October 2009: Epidemiology and Factors Associated with Fatal Cases. Euro Surveill. 2009;14(42):pii, 19369. doi: 10.2807/ese.14.42.19369-en. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19369. [DOI] [PubMed] [Google Scholar]
- 6.Louie JK, Acosta M, Winter K, et al. Factors Associated with Death or Hospitalization Due to Pandemic 2009 Influenza A (H1N1) Infection in California. JAMA. 2009;302(17):1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 7.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 Influenza A (H1N1) Virus Illness Amount Pregnant Women in the United States. JAMA. 2010;303(15):1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creanga AA, Johnson TF, Graitcer SB, Hartman LK, Al-Samarrai T, Schwarz AG. Severity of 2009 Pandemic Influenza A (H1N1) Virus Infection in Pregnant Women. Obstet Gynecol. 2010;115(4):717–726. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- 9.Miller AC, Safi F, Hussain S, Subramanian RA, Elamin EM, Sinert R. Novel Influenza A (H1N1) Virus Among Gravid Admissions. Arch Intern Med. 2010;170:10868–73. doi: 10.1001/archinternmed.2010.126. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention 2009 Pandemic Influenza A (H1N1) in Pregnant Women Requiring Intensive Care—New York City, 2009. MMWR. 2010;59(11):321–325. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention [Accessed June 4, 2010];Updated Interim Recommendations for Obstetric Health Care Providers Related to Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009–2010 Season. Available at: http://www.cdc.gov/h1n1flu/pregnancy/antiviral_messages.htm.
- 12.Brewster M, Smith JR, Dutkowski R, Robson R. Active metabolite from Tamiflu® solution is bioequivalent to that from capsule delivery in healthy volunteers: A crossover, randomized, open-label study. Vaccine. 2006;24:6660–6663. doi: 10.1016/j.vaccine.2006.05.080. [DOI] [PubMed] [Google Scholar]
- 13.Shentag JJ, Hill G, Chu T, Rayner CR. Similarity in pharmacokietnics of oseltamivir and oseltamivir carboxylate in Japanese and Caucasian subjects. J Clin Pharmacol. 2007;47(6):689–96. doi: 10.1177/0091270007299761. [DOI] [PubMed] [Google Scholar]
- 14.He G, Massarell J, Ward P. Clinical Pharmacokinetics of the Prodrug Oseltamivir and its Active Metabolie Ro 64-0802. Clin Pharmacokinet. 1999;37(6):471–484. doi: 10.2165/00003088-199937060-00003. [DOI] [PubMed] [Google Scholar]
- 15.Massarella JW, He GZ, Dorr A, Neiforth K, Ward P, Brown A. The Pharmacokinetics and Tolerability of the Oral Neuraminidase Inhibitor Oseltamivir (Ro 64-0796/GS4104) in Healthy Adult and Elderly Volunteers. J Clin Pharmacol. 2000;40:836–843. doi: 10.1177/00912700022009567. [DOI] [PubMed] [Google Scholar]
- 16.Davies BE. Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J Antimicrob Chemother. 2010;65(supp 2):ii5–ii10. doi: 10.1093/jac/dkq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wentges-Van Holthe N, van Eijkeren M, van der Laan JW. Oseltamivir and Breastfeeding. Int J Infect Dis. 2008;12:451. doi: 10.1016/j.ijid.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Kimberlin DW, Shalabi M, Abzug MJ, et al. Safety of oseltamivir compared with the adamantanes in children less than 12 months of Age. Ped Infect Dis J. 2010;29:195–198. doi: 10.1097/INF.0b013e3181bbf26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roche FH-L. [Accessed May 1, 2010];Tamiflu oseltamivir phosphate: product information. 2004 Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/DrugShortages/UCM183850.pdf.


