SUMMARY
Genetic studies of lung disease in Cystic Fibrosis are hampered by the lack of a severity measure that accounts for chronic disease progression and mortality attrition. Further, combining analyses across studies requires common phenotypes that are robust to study design and patient ascertainment.
Using data from the North American Cystic Fibrosis Modifier Consortium (Canadian Consortium for CF Genetic Studies, Johns Hopkins University CF Twin and Sibling Study, and University of North Carolina/Case Western Reserve University Gene Modifier Study), the authors calculated age-specific CF percentile values of FEV1 which were adjusted for CF age-specific mortality data.
The phenotype was computed for 2061 patients representing the Canadian CF population, 1137 extreme phenotype patients in the UNC/Case Western study, and 1323 patients from multiple CF sib families in the CF Twin and Sibling Study. Despite differences in ascertainment and median age, our phenotype score was distributed in all three samples in a manner consistent with ascertainment differences, reflecting the lung disease severity of each individual in the underlying population. The new phenotype score was highly correlated with the previously recommended complex phenotype, but the new phenotype is more robust for shorter follow-up and for extreme ages.
A disease progression and mortality adjusted phenotype reduces the need for stratification or additional covariates, increasing statistical power and avoiding possible distortions. This approach will facilitate large scale genetic and environmental epidemiological studies which will provide targeted therapeutic pathways for the clinical benefit of patients with CF.
Keywords: Forced Expiratory Volume, Age Effects, Severity of Illness Index
INTRODUCTION
Although cystic fibrosis (CF) is a classic Mendelian disease, there is great variability in the chronic progressive lung disease which is the principal cause of early mortality. Variation in lung function, even among patients with the same CFTR mutations, is largely heritable, and modifier genes are known to influence pulmonary disease severity in CF1-4. Large scale studies of the role of genetic variation in the severity of CF will potentially lead to novel therapeutic targets. However, for powerful analysis across the entire genome a phenotypic measure is required that will accurately represent individual disease severity for all ages, and across different populations and study designs. The pulmonary disease phenotype is usually based on forced expiratory volume in one second (FEV1), the most clinically useful measure of lung function and a known predictor of survival5-7. FEV1 is the volume of air (in liters) that can be exhaled in one second (after maximal inhalation)8 and is generally reported as a percent predicted (FEV1pp) based on age, sex, and height9-13. To address the concern that standard norms may be invalid in CF patients because of stunting and/or delayed puberty, Kulich et al. designed CF specific percentiles (CF FEV1%ile) to measure lung function relative to other CF patients of the same age, sex, and height14. Neither FEV1pp nor CF FEV1%ile account for mortality attrition; patients are compared only to those still alive, excluding patients from the same birth cohort who have died (usually because of more severe lung disease).
To derive a measure that accounted for mortality, Schluchter and colleagues developed a model combining mixed model regression and survival analysis, in which estimates of slope and intercept for an individual patient’s rate of decline in FEV1pp could be used to predict the age at death for that patient5. In an application of this complex model, Schluchter et al. concluded that estimated FEV1pp at age 20 based on a mixed model approach, wherein longitudinal FEV1pp values of many patients are analyzed together in a single regression model, was the most suitable of several phenotypes considered for modifier studies, equivalent to estimated percentile of predicted age of death15. Although this approach accounts for survival and has been useful, it has limitations for general application. These estimates are critically related to the number of measures of lung function, the number of years over which they were collected, the make-up of the study population, and the changing patterns of CF survival and lung function in recent decades.
In this paper we describe a novel approach to the development of a robust pulmonary disease phenotype that allows for both the effects of chronic disease progression and mortality attrition, and allows the use of less extensive longitudinal data.
METHODS
Patient study samples
We apply our approach to the North American CF Modifier Consortium which consists of three study groups whose ascertainment schemes differed. The Canadian Consortium for CF Genetic Studies (CGS) was designed to recruit a representative sample of the Canadian CF population to identify modifier genes. This sample of 2473 patients was collected from 37 Canadian CF centers between 2000 and 200416. Children under the age of 2 years were excluded from the study to avoid enrolling newly diagnosed infants in a period of stress and variable compromise. Longitudinal data from each clinic visit in the three years preceding entry into the study included FEV1, age, height and weight.
The Johns Hopkins University CF Twin and Sibling Study (TSS) was family-based and included 1323 patients from 693 families who were recruited from 71 CF centers in the US and one in Australia4. Individuals with CF who had one or more living siblings with CF were eligible for inclusion. Patient samples were collected over a period of several years, most in 2002-2007. Although lifetime clinical information was collected, only the three years preceding study entry were analyzed herein.
The third sample, from the University of North Carolina at Chapel Hill/Case Western Reserve University Gene Modifier Study (GMS)2, was an extremes of phenotype design. The sample included 1137 patients who were homozygous for F508del from 44 US CF centers who were enrolled between 2000 and 2006. Using data from the US CF Foundation Patient Registry to define quartiles of FEV1pp using the Knudson equations17, patients were recruited from one of three groups – severe (lowest quartile for age; 8-25 years); younger mild (highest quartile for age; 15-28 years); and older mild (highest quartile for ages 29-33 years plus all those at least 34 years, considered mild by virtue of survival). For each patient, data for the previous five years were collected, but only three years were used in the present analysis.
Entry criteria for subjects in all three studies included verification of CF diagnostic information, including elevated sweat tests and CFTR mutation analysis, according to standard guidelines18.
Phenotype derivation
Age-specific percentile scores
For the population-based CGS sample, mean age and mean FEV1pp were calculated using data from the three years prior to study enrollment. Pediatric (Wang et al.13) and adult (Hankinson et al9) reference equations were used as recommended by the US CF consensus group19 to calculate FEVpp at each visit. Residual values from a non-linear regression of mean FEV1pp vs. mean age (see Figure 1) were converted to rank percentile values to characterize the lung disease severity of each individual in the surviving population.
Figure 1.
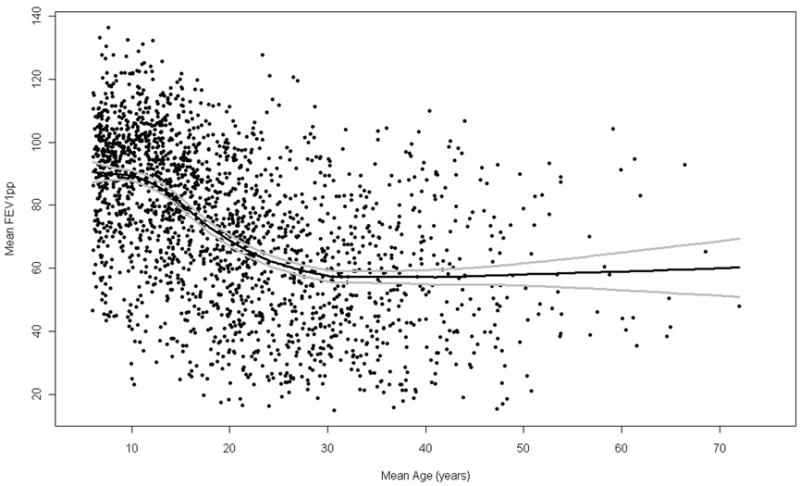
Cross-sectional plot of mean FEV1pp by mean age for 2061 CGS patients. Non-linear smoothed regression is plotted with 2 standard errors of the mean. Mean is based on the mean value of three years of data preceding study enrollment.
Because the TSS and GMS samples were not population based, we adapted the method described above, to account for variable designs and ascertainment schemes. We calculated the CF FEV1%ile (Kulich et al.) at each patient visit, and used the mean CF FEV%ile for each individual to rank him/her among CF survivors.
Computation of cohort survival estimates
The Canadian CF Patient Data Registry (CPDR) has collected prospective data on all Canadian CF patients since 197320. Patients included in the CPDR come from a network of audited CF care centers where the criteria for diagnosis of CF include elevated sweat chloride tests and/or identified CFTR mutations, and documentation of classic CF symptoms. Virtually all CF patients in Canada are captured in the CPDR, and the CGS sample is a representative subset. In order to compute birth cohort survival curves, de-identified birth and death dates, as well as date of last reported visit were requested on all patients in the 2002 CPDR, following ethics review. For patients born in 1973 or later, survival curves based on two year birth cohorts from the CPDR were created with a censoring date of 31 December 2002, the approximate midpoint of CGS data collection. Cohort cumulative survival probabilities on this date were used to create the survival estimates. For patients born before 1973, survival estimates were created by dividing the number of CF patients from each birth cohort alive on 31 December 2002 by the expected number of CF births (Canadian births x 1/2500). Local area regression was used to compute smoothed estimates of survival across the birth cohorts.
Adjustment of FEV1 percentiles for mortality
An individual’s FEV1 percentile as computed above gave an estimate of the proportion of surviving patients of the same age, ie from the same birth cohort, whose lung function was below that individual’s. Multiplying this number by the estimated survival percentile for that age provided the estimated proportion of patients from the entire original birth cohort who were surviving with FEV1 values below that of the individual. Adding this value to the proportion of patients from the original birth cohort who did not survive (1 minus the estimated survival percentile), gave an estimate of the proportion of patients from the individual’s original birth cohort who had FEV1 values below that of the individual or who had died, presumably with more severe lung disease. Transformation of this mortality adjusted cohort percentile to a standard normal score yielded the Normal Residual Mortality Adjusted (NoRMA) lung disease phenotype. Application of the survival adjustment to mean CF FEV1%ile and transformation to a normal score produced the Kulich NoRMA (KNoRMA) phenotype. This general framework (Table 1) for FEV1 could be applied to any quantitative measure that varies with age and whose distribution suffers from mortality attrition.
Table 1.
Generic Framework on Which the FEV1-Based Phenotype was Created.
|
Statistical methods
Local area regression (PROC LOESS in SAS version 9.1) was used to define age-specific non-linear regression estimates of FEV1pp for NoRMA in the CGS sample, and to create the final smoothed age-specific survival estimates. Local area regression computes least squares regression of a dependent variable (here FEVpp) on an independent variables (here age) in successive small neighbourhoods of the dependent variable and then splices these small linear regression lines together to produce a curve that is not constrained to any particular shape. FEV1pp residuals from the LOESS curve were normally distributed at all ages and showed no age-specific patterns (data not shown), justifying the conversion of the mortality-adjusted percentile values to normal deviates using SAS PROC PROBIT. SAS PROC LIFETEST was used to compute birth cohort survival curves from the CPDR data. The three years of data that were used to create NoRMA and KNoRMA were also used in a mixed model analysis (PROC MIXED) to compute two phenotypes proposed by Schluchter: the predicted value of FEV1pp at age 20 (FEVest20) and the predicted age at death percentile (AgeDied%ile)5, 15 based on patient specific slope and intercept. The MIXED procedure is a combination regression model allowing repeated measures for individuals, providing fixed effect coefficients for the overall effects of intercept and slope as well as random effect coefficients to calculate subject specific values for intercept and slope. Correlations between the phenotypes were investigated using PROC CORR.
Estimate of heritability for KNoRMA
Heritability was estimated using the TSS sample, which included twins as well as siblings. Heritability was calculated from 67 pairs of monozygous twins and 138 pairs of same-sex dizygous twins and non-twin siblings born within 3 years of each other. The pairwise correlation coefficient was calculated for each group, with assignment of twins or siblings as “A” or “B” permuted 106 times. The average of the 106 estimates for monzygous twins and for same sex dizygous twins or sibs was a robust estimate of the true correlation in each group, avoiding any bias that may have been introduced by arbitrary or systematic assignments to A and B status. Heritability was estimated to be twice the difference in the group mean coefficients4.
RESULTS
Population based CGS patient sample
Representativeness of the CGS sample was assessed by comparison to the 2002 CPDR Report (Table 2).
Table 2.
Comparison of CGS Sample to the Overall Canadian CF Population.
| CGS Sample (subset of CPDR) | CPDR | |
|---|---|---|
| Number of Patients | 2473 | 3500 |
| Age (Mean) | 19.6a | 18.8 |
| Sex, %male | 53.4% | 52.4% |
| FEV1pp (Mean) | 72.2 | 74.1 |
| %Pancreatic Insufficient | 88% | Not reportedb |
| %F508del/F508delc | 52.4% | 52.5% |
| %F508del/Other | 37.2% | 35.9% |
Patients under 2 years of age were excluded from the CGS sample producing a slightly higher mean.
Although not included in the CPDR report, the prevalence of 88% for PI is consistent with previous reports23.
F508del is the most common CF-causing mutation.
Reproducible and reliable FEV1 measurements are typically only obtained in patients six years and older. Thus, development of the NoRMA phenotype was based on 2061 patients (1104 males; 957 females), six years of age and older, with 1 to 65 visits with a mean of 9.1 (SD=5.6). The mean age of FEV1 measurements was 20.5 (SD=17.9), ranging from 6.0 to 68.6. FEV1pp ranged from 14.8 to 136.5 with a mean of 73.5 (SD=25.0). Although local area regression showed a decline in lung function with age, FEV1pp was highly variable at all ages, and average values at older ages showed no age-related pattern because of mortality attrition (Figure 1). The age-specific survival estimates used for mortality adjustment are provided as supplemental information; there was minimal mortality in children (>90% survival under age 18 years) but survival estimates declined steeply throughout the twenties and roughly logarithmically after age 30. The dramatic effect of adjusting for mortality attrition can be seen in Figure 2 which shows the distribution of NoRMA scores across the age range.
Figure 2.
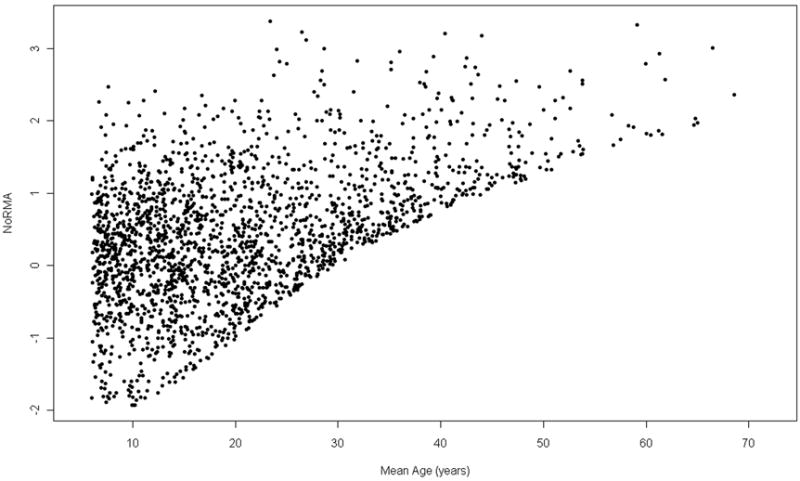
Cross-sectional plot of NoRMA score by mean age for 2061 CGS patients.
To illustrate the impact of these steps in calculating the severity scores, we compare two patients, a 10-year-old and a 30-year-old each with FEV1 of 60% of predicted (Table 3). The 10-year-old had an age-specific percentile score (based on residuals from the LOESS curve) of 0.10 and the 30-year-old had a score of 0.52. Adjusting for mortality attrition further improves the score for the 30-year old who is seen to have relatively mild CF lung disease while the 10-year-old is severely affected.
Table 3.
Effect of NoRMA Calculations in Two Patients (10 Year and 30 Years of Age) – With the Same FEV1pp Values.
| Younger Patient | Older Patient | |
|---|---|---|
| Age | 10 years | 30 years |
| FEV1pp | 60 | 60 |
| Age-specific FEV1pp Percentile | 0.10 | 0.52 |
| Estimated Survival Percentile | 0.97 | 0.49 |
| Survival Adjusted FEV1 Percentile | 0.12 | 0.76 |
| NoRMA | -1.14 | 0.71 |
The two phenotypes based on mixed model analysis 5, 15 were compared to NoRMA in the CGS sample (1435 unrelated patients). AgeDied%ile was normally distributed with a mean of 0.50 (SD=0.29). FEVest20 ranged from -31.95% to 135.84% with a mean of 72.56 (SD=20.21). Fifteen patients had FEVest20 less than 15% while four patients had estimates over 130%. Correlation coefficients for NoRMA, AgeDied%ile and FEVest20 (Table 4) were higher in the younger subset, reflecting the limitations of the mixed model estimates at ages further from age 20, when using only three years of data for each subject.
Table 4.
Correlation Coefficients for Three Measures of Lung Disease Severity in 1435 Unrelated CGS Patients (Upper Section) and in a Subset of 917 Unrelated CGS Patients Aged 10-30 Years (Lower Section).
| CGS Patients Aged 6-50 Years (n = 1435) | NoRMA | AgeDied%ile | FEVest20 | |
|---|---|---|---|---|
| CGS Patients Aged 10-30 Years (n = 917) | ||||
| NoRMA | 0.85 | 0.85 | ||
| AgeDied%ile | 0.90 | 0.94 | ||
| FEVest20 | 0.91 | 0.94 |
Generalizing beyond a population-based design (KNoRMA)
Since the GMS and TSS samples were not population based, population percentiles and the NoRMA phenotype as described above could not be computed. Age-specific CF percentiles for FEV1 developed by Kulich were used to compute KNoRMA in the three samples (Table 5).
Table 5.
Comparison of Patient Characteristics in the CGS, GMS, and TSS Samples.
| CGS | GMS | TSS | |
|---|---|---|---|
| Number of Patients | 2061 | 1137 | 1323 |
| Age, mean (SD) | 20.5 (11.4) | 23.1 (10.2) | 16.3 (9.1) |
| Sex, %male | 53 | 53 | 52 |
| %F508del/F508del | 53.0 | 100 | 55.5 |
| Number of useable visits per patient, mean (SD) | 9.1 (5.6) | 15.2 (11.9) | 14.4 (9.8) |
| KNoRMA, mean (SD) | 0.56 (0.85) | 0.54 (0.96) | 0.47 (0.79) |
Mean age differed significantly across sites (ANOVA P<0.001). The TSS sample had a younger mean age than both GCS and GMS (P<0.001). This is consistent with previous sibling studies, which tend to enroll younger patients than those using a population based approach 21. Conversely, GMS had an older mean age than the other two groups (P<0.001), reflecting the fact that mild patients under the age of 15 years were excluded from this study. There was no significant difference in the sex distributions.
Mean KNoRMA scores of CGS and GMS were not significantly different from each other, yet both were significantly higher than TSS (P=0.003 and P=0.04, respectively). The frequency distributions of KNoRMA for each of the three groups are shown in Figure 3. The distributions in TSS and CGS are very similar, whilst the distribution in GMS was bimodal, reflecting the ascertainment of patients with extreme lung phenotypes, and the recruitment of twice as many subjects in the “mild” range of lung disease compared to those in the “severe” range.
Figure 3.
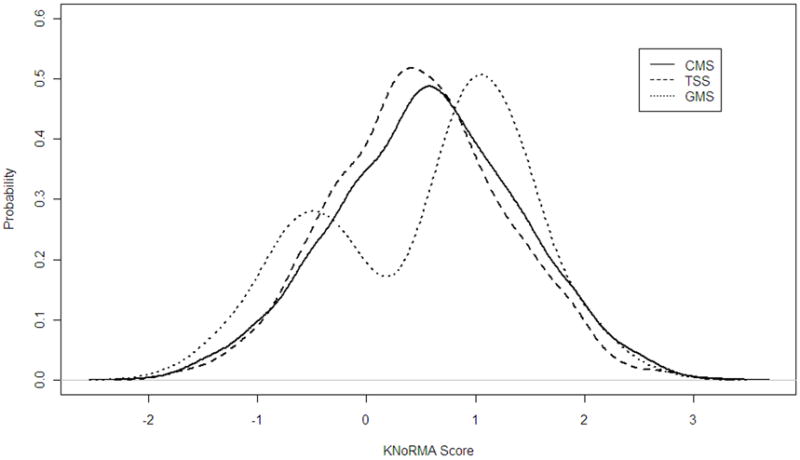
Frequency distributions of KNoRMA in the three study samples: 1137 GMS patients, 1323 TSS patients, and 2061 CGS patients.
Correlation coefficients between KNoRMA and the mixed model phenotypes in TSS and GMS are shown in Table 6. Lower correlations in TSS likely reflect the fact that the TSS sample is younger and further away from the age of 20. Results were unchanged when only one sibling from each TSS family was used in analysis (data not shown).
Table 6.
Correlation Coefficients Between Three Measures of Lung Disease Severity in 1323 TSS Patients (Upper Section) and 1137 GMS Patients (Lower Section).
| TSS Patients (n = 1323) | KNoRMA | AgeDied%ile | FEVest20 | |
|---|---|---|---|---|
| GMS Patients (n=1137) | ||||
| KNoRMA | 0.67 | 0.57 | ||
| AgeDied%ile | 0.79 | 0.87 | ||
| FEVest20 | 0.81 | 0.89 |
Heritability estimate for KNoRMA
The correlation coefficient for KNoRMA in monozygous twins was 0.79 while it was 0.53 for the dizygous twin/sib group. Thus, the heritability estimate was 0.51 (estimated 95% confidence interval: 0.28, 0.77), similar to that previously reported for the Kulich percentile alone without mortality adjustment in the TSS cohort4.
DISCUSSION
We used age-adjusted severity disease severity and age-specific survival estimates to define a novel framework for deriving phenotypes in CF pulmonary disease. One phenotype (NoRMA) was based on a population based sample, while the second (KNoRMA) used previously published CF-specific norms for applicability across variable study designs. These measures of disease severity can be compared across a range of ages, do not require data from a long period of follow up, and can be used across disparate populations. Accounting for attrition due to disease-related mortality removes the penalty that occurs when older patients are compared only against other survivors using simply FEV1pp or CF FEV1%ile.
The mixed model estimate of FEV1 at age 20 was also designed to adjust for disease progression and mortality attrition. However, it requires extrapolation of data for patients older or younger than 20 years of age, meaning that the further from age 20, the less reliable the estimate. Use of mixed model estimates of slope and intercept to provide estimated death age percentile is also less robust in the youngest and oldest subjects where flat regression lines may yield inappropriate estimates beyond the observed measurements. This is particularly problematic when combining study samples whose age distributions differ. These methods were designed for use in studies with extensive longitudinal data. With limited longitudinal data or cross-sectional data, these disease severity estimates will be seriously biased for many subjects, while NoRMA and KNoRMA provide valid estimates based on specific age at time of measurement for each individual. Multiple measurements can improve precision, but a single measurement is acceptable, so that subjects need not be deleted from analysis based on arbitrary rules of data availability.
Although the ascertainment schemes of the three CF samples differed drastically, KNoRMA provided a phenotype that was comparable across the sites. An additional benefit to KNoRMA (compared to mixed model methods and NoRMA) is that the phenotype estimate for each subject is independent. With the mixed model and NoRMA, lung function scores are based on the study sample; adding patients (for example, when working in a consortium) or removing patients from the sample would affect the results of all other patients. Since KNoRMA estimates are based on CF population based reference equations, addition or removal of patients from the analysis has no effect on values for the remaining patients.
There are limitations with this process that must be considered in future studies. The Kulich percentiles were based on a cohort gathered between 1994 and 200114. It is known that lung function has improved for CF patients since that time. Therefore, lung function scores derived using the Kulich reference equations14 were expected to be higher than those derived by LOESS in the CGS sample. However, this is a known bias, in a known direction, and we hypothesize that all patients will be affected equally and the analysis of association with genetic factors should be unbiased. The Kulich scores adjust for lung function differences between the sexes, which may affect the estimation of potential sex specific effects in genetic analysis.
Another possible limitation is our use of Canadian-based mortality rates for all samples, including the US-based study samples recruited by GMS and TSS. However, data from the US Cystic Fibrosis Foundation’s registry22, from which mortality for birth cohorts from 1985 onwards could be computed, indicated no significant differences in mortality estimates to age 22 (data not shown). Therefore, we do not believe that our use of Canadian-based mortality rates introduced a significant bias. Survival estimates were calculated without accounting for sex. Given historically discordant survival between males and females, future studies with separate survival estimates may be appropriate. The assumption that all CF patients who died before a given age had worse lung disease than those who survived to that age results in a small overestimate of mortality due to lung disease but one that is probably well within the margin of error of the estimates themselves since lung disease is the primary cause of death in CF. As national CF data registries around the world become more extensive, future studies may refine the CF mortality estimates in many ways – by sex, by country, by precise age, by cause of death – or may develop parametric survival models that incorporate these factors.
The principles behind this phenotype can be applied to any disease or quantitative phenotype for which a common measure for the study subjects, compared to the overall patient population, can be identified and augmented by suitable population mortality rates. Although our application is to CF, the approach can be used for any trait that is age dependent and for which cohort survival data are available. For example, in a survey of Canadians in 2005, 4.4% of those 35 years or older reported that they had been diagnosed by a health professional with chronic obstructive pulmonary disease (COPD)23. The main measure of disease severity in COPD is FEV1, which is predictive of other complications such as cardiovascular disease24, but as lung function progressively worsens in COPD patients, mortality attrition produces a bias in the comparison of younger versus older patients. In cross-sectional studies of FEV1 in COPD, the use of published FEV1 norms and mortality rates could be used to create a mortality adjusted phenotype which could be used to find genes that may modify the progression of COPD.
The use of a disease progression and a mortality adjusted phenotype reduces the need for stratification or additional covariates in large scale genetic and epidemiological studies, increasing the power of analysis and avoiding possible distortions. This advantage will be critical25 for the identification of true genetic influences that will translate to targeted therapies for individuals with high risk genotypes. In future, the inclusion of other factors related to disease progression or mortality, such as sex or early events such as poor growth or age of first serious infection, could be incorporated into the severity score, to provide a more refined measure to investigate prognosis and treatment.
Acknowledgments
This project was supported by Genome Canada through the Ontario Genomics Institute as per research agreement 2004-OGI-3-05 (PD), the Canadian Cystic Fibrosis Foundation (MC, LJS, PD), the Ontario Research Foundation (LJS, PD), the Natural Sciences and Engineering Research Council of Canada (LJS, LS), the National Institute of Health grant HG-004314 (LJS), CFF DRUMM00A0 (MLD), P30 DK27651 (MLD), HL68890 (MLD), CFF KNOWLE00A0 (MRK), RR00046 (MRK), RR00059 (MRK), NHLBI Grant HL68927 and FAMRI 062553 (GRC).
The Canadian Consortium for CF Genetic Studies
St. Paul’s Adult CF Clinic, Vancouver, BC, Canada E. M. Nakielna M.D., J. Hopkins; BC Children’s Hospital, Vancouver, BC , G. Davidson M.D. , A. Gravelle; Alberta Children’s Hospital, Calgary, AB, M. Montgomery M.D., Lisa Semple; Royal University Hospital Adult Clinic, Saskatoon, SK, J. Gjevre M.D., C. Brunoro; Royal University Hospital Pediatric Clinic, Saskatoon, SK; Dr. K.Ramlall, C. Turtle; Regina General Hospital, Regina, Saskatoon, SK; S.B.Holmes M.D., B. Beaurivage; Royal Jubilee Hospital, Victoria, BC, I. Waters M.D., S. Wiltse; Victoria General Hospital, Victoria, BC, B. Habbick M.D.,J. Jacob,S. Bourgh; Health Sciences Centre, Winnipeg MB –Adult Clinic, W.Kepron M.D., T. Wells; Children’s Hospital of Winnipeg, Winnipeg, MB- Pediatric Clinic, H. Pasterkamp M.D., M. Lowe; University of Alberta Adult Clinic, Edmonton, AB, N. E. Brown M.D., J. Salgado; University of Alberta Pediatric Clinic, Edmonton, AB, P. Zuberbuhler M.D., J. Tabak; Hospital for Sick Children, Toronto, ON, M. Solomon M.D., L. Taylor; St. Michael’s Hospital, Toronto, ON; E. Tullis M.D. A. Tsang; Children’s Hospital of Western Ontario, London, ON, B. Lyttle M.D. and N. Patterson/Patrice Kean; Hamilton Health Science Centre, Hamilton, ON - L. Pedder (Pediatric), A. Freitag (Adult), R. Hennessey; Hotel Dieu Hospital, Kingston, ON, R. van Wylick (M.D.), D. Lougheed (M.D.), D. McCulloch; Grand River Hospital, Kitchener, ON, K. Malhotra M.D., M. Jackson M.D.,L. Peterson; Children’s Hospital of Eastern Ontario, Ottawa, ON, M.Boland M.D., T. Kovesi M.D.,A. Smith; Sudbury Regional Hospital, Sudbury, ON, V.J.Kumar M.D., W.Gervais, C. Piche; Ottawa Genera Hospital, Ottawa, ON, S. Aaron M.D., K. Devesceri, K. Vandamheen; IWK Health Centre, Halifax, NS – Pediatric, D. Hughes M.D., P. Barrett; Queen Elisabeth Health Sciences Centre, Halifax, NS. –Adult, R.T. Michael M.D., F. Gosse, A. Dale; Janeway Children’s Health Centre, St. John’s, NL, R. Morris M.D., E. Sheppard; St. John Regional Hospital. St. John, NB, D.N.Garey M.D., R. Stackhouse; Hopital Sainte-Justine, Montreal, QC, J.E. Marcotte M.D., V. Fauvel, N Bureau, R. Dicaire; Hopital de Chicoutimi, Chicoutimi, QC, F. Simard, M.D., C. Martineau ; Centre Hospitalier Rouyn-Noranda, Rouyn-Noranda, QC ; N. Petit M.D., L. Boucher ; Centre Hospitalier Regional de L’Outaouais, QC, G. Cote M.D., M. LaPerriere; Centre Hospitalier de Gatineau. Gatineau, QC, L. Charette M.D., S. Marsolais; Centre Hospitalier Regional de Rimouski QC, J. Boucher M.D., F. Raymong ; Centre Universitaire de Sante de L’Estrie QC, L. Rivard, A. Cantin M.D., M. Plante ; Centre Hospitalier de L’Universite, Laval, QC, P. Bigonesse M.D., G. Rivard M.D., M. Ruel M.D., F. Brosseau, M. Roussin ; Montre Chest Institute, Montreal, QC, E. Matouk M.D., F. Paquet; Hotel Dieu de Montreal, Montreal, QC. Y. Berthiaume, A. Jeanneret M.D. ,M. van Spall M.D., R. Levesque; Montreal Children’s Hospital, Montreal, QC, L. Lands M.D., M. Gaul.
The University of North Carolina at Chapel Hill/Case Western Reserve Univeristy Gene Modifier Study Group
Alabama: University of Alabama at Birmingham (J.P. Clancy); Arizona: Phoenix Children’s Hospital, Phoenix (P.J. Radford, N. Argel); Arkansas: University of Arkansas Medical Sciences, Little Rock (D.E. Schellhase, P. Anderson, A. Taggart); California: Northern California Kaiser Permanente Medical Care Program, Oakland (G.F. Shay); California: Bay Area Pediatric Pulmonary, Oakland (K.A. Hardy); California: California Pacific Medical Center, San Francisco (N.R. Henig); California: Children’s Hospital of LA, Los Angeles (A.C.G. Platzker, M.S. Woo, L. Fukushima, E. Hsu); California: Lucile Packard Children’s Hospital, Stanford University Medical Center, Palo Alto (R.B. Moss, C.E. Dunn); California: University of California, San Francisco (D.W. Nielson); California: University of California-San Diego, Children’s Hospital (M.S. Pian); Colorado: Denver Children’s Hospital, Denver (F.J. Accurso); Florida: Nemours Children’s Clinic–Orlando (D.E. Geller); Florida: University of Florida, Gainesville (E.L. Olson); Georgia: Emory University Cystic Fibrosis Center, Atlanta (A.A. Stecenko); Georgia: Medical College of Georgia, Augusta (M.F. Guill); Illinois: Northwestern University Feinberg School of Medicine, Chicago (S.A. McColley); Children’s Memorial Hospital, Chicago (S.A. McColley, E.M. Potter); Indiana: Riley Hospital for Children, Indianapolis (M.S. Howenstine); Iowa: University of Iowa, Iowa City (R.C. Ahrens, M. Teresi); Maryland: Johns Hopkins University School of Medicine, Baltimore (P.L. Zeitlin, L. Brass); Massachusetts: Children’s Hospital, Boston (H. Levy, I. Huntington); Michigan: Michigan State University Cystic Fibrosis Center, East Lansing (R.E. Honicky); Michigan: Children’s Hospital of Michigan–Wayne State, Detroit (D.S. Toder); Michigan: University of Michigan Health System, Ann Arbor (R.H. Simon, S.Z. Nasr, C.N. Lumeng, M.E. Ball); Minnesota: University of Minnesota, Minneapolis (W.E. Regelmann, J.R. Phillips); Mississippi: University of Mississippi Medical Center, Jackson (F.E. Ruiz, K.G. Adcock); Missouri: Saint Louis University, St Louis (B.E. Noyes, V.L. Kociela); Missouri: University of Missouri, Columbia (P. Konig); Missouri: Washington University School of Medicine, St Louis (T. Ferkol Jr, M. Boyle); Nebraska: University of Nebraska Medical Center, Omaha (J.L. Colombo); New Hampshire: Dartmouth Hitchcock Medical Center, Lebanon (H.W. Parker); New Jersey: Atlantic Health/Morristown Memorial Hospital, Morristown (S.B. Fiel, P. Lomas).; New Jersey: Monmouth Medical Center, Long Branch (R.L. Zanni); New Mexico: University of New Mexico, Albuquerque (J. Taylor-Cousar); New York: Columbia University, New York (E.A. DiMango); New York: Schneider Children’s Hospital, New Hyde Park (J.K. DeCelie-Germana); New York: St. Vincent’s Hospital, New York (P.A. Walker, M.N. Berdella, E. Langfelder-Schwind); New York: The Women and Children’s Hospital of Buffalo, Buffalo (D. Borowitz); New York: University of Rochester, Rochester (C.L. Ren, A.K. Rovitelli); New York: Upstate Medical University, SUNY, Syracuse (R.D. Anbar, D.M. Lindner); North Carolina: Duke University, Durham (J. Taylor-Cousar, J.A. Voynow, K.J. Auten); North Carolina: University of North Carolina at Chapel Hill (M.R. Knowles, M.W. Leigh); North Carolina: Wake Forest University Baptist Medical Center, Winston-Salem (M.S. Schechter); Ohio: Akron Children’s Hospital, Akron (G.J. Omlor, D.A. Ouellette); Ohio: Cincinnati Children’s Hospital, Cincinnati (C.L. Karp); Ohio: Nationwide Children’s Hospital, Columbus (K.S. McCoy); Ohio: Rainbow Babies and Children’s Hospital, CWRU, Cleveland (M.W. Konstan); Ohio: Toledo Children’s Hospital, Toledo (P.A. Vauthy, M.L. Vauthy); Ohio: University of Cincinnati Medical Center, Cincinnati (P.M. Joseph); Pennsylvania: Children’s Hospital of Philadelphia–University of Pennsylvania, Philadelphia (T.F. Scanlin, R. Rubenstein, C. Murray, M. Skotleski); Pennsylvania: Drexel University College of Medicine, Philadelphia (S.B. Fiel, W.P. Sexauer, A. Ko, J. Hillman); Pennsylvania: Penn State Children’s Hospital and Penn State University College of Medicine, Hershey (N.J. Thomas, J.C. Hess); Pennsylvania: University of Pittsburgh, Pittsburgh (D.M. Orenstein); Rhode Island: Rhode Island Hospital, Providence (M.S. Schechter); South Carolina: Medical University of South Carolina, Charleston (P.A. Flume); Tennessee: Vanderbilt University Medical Center, Nashville (P.E. Moore, B. Slovis); Tennessee: University of Tennessee Health Science Center, Memphis (R. Schoumacher, B. Culbreath); Texas: Texas Children’s Hospital–Baylor, Houston (P.W. Hiatt); Texas: University of Texas Health Science Center, Tyler (R. Amaro, L. Macleod); Texas: Wilford Hall Medical Center, San Antonio (K.N. Olivier); Utah: University of Utah Pulmonary Division, Salt Lake City (T.G. Liou); Virginia: University of Virginia Medical Center, Charlottesville (D.K. Froh); Washington: Seattle Children’s Hospital/University of Washington, Seattle (R.L. Gibson, S. McNamara, K. Worrell); University of Washington School of Medicine, Seattle (S.M. Moskowitz); West Virginia: West Virginia University, Morgantown (K.S. Moffett, L.S. Baer).; Wisconsin: Children’s Hospital of Wisconsin, Milwaukee (T. Miller); Wisconsin: Medical College of Wisconsin, Milwaukee (J. Biller); Wisconsin: University of Wisconsin Adult Cystic Fibrosis Center, Madison (G.A. do Pico, L.M. Makholm); University of Wisconsin, CF-Pediatric Pulmonary Center, Madison (M.J. Rock, S.R. Osmond).
The Johns Hopkins University CF Twin and Sibling Study
AK: Providence Medical Center, Anchorage CF Clinic, Anchorage (Dion Roberts, MD, Vicki Roberts); AL: University of Alabama at Birmingham, Children’s Hospital, Birmingham (Hector Gutierrez, MD, Valerie Eubanks Tarn); AR: Sparks Regional Medical Center , Fort Smith (Louay Nassri, MD); AZ: Phoenix Children’s Hospital, CF Center, Phoenix (Gerald Gong, MD, Natalia Argel); AZ: Tuscon Cystic Fibrosis Center , Tucson (Wayne Morgan, MD); CA: Sutter Medical Center , Sacramento (Bradley Chipps, MD, Kasey Pearson); CA: Children’s Hospital - Oakland, Pediatric Pulmonary Center, Oakland (Karen Hardy, MD); CA: Children’s Hospital of Orange County , Orange (David Hicks, MD); CA: Pediatric Diagnostic Center , Ventura (Chris Landon, MD); CA: Kaiser Permanente Southern California, Kaiser-Permanente, Panorama City (Allan Lieberthal, MD, Joan Franco); CA: Stanford University Medical Center, Pediatric Pulmonary Medicine, Palo Alto (Richard Moss, MD, Coleen Dunn); CA: University of California at San Francisco, Cystic Fibrosis Center, San Francisco (Dennis Nielson, MD); CA: Children’s Hospital of Los Angeles, University of Southern California, Los Angeles (Arnold Platzker, MD); CA: Kaiser Permanente Medical Center, Department of Pediatrics, Oakland (Gregory Shay, MD, Mary Seastrand); CA: Naval Medical Center - San Diego, Attn: Department of Pediatric Pulmonary, San Diego (Henry Wojtczak, MD); CO: University of Colorado Health Sciences Center, Children’s Hospital of Denver, Denver (Frank Accurso, MD, Shelley Mann); CT: Yale University SOM, Pediatric Respiratory Medicine, New Haven (Marie Egan, MD, Jane Young); CT: Connecticut Children’s Medical Center, Central Connecticut Cystic Fibrosis Center, Hartford (Craig Lapin, MD, Ginnie Drapeau); FL: University of Florida, CF and Pediatric Pulmonary Disease Center, Gainesville (Veena Antony, MD); FL: St. Mary’s Medical Center, CF Center, West Palm Beach (Maurice Cruz, MD, Lisa Middleton); FL: Miami Children’s Hospital , Miami (Maria Franco, MD, Hilda Santrock); FL: University of South Florida, All Children’s Hospital, CF Center, St. Petersburg (Magdalen Gondor, MD, Jennifer Flanery); FL: Joe DiMaggio Children’s Hospital, Pulmonary Center, Hollywood (Morton Schwartzman, MD); GA: Medical College of GA , Augusta (Margaret Guill, MD, Kathy Dyer); GA: Emory University School of Medicine, Department of Pediatrics, Atlanta (Michael Schechter, MD); GA: Georgia Pediatric Pulmonology Assoc., PC, Children’s Health Care of Atlanta at Scottish Rite, Atlanta (Peter Scott, MD); IA: University of Iowa Hospitals & Clinics , Iowa City (Richard Ahrens, MD, Mary Teresi and Jean Frauenholtz); IA: University of Iowa Hospitals & Clinics, Adult CF Program, Iowa City (Douglas Hornick, MD); ID: St. Luke’s CF Clinic, Children’s Special Health Program, Boise (Henry Thompson, MD, Mary Nelsen); IL: Lutheran General Children’s Hospital, Nesset Health Center, Park Ridge (Gabriel Aljadeff, MD); IL: Children’s Asthma Respiratory&Exercise Specialists , Glenview (Steven Boas, MD); IL: University of Chicago Children’s Hospital, CF Center, Chicago (Lucille Lester, MD); IL: Children’s Memorial Hospital & Northwestern University , Chicago (Susanna McColley, MD, Eileen Potter and Cathy Powers); IL: University of Chicago Hospitals , Chicago (Edward Naureckas, MD); IN: Riley Hospital for Children, Indiana University SOM, Indianapolis (Michelle Howenstine, MD, Mary Blagburn); IN: Lutheran Hospital, Cystic Fibrosis and Pediatric Pulmonary Clinic, Fort Wayne (Pushpom James, MD); KS: Via Christi, St. Francis Campus , Wichita (Daniel Doornbos, MD); KS: University of Kansas Medical Center, CF Center, Kansas City (Gayln Perry, MD); KS: Via-Christi- St. Francis Campus, CF Care and Teaching Center, Wichita (Maria Riva, MD, Janet Messamore); KY: University of Kentucky, Department of Pediatric Pulmonology, Lexington (Jamshed Kanga, MD); MA: University of Massachusetts Memorial Health Care , Worcester (Brian O’Sullivan, MD, Dawn Baker); MA: Children’s Hospital of Boston, Pulmonary Division, Boston (Terry Spencer, MD, Magen Lorenzi and others); MA: New England Medical Center, CF Center, Boston (William F. Yee, MD, Corrie DeDomenico); MD: Johns Hopkins Hospital , Baltimore (Michael Boyle, MD); MD: National Naval Medical Center , Bethesda (Andrew Lipton, MD); MD: Johns Hopkins Hospital, CF Center, Baltimore (Pamela Zeitlin, MD); ME: Eastern Maine Medical Center, Pennobscott Pediatrics, Bangor (Thomas Lever, MD, Rebecca Welsh); MI: University of Michigan Medical Center , Ann Arbor (Samya Nasr, MD, Dawn Kruse); MN: University of Minnesota, CF Center, Minneapolis (Jordan Dunitz, MD, Brooke Noren); MN: Children’s Hospitals and Clinics of Minneapolis , Minneapolis (John McNamara, MD, Mahrya Johnson); MN: University of Minnesota , Minneapolis (Warren Regelmann, MD, Brooke Noren); MO: Children’s Mercy Hospital, University of Missouri at Kansas City, Kansas City (Philip Black, MD, Jaylene Wiegel); MO: St. Louis Children’s Hospital, Washington University School of Medicine, St. Louis (Thomas Ferkol, MD, Mary Boyle); MS: University of Mississippi Medical Center, Pediatric Pulmonary Division Adult CF Center, Jackson (Suzanne Miller, MD); MS: Memphis Lung Physicians , Southhaven (William Richards, MD); MT: Great Falls Clinic , Great Falls (Jerald Eichner, MD); NC: Asthma & Allergy Specialists, PA , Charlotte (Hugh Black, MD 2); NC: UNC at Chapel Hill, CF Pulmonary Research and Treatment Center, Chapel Hill (Michael Knowles, MD); NC: UNC at Chapel Hill, University of North Carolina, Chapel Hill (George Retsch-Bogart, MD); ND: St. Alexius Heart & Lung CF Clinic , Bismarck (Carla Zacher, MD, Deb McPherson); NE: University of Nebraska Medical Center , Omaha (John L. Colombo, MD, Dee Acquazino); NE: University of Nebraska Medical Center , Omaha (Peter Murphy, MD); NH: Dartmouth-Hitchcock Medical Center , Lebanon (H. Worth Parker, MD); NJ: Morristown Memorial Hospital , Morristown (Stanley Fiel, MD, Paula Lomas); NJ: University of Medicine & Dentistry of NJ, Cystic Fibrosis Center, Newark (Nelson Turcios, MD); NJ: Monmouth Medical Center , Long Branch (Robert Zanni, MD, Bridget Marra); NM: University of New Mexico, SOM, Pediatrics/Pulmonary, Albuquerque (Lea Davies, MD); NY: SUNY Upstate Medical University , Syracuse (Ran Anbar, MD, Donna Lindner); NY: Saint Vincents Hospital & Medical Center, CF Center, New York (Maria Berdella, MD, Elinor Langfelder-Schwind); NY: Women & Children’s Hospital of Buffalo, Cystic Fibrosis Center at SUNY, Buffalo (Drucy Borowitz, MD, Jeanne Smith); NY: Women & Children’s Hospital of Buffalo, Cystic Fibrosis Center at SUNY, Buffalo (Joe Cronin, MD); NY: Schneider Children’s Hospital, North Shore Long Island Jewish Health system, New Hyde Park (Joan DeCelie-Germana, MD, Sue Galvin); NY: New York Medical College/Westchester Medical Center, Pediatric Pulmonology, Valhalla (Allen Dozor, MD, Ingrid Gherson); NY: Long Island College Hospital, Pediatric CF Center, Brooklyn (Robert Giusti, MD); NY: University of Rochester Medical Center, Strong Memorial Hospital, Division of pediatric Pulmonology & Allergy, Rochester (Clement Ren, MD, Marissa Dixon); NY: Albany Medical College, Adult CF Program, Albany (Jonathan Rosen, MD, Kathy Mokhiber); NY: Long Island College Hospital, Adult CF Center, Brooklyn (Peter Smith, MD); OH: Cincinatti Children’s Hospital and Medical Center, Division of Pulmonary Medicine, C5, Cincinnati (James Acton, MD, Lorrie Duan); OH: Rainbow Babies and Children’s Hospital, Pediatric Pulmonary Division, Cleveland (Michael Konstan, MD, Colette Bucur); OH: Children’s Hospital Medical Center of Akron, Lewis H Walker CF Center, Akron (Nathan Kraynack, MD, Debbie Oulette); OH: Toledo Children’s Hospital, Adult CF Program, Toledo (Jeff Lewis, MD); OH: Columbus Children’s Hospital, Pulmonary Department, Columbus (Karen McCoy, MD, Debbi Johnson); OH: Children’s Hospital Medical Center of Akron, Adult Program, Akron (Titus Sheers, MD); OH: Toledo Children’s Hospital , Toledo (Pierre Vauthy, MD, Mary Vauthy); OK: Oklahoma Cystic Fibrosis Center, Tulsa, Pediatric Clinic, Tulsa (John Kramer, MD); OK: Childrens Hospital of Oklahoma, Univ. of Oklahoma Health Sciences Ctr., Oklahoma City (James Royall, MD); OR: Kaiser Permanente, Northwest Region, Portland (Richard Cohen, MD , Megan Buckholtz); PA: University of Pennsylvania , Philadelphia (Douglas Holsclaw, MD, Marianne Ferrin); PA: Children’s Hospital of Pittsburgh, University of Pittsburgh, Pittsburgh (David Orenstein, MD, Sandra Hurban); PA: Children’s Hospital of Philadelphia, CHOP, Philadelphia (Ron Rubenstein, MD); PA: St. Christopher’s Hospital for Children, Cystic Fibrosis Center, Philadelphia (Laurie Varlotta, MD); RI: Brown University Medical School Rhode Island Hospital, CF Center, Providence (Mary Passero, MD); SC: Pediatric Pulmonary Associates. , Columbia (Daniel Brown, MD); SC: Medical University of South Carolina, Division of Pulmonary Care and Critical Care, Charleston (Patrick Flume, MD, Sue Gray); TN: University of Tennessee , Memphis (Richard Boswell, MD); TN: TC Thompson Children’s Hospital , Chattanooga (Joel Ledbetter, MD); TN: Vanderbilt University Medical Center, Vanderbilt Children’s Hospital, Nashville (Elizabeth Perkett, MD, Cathy Tuzeneu); TN: University of Tennessee, LeBonheur Children’s Medical Center, Memphis (Dennis/Robert Stokes, MD , Barbara Culbreath); TX: University of Texas at Tyler Health Center, Pediatric Pulmonology, Tyler (Rodolfo Amaro, MD, Janice Hoeft); TX: Cook Children’s Medical Center , Fort Worth (James Cunningham, MD, Sara Scott); TX: Texas Children’s Hospital, Baylor CF Care Center, Houston (Peter Hiatt, MD, Charlene Hallmark); TX: Baylor College of Medicine, The Methodist Hospital, Houston (Marcia Katz, MD); TX: Methodist Children’s Hospital , San Antonio (Martha Morse, MD); TX: Children’s Medical Center of Dallas, CF Center, Dallas (Claude Prestidge, MD, Andrew Hebert); UT: University of Utah Health Sciences Center, Intermountain Pediatric CF Center, Salt Lake City (Barbara Chatfield, MD, Jane Vroom); VA: University of Virginia Health System , Charlottesville (Deborah Froh, MD, Lauren Ahrens); VA: Naval Medical Center - Portsmouth, Department of Pediatrics, Portsmouth (Reese Lee, MD); VT: Fletcher Allen Health Care, CF Center, Burlington (Thomas Lahiri, MD, Jackie Swartz); WA: Childrens Hospital & Regional Medical Center, Division of Pulmonary Medicine, Mailstop CH-68, Seattle (Ronald Gibson, MD, Alan Genatassio); WI: Children’s Hospital of Wisconsin, Froedert and Medical College of Wisconsin, Milwaukee (Julie Biller, MD); WI: Children’s Hospital of Wisconsin, Children’s Hospital and Health System, Millwaukee (William Gershan, MD, Tami Miller); WI: St. Vincent Hospital - Genetics , Green Bay (Peter Holzwarth, MD, Sumedha Ghate); WI: University of Wisconsin Hospital , Madison (Michael Rock, MD, Linda Makholm); WI: Marshfield Clinic, 1000 North Oak St, Marshfield (Bradley Sullivan, MD, Christina Zaleski); WV: West Virginia University, Mountain State Cystic Fibrosis Center, Morgantown (Kathryn Moffett, MD, Linda Baer); Australia: Royal Children’s Hospital , Brisbane, Queensland (Claire Wainwright, Joyce Cheney and Mary Jackson); Australia: The Prince Charles Hospital , Brisbane, Queensland (Scott Bell, MD, Michelle Wood); Scotland: Royal Hospital for Sick Children , Scotland (Ann Devenny, MD)
Footnotes
Additional Contributions: The authors would like to thank Mary Christofi, Jennifer Breaton, Nicole Anderson and Katherine Keenan for coordinating patient recruitment and phenotype verification; Andreea Cojocarru for data analysis; Johanna Rommens and Lei Sun for their helpful discussions and comments on study direction.
We thank Lori Vanscoy MD, Deanna Green MD, Vishal Doshi and Lindsay Henderson for contributions to the operation of the CF Twin and Sibling Study.
We are indebted to the University of North Carolina Center for Bioinformatics (Airong Xu, MD, MSIS and Hemant Kelkar, PhD) for their support and assistance. We thank Sarah Norris, Patricia Miller, Leia Charnin, Sally Wood, Sonya Adams, Colette Bucur and John Dunn for coordinating patient recruitment and phenotype verification. We are also indebted to Wanda O’Neal, PhD, Molecular Biology Core Laboratory for the Cystic Fibrosis/Pulmonary Research and Treatment Center at the University of North Carolina at Chapel Hill, for thoughtful discussion. The authors express deep gratitude to all CF patients and their families.
There are no competing interests to disclose.
Reference List
- 1.Collaco JM, Cutting GR. Update on gene modifiers in cystic fibrosis. Curr Opin Pulm Med. 2008;14(6):559–566. doi: 10.1097/MCP.0b013e3283121cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drumm ML, Konstan MW, Schluchter MD, et al. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med. 2005;353(14):1443–1453. doi: 10.1056/NEJMoa051469. [DOI] [PubMed] [Google Scholar]
- 3.Hull J, Thomson AH. Contribution of genetic factors other than CFTR to disease severity in cystic fibrosis. Thorax. 1998;53(12):1018–1021. doi: 10.1136/thx.53.12.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanscoy LL, Blackman SM, Collaco JM, et al. Heritability of lung disease severity in cystic fibrosis. Am J Respir Crit Care Med. 2007;175(10):1036–1043. doi: 10.1164/rccm.200608-1164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schluchter MD, Konstan MW, Davis PB. Jointly modeling the relationship between survival and pulmonary function in cystic fibrosis patients. Stat Med. 2002;21(9):1271–1287. doi: 10.1002/sim.1104. [DOI] [PubMed] [Google Scholar]
- 6.Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992;326(18):1187–1191. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 7.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131(6):809–814. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 8.Nelson SB, Gardner RM, Crapo RO, Jensen RL. Performance evaluation of contemporary spirometers. Chest. 1990;97(2):288–297. doi: 10.1378/chest.97.2.288. [DOI] [PubMed] [Google Scholar]
- 9.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 10.Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis. 1976;113(5):587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 11.Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med. 2008;177(3):253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang SS, O’Leary LA, Fitzsimmons SC, Khoury MJ. The impact of early cystic fibrosis diagnosis on pulmonary function in children. J Pediatr. 2002;141(6):804–810. doi: 10.1067/mpd.2002.129845. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15(2):75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 14.Kulich M, Rosenfeld M, Campbell J, et al. Disease-specific reference equations for lung function in patients with cystic fibrosis. Am J Respir Crit Care Med. 2005;172(7):885–891. doi: 10.1164/rccm.200410-1335OC. [DOI] [PubMed] [Google Scholar]
- 15.Schluchter MD, Konstan MW, Drumm ML, Yankaskas JR, Knowles MR. Classifying severity of cystic fibrosis lung disease using longitudinal pulmonary function data. Am J Respir Crit Care Med. 2006;174(7):780–786. doi: 10.1164/rccm.200512-1919OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor C, Corey M, Breaton J. The Canadian CF Modifier Gene Project: A nationally representative DNA and phenotype resource. Pediatr Pulmonol. 2006;29(Suppl):362. [Google Scholar]
- 17.Knudson RJ, Burrows B, Lebowitz MD. The maximal expiratory flow-volume curve: its use in the detection of ventilatory abnormalities in a population study. Am Rev Respir Dis. 1976;114(5):871–879. doi: 10.1164/arrd.1976.114.5.871. [DOI] [PubMed] [Google Scholar]
- 18.Farrell PM, Rosenstein BJ, White TB, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153(2):S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cystic Fibrosis Foundation registry committee task force to evaluate choice of spirometric reference equations for the National Patient Registry. Summary and Recommendations. 2009 [Google Scholar]
- 20.Canadian Cystic Fibrosis Foundation, Report of the Canadian Patient Data Registry. 2002 [Google Scholar]
- 21.Zammit S, Lewis G, Thapar A, et al. Phenotypic variation between parent-offspring trios and non-trios in genetic studies of schizophrenia. J Psychiatr Res. 2006;40(7):622–626. doi: 10.1016/j.jpsychires.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Cystic Fibrosis Foundation Patient Registry 2005 Annual Data Report to the Center Directors. 2005 [Google Scholar]
- 23.Chen JC, Mannino DM. Worldwide epidemiology of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 1999;5(2):93–99. doi: 10.1097/00063198-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Malerba M, Romanelli G. Early cardiovascular involvement in chronic obstructive pulmonary disease. Monaldi Arch Chest Dis. 2009;71(2):59–65. doi: 10.4081/monaldi.2009.362. [DOI] [PubMed] [Google Scholar]
- 25.Naylor MG, Weiss ST, Lange C. Recommendations for using standardised phenotypes in genetic association studies. Hum Genomics. 2009;3(4):308–319. doi: 10.1186/1479-7364-3-4-308. [DOI] [PMC free article] [PubMed] [Google Scholar]


