Abstract
In this study, the use-dependent, nicotinic receptor antagonist bis (2, 2, 6, 6-tetramethyl-4-piperidinyl) sebacate (BTMPS) was evaluated for its ability to attenuate the adverse consequences associated with morphine in rats in all three phases of an abstinence model of drug seeking: self-administration, acute withdrawal, and delayed test of drug seeking. Rats were allowed to self-administer morphine (FR1 schedule) with an active response lever, on a 24hr basis inside operant chambers, for 14 days. Each rat was subsequently evaluated for stereotypical behaviors associated with spontaneous morphine withdrawal. Rats were then placed in standard housing cages for a six week period of protracted abstinence from morphine. After this period, each rat was placed back into its respective operant chamber for a 14 day assessment of unrewarded drug seeking responses. BTMPS was administered to the animals in all three clinically relevant phases in three separate sets of experiments. BTMPS treatment during the self-administration phase resulted in up to a 34% reduction of lever responses to morphine when compared to vehicle treated control animals, as well as a 32% reduction in the dose of morphine self-administered. When given during self-administration and acute withdrawal, BTMPS treatment decreased acute withdrawal symptoms (up to 64%) of morphine use and reduced (up to 45%) drug seeking responses after six weeks of protracted withdrawal compared to control animals. BTMPS treatment after six weeks of abstinence from morphine had no effect. These results offer insight into the role of central cholinergic receptors in the onset and maintenance of drug addiction.
Keywords: Self-administration, 24 hr-access, BTMPS, morphine, withdrawal, recidivism
Introduction
As substance abuse continues to pose serious problems for society, both medical and financial, there is an increasing need to develop more effective pharmacological treatment strategies for the many aspects of addiction. The somatic and affective symptoms of acute drug withdrawal are powerful motivators for addicted individuals to continue to use drugs even in the face of adverse consequences. This involves a shift from positive reinforcement to negative reinforcement whereby an addict will continue to use drugs to take away the aversive stimulus of withdrawal (Koob and Le Moal, 1997). Of particular interest for addiction researchers and treatment providers is relapse behavior. Relapse is known to be heavily cue and context-dependent (Drummond et al, 1995), and the cravings induced by these cues diminish little over time (Aston-Jones and Harris, 2004; Koob and Le Moal, 2001; Shalev et al, 2002). The contributions of drug-associated cues and context to cravings and relapse has been observed in both animals and humans (Wing and Shoiab, 2008; Kilts et al, 2001). Any potential pharmacological intervention strategy must be capable of overcoming these important obstacles for the successful treatment of addicts. While current treatment strategies and therapies for substance abuse have made remarkable strides in reducing the incidence of relapse among drug users, the rates of relapse still remain between 40-60% of all treated users (McLellan, et al, 2000).
Among the major classes of drugs of abuse, opiates account for the largest number of (non-alcohol related) admissions to substance abuse treatment facilities (NIDA, 2008). The current drug replacement therapy related to opiate addiction (such as administration of methadone) remains controversial owing to side effect issues, and more importantly, some critics’ claim that it is simply replacing one dangerous drug with another (Greenberg, et al, 2007). The use of naltrexone, a μ-opioid receptor antagonist, has been met with patient compliance issues (Krupitsky, et al, 2010). Compounding this problem has been the low level of participation of the pharmaceutical industry in pursuing the development of novel pharmacotherapies for addiction treatment, most likely due to the societal stigma of drug addiction as well as reimbursement issues from the insurance industry (Volkow and Li, 2005).
Bis (2,2,6,6-tetramethyl-4-piperidinyl) sebacate (BTMPS) is a compound originally developed as a commercial additive to plastics that was subsequently shown to be a use-dependent, non-subtype selective nicotinic receptor (nAChR) antagonist (Papke, et al, 1994). The compound has a high affinity for nicotinic receptors (KD~200nM), and shows greater preference for neuronal nicotinic receptors (Papke, et al, 1994). The compound has been shown in behavioral studies to functionally antagonize nAChRs in the CNS (Graham, et al, 2005). The compound requires an open, activated channel to bind to the receptor, after current has been initiated, and its use-dependence property refers to the compound having greater antagonistic effects as greater concentrations of agonist are applied to the receptor (Papke et al, 1994). In two previous studies, we have shown that administration of BTMPS reduces self-administration of nicotine in rats as well as drug seeking responses to nicotine after a period of protracted abstinence (Hall, et al, 2010a) and that BTMPS reduces withdrawal behaviors in the rat after chronic administration of cocaine (Hall, et al, 2010b). The administration of BTMPS does not appear to elicit the typical side effects observed using classical nicotinic antagonists such as decreasing resting blood pressure, demonstrating a lack of ganglionic effect under basal conditions (Graham, et al, 2005). We have also shown that the compound does not suppress the motivation to obtain food in rodents via food-rewarded lever responding (Hall, et al, 2010a).
In the present study, we hypothesized that administration of a compound like BTMPS could reduce the self-administration of morphine, attenuate withdrawal symptoms associated with its use, as well as reduce recidivistic-like behavior to morphine in our abstinence model of drug seeking: 24 hr self-administration, acute withdrawal, and delayed test of drug seeking (Buccafusco and Bain, 2007). These three stages of the addiction cycle have been referred to as binge-intoxication, withdrawal-negative affect, and preoccupation-anticipation (Koob, et al, 2009). Rats were allowed to self administer morphine via lever pressing on a 24hr basis for a period of 14 days (binge-intoxication). After completing the self-administration phase the rats were evaluated for stereotypical signs of spontaneous morphine withdrawal (withdrawal-negative affect), followed by a six week period of standard housing. At the end of the six week period of protracted abstinence from morphine, each rat was placed back in its respective operant chamber for a 14 day assessment of their drug seeking responses (preoccupation-anticipation). BTMPS was administered subcutaneously in two doses (0.1 mg/kg, 0.5mg/kg). Treatment intervention with BTMPS occurred during three different dose regimens. During the first regimen BTMPS was administered during the initial self-administration phase of the study. In the second dose regimen BTMPS intervention occurred during the spontaneous morphine withdrawal phase, to examine the compound’s potential to reduce the withdrawal symptoms and drug seeking responses to morphine without having first inhibited self-administration. Finally, BTMPS intervention was delayed until the final drug seeking phase of our model, to assess its effects on responding after six weeks of abstinence from drug.
Materials and Methods
Male Wistar rats, weighing 300-330 grams at the time of study were purchased from Harlan, Sprague-Dawley, Inc. The animals were housed in the animal care facility of the VA Medical Center, Augusta, GA, kept on a regular 12 hr light/dark cycle, and were provided with food and water available ad libitum. All procedures performed during the course of this study were pre-approved by the Veterans Administration Institutional Animal Care and Use Committee, and conducted according to AAALAC guidelines.
Morphine Sulfate was purchased from Paddock Laboratories, Inc. (Minneapolis MN). Bis (2, 2, 6, 6-tetramethyl-4-piperidinyl) sebacate (BTMPS) was provided by Ciba-Geigy (Summit, NJ). Morphine used in the study was dissolved in 0.9% sterile saline solution purchased from Baxter (Deerfield, IL). BTMPS was dissolved in a vehicle solution containing equal parts sterile saline and ethyl alcohol.
Surgical Procedures
Rats were anesthetized using an isoflurane gas delivery system. Using aseptic technique, a midline incision was made ventrally over the neck and the jugular vein of the animal was exposed by blunt dissection. A small nick was made in the vein to allow for the introduction of sterilized catheter tubing, which was inserted until the tip reached the atrium. The tubing was secured to the jugular vein using silk suture. The opposing end of the catheter tubing was routed subcutaneously through the back of the animal to emerge at the nape of the neck. The tubing was stabilized to the back of the neck by using a sterile plastic anchor button sutured in with Vicryl® (Ethicon, Inc). The tubing was then connected to a water-tight swivel that was connected to an infusion pump controlled by Graphic State 3 software (Coulbourn Instruments, Whitehall, PA). The tubing was filled with heparin-saline solution until the start of morphine self-administration.
Self-administration phase
Animals self-administered morphine 24 hr/day using methods described previously (Buccafusco and Bain, 2007; Hall, et al, 2010a). Rats were maintained in operant chambers (Coulbourn Instruments, Whitehall, PA) on a regular light cycle and allowed to self-administer morphine via lever pressing on a 24 hr basis for a period of 14 days. The animals received no lever response training prior to testing sessions. The 24 hr test sessions were not interrupted except for brief periods to administer BTMPS or vehicle injections, and twice weekly waste-pan changes. All lever responses were recorded using Graphic State 3 software (Coulbourn Instruments, Whitehall, PA). A panel above the response lever, containing illuminated red, green, and yellow lights, signaled an active lever. Rats self-administered morphine under an FR1 schedule of reinforcement with unrestricted access to the drug, except for timeout periods of 120 seconds after each infusion to prevent overdose. During the timeout period, the cue lights were extinguished and lever responses were recorded, but no morphine was delivered (response levers became inactive). The dosage of morphine delivered per infusion was increased stepwise over the 14 day self-administration phase. During the first 3 days of the phase, the dose of morphine delivered was 0.25 mg/kg. The following three days the dose was increased to 0.5 mg/kg. For the final eight days, the dose of morphine delivered per infusion was 1.0 mg/kg.
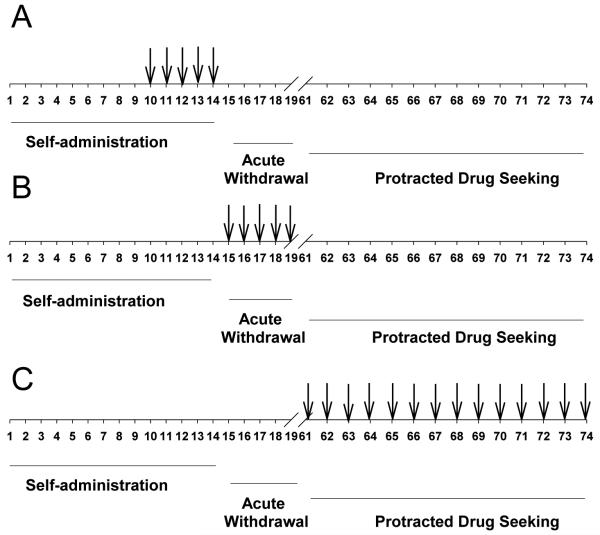
BTMPS was administered s.c. in two doses, 0.1 mg/kg and 0.5 mg/kg, during three separate experiments using different dose regimens. The treatment regimens for the three separate experiments are illustrated in Table 1. Doses for BTMPS were selected based on previous studies where the compound elicited dose-dependent decreases in self-administration of nicotine in rodents (Hall, et al, 2010a). During the first experiment BTMPS was administered daily starting day 10 of the self-administration phase and treatment was terminated on day 14, the last day of the phase. During the second experiment, BTMPS was administered starting on the last day of self- administration (Day 14) and continued for 5 days post-self-administration into the acute withdrawal phase. In the third set of experiments BTMPS treatment occurred starting on day 1 of the protracted abstinence/drug seeking phase and continued throughout the 14 day phase. Control animals for all experiments received vehicle injections.
Table 1.
Schematic of BTMPS treatment in each addiction cycle phase during three separate experiments. (A) In the first series of experiments, BTMPS treatment occurred during days 10-14 of the self-administration phase. (B) In the second series of experiments BTMPS treatment occurred on the last day of self-administration and continued for 5 days into the acute withdrawal phase of the study. (C) Final series of experiments in which BTMPS treatment was delayed until the protracted drug seeking phase of the study. Numbers indicate experimental day of the study.
|
Spontaneous Morphine Withdrawal Phase
At the end of the last day of the initial self-administration phase all animals were taken out of the operant chambers, and they were placed into standard housing cages. After twelve hours the rats were evaluated for signs associated with morphine withdrawal, using methods previously described (Buccafusco et al, 1984; Marshall and Buccafusco, 1985). Signs associated with spontaneous morphine withdrawal included “wet dog” shakes, writhing, diarrhea, teeth chattering, escape attempts, and chromodacryorrhea around the eyes and nose. The experiments were conducted in open field boxes measuring 16″ × 16″ × 16″. Each animal was evaluated for these withdrawal symptoms during four consecutive 30 min sessions, each spaced two hours apart. Withdrawal symptoms for each rat were totaled into a score for that animal. Withdrawal scores from the animals were then averaged for their respective treatment groups. Animals were then returned to standard home cages for a six week period of protracted abstinence from morphine.
Protracted Abstinence/Drug Seeking Phase
At the end of the six week post- withdrawal period all animals used in the previous experiments were returned to their respective operant chambers for assessment of their drug seeking behavior. The protracted abstinence/drug seeking phase of the study was conducted for 14 days. Conditions of this phase of the study were identical to those of the self-administration phase; except lever responses received no drug reward. All cues related to previous drug rewards were present during this phase (i.e., cue lights, house lights etc.)
Saline Self-administration
A separate cohort of animals was surgically prepared for self-administration studies in which all the animals were allowed to self-administer sterile saline. This group of animals self-administered saline under conditions identical to the morphine self-administration studies, and were subsequently evaluated for spontaneous withdrawal and drug seeking responses post-6 week abstinence. Animals in this cohort were divided into two groups and given s.c. injections of either vehicle or 0.5 mg/kg BTMPS starting day 10 of self-administration and ending on day 14.
Statistical Analysis
Averaged data sets are presented as the mean±S.E.M. Data for self-administration and protracted drug seeking experiments were analyzed using two-way ANOVA with a repeated measures design. Data for spontaneous morphine withdrawal were analyzed using a one-way ANOVA. All post hoc analyses were conducted using Tukey’s HSD test for multiple comparisons. Statistical analyses were performed using JMP software (SAS Institute Inc., Cary, NC). Differences between means were considered significant at p<0.05 level.
Results
BTMPS intervention days 10-14 of self-administration phase
Self-administration phase
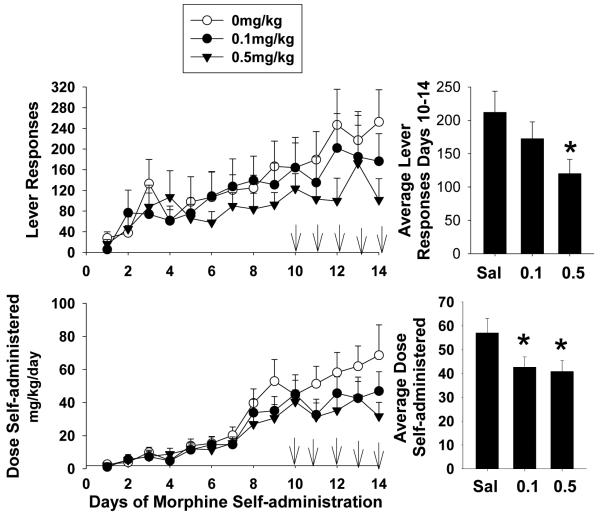
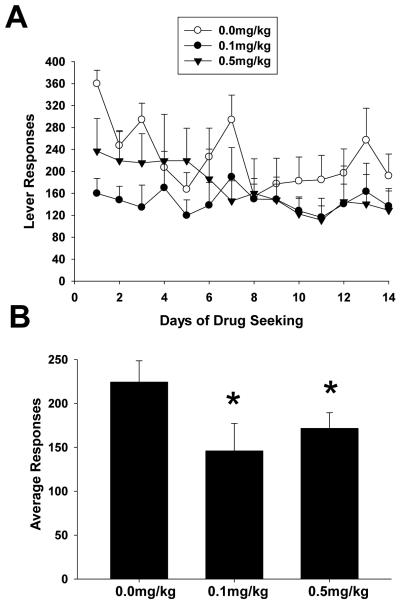
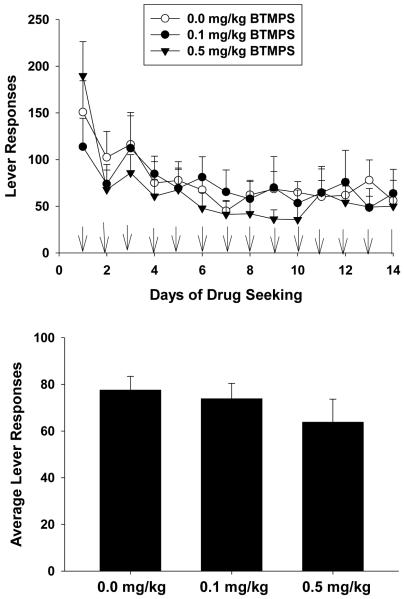
In this series of experiments three separate groups of rats were given either s.c. injections of BTMPS at doses of 0.1 mg/kg or 0.5 mg/kg or vehicle starting on day 10 of self-administration and ending day 14. Figure 1 left panel shows the response profile of each treatment group of rats over the 14 day self-administration period. In this series, each group showed a steady increase in lever responses to morphine until the start of treatment intervention on day 10. Once treatment was begun, the response profile of rats treated with BTMPS showed an attenuation of their daily response rate compared to control animals which continued to increase their responding. Two-way analysis of variance for days 10-14 of treatment revealed significant differences of the dose of BTMPS on lever responding (Group F2, 125 =3.08, p<0.05; Time F4=0.4167, p>0.05; Group x Time F8=0.2395, p>0.05). Post hoc analysis showed a significant difference in average lever responses by rats treated with 0.5mg/kg BTMPS compared to vehicle treated animals (p<0.05). These data are presented in the top right panel of Fig. 1. No significant differences in lever responding were observed between groups of rats for days 7-9 of self-administration, before treatment intervention was begun. The response profile of the dose of morphine self-administered for each treatment group mirrored that of their respective lever response profiles. These data are presented in the bottom left panel of Fig. 1. The dose of morphine self-administered for each group showed a steady increase from day 1 through day 9, however once treatment was begun animals treated with BTMPS exhibited a plateau in their dose of morphine self-administered over the 5 days of treatment, whereas the amount of morphine self-administered for control animals continued to increase. A significant effect of the dose of BTMPS (Group F2, 125 = 4.585, p<0.05; Time F4, 125=0.5119, p>0.05, Group x Time F8, 125=0.3565, p>0.05) on the average dose of morphine self-administered was observed using a two-way ANOVA. Post-hoc analysis revealed significant differences between both BTMPS treatment groups versus controls (0.1 mg/kg: p<0.05; 0.5 mg/kg: p<0.05). These data are presented in the bottom right panel of Fig. 1.
Figure 1. BTMPS administration days 10-14 of self-administration: Self-administration Phase.
Left Panel: The lever response profile and respective dose of morphine self-administered over 14 days by rats given s.c. injections of either vehicle (clear circles, n=8), 0.1 mg/kg BTMPS (black circles, n=9), or 0.5 mg/kg BTMPS (black triangles, n=12). Treatment intervention began on day 10 of self-administration and ended day 14 (arrows). Right Panel: Average daily lever responses and resultant average dose of morphine self-administered of treatment days 10-14 of the self-administration phase. *Significantly different (p<0.05) from vehicle treated (0 mg/kg) controls.
Spontaneous Morphine Withdrawal Phase
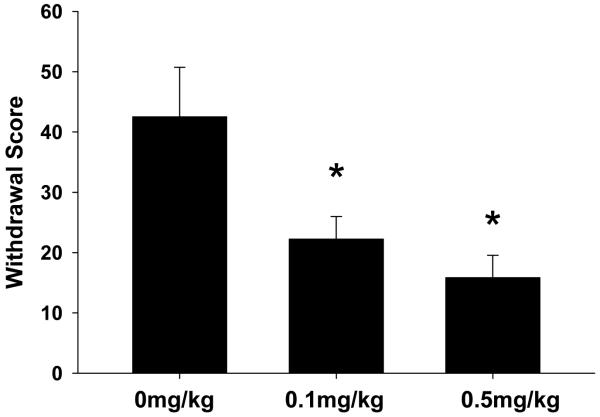
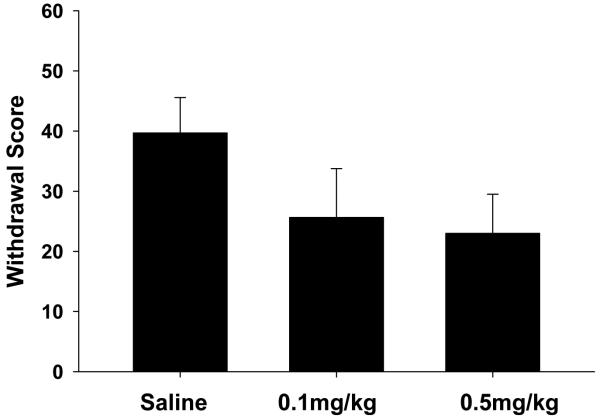
Twelve hours after the final day of self-administration, animals in each treatment group were evaluated for morphine withdrawal symptoms. The results of these experiments are presented in Fig 2. Withdrawal scores represent averaged totals for animals in each treatment group based on characteristic behaviors (as described in methods) associated with morphine withdrawal. Treatment with BTMPS resulted in a dose-dependent reduction of spontaneous morphine withdrawal scores for both treatment groups compared to vehicle treated controls (F2 = 6.88, p<0.05; Post hoc: 0.1 mg/kg p<0.05; 0.5 mg/kg p<0.05).
Figure 2. BTMPS administration days 10-14 of self-administration: Spontaneous Morphine Withdrawal Phase.
Effect of s.c. vehicle (0 mg/kg) or BTMPS (0.1 mg/kg or 0.5 mg/kg) intervention days 10-14 of morphine self-administration on symptoms associated with spontaneous morphine withdrawal. The average withdrawal scores for each treatment group based on stereotypical behaviors are shown. Treatment with BTMPS resulted in a dose-dependent reduction in withdrawal scores compared with vehicle treated counterparts. *Significantly different (p<0.05) from vehicle treated controls (0 mg/kg)
Protracted Abstinence/Drug Seeking Phase
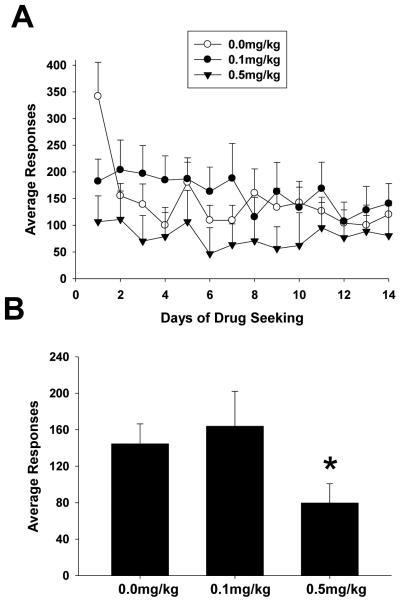
Figure 3 shows the results of the 14 day protracted abstinence/drug seeking phase for each group. Initially control animals on day 1 appeared to display greater lever responses than BTMPS treated animals after which, control animal responses decreased and leveled off. Statistical analysis for the 14 day drug seeking phase showed a significant effect of the dose of BTMPS on lever responses (Group F2, 350=22.47, p<0.05; Time F13, 350=1.899, p<0.05; Group x Time F26, 350=0.97, p>0.05)). Post-hoc analysis showed significant reductions in the average lever responses over the protracted abstinence/drug seeking phase of animals treated with 0.5 mg/kg BTMPS compared to vehicle treated controls (p<0.05).
Figure 3. BTMPS administration days 10-14 of self-administration: Protracted Abstinence/Drug Seeking Phase.
Unrewarded drug seeking lever responses for each treatment group after six weeks of protracted abstinence from morphine. (A) Lever response profile for each treatment group over the 14 day period. (B) Average lever responses for each treatment group over the 14 day period. Statistical analysis revealed a significant reduction of lever responses compared to controls for rats treated with 0.5 mg/kg BTMPS. *Significantly different (p<0.05) from vehicle treated controls (0 mg/kg).
BTMPS intervention during acute withdrawal from morphine
Self-administration phase
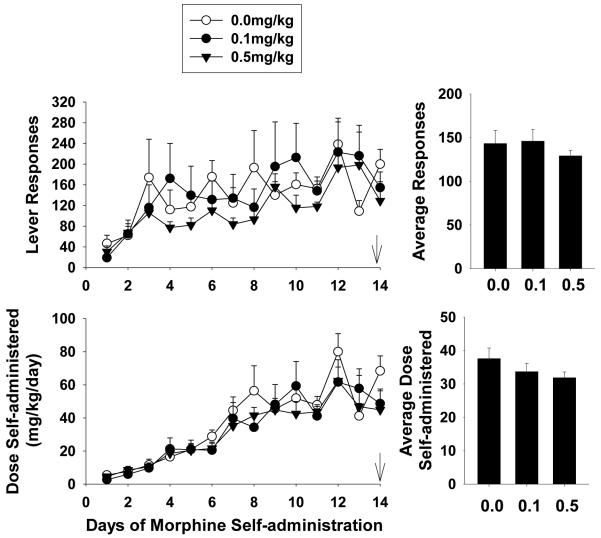
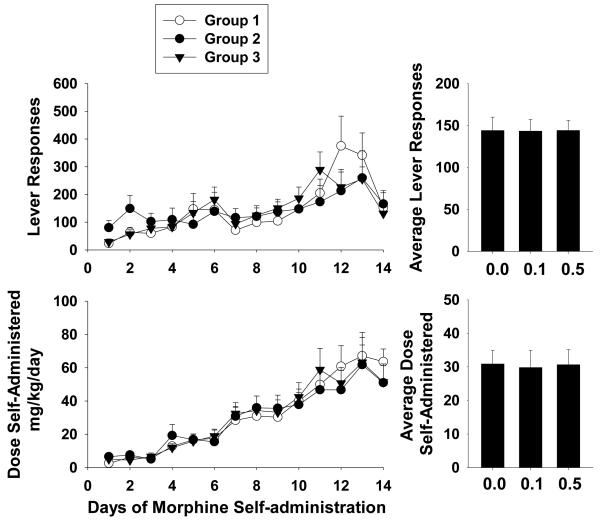
During this series of experiments, BTMPS intervention occurred on the last day of self-administration (Day 14) and continued for 5 days into the acute withdrawal phase of the study. Figure 4 left panel shows the lever response profile and resultant dose of morphine self-administered of the self-administration phase for each treatment group. The average lever responses and dose self-administered for each group is shown in the right panel of Fig. 4. Each group displayed a steady increase in their respective rates of responding as well as the dose of morphine self-administered over the 14 days. No significant differences in average lever responses or the dose of morphine self- administered were observed for any group.
Figure 4. BTMPS intervention during acute withdrawal from morphine: Self-administration Phase.
Left Panel: The lever response profile and resultant dose of morphine self-administered over 14 days by rats given s.c. injections of either vehicle (clear circles, n=6), 0.1 mg/kg BTMPS (black circles, n=8), or 0.5 mg/kg BTMPS (black triangles, n=6). Treatment with BTMPS in this series began on day 14 of self-administration (arrows) and continued for 5 days into the acute withdrawal period. Right Panel: Average daily lever responses and resultant average dose of morphine self-administered over the entire 14 day self-administration phase. No significant differences between treatment groups were observed for average lever responses or the average dose of morphine self-administered.
Spontaneous Morphine Withdrawal Phase
BTMPS was administered to treated animals throughout the acute withdrawal phase of this series of experiments. Control animals received vehicle injections. The results of the spontaneous morphine withdrawal evaluations are presented in Fig. 5. Although no significant differences were observed for withdrawal scores between either treatment group versus controls, BTMPS treatment was associated with a non-significant trend (p=0.08 for 0.5 mg/kg group) toward a dose-dependent reduction in these behaviors.
Figure 5. BTMPS intervention during acute withdrawal from morphine: Spontaneous Morphine Withdrawal Phase.
Spontaneous morphine withdrawal scores for rats receiving s.c. injections of vehicle (0 mg/kg) or BTMPS (0.1 mg/kg or 0.5 mg/kg) starting day 14 of the self-administration phase and continuing treatment for 5 days into acute withdrawal. Treatment with BTMPS tended toward a dose dependent decrease in withdrawal scores compared to control animals, however no significant differences were observed.
Protracted Abstinence/Drug Seeking Phase
The data for the protracted abstinence/drug seeking phase of this series of animals are presented in Fig. 6. Similar to the previous experiment, control animals in this series appeared to exhibit greater lever responses on the first day of testing. The total response profiles for the 14 days for each group are shown in Fig. 6A. Two-way ANOVA revealed significant differences in lever responses for the dose of BTMPS over the 14 day period (Group F2, 350=8.19, p<0.05; Time F13, 350=1.14, p>0.05; Group x Time F26, 350=0.455, p>0.05). Post-hoc analysis showed significantly reduced average lever responses of both BTMPS treatment groups compared to controls (0.1mg/kg: p<0.05; 0.5mg/kg: p<0.05).
Figure 6. BTMPS intervention during acute withdrawal from morphine: Protracted Abstinence/Drug Seeking Phase.
Unrewarded drug seeking responses for each group of animals treated with vehicle or BTMPS during acute withdrawal. (A) Lever response profile for each treatment group over the entire 14 day period. (B) Average lever responses for each treatment group for the entire 14 day period. Statistical analysis revealed significant differences in average lever responses for both BTMPS treatment groups compared to vehicle treated controls. *Significantly different (p<0.05) from controls (0 mg/kg).
BTMPS intervention after six weeks of abstinence from morphine
Self-administration phase
During these experiments BTMPS intervention was delayed until the final protracted abstinence/drug seeking phase of the study. Figure 7 left panel shows the lever response profile and resultant dose of morphine self-administered for three separate groups of animals. The average lever responses and dose of morphine self-administered for each group is shown in the right panel of Fig. 7. Statistical analysis revealed no significant differences in either average lever responses or average dose of morphine self-administered between any groups of animals during this phase.
Figure 7. BTMPS intervention after six weeks of protracted abstinence from morphine: Self-administration phase.
Left Panel: The lever response profile and respective dose of morphine self-administered over 14 days by three separate groups of rats (for each group, n=10). Right Panel: Average daily lever responses and resultant average dose of morphine self-administered over the entire 14 day self-administration phase. No significant differences between groups were observed for average lever responses or the average dose of morphine self-administered.
Spontaneous Morphine Withdrawal Phase
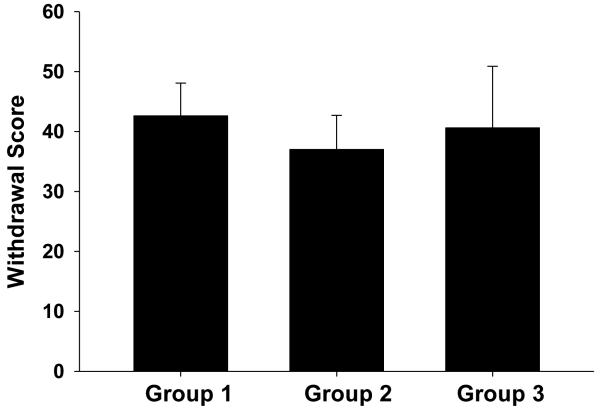
The spontaneous morphine withdrawal scores for each untreated group of animals in this series of experiments are shown in Fig. 8. No significant differences were observed for withdrawal scores between any two groups of animals in this series.
Figure 8. BTMPS intervention after six weeks of protracted abstinence from morphine: Spontaneous Morphine Withdrawal Phase.
Withdrawal scores for each group of animals to be receive treatment after six weeks of abstinence from morphine. No significant differences were observed between groups for spontaneous morphine withdrawal scores.
Protracted Abstinence/Drug Seeking Phase
The results of BTMPS treatment during the protracted abstinence/drug seeking phase of the study are shown in Fig. 9. BTMPS treatment was begun on day 1 of the phase and continued throughout the 14 days. Fig 9A shows the lever response profiles of each group of animals. The response profile for each treatment group showed higher initial responses on day 1 followed by a gradual decrease in responses that leveled off to a somewhat asymptotic level of responding for the remainder of the 14 days. The average lever responses for each group are shown in 9B. Statistical analysis revealed no significant differences between any two treatment groups during the 14 day phase.
Figure 9. BTMPS intervention after six weeks of protracted abstinence from morphine: Protracted Abstinence/Drug Seeking Phase.
Unrewarded drug seeking responses for each group of animals treated with vehicle or BTMPS during the drug seeking phase. BTMPS intervention began on day 1 of the drug seeking phase and continued throughout testing. (A) Lever response profile for each treatment group over the entire 14 day period. (B) Average lever responses for each treatment group for the entire 14 day period. Statistical analysis revealed no significant differences in average lever responses for BTMPS treatment groups compared to vehicle treated controls (0.0 mg/kg).
Saline Self-administration
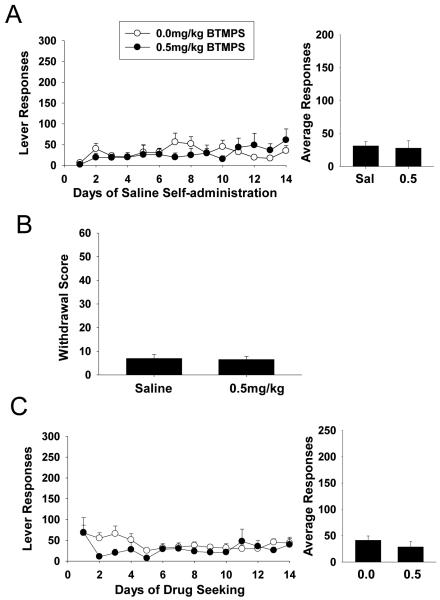
A separate group of animals were allowed to self-administer saline to serve as a negative control for the previous experiments utilizing morphine. The conditions of self-administration and subsequent withdrawal and protracted abstinence/drug seeking tests in these experiments were otherwise identical to the morphine studies. Animals were administered vehicle or BTMPS via s.c. injection starting day 10 of self-administration and terminating on day 14. The results of saline self-administration are presented in Fig. 10A. No significant differences in lever responding were observed between the two groups. Figure 10B shows the withdrawal scores for both sets of animals. No significant differences in withdrawal scores were observed for the two sets of animals. Finally, Fig. 10C shows the results of drug seeking responses after six weeks. Again, no significant differences were observed for drug seeking behavior in animals receiving either BTMPS or vehicle injections.
Figure 10. Saline self-administration.
All three phases of testing are shown of animals self-administering sterile saline. (A) Lever response profile and resultant average lever responses of animals self-administering saline 24 hr/day for 14 days. Animals were administered either vehicle or BTMPS (0.5 mg/kg) by s.c. injection starting day 10 and terminating day 14. No significant differences were observed for average lever responses to saline between these two groups. (B) Spontaneous withdrawal scores for each treatment group. No significant differences were observed for withdrawal scores between treatment groups. (C) Protracted abstinence/drug seeking response profile and average lever responses for animals previously self-administering saline. No significant differences in lever responses were observed between treatment groups.
Discussion
The present study assessed the ability of the use-dependent, nAChR antagonist BTMPS to reduce the adverse consequences of morphine self-administration in a clinically relevant abstinence model of drug seeking in rodents. We attempted to study each aspect of the addiction process in the same animals from 24 hr self-administration to acute withdrawal to drug seeking behavior after protracted abstinence from drug. Importantly, the animals in this study were not trained in the operant task of lever pressing for other reinforcers. It has even been suggested that this step is perhaps unnecessary for the study of reinstatement behavior in rodents after a period of self-administration (Bongiovanni and See, 2008). In our model, BTMPS attenuated self-administration of morphine in rats, and reduced acute morphine withdrawal symptoms as well as protracted drug seeking behavior. Intervention with the compound occurred during three clinically relevant time periods: 1) during self-administration, 2) upon termination of self-administration, and 3) upon reintroduction to the drug seeking environment. In the first two cases, BTMPS treatment was effective in alleviating the negative downstream effects of morphine self-administration. Delaying treatment until the final phase had no effect.
A growing body of evidence supports the argument that central cholinergic receptors and pathways play an important role in the onset and maintenance of drug addiction (Kelley, 2002a; Williams and Adinoff, 2008). Cholinergic activation of the ventral tegmental area (VTA) has been shown to increase the release of dopamine and induce dopamine neuron firing, activity which was attenuated by the nicotinic antagonist mecamylamine (Grenhoff, et al, 1986). Both nicotinic and muscarinic cholinergic receptors have been shown to contribute to the neuro-adaptive changes associated with the chronic abuse of drugs (Macedo, et al, 2004; Schoffelmeer, 2002; Zhang and Buccafusco, 2000). Indeed, the administration of cholinergic agents has been shown in rodent models to reduce the self-administration of cocaine and morphine (Hansen and Mark, 2007; Levin, et al, 2000; Glick et al, 1996) as well as attenuate cocaine and morphine withdrawal (Taraschenko, et al, 2000). Nicotinic receptor blockade has been also been shown to reduce both cocaine and morphine-induced conditioned place preference in rodents (Zachariou, et al, 2001; Zarrindast, et al, 2003). This evidence suggests that cholinergic receptors are promising targets for the treatment of substance abuse.
Animals treated with BTMPS on days 10-14 of self-administration exhibited significantly reduced withdrawal scores compared to control animals, in a dose-dependent manner. This attenuation of the somatic symptoms associated with morphine withdrawal was not completely unexpected, considering that interactions between nicotine and morphine have been previously reported (Berrendero et al, 2002; Zarrindast et al, 1999), and that nicotine and nicotinic compounds have been shown to alter behavioral responses to morphine (Biala and Staniak, 2010; Zarrindast and Farzin, 1996). However, the effects of BTMPS treatment in these experiments highlights the potential of compounds in this pharmacological class as agents to relieve these devastating withdrawal symptoms, which serve as powerful negative reinforcers for opiate addicts. Indeed, though statistically insignificant, animals treated with BTMPS on the last day of self-administration and throughout acute withdrawal showed a clear trend toward a reduction in withdrawal scores, even when examined after only a single day of treatment.
Treatment with BTMPS resulted in attenuated withdrawal symptoms and reduced drug seeking responses in rats self-administering morphine even when intervention was delayed until completion of the self-administration phase. This occurred in groups of animals that, statistically, self-administered similar dosages of morphine compared to controls. From these observations it can be concluded that the behavioral results obtained in the acute withdrawal tests and post-6 week drug seeking tests were the result of intervention with BTMPS and not simply a result of reduced self-administration of morphine. As such, the results of the second series of experiments in this study validate the results of the first series. There appears to be a pharmacological dissociation between lever responding in the initial self-administration phase of the study and responding during the post-6 week drug seeking phase, perhaps suggesting that treatment with BTMPS blunts the effects of contextual cues on drug seeking. Although it has been shown that nAChRs in the VTA as well as the hippocampus are involved in morphine-state-dependent learning and memory retrieval (Khajehpour, et al, 2008; Rezayof, et al, 2008), any effects BTMPS may have on the learning process or encoding of contextual cues will require further investigation.
The positive aspects of employing use-dependent compounds like BTMPS are underscored in the results of our 24hr saline self-administration experiments. These experiments were performed to serve as a positive control for our morphine experiments. Two important insights are gained from these studies. First, the results from the saline self-administration experiments demonstrate that the results observed in our morphine model are not merely artifacts or random chance. The animals self-administering saline elicited a low level of lever responding when left in operant chambers for 24 hours per day, and did not increase to the level of the responding to morphine. The same basal effects were observed in the subsequent withdrawal and protracted drug seeking experiments. The second major insight from these control studies is further confirmation that under basal levels of neuronal activity, BTMPS is largely an inert compound. BTMPS alone had no significant effect on initial lever responses to saline, showing there was no rate-suppressing effect on the animals. The compound also had no significant effect on its own in the withdrawal tests or protracted drug seeking tests. It should be noted that the low level of responding and low withdrawal scores could also represent a floor effect in this series of experiments.
It is important to note that, despite the positive outcomes of these experiments, there are also some limitations with regard to the conclusions of the present study. The results of the first series of self-administration experiments demonstrate that administration of BTMPS reduces self-administration of morphine in rats. What is not completely clear is exactly how BTMPS is causing this reduction. Animals treated with BTMPS in this experiment display a plateau-like effect in their respective self-administration of morphine. There are at least two distinct possibilities underlying the reason for this. One possible explanation is that administration of BTMPS reduces the rewarding properties of morphine. However, the typical effect on self-administration seen by the introduction of an antagonist during stable responding conditions is a compensatory increase in lever responding (Ranaldi and Wise, 2001). A second possible explanation is that administration of BTMPS interferes with the development of tolerance for morphine. The data show clearly that control animals show a steady increase in morphine intake, whereas treated animals do not. While the stepwise increase in the dose of morphine delivered was intended to control in part for tolerance buildup, this latter explanation of the compound’s effects is certainly plausible. Studies examining the effects of BTMPS on stable responding conditions will be needed to fully explore these possibilities.
There are also limitations concerning conclusions drawn from the animals’ protracted drug seeking experiments. Although, as previously mentioned, one possible interpretation of the results could be that BTMPS treatment blunts the effects of contextual cues associated with the drug reward, there are alternative explanations. The drug could be interfering with memory consolidation, specifically reinforcement learning. Another explanation is the possibility that the drug reduces cravings, which results in reduced drug seeking responses. The observation that administration of BTMPS during delayed extinction testing had no significant effect on drug seeking behavior likely supports the former explanation. Future studies targeting these questions will determine the precise mechanism by which BTMPS reduces protracted drug seeking behavior. It should also be noted that we did not observe typical extinction responding in these experiments. Although some groups do exhibit higher responding initially followed by a decline in responses, they do not decline to the level of our saline self-administration negative control experiments. However, we also did not observe typical extinction responding in our previously published work using the current methodology (Buccafusco and Bain, 2007; Hall et al, 2010). Whether this is due to methodological differences in our studies compared to others, we cannot say; however, a recent study suggests that prolonged training on a response lever results in habitual drug seeking behavior in rats, even after the reward associated with the lever has been devalued (Zapata et al, 2010). While animals in this study were not formally trained to press levers for drug (i.e., no shaping procedure was initially employed), they had access to the lever 24 hrs/day for 14 days, and certainly learned the lever/reward association. Nonetheless, the animals in this study (especially controls) do show a decrease in responding from initial levels on day one, and reach a somewhat asymptotic level of responding thereafter.
The contributions of central cholinergic receptors and pathways to addiction have long been known and much literature has been published to this effect. However, the prospect of targeting these receptors (particularly nicotinic cholinergic receptors) as a specific therapeutic strategy has typically been overlooked. We have shown in this study that BTMPS, a use-dependent, non-selective nicotinic receptor antagonist, can reduce both self-administration as well as the adverse consequences of self-administration (e.g., withdrawal symptoms, recidivistic-like behaviors) of morphine in rodents. These data support an alternative approach to the treatment of addiction-related disorders of the mesolimbic system through nAChR modulation (Janhunen and Ahtee, 2007).
Research Highlights.
BTMPS reduces self-administration and downstream effects of morphine in rats
Cholinergic agents can be used to treat substance abuse
24hr access self-administration model properly mimics the human condition
Acknowledgments
This study was supported by a Merit Review Award from the Office of Research and Development of the Department of Veterans Affairs and the National Institute of Drug Abuse (DA029127). We would also like to further acknowledge our dear friend, mentor and colleague, the late Dr. Jerry J. Buccafusco, Regents Professor of Pharmacology and Toxicology, Medical College of Georgia and Director of the Neuropharmacology Laboratory at the Charlie Norwood Veterans Administration Medical Center, Augusta, Georgia. Dr. Buccafusco’s contributions to the field of drug abuse-related research spanned more than thirty years.
Footnotes
Conflicts of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacol. 2004;47(1):167–79. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Keiffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in μ-opioid receptor knock-out mice. J Neurosci. 2002;22:10935–40. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G, Staniak N. Varenicline and mecamylamine attenuate locomotor sensitization and cross-sensitization induced by nicotine and morphine in mice. Pharmacol, Biochem & Behav. 2010;96:141–7. doi: 10.1016/j.pbb.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Bongiovanni M, See RE. A comparison of the effects of different operant training experiences and dietary restriction on the reinstatement of cocaine-seeking rats. Phamacol Biochem & Behav. 2008;89:227–233. doi: 10.1016/j.pbb.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Marshall DC, Turner RM. A comparison of the inhibitory effects of clonidine and guanfacine on the behavioral and autonomic components of morphine withdrawal in rats. Life Sci. 1984;35:1401–1408. doi: 10.1016/0024-3205(84)90398-9. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Bain JN. A 24-h access i.v. self-administration schedule of morphine reinforcement and the estimation of recidivism: pharmacological modification by arecoline. Neuroscience. 2007;149:487–498. doi: 10.1016/j.neuroscience.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Tiffany ST, Glautier S, Remington B. Cue exposure in understanding and treating addictive behavior. In: Drummond DC, Tiffany ST, Glautier S, Remington B, editors. Addictive Behaviors: Cue Exposure Therapy and Practice. Wiley & Sons; London: 1995. pp. 1–17. [Google Scholar]
- Glick SD, Kuehne ME, Maisonneuve IM, Bandarage UK, Molinari HH. 18-methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and mesolimbic dopamine release in rats. Brain Res. 1996;719:29–35. doi: 10.1016/0006-8993(96)00056-x. [DOI] [PubMed] [Google Scholar]
- Graham J, Papke R, Buccafusco JJ. Functional central nicotinic acetylcholine receptor antagonism by systemic administration of Tinuvin 770 (BTMPS) Cur Alzh Res. 2005;2:141–147. doi: 10.2174/1567205053585747. [DOI] [PubMed] [Google Scholar]
- Greenberg B, Hall D, Sorensen J. Methadone maintenance therapy in residential community settings: challenges and promise. J Psychoact Dr. 2007;39(3):203–208. doi: 10.1080/02791072.2007.10400606. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Pearson LS, Buccafusco JJ. Effect of administration of the nicotinic acetylcholine receptor antagonist BTMPS, during nicotine self-administration, on lever responding induced by context long after withdrawal. Neuropharmacology. 2010a;58(2):429–435. doi: 10.1016/j.neuropharm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Hall BJ, Pearson LS, Buccafusco JJ. Effect of the use-dependent, nicotinic receptor antagonist BTMPS in the forced swim test and elevated plus maze after cocaine discontinuation in rats. Neuroscience Letters. 2010b;474(2):84–87. doi: 10.1016/j.neulet.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Hansen ST, Mark GP. The nicotinic acetylcholine receptor antagonist mecamylamine prevents escalation of cocaine self-administration in rats with extended daily access. Psychopharmacology. 2007;194:53–61. doi: 10.1007/s00213-007-0822-z. [DOI] [PubMed] [Google Scholar]
- Janhunen S, Ahtee L. Differential nicotinic regulation of the nigrostriatal and mesolimbic dopaminergic pathways: Implications for drug development. Neuroscience and Biobehavioral Reviews. 2007;31:287–314. doi: 10.1016/j.neubiorev.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Nicotinic receptors: addiction’s smoking gun? Nature Medicine. 2002a;8(5):447–449. doi: 10.1038/nm0502-447. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Fabe TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psych. 2001;58:334en. doi: 10.1001/archpsyc.58.4.334. Ps. [DOI] [PubMed] [Google Scholar]
- Khajehpour L, Rezayof A, Zarrindast M. Involvement of dorsal hippocampal nicotinic receptors in the effect of morphine on memory retrieval in passive avoidance task. Eur J Pharmacol. 2008;584:343–351. doi: 10.1016/j.ejphar.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Lloyd GK, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta Stone approach. Nat Rev Drug Dis. 2009;8(6):500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E, Zvartau E, Woody G. Use of naltrexone to treat opioid addiction in a country in which methadone and buprenorphine are not available. Curr Psychiatry Rep. 2010 doi: 10.1007/s11920-010-0135-5. DOI 10.1007/s11920-010-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiol Behav. 2000;71:565–570. doi: 10.1016/s0031-9384(00)00382-6. [DOI] [PubMed] [Google Scholar]
- Macedo DS, Correia EE, Vasconcelos SM, Aguiar LM, Viana GS, Sousa FC. Cocaine treatment causes early and long-lasting changes in muscarinic and dopaminergic receptors. Cell Mol Neurobiol. 2004;24:129–136. doi: 10.1023/B:CEMN.0000012718.08443.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Marshall DC, Buccafusco JJ. A comparison of the cardiovascular and the behavioral changes following naloxone as measures of the degree of physical dependence on morphine in rats. Drug Dev Res. 1985;5:271–280. [Google Scholar]
- National Institute on Drug Abuse NIDA InfoFacts: Treatment Statistics. Available at http://drugabuse.gov/infofacts/treatmenttrends.html.
- Papke RL, Craig G, Heinemann S. Inhibition of nicotinic acetylcholine receptors by bis (2,2,6,6-tetratmethyl-4-piperidinyl) sebacate (Tinuvin 770), an additive to medical plastics. J Pharmacol Exp Ther. 1994;268(2):718–726. [PubMed] [Google Scholar]
- Ranaldi R, Wise RA. Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: possible role for dendritically released dopamine. J Neurosci. 2001;21(15):5841–6. doi: 10.1523/JNEUROSCI.21-15-05841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezayof A, Darbandi N, Zarrindast M. Nicotinic acetylcholine receptors of the ventral tegmental area are involved in mediating morphine-state-dependent learning. Neurobiology of Learning and Memory. 2008;90:255–260. doi: 10.1016/j.nlm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm J, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, De Vries TJ, Wardeh G, van de Ven HWM, Vanderschuren LMJ. Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J. Neurosci. 2002;22:3269–3276. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraschenko OD, Shulan JM, Maisonneuve IM, Glick SD. 18-MC acts in the medial habenula and interpeduncular nucleus to attenuate dopamine sensitization to morphine in the nucleus accumbens. Synapse. 2007;61:547–560. doi: 10.1002/syn.20396. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drugs and alcohol: treating and preventing abuse, addiction and their medical consequences. Pharmacol & Ther. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008;33:1779–1797. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Shoaib M. Contextual stimuli modulate extinction and reintroduction in rodents self-administering intravenous nicotine. Psychopharmacology. 2008;200:357–365. doi: 10.1007/s00213-008-1211-y. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Caldarone BJ, Weathers-Lowin A, George TP, Elsworth JD, Roth RH, Changeux J, Picciotto MR. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24(5):576–589. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]
- Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30(46):15457–63. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Farzin D. Nicotine attenuates naloxone-induced jumping behavior in morphine-dependent mice. Eur J Pharmacol. 1996;298:1–6. doi: 10.1016/0014-2999(95)00761-x. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Khoshayand MR, Shafaghi B. The development of cross-tolerance between morphine and nicotine in mice. Eur Neuropsychopharmacol. 1999;9:227–33. doi: 10.1016/s0924-977x(98)00030-3. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Faraji N, Rostami P, Sahraei H, Ghoshouni H. Cross-tolerance between morphine and nicotine-induced conditioned place preference in mice. Pharmacol Biochem Behav. 2003;74:363–369. doi: 10.1016/s0091-3057(02)01002-x. [DOI] [PubMed] [Google Scholar]
- Zhang LC, Buccafusco JJ. Adaptive changes in M1 muscarinic receptors localized to specific rostral brain regions during and after morphine withdrawal. Neuropharmacology. 2000;39:1720–1731. doi: 10.1016/s0028-3908(00)00012-5. [DOI] [PubMed] [Google Scholar]