Abstract
Stimulus evoked neurotransmitter release requires that Na+ channel-dependent nerve terminal depolarization be transduced into synaptic vesicle exocytosis. Inhaled anesthetics block presynaptic Na+ channels and selectively inhibit glutamate over GABA release from isolated nerve terminals, indicating mechanistic differences between excitatory and inhibitory transmitter release. We compared the effects of isoflurane on depolarization-evoked [3H]glutamate and [14C]GABA release from isolated nerve terminals prepared from four regions of rat CNS evoked by 4-aminopyridine (4AP), veratridine (VTD), or elevated K+. These mechanistically distinct secretegogues distinguished between Na+ channel- and/or Ca2+ channel-mediated presynaptic effects. Isoflurane completely inhibited total 4AP-evoked glutamate release (IC50=0.42 ± 0.03 mM) more potently than GABA release (IC50=0.56 ± 0.02 mM) from cerebral cortex (1.3-fold greater potency), hippocampus and striatum, but inhibited glutamate and GABA release from spinal cord terminals equipotently. Na+ channel-specific VTD-evoked glutamate release from cortex was also significantly more sensitive to inhibition by isoflurane than was GABA release. Na+ channel-independent K+-evoked release was insensitive to isoflurane at clinical concentrations in all four regions, consistent with a target upstream of Ca2+ entry. Isoflurane inhibited Na+ channel-mediated (tetrodotoxin-sensitive) 4AP-evoked glutamate release (IC50=0.30 ± 0.03 mM) more potently than GABA release (IC50=0.67 ± 0.04 mM) from cortex (2.2-fold greater potency). The magnitude of inhibition of Na+ channel-mediated 4AP-evoked release by a single clinical concentration of isoflurane (0.35 mM) varied by region and transmitter: Inhibition of glutamate release from spinal cord was greater than from the three brain regions and greater than GABA release for each CNS region. These findings indicate that isoflurane selectively inhibits glutamate release compared to GABA release via Na+ channel-mediated transduction in the four CNS regions tested, and that differences in presynaptic Na+ channel involvement determine differences in anesthetic pharmacology.
Keywords: Na+ channels, glutamate, GABA, nerve terminal, tetrodotoxin, rat
1. Introduction
The synaptic mechanisms of general anesthetics are not clearly understood (Hemmings, 2009). Inhaled anesthetics selectively inhibit release of the excitatory transmitter glutamate compared to the inhibitory transmitter GABA from cerebral cortex nerve terminals (Westphalen and Hemmings, 2006b). Most inhaled anesthetics potentiate inhibitory transmission by potentiating postsynaptic GABAa receptors (Hemmings et al., 2005a) and depress excitatory transmission by blocking postsynaptic glutamate receptors (Franks, 2006). Thus, selective presynaptic inhibition of excitatory compared with inhibitory neurotransmitter release could provide a pivotal role in anesthetic depression of CNS activity. The relevant molecular sites of action by which anesthetics selectively inhibit neurotransmitter release have not been fully elucidated. Identification of these sites is essential in understanding the balance of anesthetic effects on excitatory and inhibitory synaptic transmission.
Neurotransmitter release requires the coordinated actions of voltage-gated Na+, K+ and Ca2+ channels, Ca2+-sensors coupled to the synaptic vesicle release machinery, and vesicle exocytosis/endocytosis mechanisms (Dittman and Ryan, 2009; Südhof, 2004). Nerve terminal-specific differences in the presynaptic mechanisms regulating transmitter release could lead to transmitter-selective effects on release. Presynaptic specializations in ion channel subtype expression (Bowman et al., 1993; Mechaly et al., 2005; Reid et al., 1997), ion channel modulation (Hemmings, 1998; MacDermott et al., 1999), excitation-exocytosis coupling (Prakriya and Mennerick, 2000), and/or synaptic vesicle fusion machinery (Herring et al., 2009; Südhof, 2004) could determine nerve terminal- and transmitter-specific pharmacological differences.
We tested the hypothesis that inhaled anesthetics affect the release of specific neurotransmitters through nerve terminal-specific presynaptic mechanisms by comparing the effects of the model inhaled anesthetic isoflurane on glutamate and GABA release from nerve terminals isolated from four neurochemically and functionally distinct regions of rat CNS. These neurochemical differences could underlie region-selective anesthetic effects on neuronal activity (Rudolph and Antkowiak, 2004; Veselis et al., 2002; White and Alkire, 2003).
2. Methods
2.1 Materials
Isoflurane was from Abbott Laboratories (North Chicago, IL). 4-Aminopyridine (4AP), veratridine (VTD) and buffer constituents were from Sigma-Aldrich Chemical Co. (St. Louis, MO). L-[3H]Glutamate (42 Ci/mmol) was from Amersham Radiochemical Centre (Buckinghamshire, UK), and [14C]GABA (0.24 Ci/mmol) was from PerkinElmer Inc. (Boston, MA) or American Radiolabel Chemicals Inc. (St. Louis, MO).
2.2 Nerve terminal preparation and neurotransmitter loading
Experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals as approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee. Synaptosomes were prepared from adult (200–300 g) male Sprague-Dawley rat (Charles River Laboratories, Troy, NY) cerebral cortex, hippocampus, striatum or spinal cord (Westphalen and Hemmings, 2006a; Westphalen et al., 2010). Demyelinated synaptosomes were incubated with 5–10 nM L-[3H]glutamate and 400–500 nM [14C]GABA for 15 min at 30°C in 10 ml Krebs-HEPES buffer (KHB, composition in mM: NaCl 140, KCl 5, HEPES 20, MgCl2 1, Na2HPO4 1.2, NaHCO3 5, EGTA 0.1, and D-glucose 10, pH 7.4 with NaOH). Synaptosomes were collected by centrifugation for 10 min at 20,000 × g at 4°C, resuspended in ice-cold 0.32 M sucrose, and loaded into release chambers.
2.3 Glutamate and GABA release
Simultaneous release of glutamate and GABA was assayed as described (Westphalen and Hemmings, 2006a) by superfusion at 0.5 ml/min with KHB plus 0.1 mM EGTA at 37°C using a customized Brandel SF12 superfusion ap paratus (Gaithersburg, MD) set to collect 1 min fractions. Stock solutions of isoflurane (~12 mM) were prepared in KHB, diluted to aqueous concentrations equivalent to 0.1 – 8 times minimum alveolar concentration (MAC = 0.35 mM for isoflurane in rat at 37°C, Taheri et al., 1991). Release experiments were performed in the absence or presence of 1.9 mM free extracellular Ca2+ (by the addition of 2 mM CaCl2). To avoid basal effects of TTX and/or high concentrations of isoflurane, anesthetic solutions ± 1 μM tetrodotoxin (TTX) were perfused for 12 min prior to, as well as during and following depolarizing pulses of secretegogues. After 2 minute pulses of 1 mM 4AP, 10 μM VTD, 15 mM KCl or 30 mM KCl (with KCl replacing equimolar NaCl in KHB) and the return to baseline release, experiments were terminated by synaptosomal lysis by perfusion with 0.2 M perchloric acid. Radioactivity in each 1 min fraction was quantified by liquid scintillation spectrometry with dual isotope quench correction (Beckman Coulter LS 6000IC, Fullerton, CA) using BioSafe II scintillation cocktail (RPI, Mt. Prospect, IL). Solutions were sampled upon exit from the release chambers into a gas-tight glass microsyringe at the end of each experiment for isoflurane quantification by gas chromatography (Ratnakumari and Hemmings, 1998).
2.3 Data analysis
Release of transmitter in each 1 min fraction of perfusate was expressed as a fraction of synaptosomal content of labeled transmitter prior to that fraction (fractional release [FR], Westphalen and Hemmings, 2003) The magnitude of evoked release was determined by subtracting baseline release (average of basal FR before and after stimulation) from cumulative FR values for each evoked release pulse (sum ΔFR). Sum ΔFR data from each experiment were normalized by the ratio of within assay control to overall mean control release in the presence of Ca2+ prior to curve fitting and/or statistical analysis. Data for inhibition of evoked release were fitted to concentration-effect curves by least-squares analysis to estimate Imax and IC50 (Prism 5.0; GraphPad Software, San Diego CA). Curve fits were tested for differences between the lower plateau of the curve (Imax) and zero sum ΔFR, and between the upper plateau of the curve (I0) and control sum ΔFR. If significant differences were not found, curve fits were performed with Imax constrained to zero sum ΔFR and I0 constrained to control, respectively.
Isoflurane effects on 4AP-evoked release in the presence of TTX were analyzed to determine the sum ΔFR value of TTX-insensitive 4AP-evoked release compared to isoflurane effects on 4AP-evoked release in the absence of TTX at equal isoflurane concentrations. Isoflurane effects on TTX-sensitive 4AP-evoked release were derived by subtraction of TTX-insensitive release values from 4AP-evoked release in the absence of TTX. Sum ΔFR values for isoflurane effects on TTX-sensitive 4AP-evoked release were fitted as above to estimate IC50.
Significant differences between IC50 values were determined by F-test comparisons between best-fit values derived from separate curve fits to values derived from global fits with the parameter shared. Control evoked release and anesthetic effects on K+-evoked glutamate and GABA release were analyzed by one-way ANOVA with Tukey post hoc test for multiple comparisons. Inhibition by isoflurane of TTX-sensitive glutamate and GABA release from the same preparations of nerve terminals were compared by paired t-tests.
3. Results
Depolarization by 4AP, VTD, or elevated K+ were used to distinguish between Na+ channel- and/or Ca2+ channel-mediated pharmacological effects on transmitter release from isolated nerve terminals (Farrag et al., 2008; Tibbs et al., 1989).
3.1 Release evoked by 4-aminopyridine
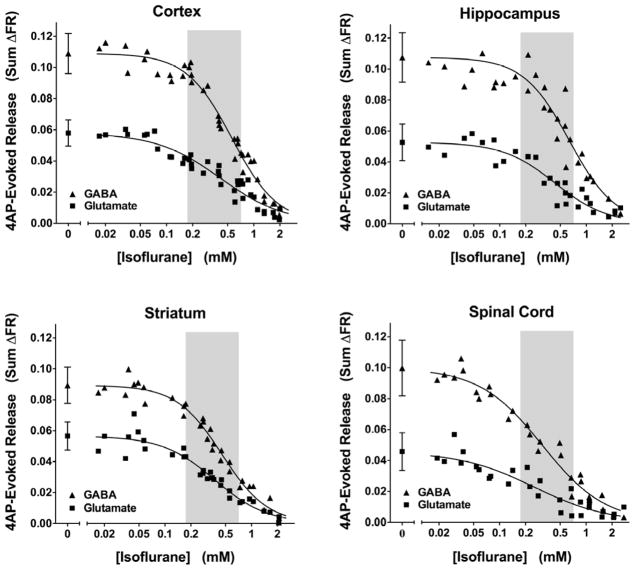
The K+ channel blocker 4AP evokes transmitter release that requires activation of both voltage-gated Na+ (Nav) and Ca2+ (Cav) channels following transient depolarizations based on its sensitivity to specific channel blockers (Tibbs et al., 1989). Stimulation of nerve terminals isolated from rat cerebral cortex, hippocampus, striatum or spinal cord with a 2 min pulse of 4AP in the presence of extracellular Ca2+ evoked release of both glutamate and GABA (Fig. 1). Fractional GABA release (sum ΔFR) was greater than glutamate release for all four CNS regions (P < 0.001). Glutamate release from cortical (n= 32), hippocampal (n=21), or striatal (n=24) nerve terminals was similar, and was greater than that from spinal cord terminals (n = 21; P < 0.001 vs. cortex). GABA release from cortical and hippocampal nerve terminals was similar, and was greater than that from striatum and spinal cord terminals (P < 0.001 vs. cortex).
Figure 1.
Inhibition by isoflurane of 4-aminopyridine-evoked transmitter release in various CNS regions. Isoflurane completely inhibited total glutamate and GABA release from rat cortical, hippocampal, striatal, and spinal cord nerve terminals (n=28–35). Data were fitted to sigmoidal concentration-effect curves (IC50 values are reported in Fig. 2). Control data (no isoflurane) are presented as mean±SD (n=7–16). Shaded areas indicate the clinical concentration range of isoflurane in rat (0.5–2 times MAC, Taheri et al., 1991)
Isoflurane completely inhibited both glutamate and GABA release evoked by 4AP from nerve terminals prepared from all four regions of the CNS (Fig. 1). These effects occurred at concentrations relevant to clinical anesthesia (Fig. 1A & 2A). The potency of isoflurane for inhibition of glutamate release was greater for spinal cord than for cortex (P=0.0009) and other brain regions (Fig. 2A). Isoflurane inhibited GABA release from striatal and spinal cord terminals more potently than from cortical and hippocampal terminals. Isoflurane inhibited glutamate release more potently than GABA release from cortical, hippocampal, and striatal nerve terminals, but inhibited glutamate and GABA release from spinal cord terminals equipotently (Fig. 2A).
Figure 2.
Isoflurane potency for inhibition of total 4-aminopyridine-evoked glutamate and GABA release from isolated nerve terminals. Data for inhibition of (A) 4AP-evoked release and (B) veratridine (VTD)-evoked release (by 10 μM or 20 μM VTD) were fitted to sigmoidal concentration-effect curves to determine IC50 values (mean ± SEM). Statistical comparisons between cerebral cortex (Cx), hippocampus (Hip), striatum (Str) and spinal cord (SC) (***P < 0.001), between glutamate and GABA release (†P < 0.05; ††P < 0.01; †††P < 0.001), and between 4AP- and VTD-evoked release from cortex (n.s.) were analyzed by comparing logIC50 values of sigmoidal curve fits by F-test.
3.2 Release evoked by veratridine
Veratridine (VTD) evokes transmitter release from isolated nerve terminals by direct interaction with TTX-sensitive Nav to impair channel inactivation resulting in sustained depolarization (Farrag et al., 2008; Ulbricht, 1998). Stimulation of cortical nerve terminals with a 2 min pulse of VTD in the presence of Ca2+ led to equivalent release (sum ΔFR) of glutamate (0.20 ± 0.01) and GABA (0.24 ± 0.01) (n=30). Isoflurane completely inhibited VTD-evoked release of both glutamate and GABA from cortical nerve terminals (data not shown) at clinically relevant concentrations (Fig. 2B); its potency was similar to that for inhibition of 4AP-evoked release (Fig. 2A). Isoflurane inhibited VTD-evoked glutamate release more potently (1.2-fold) than GABA release (Fig. 2B).
A higher concentration of VTD (20 μM) as a stronger depolarizing stimulus increased release (sum ΔFR) of glutamate (0.39 ± 0.13) and GABA (0.47 ± 0.14) from cortical nerve terminals (n=12). Isoflurane inhibited glutamate release evoked by 20 μM VTD more potently (1.3-fold) than GABA release (P=0.005) at potencies similar to those for release evoked by 10 μM VTD (Fig. 2C).
3.3 Release evoked by elevated extracellular K+ concentration
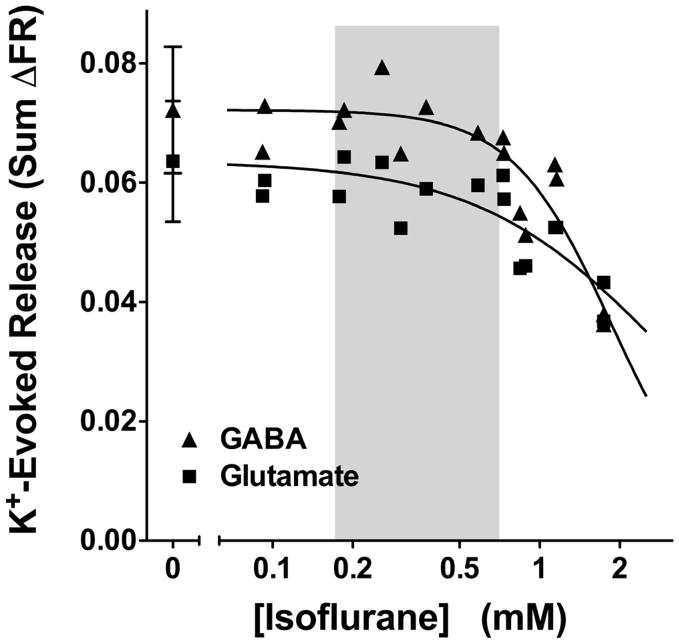
Depolarization of isolated nerve terminals by elevated extracellular K+ concentration evokes Ca2+-dependent transmitter release that requires activation of Cav but is independent of Nav activation (Tibbs et al., 1989). A 2 min pulse of 15 mM KCl in the presence of extracellular Ca2+ stimulated comparable release (sum ΔFR) of glutamate (0.058 ± 0.011) and GABA (0.069 ± 0.011; n=18) from rat cortical nerve terminals (Fig 3). K+-evoked release of glutamate or GABA from cortical nerve terminals was not inhibited by isoflurane at clinical concentrations; significant inhibition occurred only at supratherapeutic (>1 mM) concentrations (Fig. 3).
Figure 3.
Inhibition by isoflurane of elevated K+-evoked transmitter release from rat cortical nerve terminals. Isoflurane significantly inhibited glutamate and GABA release evoked by 15 mM KCl only at concentrations >2 times MAC (n=15–16). Control data are presented as mean ± SD (n=5). Shaded area indicates the clinical concentration range of isoflurane in rat (0.5–2 times MAC, Taheri et al., 1991).
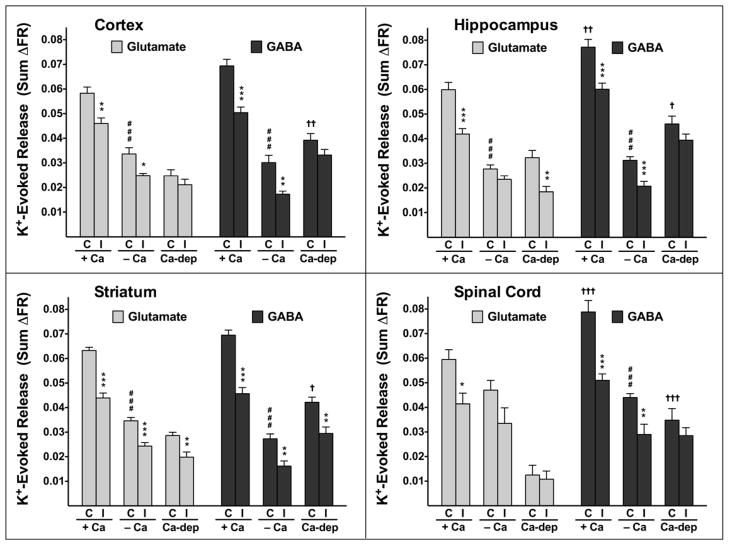
In the presence of Ca2+, K+-evoked glutamate release was equivalent to GABA release from cortical (n=18) and striatal (n=11) nerve terminals, while GABA release was greater than glutamate release from hippocampal (n=11) and spinal cord (n=14) nerve terminals (Fig. 4). Control glutamate or GABA release did not differ between any CNS region (Fig. 4). K+-evoked release of both glutamate and GABA was significantly greater in the presence (n=11–18) than in the absence (n=9–13) of Ca2+, except that glutamate release in spinal cord (n=11–13) was not Ca2+-dependent (Fig. 4). The Ca2+-dependent portion of K+-evoked glutamate or GABA release was comparable between all three brain regions. The Ca2+-dependent portion of K+-evoked GABA release was significantly higher than that of glutamate release in all CNS regions tested.
Figure 4.
Inhibition by isoflurane of elevated K+-evoked glutamate and GABA release in various CNS regions. Release was evoked by 15 mM KCl in the presence (+Ca) or absence (−Ca) of 1.9 mM free Ca2+ as indicated. Ca2+-dependent release (Ca-dep) was calculated by subtracting −Ca2+ values from +Ca2+ values. Isoflurane effects on sum ΔFR values (mean ± SEM) in the presence (1.0–1.2 ± 0.06 mM isoflurane; n=8–13) or absence (1.0–1.2 ± 0.08 mM isoflurane; n=5–6) of Ca2+ were compared to control (*P < 0.05; **P < 0.01; ***P < 0.001) by one-way ANOVA. Control glutamate and GABA sum ΔFR values (†P < 0.05; ††P < 0.01; †††P < 0.001), and control sum ΔFR values in the presence or absence of Ca2+ (###P < 0.001) were compared by one-way ANOVA.
The effects isoflurane on K+-evoked glutamate and GABA release were determined by testing a high concentration (~3 times MAC) for comparison to its effects on 4AP-evoked release. Isoflurane inhibited K+-evoked glutamate and GABA release in the presence of Ca2+ from all CNS regions tested (n=7–13). Isoflurane also inhibited K+-evoked glutamate release in the absence of Ca2+ in cortex (n=6) and striatum (n=6), but not in hippocampus (n=6) and spinal cord (n=5). Isoflurane inhibited Ca2+-dependent glutamate release in hippocampus and striatum, but not in cortex and spinal cord, and only inhibited Ca2+-dependent GABA release in striatum. Similar effects were observed for an increased depolarizing stimulus of 30 mM K+ (data not shown).
3.4 Na+ channel-mediated release
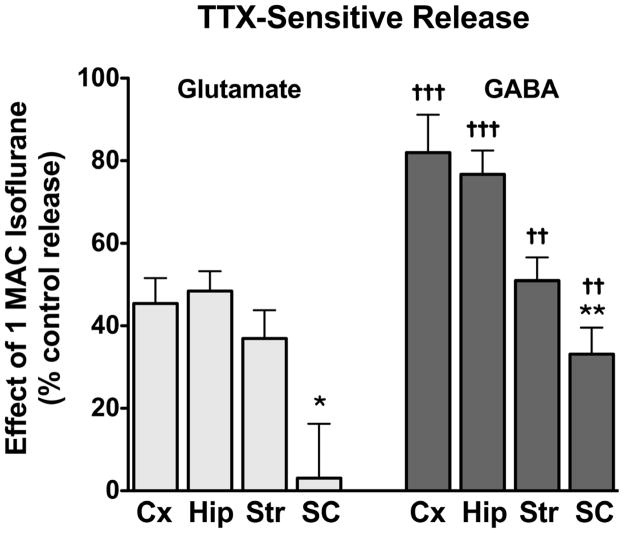
The effects of isoflurane on Na+ channel-mediated 4AP-evoked release (defined as the TTX-sensitive component of total release) were isolated using the specific Na+ channel blocker TTX (Stuart and Sakmann, 1994). In the presence of 1 μM TTX, isoflurane inhibited 4AP-evoked glutamate release (TTX-insensitive; IC50 = 0.92 ± 0.2 mM) with lower potency (p = 0.002) than total 4AP-evoked glutamate release (IC50 = 0.42 ± 0.03 mM; Fig. 5A). Isoflurane inhibited TTX-insensitive 4AP-evoked GABA release (IC50 = 0.40 ± 0.04 mM) more potently (1.3-fold) than total 4AP-evoked GABA release (IC50 = 0.56 ± 0.02 mM; Fig. 5B). The effects of isoflurane on TTX-sensitive 4AP-evoked release were determined by subtraction of TTX-insensitive 4AP-evoked release from total 4AP-evoked release to give Na+ channel-mediated release (Fig 5C). Isoflurane inhibited TTX-sensitive 4AP-evoked glutamate release from cortical nerve terminals (IC50 = 0.30 ± 0.03 mM) more potently (p = 0.003) than total 4AP-evoked glutamate release. Conversely, isoflurane inhibited TTX-sensitive 4AP-evoked GABA release from cortical nerve terminals (IC50 = 0.67 ± 0.04 mM) less potently (p = 0.011) than total 4AP-evoked GABA release. This resulted in a 2.2-fold greater potency for isoflurane inhibition of Na+ channel-mediated glutamate release than of GABA release in cortex. The effects of a clinical concentration of isoflurane (0.35 mM; 1xMAC) on Na+ channel-mediated release form each of the four CNS regions are compared in Fig. 6. Isoflurane has a significantly greater effect on spinal cord release of both glutamate and GABA, and inhibition of glutamate release was greater than of GABA release in all four regions.
Figure 5.

Inhibition by isoflurane of 4-aminopyridine-evoked glutamate and GABA release from cortical nerve terminals in the absence or presence of tetrodotoxin (TTX). Data for isoflurane inhibition of glutamate (A) and GABA (B) release evoked by 4AP alone (− TTX) or in the presence of 1 μM TTX (+ TTX; TTX-insensitive) were fitted to sigmoidal concentration-effect curves. TTX-sensitive release of glutamate and GABA (C) was derived by subtraction of the effect of each isoflurane concentration on TTX-insensitive release from that on total release evoked by 4AP alone (−TTX). Control data (−TTX) (n=16), + TTX (n=15), and the difference (n=16) are presented as mean ± SD. Statistical comparisons of isoflurane effects on evoked release between −TTX and + TTX, and between TTX-sensitive glutamate and GABA release (*P < 0.05; **P < 0.01) were analyzed by comparing logIC50 values of sigmoidal curve fits by F-test.
Figure 6.
Inhibition by isoflurane of tetrodotoxin-sensitive 4AP-evoked glutamate and GABA release. The effects of isoflurane at 1 MAC on TTX-sensitive 4AP-evoked release from cortical (Cx, 0.36 ± 0.03 mM isoflurane, n = 8), hippocampal (Hip, 0.36 ± 0.03 mM isoflurane, n = 8), striatal (Str, 0.35 ± 0.09 mM isoflurane, n = 8), and spinal cord (SC, 0.35 ± 0.04 mM isoflurane, n = 16) nerve terminals were determined by subtracting release in the presence of 1 μM TTX from release at the same concentration of isoflurane in the absence of TTX (see Methods for details). Inhibition by isoflurane of TTX-sensitive glutamate and GABA release from the same preparations of nerve terminals (mean ± SEM) were compared by paired t-tests (††P < 0.01; †††P < 0.001). Comparisons between CNS regions (***P < 0.001), (*P < 0.05; **P < 0.01) were analyzed by one-way ANOVA.
4. Discussion
Nerve terminal-specific specializations lead to regional differences in pharmacological sensitivity of neurotransmitter release (Bowman et al., 1993; Meir et al., 1999; Reid et al., 1997). We found regional neurochemical differences in the presynaptic sensitivity of neurotransmitter release to inhibition by the inhaled general anesthetic isoflurane. We also demonstrated that the greater potency of isoflurane for inhibition of glutamate release over GABA release was more marked when effects on Na+ channel-mediated transmitter release were analyzed. Together, our results suggest that the role played by Na+ channels in depolarization-evoked transmitter release varies between nerve terminals in a transmitter-specific and CNS region-specific manner. These findings support the hypothesis that neurochemical specializations result in differential pharmacological sensitivities of glutamate and GABA release between CNS regions relevant to presynaptic anesthetic actions.
Since evidence supports region-specific anesthetic effects on CNS function (Rudolph and Antkowiak, 2004), we compared anesthetic effects on nerve terminals isolated from the functionally and neurochemically distinct CNS regions cerebral cortex, hippocampus, striatum and spinal cord. Depolarization-evoked glutamate release from rat cortical nerve terminals is ~95% Na+ channel- and extracellular Ca2+-dependent, while GABA release is only ~70% Na+ channel- and Ca2+-dependent (Westphalen and Hemmings, 2006b; Westphalen et al., 2010). The greater involvement of voltage-gated Na+ channels (Nav) in glutamate release (Westphalen et al., 2010) and the greater potency of isoflurane for inhibition of glutamate than GABA release (Westphalen and Hemmings, 2003, 2006b) led us to investigate possible differences in anesthetic sensitivity between these transmitters across CNS regions.
Like cerebral cortex, hippocampus and striatum receive extensive glutamatergic innervation, but they differ in nerve terminal morphology, modulation by presynaptic receptors, and other presynaptic specializations (MacDermott et al., 1999) that might affect sensitivity to anesthetics. The hippocampus is critically involved in learning and memory processes (Lisman and Grace, 2005), and has been proposed as an important locus for anesthetic action (Pearce et al., 1989; Westphalen et al., 2009). Modulation of specific glutamatergic and dopaminergic signaling pathways supports anesthetic effects on transmitter release in mouse striatum in vivo (Snyder et al., 2007). The spinal cord is the principal locus for the immobilizing actions of inhaled anesthetics (determined as MAC), which likely involve depression of spinal glutamatergic transmission (Collins et al., 1995; Sonner et al., 2003).
Release of glutamate and GABA evoked by 4AP or VTD, which involve Nav and Cav activation, were similarly sensitive to inhibition by isoflurane, and much more sensitive than release evoked by elevated K+, which requires Cav but not Nav activation (Tibbs et al., 1989). The relatively small but significant effects of 1.0–1.2 mM isoflurane on Ca2+-independent K+-evoked glutamate and GABA release are similar to effects observed with tetrodotoxin (Westphalen and Hemmings, 2003b), consistent with Na+ channel-mediated mechanisms. Such supra-clinical concentrations of isoflurane can also inhibit glutamate and GABA transporters (Westphalen and Hemmings, 2003a), which might contribute by reducing Ca2+-independent reverse transport (Lester, 1994). Relative insensitivity of isoflurane to the Na+ channel-independent secretegogue indicates that targets upstream of Cav activation, including but not limited to Nav inhibition, are critical to the presynaptic actions of inhaled anesthetics in all four CNS regions studied. This conclusion is supported by electrophysiological evidence showing greater anesthetic sensitivity of Nav than Cav (Hemmings, 2009). Additional support for presynaptic anesthetic actions upstream of Ca2+ entry has been reported for several preparations by multiple independent methods (Lingamaneni et al., 2001) (Wu et al., 2004).
Transmitter-specific differences in the Cav subtypes coupled to transmitter release have been reported (Bowman et al., 1993; Meir et al., 1999; Reid et al., 1997), but it is unlikely that these differences have a major impact given the relatively minor contribution of presynaptic Cav to anesthetic effects. The major Nav subtypes examined to date are all inhibited by inhaled anesthetics, but there are subtype- and agent-specific differences in the degree and voltage-dependence of Nav inhibition (Herold et al., 2009; Ouyang et al., 2009). We recently identified regional- and/or transmitter-specific differences in the sensitivity of glutamate and GABA release to the specific Na+ channel blocker TTX, and differences in the expression of various Nav subtypes in nerve terminal preparations of cortex, hippocampus, striatum and spinal cord determined by immunoblotting (Westphalen et al., 2010). The potency of TTX for inhibition of 4AP-evoked release between CNS regions correlated with the expression of total Nav1 (all subtypes) and specifically of the Nav1.2 subtype for glutamate release but not for GABA release. This suggests a dominant role for Nav in glutamate compared to GABA release, which is supported by greater efficacy for TTX inhibition of glutamate release compared to GABA release in cortex, hippocampus and striatum (Westphalen et al., 2010). Regional and transmitter-specific variation in Nav expression is thus a plausible mechanism for region- and transmitter-specific differences in isoflurane potency for inhibition of transmitter release.
Pharmacological effects mediated by TTX-sensitive (TTX-s) and TTX-resistant (TTX-r) Nav subtypes can be differentiated by comparing depolarization by 4AP, which indirectly activates both TTX-s and TTX-r channels, to depolarization by VTD, which selectively activates TTX-s channels (Farrag et al., 2008). Isoflurane preferentially inhibited glutamate compared to GABA release evoked by either 4AP or VTD such that the observed differences in potencies are not secretegogue-dependent, and thus probably do not involve a major contribution from TTX-r channels. In contrast, TTX inhibits 4AP-evoked glutamate release less potently than GABA release from spinal cord nerve terminals, where TTX-r Nav are functionally expressed in glutamatergic, but not GABAergic terminals in spinal cord (Westphalen et al., 2010). The sensitivity to TTX of 4AP-evoked, but not of VTD-evoked (TTX-s specific), glutamate release varied between CNS regions, consistent with TTX-r Nav involvement in glutamatergic spinal cord nerve terminals (Westphalen et al., 2010). Greater anesthetic potency for inhibition of Na+ channel-mediated glutamate release in spinal cord is consistent with a role for this action in immobility.
Other ion channel differences could also contribute to presynaptic differences in anesthetic effects. Knockout of the TREK-1 two-pore domain K+ channel, which reduces the potency of halothane in vivo, reduces the potency of halothane as an inhibitor of glutamate release, and to a lesser extent of GABA release, in mouse cerebral cortex (Westphalen et al., 2007). The role of anesthetic modulation of other K+ channels (e.g. TASK), metabotropic glutamate, GABAA, GABAB, and/or NMDA receptors affected by anesthetics (Carlen et al.; Ishizaki et al., 1999; Lazarenko et al., 2010; Nishikawa and MacIver, 2000) in modulating transmitter release is unknown.
TTX-insensitive 4AP-evoked GABA release, which is inhibitable by isoflurane (Westphalen and Hemmings, 2006b), could contribute to the transmitter-selective effect of isoflurane on total release since, in contrast to GABA release, no residual Ca2+-dependent 4AP-evoked glutamate release is detectable in the presence of TTX (Westphalen and Hemmings, 2003; Westphalen et al., 2010). TTX-insensitive 4AP-evoked GABA release in cortex was inhibited by isoflurane more potently than total 4AP-evoked GABA release, an effect not attributable to TTX-r channels because these were identified only in glutamatergic terminals of spinal cord (Westphalen et al., 2010). Moreover, TTX-insensitive glutamate release was inhibited by isoflurane less potently than total 4AP-evoked glutamate release. These findings suggest that the potency of isoflurane for inhibition of TTX-s Nav mediated release is greater for glutamate and less for GABA than reported previously (Westphalen and Hemmings, 2006b). Isoflurane selectivity for inhibition of glutamate release was more apparent when the effects on TTX-insensitive release were eliminated to reveal effects on TTX-sensitive (Na+ channel-mediated) release. This indicates that Nav block is critical to selective inhibition of glutamate release by isoflurane. Differences in presynaptic pharmacology have also been reported for other Nav blockers such as lamotrigine (Ahmad et al., 2004), phenytoin (Ahmad et al., 2005), and riluzole (Prakriya and Mennerick, 2000), drugs known to block Nav (Obrenovitch and Urenjak, 1998) and selectively inhibit glutamate release (Sitges et al., 2007; Westphalen and Hemmings, 2003). These similarities suggest overlap in the mechanisms of multiple distinct drug classes known to depress CNS function.
Release evoked by VTD is completely TTX-sensitive (Westphalen et al., 2010), confirming an absolute requirement for activation of presynaptic Na+ channels in release evoked by this secretegogue. The similar inhibition potencies of isoflurane at two concentrations of VTD support a non-competitive mechanism, which suggests that inhibition does not involve direct competition at the VTD binding site. Although both VTD-evoked and TTX-sensitive 4AP-evoked release are Nav specific, the incomplete Ca2+ dependence of VTD-evoked release (Bicalho et al., 2002) makes it an inferior model of depolarization-evoked transmitter release. On the other hand, 4AP induces transmitter release by mimicking action potential-evoked depolarizations (Tibbs et al., 1989), such that 4AP-evoked release models Nav-dependent transmitter release.
This study focused on the release of glutamate and GABA, the most abundant excitatory and inhibitory CNS transmitters in the CNS, respectively (Krnjević, 2009). It is likely that regional differences in anesthetic sensitivity also exist for other transmitters based on their distinct presynaptic regulatory mechanisms (Snyder et al., 2007; Verhage et al., 1992). This is exemplified by the more potent inhibition by isoflurane of acetylcholine-evoked norepinephrine release from hippocampal nerve terminals mediated by presynaptic nicotinic acetylcholine receptors (Westphalen et al., 2009), and by the lesser anesthetic sensitivity of neuropeptide transmitter release from large dense core vesicles (Pashkov and Hemmings, 2002).
Our findings indicate that selective effects of isoflurane on transmitter release occur as a result of Nav inhibition due to transmitter- and region-dependent differences in the Nav dependence of release. Nerve terminal-specific anesthetic effects on transmitter release could underlie differences in anesthetic actions observed in functional neuroimaging studies of regional brain activity (Alkire, 2008; Veselis et al., 2002) and neurophysiological studies of synaptic transmission (Pittson et al., 2004). Differences in presynaptic anesthetic effects on neurotransmitter release likely reflect molecular specializations of nerve terminals due to differential expression of presynaptic anesthetic targets (Franks, 2006; Hemmings et al., 2005a; Rudolph and Antkowiak, 2004). Preferential inhibition of glutamate over GABA release by inhaled anesthetics in the CNS should potentiate postsynaptic depression of excitatory neurotransmission by glutamate receptor blockade (Franks, 2006) to produce CNS depression. An important goal in understanding the synaptic effects of general anesthetics is to resolve their effects on these specific presynaptic targets. Further progress in identifying nerve terminal-specific anesthetic actions will require higher resolution techniques that allow molecular phenotyping and analysis of specific neuronal populations (Hemmings et al., 2005b).
Research Highlights.
The effects of isoflurane on transmitter release were pharmacologically isolated.
Selective inhibition of glutamate release involved differential Na+ channel inhibition.
Glutamate release was inhibited more than GABA release across multiple CNS regions.
Inhibition potency was greatest in spinal cord, a region involved in immobilization.
Acknowledgments
Supported by National Institutes of Health grant GM 58055.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad S, Fowler LJ, Whitton PS. Effects of acute and chronic lamotrigine treatment on basal and stimulated extracellular amino acids in the hippocampus of freely moving rats. Brain Res. 2004;1029:41–47. doi: 10.1016/j.brainres.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Fowler LJ, Whitton PS. Lamotrigine, carbamazepine and phenytoin differentially alter extracellular levels of 5-hydroxytryptamine, dopamine and amino acids. Epilepsy Res. 2005;63:141–149. doi: 10.1016/j.eplepsyres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Alkire MT. Probing the mind: anesthesia and neuroimaging. Clin Pharmacol Ther. 2008;84:149–152. doi: 10.1038/clpt.2008.75. [DOI] [PubMed] [Google Scholar]
- Bicalho AF, Guatimosim C, Prado MA, Gomez MV, Romano-Silva MA. Investigation of the modulation of glutamate release by sodium channels using neurotoxins. Neuroscience. 2002;113:115–123. doi: 10.1016/s0306-4522(02)00139-2. [DOI] [PubMed] [Google Scholar]
- Bowman D, Alexander S, Lodge D. Pharmacological characterisation of the calcium channels coupled to the plateau phase of KCl-induced intracellular free Ca2+ elevation in chicken and rat synaptosomes. Neuropharmacology. 1993;32:1195–1202. doi: 10.1016/0028-3908(93)90013-s. [DOI] [PubMed] [Google Scholar]
- Carlen PL, Gurevich N, Davies MF, Blaxter TJ, O’Beirne M. Enhanced neuronal K+ conductance: a possible common mechanism for sedative-hypnotic drug action. Can J Physiol Pharmacol. 63:831–837. doi: 10.1139/y85-137. [DOI] [PubMed] [Google Scholar]
- Collins JG, Kendig JJ, Mason P. Anesthetic actions within the spinal cord: contributions to the state of general anesthesia. Trends Neurosci. 1995;18:549–553. doi: 10.1016/0166-2236(95)98377-b. [DOI] [PubMed] [Google Scholar]
- Dittman J, Ryan TA. Molecular circuitry of endocytosis at nerve terminals. Annu Rev Cell Dev Biol. 2009;25:133–160. doi: 10.1146/annurev.cellbio.042308.113302. [DOI] [PubMed] [Google Scholar]
- Farrag KJ, Bhattacharjee A, Docherty RJ. A comparison of the effects of veratridine on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in isolated rat dorsal root ganglion neurons. Pflugers Arch. 2008;455:929–938. doi: 10.1007/s00424-007-0365-5. [DOI] [PubMed] [Google Scholar]
- Franks NP. Molecular targets underlying general anaesthesia. Br J Pharmacol. 2006;147:S72–S81. doi: 10.1038/sj.bjp.0706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005a;26:503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Yan W, Westphalen RI, Ryan TA. The general anesthetic isoflurane depresses synaptic vesicle exocytosis. Mol Pharmacol. 2005b;67:1591–1599. doi: 10.1124/mol.104.003210. [DOI] [PubMed] [Google Scholar]
- Hemmings HCJ. General anesthetic effects on protein kinase C. Toxicol Lett. 1998;100–101:89–95. doi: 10.1016/s0378-4274(98)00170-2. [DOI] [PubMed] [Google Scholar]
- Hemmings HJ. Sodium channels and the synaptic mechanisms of inhaled anaesthetics. Br J Anaesth. 2009;103:61–69. doi: 10.1093/bja/aep144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KF, Nau C, Ouyang W, Hemmings HCJ. Isoflurane inhibits the tetrodotoxin-resistant voltage-gated sodium channel Nav1.8. Anesthesiology. 2009 doi: 10.1097/ALN.0b013e3181af64d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BE, Xie Z, Marks J, Fox AP. Isoflurane inhibits the neurotransmitter release machinery. J Neurophysiol. 2009;102:1265–1273. doi: 10.1152/jn.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki K, Sasaki M, Karasawa S, Obata H, Nara T, FG Intrathecal co-administration of NMDA antagonist and NK-1 antagonist reduces MAC of isoflurane in rats. Acta Anaesthesiol Scand. 1999;43:753–759. doi: 10.1034/j.1399-6576.1999.430711.x. [DOI] [PubMed] [Google Scholar]
- Krnjević K. When and why amino acids? J Physiol. 2009;588:33–44. doi: 10.1113/jphysiol.2009.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko RM, Willcox SC, Shu S, Berg AP, Jevtovic-Todorovic V, Talley EM, Chen X, Bayliss DA. Motoneuronal TASK channels contribute to immobilizing effects of inhalational general anesthetics. J Neurosci. 2010;30:7691–7704. doi: 10.1523/JNEUROSCI.1655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingamaneni R, Birch ML, Hemmings HC., Jr Widespread inhibition of sodium channel-dependent glutamate release from isolated nerve terminals by isoflurane and propofol. Anesthesiology. 2001;95:1460–1466. doi: 10.1097/00000542-200112000-00027. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- Mechaly I, Scamps F, Chabbert C, Sans A, Valmier J. Molecular diversity of voltage-gated sodium channel alpha subunits expressed in neuronal and non-neuronal excitable cells. Neuroscience. 2005;130:389–396. doi: 10.1016/j.neuroscience.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Meir A, Ginsburg S, Butkevich A, Kachalsky SG, Kaiserman I, Ahdut R, Demirgoren S, Rahamimoff R. Ion channels in presynaptic nerve terminals and control of transmitter release. Physiol Rev. 1999;79:1019–1088. doi: 10.1152/physrev.1999.79.3.1019. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, MacIver MB. Excitatory synaptic transmission mediated by NMDA receptors is more sensitive to isoflurane than are non-NMDA receptor-mediated responses. Anesthesiology. 2000;92:228–236. doi: 10.1097/00000542-200001000-00035. [DOI] [PubMed] [Google Scholar]
- Obrenovitch TP, Urenjak J. Glutamate release inhibitors: a critical assessment of their action mechanism. Amino Acids. 1998;14:143–150. doi: 10.1007/BF01345255. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Herold KF, Hemmings HCJ. Comparative effects of halogenated inhaled anesthetics on voltage-gated Na+ channel function. Anesthesiology. 2009;110:582–590. doi: 10.1097/ALN.0b013e318197941e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashkov VN, Hemmings HC., Jr The effects of general anesthetics on norepinephrine release from isolated rat cortical nerve terminals. Anesth Analg. 2002;95:1274–1281. doi: 10.1097/00000539-200211000-00032. [DOI] [PubMed] [Google Scholar]
- Pearce RA, Stringer JL, Lothman EW. Effect of volatile anesthetics on synaptic transmission in the rat hippocampus. Anesthesiology. 1989;71:591–598. doi: 10.1097/00000542-198910000-00019. [DOI] [PubMed] [Google Scholar]
- Pittson S, Himmel AM, MacIver MB. Multiple synaptic and membrane sites of anesthetic action in the CA1 region of rat hippocampal slices. BMC Neurosci. 2004;3:52–61. doi: 10.1186/1471-2202-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Mennerick S. Selective depression of low-release probability excitatory synapses by sodium channel blockers. Neuron. 2000;26:671–682. doi: 10.1016/s0896-6273(00)81203-9. [DOI] [PubMed] [Google Scholar]
- Ratnakumari L, Hemmings HC., Jr Inhibition of presynaptic sodium channels by halothane. Anesthesiology. 1998;88:1043–1054. doi: 10.1097/00000542-199804000-00025. [DOI] [PubMed] [Google Scholar]
- Reid CA, Clements JD, Bekkers JM. Nonuniform distribution of Ca2+ channel subtypes on presynaptic terminals of excitatory synapses in hippocampal cultures. J Neurosci. 1997;17:2738–2745. doi: 10.1523/JNEUROSCI.17-08-02738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- Sitges M, Guarneros A, Nekrassov V. Effects of carbamazepine, phenytoin, valproic acid, oxcarbazepine, lamotrigine, topiramate and vinpocetine on the presynaptic Ca2+ channel-mediated release of [3H]glutamate: comparison with the Na+ channel-mediated release. Neuropharmacology. 2007;53:854–862. doi: 10.1016/j.neuropharm.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Galdi S, Hendrick JP, Hemmings HJ. General anesthetics selectively modulate glutamatergic and dopaminergic signaling via site-specific phosphorylation in vivo. Neuropharmacology. 2007;53:619–630. doi: 10.1016/j.neuropharm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Sonner JM, Zhang Y, Stabernack C, Abaigar W, Xing Y, Laster MJ. GABA(A) receptor blockade antagonizes the immobilizing action of propofol but not ketamine or isoflurane in a dose-related manner. Anesth Analg. 2003;96:706–712. doi: 10.1213/01.ANE.0000048821.23225.3A. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Taheri S, Halsey MJ, Liu J, Eger EI, 2nd, Koblin DD, Laster MJ. What solvent best represents the site of action on inhaled anesthetics in humans, rats, and dogs. Anesth Analg. 1991;72:627–634. doi: 10.1213/00000539-199105000-00010. [DOI] [PubMed] [Google Scholar]
- Tibbs GR, Barrie AP, Van Mieghem FJE, McMahon HT, Nicholls DG. Repetitive action potentials in isolated nerve terminals in the presence of 4-aminopyridine: Effects on cytosolic free Ca2+ and glutamate release. J Neurochem. 1989;53:1693–1699. doi: 10.1111/j.1471-4159.1989.tb09232.x. [DOI] [PubMed] [Google Scholar]
- Ulbricht W. Effects of veratridine on sodium currents and fluxes. Rev Physiol Biochem Pharmacol. 1998;133:1–54. doi: 10.1007/BFb0000612. [DOI] [PubMed] [Google Scholar]
- Verhage M, Sandman H, Mosselveld F, van de Velde M, Hengst PA, Lopes da Silva FH, Ghijsen EJM. Perfusion of immobilized isolated nerve terminals as a model for the regulation of transmitter release: Release of different, endogenous transmitters, repeated stimulation, and high time resolution. J Neurochem. 1992;58:1313–1320. doi: 10.1111/j.1471-4159.1992.tb11344.x. [DOI] [PubMed] [Google Scholar]
- Veselis RA, Reinsel RA, Feshchenko VA, Dnistrian AM. A neuroanatomical construct for the amnesic effects of propofol. Anesthesiology. 2002;97:329–337. doi: 10.1097/00000542-200208000-00008. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Gomez RS, Hemmings HJ. Nicotinic receptor-evoked hippocampal norepinephrine release is highly sensitive to inhibition by isoflurane. Br J Anaesth. 2009;102:355–360. doi: 10.1093/bja/aen387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr Selective depression by general anesthetics of glutamate versus GABA release from isolated cortical nerve terminals. J Pharmacol Exp Ther. 2003;304:1188–1196. doi: 10.1124/jpet.102.044685. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: basal release. J Pharmacol Exp Ther. 2006a;316:208–215. doi: 10.1124/jpet.105.090647. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: four-aminopyridine evoked release. J Pharmacol Exp Ther. 2006b;316:216–223. doi: 10.1124/jpet.105.090662. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Krivitski M, Amarosa A, Guy N, Hemmings H., Jr Reduced inhibition of cortical glutamate and GABA release by halothane in mice lacking the K+ channel, TREK-1. Br J Pharmacol. 2007;152:939–945. doi: 10.1038/sj.bjp.0707450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen RI, Yu J, Krivitski M, Jih TY, Hemmings HCJ. Regional differences in nerve terminal Na+ channel subtype expression and Na+ channel-dependent glutamate and GABA release in rat central nervous system. J Neurochem. 2010;113:1611–1620. doi: 10.1111/j.1471-4159.2010.06722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. Neuroimage. 2003;19:402–411. doi: 10.1016/s1053-8119(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Wu XS, Sun JY, Evers AS, Crowder M, Wu LG. Isoflurane inhibits transmitter release and the presynaptic action potential. Anesthesiology. 2004;100:663–670. doi: 10.1097/00000542-200403000-00029. [DOI] [PubMed] [Google Scholar]