Abstract
In two experiments, the ability to use multisensory information (haptic information, provided by lightly touching a stationary surface, and vision) for quiet standing was examined in typically developing (TD) children, adults, and in 7-year-old children with Developmental Coordination Disorder (DCD). Four sensory conditions (no touch/no vision, with touch/no vision, no touch/with vision, and with touch/with vision) were employed. In experiment 1, we tested 4-, 6- and 8-year-old TD children and adults to provide a developmental landscape for performance on this task. In experiment 2, we tested a group of 7-year-old children with DCD and their age-matched TD peers. For all groups, touch robustly attenuated standing sway suggesting that children as young as 4 years old use touch information similarly to adults. Touch was less effective in children with DCD compared to their TD peers, especially in attenuating their sway velocity. Children with DCD, unlike their TD peers, also benefited from using vision to reduce sway. The present results suggest that children with DCD benefit from using vision in combination with touch information for standing control possibly due to their less well developed internal models of body orientation and self-motion. Internal model deficits, combined with other known deficits such as postural muscles activation timing deficits, may exacerbate the balance impairment in children with DCD.
Keywords: Developmental Coordination Disorder (DCD), Standing Balance Multisensory, Light Touch, Vision
Introduction
Approximately 6% of school-aged children are affected by Developmental Coordination Disorder (DCD), which interferes with their activities of daily living due to motor dyscoordination1, including compromised standing balance2-5. Here we focus on whether multisensory integration deficits play a role in the poor standing balance of children with DCD. Combining information from multiple sensory modalities is critical for balance control6,7 and development8, and deficits in multisensory integration have been observed in a hand target matching task in children with DCD9,10. However, few studies have investigated whether deficits in multisensory integration may contribute to poor standing balance in children with DCD4,5. Here we address this issue by experimentally manipulating stationary light touch and vision, a protocol commonly used in balance studies6,7,11 that has never been investigated in children with DCD.
Providing light touch through contact of the fingertip to a stationary surface has proven to be a powerful sensory input that stabilizes standing in adults12 and infants13,14. While light touch is not the natural condition for standing, individuals with normal balance seek light touch information under reduced sensory conditions and those with poor balance benefit from touch, an enriched sensory information. Moreover, an advantage of using light touch contact experimentally is that, like vision, it is easily manipulated (i.e., it is easy to add and remove), making it possible to precisely vary vision and touch relative to one another and study their interaction in balance.
With adults, investigations of multisensory processing have shown that stationary touch and vision provide equivalent information for standing control11. However, while touch effectively attenuates sway in infants13,14, vision does not always reduce sway15,16. This suggests that the use of sensory modalities for standing control develops differentially and may influence how they combine for estimation of self-motion. There is no existing study that concurrently manipulates the availability of stationary touch and vision in children (TD or with DCD). We speculate that known deficits in multisensory integration as found in hand target matching9,10 may negatively affect standing control in children with DCD.
To investigate these issues, we conducted two experiments in which we systematically manipulated the availability of touch and vision (no touch/no vision, with touch/no vision, no touch/with vision, and with touch/with vision). Experiment 1 compared 4-, 6- and 8-year-old TD children and adults to provide a developmental landscape for this task. Experiment 2 compared 7-year-old children with DCD to their age-matched TD peers. The purpose of these experiments was two-fold: First, to describe how stationary touch and vision individually and in combination contribute to balance control in young children compared to adults; and, second, to answer how they contribute to balance control in children with DCD compared to their TD peers.
Methods
Subjects
In experiment 1, we recruited male subjects from four age groups: 4-year-old (eight subjects, 4.1 ± 0.3 years), 6-year-old (nine subjects, 6.1 ± 0.4 years), 8-year-old (nine subjects, 8.1 ± 0.3 years) TD children and adults (nine subjects, 22.8 ± 2.3 years). In experiment 2, twenty-five children with parent-reported movement dyscoordination were recruited and screened. We included eleven (eight boys, three girls; age 7.2 ± 0.5 years) children who were diagnosed with DCD by two independent tests: 1) a developmental pediatrician’s diagnosis; and, 2) the Movement Assessment Battery for Children (MABC)17 with a score at or below the 10th percentile (see Table 1). A group of TD children not tested in experiment 1 with the MABC above 35th percentile were recruited (six boys, four girls; age 7.3 ± 0.7 years). All control subjects in both experiments reported no known problems that might affect their balance. Children’s parents and adult subjects gave written informed consent according to procedures approved by the Institutional Review Board at the University of Maryland.
Table 1.
Sex, test age and MABC performance for children with DCD and TD children
| DCD | Impairment score | TD | Impairment score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Sex | Test age (year) |
Total | Balance | %ile | ID | Sex | Test age (year) |
Total | Balance | %ile |
| 1 | M | 6.2 | 20.0 | 11.0 | 1 | 1 | M | 6.0 | 6.0 | 1.5 | 36 |
| 2 | M | 6.7 | 15.5 | 4.5 | 3 | 2 | M | 6.8 | 0.0 | 0.0 | 96 |
| 3 | M | 7.0 | 20.5 | 9.5 | <1 | 3 | M | 7.3 | 0.0 | 0.0 | 96 |
| 4 | M | 7.2 | 28.0 | 7.5 | <1 | 4 | M | 7.6 | 4.0 | 0.0 | 54 |
| 5 | M | 7.6 | 23.0 | 4.0 | <1 | 5 | M | 8.0 | 5.0 | 0.0 | 45 |
| 6 | M | 7.6 | 13.0 | 4.0 | 6 | 6 | M | 8.4 | 4.5 | 0.0 | 49 |
| 7 | M | 7.6 | 13.0 | 4.5 | 6 | 7 | F | 6.4 | 6.0 | 0.0 | 39 |
| 8 | M | 8.0 | 40.0 | 15.0 | <1 | 8 | F | 7.1 | 5.0 | 0.0 | 45 |
| 9 | F | 6.7 | 25.5 | 11.5 | <1 | 9 | F | 7.2 | 5.5 | 0.0 | 40 |
| 10 | F | 7.1 | 16.5 | 2.0 | 2 | 10 | F | 7.8 | 5.0 | 0.0 | 45 |
| 11 | F | 7.2 | 16.0 | 1.0 | 2 | ||||||
|
| |||||||||||
| Mean | 7.2 | 21.0 | 6.8 | 7.3 | 4.1 | 0.2 | |||||
| SD | 0.5 | 8.0 | 4.5 | 0.7 | 2.3 | 0.5 | |||||
A high impairment score in the total (max. = 40) and the Balance (max. = 15) sub-section reflects poor motor ability. The percentile (%ile) refers to the percentile ranking derived from the MABC scoring of the overall impairment score.
Testing procedures and protocol
Participants stood in a room (1.5 m × 1.5 m) formed by black curtains. A triangular ultrasonic receiver sampled at 50Hz with 0.01 cm resolution (7 cm equilateral triangle, Logitech, Inc.) was affixed near the approximate center of mass (COM). Subjects stood with feet parallel and slightly separated (≈ 2 cm apart) in experiment 1 and in modified tandem stance (inner edges of two feet align in sagittal plane) in experiment 2. Foot position was traced on the standing surface to ensure a similar stance across trials. Although stances were different in these two experiments, they were both narrower than a normal shoulder-width stance in order to enhance medio-lateral (ML) sway8,12. We chose a slightly wider stance (≈ 2 cm apart) in experiment 1 to avoid excessive failure in testing 4-year-old children.
Instructions were to stand quietly and to follow specific directions for each condition. In conditions with touch, subjects were instructed to use their index finger to lightly touch a stationary surface at a fixed point without moving the finger on the surface and without triggering an alarm (threshold: 1 Newton vertical force). The touch device was placed at the subject’s right side in the frontal plane at hip height with subject’s elbow flexed at 165°. In conditions without touch, the subject’s arms hung freely alongside the body. In conditions with vision, subjects looked at a 12 cm × 12 cm eye-level target 1.2 m in front of them. In conditions without vision, subjects closed their eyes throughout the trial. Experiments were conducted with conditions randomized within each block. A practice trial was given for each condition. Video and touch force monitoring were used and a research assistant behind the child monitored for compliance with the instructions that the finger did not move on the touch device. The trial was stopped if the child failed to follow instructions. Only a few children needed to repeat one or two trials and no participants fell during the test. There were two 25-second trials for experiment 1 and four 60-second trials for experiment 2 for each condition. Trial number and duration were shorter in experiment 1 to avoid excessive failure in testing 4-year-old children. A brief rest was given between trials. A custom LabView™ program was used to collect kinematic data and touch force sampled at 50 Hz. Children received prizes and monetary compensation for their participation.
Analysis
Sway measures
Kinematic data of the approximate COM were analyzed in the medio-lateral (ML), anterio-posterior (AP) direction and in the horizontal plane. A 4th-order, 5-Hz low pass Butterworth filter with mean subtraction was used for data preprocessing. Two sway measures for each direction and horizontal plane were calculated for each trial according to Prieto18. The formulas for the ML measures were:
-
The mean absolute value of velocity.
This measure was chosen for its importance in balance control19 and its sensitivity to differentiate vision effects in standing between different populations (e.g., young adults and elderly)18. It is referred to as velocity hereafter.
-
The variance of the kinematic trajectory.
This measure was chosen as a general index for balance control14 as various models have predicted increased variance when sensory information was removed7. It is referred to as variance hereafter.
ML[n]: Medio-lateral kinematic data of approximate COM at nth data point.
: Mean medio-lateral kinematic trajectory value of approximate COM.
N: Number of total data points.
Measures in AP direction were calculated in a similar way to ML and measures in the horizontal plane were calculated from their ML and AP components. The average of each measure from all trials of the same condition was used for statistical analysis.
Statistical analysis
Each measure was log-transformed and analyzed using a mixed model (SAS version 9.1) with Group as a non-repeated factor and Touch and Vision as repeated factors. The model specifications were: unstructured covariance, unequal group variance, Kenward-Roger adjustment for reducing small-sample bias, and Bonferroni adjustment for multiple pairwise comparisons. Main effects, 2-, and 3-way interactions were tested. Significance level was set at p<0.05 and marginal significance at p<0.1. Note that the main touch effect (i.e., the difference between two log transformed data) corresponds to the ratio between the raw “Touch to No Touch” sway measure. Similarly, the main vision effect corresponds to the ratio between the raw “Vision to No Vision” sway measures.
Results
Regarding the direction/plane of analysis, the main touch effect was consistently significant in the ML direction and the horizontal plane but not in the AP direction. We concluded that the main touch effect in the horizontal plane was mainly due to the effect in the ML direction. This result was consistent with a previous finding that the touch effect in reducing sway was maximized when the touch surface was placed in the unstable plane of standing20. In both of our experiments, the ML direction was the unstable plane due to the narrower-than-normal stance. With the touch surface placed in the frontal plane, the main touch effect was significant in the ML direction and not in the AP direction as shown in previous studies6-8,12. Thus, we chose to present our results only from the ML direction because our testing protocol rendered it most sensitive in detecting sensory effects on balance control. This decision was further supported by the fact that all statistical results involving the touch effect were the same for the ML direction and the horizontal plane with the exception that the only significant 3-way interaction, Group × Touch × Vision, was found in the ML direction.
Experiment 1: TD children and adults
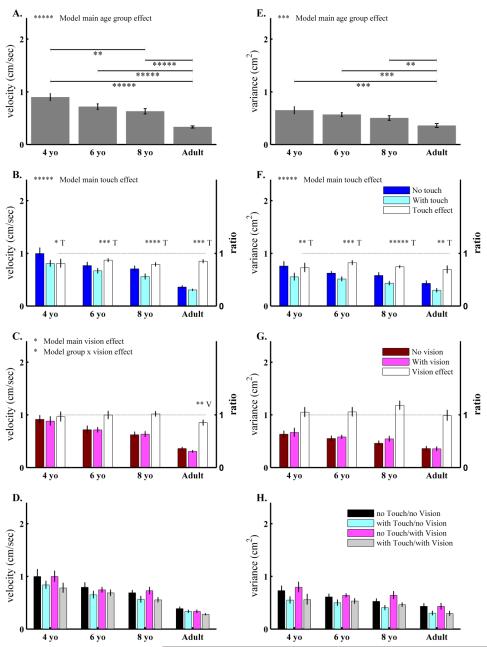
The main age group effect was significant for velocity (Fig.1A) and variance (Fig.1E). Post-hoc analysis between any two groups showed: 1) adults swayed with lower velocity and less variance than the 4-, 6- and 8-year-old groups; and, 2) among the three groups of children, only 4-year-old children swayed with higher velocity than 8-year-old children. Overall, the post-hoc analysis showed an age-related trend for improved standing balance from childhood to adulthood.
Figure 1.
Results from experiment 1 for the three groups of typically developing (TD) children (4-, 6- and 8-year-old) and adults. Two sway measures in medio-lateral (ML) direction: sway velocity (1st column: A-D), and distance variance (2nd column: E-H) are plotted for age groups (1st row: A, E), by touch availability (2nd row: B, F), by vision availability (3rd row: C, G) and by the four sensory conditions (4th row: D, H). All bars are plotted as Mean ± Standard Error. For each subplot, left y-axis shows the unit of the sway measure. For subplots B and F, the second right y-axis is the ratio of sway measure of “With touch” to “No touch” conditions. These ratios are plotted by the bars in white. A ratio of 1 (⋯⋯· line) indicates no touch effect. A ratio significantly less than 1 indicates a touch effect on sway attenuation. The smaller the ratio, the larger the touch effect is. Similarly, the 2nd right y-axis and notations are used to indicate vision effects in subplots C and G. Overall model significant main effects and interactions are labeled at the top of each subplot. Post-hoc analysis of the main age group effect is indicated by  showing significant differences between two age groups. For each group, the letter above each group plot indicates a main sensory effect (T: main touch effect, V: main vision effect) or sensory interaction (T × V for touch vision interaction). Asterisks indicate p values as: * p<0.1, ** p<0.05, *** p<0.01, **** p<0.001 and ***** p<0.0001.
showing significant differences between two age groups. For each group, the letter above each group plot indicates a main sensory effect (T: main touch effect, V: main vision effect) or sensory interaction (T × V for touch vision interaction). Asterisks indicate p values as: * p<0.1, ** p<0.05, *** p<0.01, **** p<0.001 and ***** p<0.0001.
The main touch effect was significant for velocity (Fig.1B) and variance (Fig.1F). Post-hoc analysis for each group showed that all groups attenuated sway velocity and reduced variance with touch. The Group × Touch interaction was not significant indicating that the touch effect was similar for all age groups.
The main vision effect and Group × Vision interaction were only marginally significant for the velocity measure (Fig.1C) but not for variance (Fig.1G). Post-hoc analysis showed that the main vision effect was only significant in adults. There was no evidence of a Touch × Vision interaction nor a Group × Touch × Vision interaction (Fig.1D,H). Table 2 lists the statistical results for experiment 1.
Table 2.
Statistical results for experiment 1
| Velocity | Variance | |||||||
|---|---|---|---|---|---|---|---|---|
| Main age effect | F3,15.5=37.7, p<0.0001 | F3,15.3=8.3, p=0.0017 | ||||||
| Pair-wise comparison of age effect |
Ault vs. 4 year-old F1,13.3=82.0 adjusted p<0.0001 |
Ault vs. 6 year-old F1,15.8=62.6 adjusted p<0.0001 |
Ault vs. 8 year-old F1,16=44.1 adjusted p<0.0001 |
4 year-old vs. 8 year-old F1,13.5=11.4 adjust p=0.0288 |
Ault vs. 4 year-old F1,14=18.1 adjusted p=0.0048 |
Ault vs. 6 year-old F1,14.9=22.0 adjusted p=0.0018 |
Ault vs. 8 year-old F1,15.4=9.5 adjust p=0.0435 |
|
| Main touch effect | F1,13.3=34.18, p<0.0001 | F1,18.6=49.99, p<0.0001 | ||||||
| Touch effect, each group |
4 year-old F1,7=4.6 adjusted p=0.0698 |
6 year-old F1,8=13.5 adjusted p=0.0062 |
8 year-old F1,8=26.8 adjusted p=0.0008 |
Adults F1,8=15.8 adjusted p=0.0041 |
4 year-old F1,7=10.3 adjusted p=0.0150 |
6 year-old F1,8=15.7 adjusted p=0.0042 |
8 year-old F1,8=82.3 adjusted p<0.0001 |
Adults F1,8=10.9 adjusted p=0.0109 |
| Group × Touch | F3,14.9=1.12, p=0.3711 | F3,13.6=0.93, p=0.4536 | ||||||
| Main vision effect | F1,26=3.1, p=0.0919 | F1,28.5=1.19, p=0.2845 | ||||||
| Vision effect, each group |
4 year-old F1,7=1.16 adjusted p=0.3174 |
6 year-old F1,8=0.13 adjusted p=0.7245 |
8 year-old F1,8=0.11 adjusted p=0.7535 |
Adults F1,8=10.9 adjusted p=0.0109 |
4 year-old F1,7=0.01 adjusted p=0.9180 |
6 year-old F1,8=0.43 adjusted p=0.5324 |
8 year-old F1,8=1.76 adjusted p=0.2210 |
Adults F1,8=0.05 adjusted p=8325 |
| Group × Vision | F3,15.3=2.7, p=0.0803 | F3,15.6=1.07, p=0.3906 | ||||||
| Vision × Touch | F1,19.5=0.05, p=0.8237 | F1,25.1=0.35, p=0.5587 | ||||||
| Group × Vision × Touch |
F3,14.3=0.53, p=0.6700 | F3,14.9=0.30, p=0.8271 | ||||||
Experiment 2: Children with DCD vs. TD children
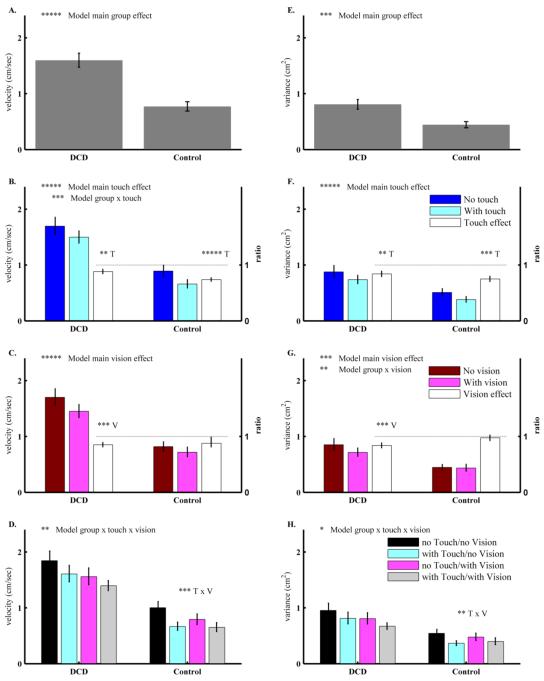
The main group effect was significant for velocity (Fig.2A) and variance (Fig.2E). Post-hoc analysis showed that children with DCD swayed with higher velocity and greater variance than TD children for every test condition. The main touch effect was significant for velocity (Fig.2B) and variance (Fig.2F). Post-hoc analysis showed that both groups attenuated sway velocity, and reduced variance with touch information. The Group × Touch interaction was significant for the velocity measure (Fig.2B) and TD children showed a significantly larger main touch effect in attenuating velocity.
Figure 2.
Results from experiment 2 with two groups of children: children with Developmental Coordination Disorder (DCD) and typically developing (TD) children. Similar to Figure 1, subplots in each column are for sway velocity and distance variance in medio-lateral (ML) direction; and subplots in each row are by group, touch, vision and four sensory conditions. All bars are plotted as Mean ± Standard Error. As in Figure 1, only subplots B, C, F and G have a 2nd right y-axis and corresponding bars in white color to indicate the main sensory effects as measured by ratio. Text and asterisks notations are the same as in Figure 1.
The main vision effect was significant for velocity (Fig.2C) and variance (Fig.2G) and it was mainly due to the vision effect in children with DCD. However, the Group × Vision interaction was only significant for variance (Fig.2G). Because our main interest was to contrast children with DCD with their TD peers on their ability to use multisensory information, we focused on the analysis of Group × Touch × Vision interaction to answer this question directly.
The Group × Touch × Vision interaction was significant for velocity (Fig. 2D) and marginally significant for variance (Fig. 2H). Post-hoc analysis showed that the Touch × Vision interaction was significant for TD children but not in children with DCD. In TD children, post-hoc analysis showed that vision reduced sway velocity only when touch was not available (F1,9=36.54, adjusted p=0.0002). In contrast, in children with DCD, both the main touch and the main vision effects were significant without a Touch × Vision interaction. Table 3 lists the statistic results for experiment 2.
Table 3.
Statistical results for experiment 2
| Velocity | Variance | |||||||
|---|---|---|---|---|---|---|---|---|
| Main group effect | F1,16.6=29.9, p<0.0001 | F1,18.3=13.3, p=0.0018 | ||||||
| Group effect, each condition |
NT/NV F1,18.2=19.64, adjusted p=0.0003 |
T/NV F1,18.1=39.38, adjusted p<0.0001 |
NT/V F1,17.8=20.02, adjusted p=0.0003 |
T/V F1,13.5=30.16, adjusted p<0.0001 |
NT/NV F1,19=10.49, adjusted p=0.0043 |
T/NV F1,18.9=21.20, adjusted p=0.0002 |
NT/V F1,18.5=8.07, adjusted p=0.0106 |
T/V F1,14.5=8.70, adjusted p=0.0102 |
| Main touch effect | F1,19=45.6, p<0.0001 | F1,18.6=29.1, p<0.0001 | ||||||
| Touch effect, each group |
TD F1,9=47.0, adjusted p<0.0001 |
DCD F1,10=7.4, adjusted p=0.0213 |
TD F1,9=20.8, adjusted p=0.0014 |
DCD F1,10=9.0, adjusted p=0.0132 |
||||
| Group × Touch | F1,19=8.2, p=0.0099 | F1,18.6=1.78, p=0.1986 | ||||||
| Main vision effect | F1,19=24.8, p<0.0001 | F1,18.9=8.3, p=0.0094 | ||||||
| Vision effect, each group |
TD F1,9=1.24, adjusted p=0.2938 |
DCD F1,10=13.2, adjusted p=0.0041 |
TD F1,9=0.25, adjusted p=0.6270 |
DCD F1,10=12.9, adjusted p=0.0050 |
||||
| Group × Vision | F1,18.7=0.77, p=0.3909 | F1,18.9=4.7, p=0.0424 | ||||||
| Group × Touch × Vision |
F1,18.4=5.3, p=0.033 | F1,17.3=3.0, p=0.0925 | ||||||
| Touch × Vision each group |
TD F1,9=17.4, adjusted p=0.0024 |
DCD F1,10=0.13, adjusted p=0.7307 |
TD F1,9=7.8, adjusted p=0.0211 |
DCD F1,10=0.04, adjusted p=0.8549 |
||||
NT/NV, T/NV, NT/V and T/V denote no touch/no vision, with touch/no vision, no touch/with vision, and with touch/with vision condition respectively. TD stands for typically developing children and DCD stands for children with Developmental Coordination Disability.
Discussion
Touch, but not vision, is robust across the lifespan and stances – implication for studying multisensory integration in developing children
Experiment 1 showed that TD children as young as 4 years old use touch as effectively as adults. This finding is similar to reports in infancy13,14, adults12 and elderly individuals21. Our data elaborate the developmental landscape to include young children and supports a robust touch effect across the lifespan. Unlike in adults, vision does not reduce sway significantly in TD children, similar to findings in a study using a normal stance16. Experiment 2 showed that while both TD children and children with DCD use touch, children with DCD use touch less effectively. Taken together, the touch effect for TD children from both experiments, we conclude that the touch effect is not only robust across the lifespan but also across stances. In contrast, vision is sensitive to the stance as seen in the results for vision in TD children across the two experiments. In experiment 1, vision does not reduce sway, while in experiment 2 TD children benefited from vision but only when touch was not available. The vision effect in experiment 2 highlights the fact that a challenging stance (such as the modified tandem stance used in experiment 2) may be needed to reveal the effect of vision in developing children. Our finding of a vision effect in TD children with a challenging stance is similar to previous findings using a compliant standing surface5. The dependency of the vision effect on the subject’s stance may explain the inconsistent findings for the effect of vision in children found in the extant literature15,16. The implication for studying developing children is that a challenging stance may be needed to study the vision interaction with another sensory modality such as touch.
Interpreting the less effective touch effect in children with DCD in the framework of multisensory integration
Our findings of a robust touch effect do not support the notion of visual dominance for balance control in children as suggested by Woollacott and her colleagues15. Nevertheless, we do not propose touch as the dominant modality either. Instead, we emphasize the integration of sensory modalities rather than the dominance of any particular modality8. That is, the utilization of any sensory information is influenced by other coexisting sensory information.
Although the touch effect is robust, interestingly, the touch effect was smaller in children with DCD than in TD children. Why is it that children with DCD do not use touch, such a robust sensory modality, effectively? At the neurophysiology level, a small case study shows that the latency of cortical somatosensory evoked potentials is delayed in children with DCD22. More importantly at the behavioral level, a meta-analysis shows that kinesthetic impairment is one of the major information processing deficits in children with DCD23. Although kinesthetic information is mainly mediated by muscle spindles24, recent studies show that cutaneous receptors also contribute significantly to position sense24.25. Specifically in our protocol, in order to maintain finger contact at a fixed point, participants also need to accommodate an arm configuration relative to their postural sway. Thus, touch information at the fingertip combined with proprioceptive information about the arm can provide information of body position relative to the contact point (i.e., body orientation)20,26. Similarly, Riley also suggested that touch provides a reference frame about the body’s orientation11. Based on the above findings, we consider that the smaller touch effect may involve a deficit in central processing of touch information in children with DCD. However, we cannot rule out the potential contribution of kinesthetic deficit at the peripheral level as we did not specifically test for this function.
Using multisensory information to construct internal models for motor control – possible internal model deficits in children with DCD
Touch information contributes significantly to body orientation11,18,26, an important internal model for balance control. However, an individual sensory modality does not always provide an accurate representation of body orientation. Instead, the central nervous system uses internal models to combine information from multiple sensory modalities27. Recently, proprioceptive function has been shown to affect multisensory-motor integration in children28. Here, we speculate that the smaller touch effect observed in children with DCD may have a negative effect on the children’s ability to combine touch information with vision to construct optimal internal models of body orientation. The possible internal model deficits may hamper feedforward as well as feedback control of balance leading to excessive sway in children with DCD.
It has been shown that children with DCD have difficulty in cross-modal judgments that require the use of visual information to guide proprioceptive judgments of limb position10. They also perform worse than their TD peers on an inter-modality matching task9. In the present experiment, we have demonstrated that children with DCD benefit from both touch and vision when performing a balance task and there is no touch and vision interaction as observed in TD children. We interpret these results as possible multisensory integration deficits. If multisensory integration is impaired in children with DCD, then they would benefit from using both touch and vision at all times in order to achieve a better estimate of their body orientation and self-motion. The internal model deficits may exacerbate the balance impairment with other coexisting deficits. For example, postural muscle activation timing deficits29 may well explain our findings that when standing with natural sensory conditions (no touch/with vision), children with DCD still sway more than their TD peers. With enriched touch information, it has been shown that muscle activation is reduced almost by half compared to no touch condition12 and the result is interpreted as that sensory information is used for better estimation to allow sway and muscle activity reduction simultaneously. If DCD children also have trouble with the relationship between estimation and muscle activity, combined muscular and internal model deficits may underscore the balance challenges that children with DCD face in everyday life.
Conclusion
In summary, we conclude that children with DCD use both touch and vision to attenuate sway in part due to their less effective use of touch information. This finding suggests a deficit in using touch information which may also contribute to deficits in multisensory integration leading to less well established internal models of body orientation and self-motion in children with DCD. These deficits lead to compromised balance control in standing that may also contribute to other motor problems observed in children with DCD.
Acknowledgements
This research was supported by NIH HD38337 (PI: Jill Whitall), NIH HD42527 (PI: Jane E. Clark), and a scholarship from the Taiwan Ministry of Education to Woei-Nan Bair. We thank Jane Jung-Potter for data collection for experiment two, Li-Chiou Chen for project coordination, Jason Metcalfe for valuable discussions, and Tim Kiemel for consultation on statistical analysis. The authors also would like to thank the children and their parents who gave willingly of their time and effort for this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association . Learning Disorders. Washington, DC: 1994. [Google Scholar]
- 2.Forseth AK. Static balance in children with hand-eye co-ordination problems. Child Care Health Dev. 2003;29(6):569–579. doi: 10.1046/j.1365-2214.2003.00378.x. [DOI] [PubMed] [Google Scholar]
- 3.Geuze RH. Static balance and developmental coordination disorder. Hum Mov Sci. 2003;22(4-5):527–548. doi: 10.1016/j.humov.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Grove CR, Lazarus JA. Impaired re-weighting of sensory feedback for maintenance of postural control in children with developmental coordination disorder. Hum Mov Sci. 2007;26(3):457–476. doi: 10.1016/j.humov.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Deconinck FJA, De Clercq D, van Coster R, Oostra A, Dewitte G, Savelsbergh GJR, Cambier D, Lenoir M. Sensory contributions to balance in boys with developmental coordination disorder. Adapt Phys Act Q. 2008;25(1):17–35. doi: 10.1123/apaq.25.1.17. [DOI] [PubMed] [Google Scholar]
- 6.Jeka JJ, Oie KS, Kiemel T. Multisensory information for human postural control: integrating touch and vision. Exp Brain Res. 2000;134(1):107–125. doi: 10.1007/s002210000412. [DOI] [PubMed] [Google Scholar]
- 7.Kiemel T, Oie KS, Jeka JJ. Multisensory fusion and the stochastic structure of postural sway. Biol Cyber. 2002;87(4):262–277. doi: 10.1007/s00422-002-0333-2. [DOI] [PubMed] [Google Scholar]
- 8.Bair WN, Kiemel T, Jeka JJ, Clark JE. Development of multisensory reweighting for posture control in children. Exp Brain Res. 2007;183(4):435–446. doi: 10.1007/s00221-007-1057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigmundsson H, Ingvaldsen RP, Whiting HTA. Inter- and intra-sensory modality matching in children with hand-eye co-ordination problems: exploring the developmental lag hypothesis. Exp Brain Res. 1997;114(3):492–499. doi: 10.1007/pl00005658. [DOI] [PubMed] [Google Scholar]
- 10.Mon-Williams MA, Wann JP, Pascal E. Visual-proprioceptive mapping in children with developmental coordination disorder. Dev Med Child Neurol. 1999;41(4):247–254. doi: 10.1017/s0012162299000523. [DOI] [PubMed] [Google Scholar]
- 11.Riley MA, Wong S, Mitra S, Turvey MT. Common effects of touch and vision on postural parameters. Exp Brain Res. 1997;(117):165–170. doi: 10.1007/s002210050211. [DOI] [PubMed] [Google Scholar]
- 12.Jeka JJ, Lackner JR. The role of haptic cues from rough and slippery surfaces in human postural control. Exp Brain Res. 1995;103(2):267–276. doi: 10.1007/BF00231713. [DOI] [PubMed] [Google Scholar]
- 13.Barela JA, Jeka JJ, Clark JE. The use of somatosensory information during the acquisition of independent upright stance. Infant Behav Dev. 1999;22(1):87–102. [Google Scholar]
- 14.Metcalfe JS, Chen LC, Chang TY, McDowell K, Jeka JJ, Clark JE. The temporal organization of posture changes during the first year of independent walking. Exp Brain Res. 2005;161(4):405–416. doi: 10.1007/s00221-004-2082-z. [DOI] [PubMed] [Google Scholar]
- 15.Woollacott MH, Debu B, Mowatt M. Neuromuscular control of posture in the infant and child: Is vision dominant? J Motor Behav. 1987;19:167–186. doi: 10.1080/00222895.1987.10735406. [DOI] [PubMed] [Google Scholar]
- 16.Riach CL, Hayes KC. Maturation of postural sway in young children. Dev Med Child Neurol. 1987;29(5):650–658. doi: 10.1111/j.1469-8749.1987.tb08507.x. [DOI] [PubMed] [Google Scholar]
- 17.Henderson SE, Sugden D. Movement Assessment Battery for Children. The Psychological Corporation; London: 1992. [Google Scholar]
- 18.Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM. Measures of postural steadiness: Differences between healthy young and elderly adults. IEEE Trans Biomed Eng. 1996;43(9):956–966. doi: 10.1109/10.532130. [DOI] [PubMed] [Google Scholar]
- 19.Jeka JJ, Kiemel T, Creath R, Horak F, Peterka R. Controlling human upright posture: velocity information is more accurate than position or acceleration. J Neurophysiol. 2004;92(4):2368–2379. doi: 10.1152/jn.00983.2003. [DOI] [PubMed] [Google Scholar]
- 20.Rabin E, Bortolami SB, DiZio P, Lackner JR. Haptic stabilization of posture: Changes in arm proprioception and cutaneous feedback for different arm orientations. J Neurophysiol. 1999;82(6):3541–3549. doi: 10.1152/jn.1999.82.6.3541. [DOI] [PubMed] [Google Scholar]
- 21.Baccini M, Rinaldi LA, Federighi G, Vannucchi L, Paci M, Masotti G. Effectiveness of fingertip light contact in reducing postural sway in older people. Age Ageing. 2007;36(1):30–35. doi: 10.1093/ageing/afl072. [DOI] [PubMed] [Google Scholar]
- 22.Bockowski L, Sobaniec W, Kulak W, Smigielska-Kuzia J. The cortical evoked potentials in children with developmental coordination disorder (DCD) Rocz Akad Med Bialymst. 2005;50(Suppl 1):87–90. [PubMed] [Google Scholar]
- 23.Wilson PH, McKenzie BE. Information processing deficits associated with developmental coordination disorder: a meta-analysis of research findings. J Child Psychol Psyc. 1998;39(6):829–840. [PubMed] [Google Scholar]
- 24.Proske U, Gandevia SC. The kinaesthetic senses. J Physiol. 2009;587(17):4139–4146. doi: 10.1113/jphysiol.2009.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol. 2005;94(3):1699–1706. doi: 10.1152/jn.00191.2005. [DOI] [PubMed] [Google Scholar]
- 26.Lackner JR, DiZio P. Vestibular, proprioceptive, and haptic contributions to spatial orientation. Annu Rev Psychol. 2005;56:115–147. doi: 10.1146/annurev.psych.55.090902.142023. [DOI] [PubMed] [Google Scholar]
- 27.Zupan LH, Park S, Merfeld DM. The nervous system uses internal models to achieve sensory integration. IEEE Eng Med Biol Soc. 2004;6(1557-170):4487–4490. doi: 10.1109/IEMBS.2004.1404247. [DOI] [PubMed] [Google Scholar]
- 28.King BR, Pangelinan MM, Kagerer FA, Clark JE. Improvements in proprioceptive functioning influence multisensory-motor integration in 7-to 13-year-old children. Neurosci Lett. 2010;483(1):36–40. doi: 10.1016/j.neulet.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston LM, Burns YR, Brauer SG, Richardson CA. Differences in postural control and movement performance during goal directed reaching in children with developmental coordination disorder. Hum Mov Sci. 2002;21(5-6):583–601. doi: 10.1016/s0167-9457(02)00153-7. [DOI] [PubMed] [Google Scholar]