Abstract
The discovery of resistin ten years ago as a fat cell-secreted factor that modulates insulin resistance suggested a link to the obesity-associated epidemics of diabetes and cardiovascular disease that are major human health concerns. While adipocyte-derived resistin is indisputably linked to insulin resistance in rodent models, the relevance of human resistin is complicated because human resistin is secreted by macrophages rather than adipocytes, and because of the descriptive nature of human epidemiology. Here we review the recent and growing evidence that human resistin is an inflammatory biomarker and potential mediator of diabetes and cardiovascular disease.
The Discovery of Resistin
Resistin is a 12.5 kDa cysteine-rich polypeptide discovered in a screen for adipocyte gene products that are downregulated by antidiabetic thiazolidinedione drugs.1 Resistin is a member of a small family of secreted proteins characterized by a unique spacing of 10–11 cysteine residues and known as Resistin-Like Molecules (RELMs)2 or as Found in Inflammatory Zone (FIZZ) proteins. The founding member, FIZZ1 (also known as RELMα), was discovered in a screen for proteins secreted by the lung in a mouse model of asthma. In rodents, resistin is an adipokine, secreted from white adipocytes.1, 3, 4
Resistin in Insulin Sensitivity and Diabetes in Rodent Models
Since rodent resistin is an adipokine found in a screen for targets of insulin-sensitizing drugs, much attention has been paid to its potential role in obesity-associated diabetes. Indeed, resistin levels are elevated in several animal models of obesity.1, 5 Administration of exogenous resistin or transgenic overexpression leads to decreased insulin sensitivity and altered glucose handling and, conversely, blocking resistin activity or genetically decreasing its levels improves insulin sensitivity and restores glucose homeostasis6 (and citations within reference 6). Thus there is strong genetic and pharmacological evidence that resistin is a mediator of insulin resistance in rodents.
Mechanisms of Resistin Action
The mechanisms by which resistin exerts its biological effects are incompletely understood. Multiple tissues in rodent and human have been reported to be responsive to resistin in vitro and in vivo, including adipose, liver, muscle, vascular endothelium, cardiomyocyte and inflammatory cells.1, 7–11 Hyperresistinemic rodents show altered glucose homeostasis and insulin resistance in liver and skeletal muscle.7–9 Resistin also decreased insulin-stimulated glucose uptake in cultured murine cardiomyocytes.10 In human macrophages, resistin has been shown to induce production of inflammatory cytokines and to drive expression of cell adhesion molecules, including vascular cell adhesion molecule-1 (VCAM-1), inter-cellular adhesion molecule-1 (ICAM-1) and monocyte chemoattractant protein-1 (MCP-1).11, 12 In vascular cells from human and animal, resistin causes vascular smooth muscle proliferation,13 leads to endothelial dysfunction,14, 15 and promotes endothelial-monocyte adhesion and infiltration.16, 17
As a secreted, circulating polypeptide, these effects may be mediated through both endocrine and paracrine modes of action, and it seems likely that resistin functions via a receptor on the surface of target cells. However, the resistin receptor remains unknown, although a recent report has suggested that resistin may bind to the endotoxin receptor Toll-Like Receptor 4.18 Downstream targets of resistin provide indirect evidence of its role in intracellular pathways involving inhibition of insulin signaling as well as response to inflammation. In rodent liver and muscle, resistin has been shown to inhibit AMP-activated kinase (AMPK).8, 19, 20 Like several other inflammatory cytokines, resistin also activates the anti-inflammatory mediator suppressor of cytokine signaling-3 (SOCS-3) in rodent adipose tissue.21 SOCS-3 is known to decrease insulin signaling in adipose and other tissues and so further connects resistin to both insulin and inflammatory pathways. In human vascular cells, the actions of resistin involve activation of p38 mitogen-activated protein kinase (MAPK) impairing insulin signaling, altering oxidative stress response and driving cell proliferation.22–24
Despite a still imprecise knowledge of resistin biology in the rodent, and predominantly in vitro data regarding resistin signaling in humans, the impact of resistin on metabolism and inflammation in several organ systems has led investigators to examine whether such effects underlie human disease as well.
Human Resistin
Resistin is detectable in human serum, but unlike rodents, human resistin is predominantly expressed in macrophages, and its expression in human adipose tissue is predominantly due to non-adipocyte resident inflammatory cells.25–29 Human and rodent resistin gene and protein sequences share about 60% identity, less than most hormones conserved across species.30 Yet, the genes are syntenic, with the gene coding resistin (Retn) on mouse chromosome 8 located a similar distance from the Insulin Receptor gene as is the Retn gene on human chromosome 19. Also, as in rodents, resistin levels are decreased with thiazolidinedione treatment in humans.6 The lack of human resistin expression in adipocytes appears to be attributable to loss of a genomic binding site for the nuclear receptor PPARγ, which controls the adipocyte-specific expression of the mouse Retn gene.31
The expression and secretion of resistin by human mononuclear cells are dramatically induced by inflammatory stimuli,32–34 which also increase circulating resistin levels.11, 35 Inflammation is increasingly recognized to play a pathogenic role in obesity and type 2 diabetes, as well as cardiovascular disease.36, 37 As discussed below, recent genetic and epidemiologic data connect resistin to these human diseases.
Epidemiologic Evidence for Resistin in Human Insulin Resistance and Diabetes
A mouse model in which human resistin is expressed primarily from monocytes and macrophages, as is the case in humans, is predisposed to developing insulin resistance, suggesting that the metabolic effects of resistin may be translatable from rodents to man.38 Given the association of resistin and insulin resistance in rodents, many studies have investigated the correlation between circulating resistin levels and type 2 diabetes. The results of initial efforts were conflicting, with some but not all studies elucidating a significant correlation between serum resistin level and insulin resistance or type 2 diabetes (summarized in reference 6). The discrepancy among study results could be related to differing demographics of the study groups, although positive correlations were seen in lean as well as obese subjects and across Caucasian and Asian populations. In addition, many of the initial studies had small sample sizes, and used non-standardized assays.
More recently, three large American case-control studies have indeed shown an increased risk of developing type 2 diabetes among those with elevated serum resistin at baseline.39, 40 Data from the Nurses’ Health Study, and both the Women’s Health Study and the Physicians’ Health Study – which followed previously healthy subjects for 12, 10 and 8 years, respectively – showed an increased risk even after adjusting for known diabetes risk factors. Higher levels of resistin were observed in diabetics versus controls, and the risk of diabetes increased with resistin levels up to an odds ratio of 1.68 in subjects in the highest quintile of resistin levels.39 At least some of the association between resistin and insulin resistance in humans seems attributable to other factors known to impact insulin sensitivity, namely obesity and inflammation. However, while most studies have analyzed whether the relative risk associated with increased resistin levels is independent of previously established risk factors, the converse analysis is rarely done, leaving open the possibility that the biological effect of these other risk factors is mediated by resistin.
Whether resistin is an active player or merely a responder in metabolic dysfunction cannot be fully determined without an understanding of the regulation of resistin itself. Genetic determinants of resistin expression may provide additional clues as to the role of resistin in human susceptibility to disease.
Genetic Evidence for Resistin in Human Insulin Resistance and Diabetes
While the ability to manipulate the mouse genome has provided an indisputable link between resistin and insulin resistance, no humans that completely lack resistin have yet been identified. Nevertheless, several single nucleotide polymorphisms (SNPs) have been shown to correlate with increased circulating resistin levels, and it has been estimated that about 70% of resistin expression can be attributed to genetic effects.41 Gene variants in the promoter region upstream of the Retn gene appear to have the strongest effect. In a Japanese population, 3 promoter SNPs correlated with elevated resistin levels, and two of these (−638 G>A and −420 C>G) together drove increased resistin expression in an in vitro transcription assay.42 The G/G genotype at SNP −420 increased Retn gene transcription through increased binding of the transcription factors Sp1 and Sp3,43 and correlated with monocyte resistin expression as well as increased serum levels in large studies of over 200 Japanese subjects.44, 45 The −420G allele, together with −537A>C, was also associated with increased resistin levels in a Korean population.46
The −420 C>G SNP associated with increased circulating resistin levels has been associated with type 2 diabetes in several studies of Asian populations. In addition to the Japanese studies described above,42–45 the G/G genotype at −420 in a Chinese population correlated with the development of both worse glucose tolerance and hyperglycemia at five year follow-up.47 Other SNPs at the Retn locus did not significantly influence diabetes risk in Asian cohorts.48
Whereas the gene dosage of the −420G allele has been robustly correlated with resistin levels and diabetes risk in Asians, the data in non-Asian populations have been conflicting. A North American cohort of Caucasian non-diabetics corroborated the association of G/G at −420 with resistin level, but saw no correlation with markers of insulin resistance,49 and the Framingham Offspring Cohort did not find significance for −420 C>G in their population although four SNPs in the 3′-untranslated region of the Retn gene did correlate with elevated resistin levels.50 In an Italian cohort, the −420G/G genotype correlated with metabolic syndrome,51 but this was not the case in a Finnish study.52
Observational studies of human Retn gene variants have also connected resistin with type 2 diabetes. In Europe and North America, several studies have documented several non-coding polymorphisms near the Retn locus that correlate with insulin resistance and/or type 2 diabetes, while in other studies Retn polymorphisms (often at different loci) have not correlated with metabolic risk, as has been reviewed elsewhere.48 Indeed, one study that showed a correlation with SNPs and obesity in French Canadians did not observe a relationship in a similar cohort of Scandinavian descent.53 These data reveal several potential genetic modulators of resistin, with strong evidence for variants in the promoter region. However, these genetic signals have not been consistently correlated with resistin levels or with metabolic outcomes across all study groups. This may be due to variations in sample size, unknown confounders in patient population and ethnic differences.
More recent studies of the complex genetic regulation of resistin expression may help to explain some of the ethnic variation. The minor allele frequencies for −638 G>A have been found to be dramatically lower in Caucasians than Japanese,54 and a case-control study associated −638 G>A with increased resistin levels.55 More strikingly, a survey of the general Japanese population found the highest resistin levels in those with both a G at −420 and an A allele at −358, the latter SNP being virtually absent in Caucasian populations.56 Indeed, published databases indicate that both −358 and −638 may be monomorphic in Caucasians.54, 56 (dbSNP www.ncbi.nlm.nih.gov/projects/SNP/; HapMap www.hapmap.org/index.html.en)
Thus, resistin expression appears to be controlled in part by genetic programming as genotype correlates with both level and disease state in some populations. Genetic analysis has given insight into the variation and complexity of the role of resistin in metabolic disease. This variability also suggests that resistin may be integral to inflammatory states beyond insulin resistance and diabetes.
Evidence for Resistin in Human Obesity
Studies of obese non-diabetic subjects have frequently noted higher serum levels of resistin as well as direct correlations between resistin level and adiposity as measured by BMI.6, 57 Using computed tomography (CT) imaging, other groups have more closely associated resistin with quantitative fat mass, in visceral and subcutaneous, and in abdominal and intrathoracic depots.58–60 As with measures of diabetes, the association of resistin and obesity may be stronger in women than men,59, 61 though not all studies agree.62
Weight loss studies have also yielded intriguing results. A regimen of diet and exercise led to decreases in resistin that paralleled reductions in BMI and fat mass after one and a half years.63 Studies of morbidly obese patients undergoing bariatric surgery showed a reduction in serum resistin levels as early as 3–6 months post-operatively.64, 65 By contrast, an earlier study showed a small increase in resistin levels with weight loss at shorter follow-up,66 and a recent report showed no change in serum resistin following bariatric surgery despite significant weight loss.67 These studies are notably small and include differing populations undergoing different types and amounts of weight loss. Additionally, varying comorbidities, medication regimens and the assays used in each study may have contributed to discrepant results. Importantly, resistin levels appear plastic and responsive to treatments aimed at improving the metabolic profile.
Thus, although human resistin is not fat-cell derived, it seems to be well connected to obesity and responsive to changes in adiposity. This connection between macrophage-derived resistin and adipose may further the understanding of obesity as an inflammatory state and implicates resistin as a potential modulator of that state.
Evidence for Resistin in Human Inflammation
Since the initial finding that resistin in humans was expressed predominantly in bone marrow-derived inflammatory cells26 and correlated with markers of inflammation,11 many epidemiological studies tying resistin to metabolic diseases have corroborated this connection. In several multivariate analyses of diabetic, obese and lean subjects, serum resistin levels correlated strongly with circulating markers of inflammation, including C-reactive protein (CRP), tumor necrosis factor-α (TNFα), and interleukin-6 (IL-6).60, 68, 69 Indeed, a follow-up analysis of the Nurses’ Health Study, which showed a correlation between resistin levels and diabetes, found that resistin levels among healthy women correlated only with TNFα and IL-6 after adjusting for age and BMI.70
Elevated resistin levels have also been shown in patients with other inflammatory diseases including rheumatoid arthritis (RA) and inflammatory bowel disease (IBD) (reviewed elsewhere)71 Interestingly, resistin appeared responsive to anti-inflammatory treatment with the anti-TNFα agent infliximab in both RA and IBD.71 In addition, an experimental model of human sepsis and studies of those critically ill from sepsis and septic shock have shown elevated resistin in response to inflammation.72–74 Resistin correlated with sepsis severity, inflammatory cytokines, and insulin resistance in the critically ill.72–74 Thus, the connection between resistin and sepsis may in fact be more robust than its tie to obesity.75 Moreover, elevated resistin in non-septic but critically ill patients was also shown to predict worse outcomes.74 Indeed, in the highly inflammatory disease of acute pancreatitis resistin levels on hospital admission correlated with disease severity, tissue necrosis, and clinical outcome whereas CRP showed no correlation.76
Evidence for Resistin in Human Atherosclerosis and Coronary Artery Disease
Building on the preclinical data that resistin alters cell signaling in endothelial cells14, 22, 77 and cardiomyocytes,10 and the finding of elevated serum resistin and the presence of resistin protein within atherosclerotic plaques in the apoE −/− mouse model,12 several groups have investigated the impact of resistin on the development of coronary artery disease (CAD) in humans. Elevated resistin has predicted the presence and severity of CAD, correlating with coronary artery calcium score in asymptomatic healthy subjects35 and in patients undergoing angiography for chest pain.78, 79 A European case-control study of 26,490 healthy individuals found a relative risk of 2.09 for the development of myocardial infarction in those in the highest quartile of resistin, adjusted for CRP.80 Moreover, a Chinese study of 225 cases and controls revealed a markedly increased risk of coronary artery disease (odds radio 3.01) in individuals with the −420 G/G genotype associated with increased resistin levels.81 Several studies have not detected a correlation between resistin and either coronary disease prevalence or outcome.82–84 This could be due to ethnic variation or to differences in study design and patient selection. Resistin has been most predictive in prospective and cohort studies which do not exclude those with known or prior CAD and may have less confounding due to medical therapy or other biases. Thus there appears to be a potential connection between resistin and CAD outcomes across a spectrum of populations.
Resistin has been correlated with the number of diseased coronary vessels85 in patients with stable CAD, was an independent risk factor for Major Adverse Cardiovascular Events, including cardiovascular death and myocardial infarction, and, in one study, correlated with rates of restenosis after stenting.86–88 Inducible ischemia and decreased exercise tolerance were correlated with elevated resistin in a recent study of patients with known CAD undergoing stress testing.89 Elevated resistin may also predict worse outcomes after stroke,90 and appears to correlate with both renal function and CAD risk in those with known end-stage renal disease.71
The relation of serum resistin levels to acute coronary events has also been addressed. Intriguingly, an unbiased gene expression analysis of patients undergoing cardiopulmonary bypass, a possible model of controlled ischemia-reperfusion, demonstrated upregulation of resistin expression in blood at 2 and 24 hours.91 Others have shown increased levels of resistin in patients with acute coronary syndromes.85, 92, 93 Resistin has been shown to be increased in the epicardial adipose of those having an MI,94 and to remain elevated up to one week after MI.93 As might be expected, resistin often correlated with markers of inflammation including CRP.92
Thus, animal and human studies have suggested a role for resistin in driving atherosclerosis and human coronary disease. While the role of resistin as an independent prognostic biomarker remains unclear, the impact of resistin on CAD and other heart disease is gaining attention.
Evidence for Resistin in Human Congestive Heart Failure
Independent of ischemia, resistin has been shown to impact cardiomyocyte function in vitro. Resistin induces insulin resistance and alters glucose handling,10 leads to hypertrophy and decreased myocyte contractility,95 and is itself expressed in response to mechanical stretch.96 Much has been reported on the impact of insulin resistance and diabetes on both ischemic and non-ischemic cardiomyopathy and has been reviewed recently.97 The hypothesis that insulin resistance leading to altered glucose handling, thus leaving the heart vulnerable to stress when it shifts to glucose as its primary energy source, could connect resistin to the development of heart failure.
Indeed, two Asian studies first suggested that elevated resistin predicted a higher event rate and worse mortality in patients with active heart failure.98, 99 More recently, two reports from large cohorts published in the same year assessed the connection between resistin and incident heart failure. The HealthABC Study included 2902 mainly white elderly subjects followed for nine years and found an increased risk for heart failure hospitalization with elevated baseline resistin (HR 1.15 per 10ng/mL resistin), after adjustment for known risk factors including CRP and adiposity.100 A similar study analyzing data from 2739 Framingham Offspring Study subjects followed for six years, found a hazard ratio of 1.26 per 7.45ng/mL of resistin, also after adjusting for known risks.101 Both studies found that the risk was independent of coronary disease, inflammation and insulin resistance. In addition, the Heart and Soul Study of American veterans with known CAD showed a significantly higher risk of heart failure and all-cause mortality in those in the highest quartile of resistin level after adjustment for age, sex and BMI.102 In this study the apparent effect of resistin was not independent of traditional heart failure risk factors, although these factors may themselves be dependent on resistin. This is underscored by a recent study in Finland, where resistin gene variation is associated with obesity and insulin resistin,103 demonstrating that a high resistin level was an independent predictor of the prevalence of metabolic syndrome with a statistical association with every criterion including waist circumference, tumor necrosis factor-α, and insulin resistance, and reduction in high-density lipoprotein cholesterol.104
Concluding remarks
The growing public health concerns surrounding diabetes, obesity and their consequences have focused attention on efforts at treatment and prevention. However, an understanding of the mechanisms underlying these epidemics is essential. Basic and clinical research have described the inflammatory nature of many metabolic derangements from diabetes to cardiovascular disease. The hormone resistin, initially described as a rodent adipokine and now understood to be predominantly macrophage-derived in humans, is a potential link between inflammation and metabolic disease. (Fig. 1) Genetic studies have helped explain ethnic variability in resistin expression and activity and have elucidated the complexity of genetic regulation of metabolic disease. Clinical, genetic, and epidemiological studies have strengthened the association between resistin and the prevalence, severity, and outcome of metabolic disease. Although in rodent models resistin is clearly a pathogenic factor in the severity of insulin resistance and abnormal glucose metabolism, the human studies are by nature descriptive and thus do not address the critical question of whether resistin is more than a biomarker for insulin resistance, diabetes, and cardiovascular disease. It will be of great interest to determine whether blockage of resistin function, either by antibody neutralization or by antagonism of the elusive resistin receptor, has sufficient therapeutic utility in pre-clinical models such as the humanized mouse38 to warrant investigation as a viable therapeutic target in humans with diabetes or cardiovascular disease.
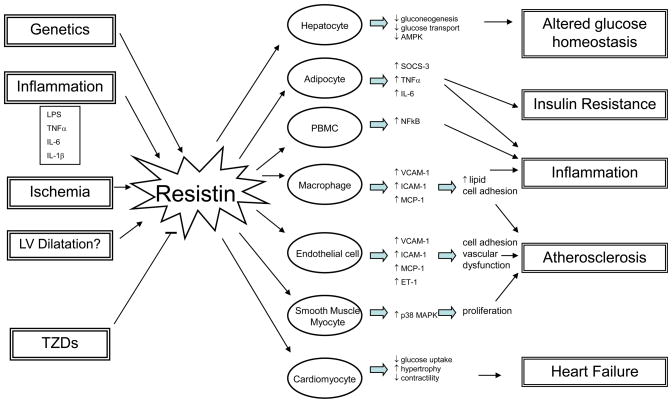
Figure 1. Potential impact of resistin on human disease.
Resistin is upregulated in response to genetic signals and various stimuli. As shown in studies of both human and animals, resistin may act on multiple cell types to promote inflammation, insulin resistance and cardiac pathology. Abbreviations: LPS, lipopolysaccharide; TNFα, tumor necrosis factor-alpha; IL-6, interleukin-6; IL-1β, interleukin-1beta; TZD, thiazolidinedione; PBMC, peripheral blood mononuclear cell; AMPK, adenosine monophosphate-activated protein kinase; SOCS-3, suppressor of cytokine signaling-3; NFkB, nuclear factor kappa B; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, inter-cellular adhesion molecule-1; MCP-1, monocyte chemoattractant protein-1; ET-1, endothelin-1; MAPK, mitogen activated protein kinase.
Acknowledgments
Research on resistin in the Lazar lab is funded by P01 DK 49210. Dr. Schwartz is funded by 5-T32 HLO007843-14. The authors apologize for not being able to cite all worthy papers due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steppan CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 2.Steppan CM, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci U S A. 2001;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim KH, et al. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J Biol Chem. 2001;276:11252–11256. doi: 10.1074/jbc.C100028200. [DOI] [PubMed] [Google Scholar]
- 4.Rajala MW, et al. Cell type-specific expression and coregulation of murine resistin and resistin-like molecule-alpha in adipose tissue. Mol Endocrinol. 2002;16:1920–1930. doi: 10.1210/me.2002-0048. [DOI] [PubMed] [Google Scholar]
- 5.Rajala MW, et al. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes. 2004;53:1671–1679. doi: 10.2337/diabetes.53.7.1671. [DOI] [PubMed] [Google Scholar]
- 6.Lazar MA. Resistin- and Obesity-associated metabolic diseases. Horm Metab Res. 2007;39:710–716. doi: 10.1055/s-2007-985897. [DOI] [PubMed] [Google Scholar]
- 7.Rajala MW, et al. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J Clin Invest. 2003;111:225–230. doi: 10.1172/JCI16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satoh H, et al. Adenovirus-mediated chronic “hyper-resistinemia” leads to in vivo insulin resistance in normal rats. J Clin Invest. 2004;114:224–231. doi: 10.1172/JCI20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rangwala SM, et al. Abnormal glucose homeostasis due to chronic hyperresistinemia. Diabetes. 2004;53:1937–1941. doi: 10.2337/diabetes.53.8.1937. [DOI] [PubMed] [Google Scholar]
- 10.Graveleau C, et al. Mouse and human resistins impair glucose transport in primary mouse cardiomyocytes, and oligomerization is required for this biological action. J Biol Chem. 2005;280:31679–31685. doi: 10.1074/jbc.M504008200. [DOI] [PubMed] [Google Scholar]
- 11.Lehrke M, et al. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004;1:e45. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnett MS, et al. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182:241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Calabro P, et al. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335–3340. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- 14.Verma S, et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 15.Kougias P, et al. Adipocyte-derived cytokine resistin causes endothelial dysfunction of porcine coronary arteries. J Vasc Surg. 2005;41:691–698. doi: 10.1016/j.jvs.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 16.Cho Y, et al. Adipokine resistin is a key player to modulate monocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J Am Coll Cardiol. 2010;57:99–109. doi: 10.1016/j.jacc.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Hsu WY, et al. Resistin induces monocyte-endothelial cell adhesion by increasing ICAM-1 and VCAM-1 expression in endothelial cells via p38MAPK-dependent pathway. J Cell Physiol. 2010 doi: 10.1002/jcp.22555. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Tarkowski A, et al. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J Cell Mol Med. 2010;14:1419–1431. doi: 10.1111/j.1582-4934.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banerjee RR, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 20.Muse ED, et al. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest. 2004;114:232–239. doi: 10.1172/JCI21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steppan CM, et al. Activation of SOCS-3 by resistin. Mol Cell Biol. 2005;25:1569–1575. doi: 10.1128/MCB.25.4.1569-1575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen YH, et al. Up-regulation of PTEN (phosphatase and tensin homolog deleted on chromosome ten) mediates p38 MAPK stress signal-induced inhibition of insulin signaling. A cross-talk between stress signaling and insulin signaling in resistin-treated human endothelial cells. J Biol Chem. 2006;281:7727–7736. doi: 10.1074/jbc.M511105200. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, et al. Resistin decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2010;299:H193–201. doi: 10.1152/ajpheart.00431.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mu H, et al. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006;70:146–157. doi: 10.1016/j.cardiores.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Savage DB, et al. Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator-activated receptor-gamma action in humans. Diabetes. 2001;50:2199–2202. doi: 10.2337/diabetes.50.10.2199. [DOI] [PubMed] [Google Scholar]
- 26.Patel L, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 27.Jung HS, et al. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc Res. 2006;69:76–85. doi: 10.1016/j.cardiores.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 28.McTernan CL, et al. Resistin, central obesity, and type 2 diabetes. Lancet. 2002;359:46–47. doi: 10.1016/s0140-6736(02)07281-1. [DOI] [PubMed] [Google Scholar]
- 29.Fain JN, et al. Resistin release by human adipose tissue explants in primary culture. Biochem Biophys Res Commun. 2003;300:674–678. doi: 10.1016/s0006-291x(02)02864-4. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh S, et al. The genomic organization of mouse resistin reveals major differences from the human resistin: functional implications. Gene. 2003;305:27–34. doi: 10.1016/s0378-1119(02)01213-1. [DOI] [PubMed] [Google Scholar]
- 31.Tomaru T, et al. Adipocyte-specific expression of murine resistin is mediated by synergism between peroxisome proliferator-activated receptor gamma and CCAAT/enhancer-binding proteins. J Biol Chem. 2009;284:6116–6125. doi: 10.1074/jbc.M808407200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu SC, et al. Lipopolysaccharide increases resistin gene expression in vivo and in vitro. FEBS Lett. 2002;530:158–162. doi: 10.1016/s0014-5793(02)03450-6. [DOI] [PubMed] [Google Scholar]
- 33.Kaser S, et al. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun. 2003;309:286–290. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Kunnari AM, et al. The expression of human resistin in different leucocyte lineages is modulated by LPS and TNFalpha. Regul Pept. 2009;157:57–63. doi: 10.1016/j.regpep.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Reilly MP, et al. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 36.Libby P, et al. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 38.Qatanani M, et al. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest. 2009;119:531–539. doi: 10.1172/JCI37273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heidemann C, et al. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med. 2008;149:307–316. doi: 10.7326/0003-4819-149-5-200809020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen BH, et al. Circulating levels of resistin and risk of type 2 diabetes in men and women: results from two prospective cohorts. Diabetes Care. 2009;32:329–334. doi: 10.2337/dc08-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menzaghi C, et al. Heritability of serum resistin and its genetic correlation with insulin resistance-related features in nondiabetic Caucasians. J Clin Endocrinol Metab. 2006;91:2792–2795. doi: 10.1210/jc.2005-2715. [DOI] [PubMed] [Google Scholar]
- 42.Azuma K, et al. Novel resistin promoter polymorphisms: association with serum resistin level in Japanese obese individuals. Horm Metab Res. 2004;36:564–570. doi: 10.1055/s-2004-825762. [DOI] [PubMed] [Google Scholar]
- 43.Osawa H, et al. The G/G genotype of a resistin single-nucleotide polymorphism at −420 increases type 2 diabetes mellitus susceptibility by inducing promoter activity through specific binding of Sp1/3. Am J Hum Genet. 2004;75:678–686. doi: 10.1086/424761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osawa H, et al. Resistin SNP-420 determines its monocyte mRNA and serum levels inducing type 2 diabetes. Biochem Biophys Res Commun. 2005;335:596–602. doi: 10.1016/j.bbrc.2005.07.122. [DOI] [PubMed] [Google Scholar]
- 45.Osawa H, et al. Plasma resistin, associated with single nucleotide polymorphism −420, is correlated with insulin resistance, lower HDL cholesterol, and high-sensitivity C-reactive protein in the Japanese general population. Diabetes Care. 2007;30:1501–1506. doi: 10.2337/dc06-1936. [DOI] [PubMed] [Google Scholar]
- 46.Cho YM, et al. Common genetic polymorphisms in the promoter of resistin gene are major determinants of plasma resistin concentrations in humans. Diabetologia. 2004;47:559–565. doi: 10.1007/s00125-003-1319-x. [DOI] [PubMed] [Google Scholar]
- 47.Xu JY, et al. Resistin gene polymorphisms and progression of glycaemia in southern Chinese: a 5-year prospective study. Clin Endocrinol (Oxf) 2007;66:211–217. doi: 10.1111/j.1365-2265.2006.02710.x. [DOI] [PubMed] [Google Scholar]
- 48.Steppan CM, Lazar MA. The current biology of resistin. J Intern Med. 2004;255:439–447. doi: 10.1111/j.1365-2796.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- 49.Qasim AN, et al. Resistin gene variation is associated with systemic inflammation but not plasma adipokine levels, metabolic syndrome or coronary atherosclerosis in nondiabetic Caucasians. Clin Endocrinol (Oxf) 2009;70:698–705. doi: 10.1111/j.1365-2265.2008.03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hivert MF, et al. Association of variants in RETN with plasma resistin levels and diabetes-related traits in the Framingham Offspring Study. Diabetes. 2009;58:750–756. doi: 10.2337/db08-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norata GD, et al. Effect of the −420C/G variant of the resistin gene promoter on metabolic syndrome, obesity, myocardial infarction and kidney dysfunction. J Intern Med. 2007;262:104–112. doi: 10.1111/j.1365-2796.2007.01787.x. [DOI] [PubMed] [Google Scholar]
- 52.Ukkola O, et al. Genetic variants at the resistin locus are associated with the plasma resistin concentration and cardiovascular risk factors. Regul Pept. 2008;149:56–59. doi: 10.1016/j.regpep.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 53.Engert JC, et al. 5′ flanking variants of resistin are associated with obesity. Diabetes. 2002;51:1629–1634. doi: 10.2337/diabetes.51.5.1629. [DOI] [PubMed] [Google Scholar]
- 54.Asano H, et al. Plasma resistin concentration determined by common variants in the resistin gene and associated with metabolic traits in an aged Japanese population. Diabetologia. 2010;53:234–246. doi: 10.1007/s00125-009-1517-2. [DOI] [PubMed] [Google Scholar]
- 55.Chen BH, et al. Association of resistin promoter polymorphisms with plasma resistin levels and type 2 diabetes in women and men. Int J Mol Epidemiol Genet. 2010;1:167–174. [PMC free article] [PubMed] [Google Scholar]
- 56.Onuma H, et al. A at single nucleotide polymorphism-358 is required for G at −420 to confer the highest plasma resistin in the general Japanese population. PLoS One. 2010;5:e9718. doi: 10.1371/journal.pone.0009718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Owecki M, et al. Serum Resistin Concentrations are Higher in Human Obesity but Independent from Insulin Resistance. Exp Clin Endocrinol Diabetes. 2010 doi: 10.1055/s-0030-1263111. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 58.Utzschneider KM, et al. Resistin is not associated with insulin sensitivity or the metabolic syndrome in humans. Diabetologia. 2005;48:2330–2333. doi: 10.1007/s00125-005-1932-y. [DOI] [PubMed] [Google Scholar]
- 59.Jain SH, et al. Cross-sectional associations between abdominal and thoracic adipose tissue compartments and adiponectin and resistin in the Framingham Heart Study. Diabetes Care. 2009;32:903–908. doi: 10.2337/dc08-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Won JC, et al. Association of plasma levels of resistin with subcutaneous fat mass and markers of inflammation but not with metabolic determinants or insulin resistance. J Korean Med Sci. 2009;24:695–700. doi: 10.3346/jkms.2009.24.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chu MC, et al. Insulin resistance in postmenopausal women with metabolic syndrome and the measurements of adiponectin, leptin, resistin, and ghrelin. Am J Obstet Gynecol. 2006;194:100–104. doi: 10.1016/j.ajog.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 62.Hansen D, et al. Plasma adipokine and inflammatory marker concentrations are altered in obese, as opposed to non-obese, type 2 diabetes patients. Eur J Appl Physiol. 2010;109:397–404. doi: 10.1007/s00421-010-1362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Azuma K, et al. Correlation between serum resistin level and adiposity in obese individuals. Obes Res. 2003;11:997–1001. doi: 10.1038/oby.2003.137. [DOI] [PubMed] [Google Scholar]
- 64.Edwards C, et al. Downregulation of leptin and resistin expression in blood following bariatric surgery. Surg Endosc. 2010 doi: 10.1007/s00464-010-1494-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Moschen AR, et al. Effects of weight loss induced by bariatric surgery on hepatic adipocytokine expression. J Hepatol. 2009;51:765–777. doi: 10.1016/j.jhep.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 66.Koebnick C, et al. Increase in serum resistin during weight loss in overweight subjects is related to lipid metabolism. Int J Obes (Lond) 2006;30:1097–1103. doi: 10.1038/sj.ijo.0803242. [DOI] [PubMed] [Google Scholar]
- 67.de Luis DA, et al. Resistin Levels in Morbid Obese Patients Following the Biliopancreatic Diversion Surgery. Horm Metab Res. 2011 doi: 10.1055/s-0030-1270529. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 68.Shetty GK, et al. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care. 2004;27:2450–2457. doi: 10.2337/diacare.27.10.2450. [DOI] [PubMed] [Google Scholar]
- 69.Qi Q, et al. Associations of resistin with inflammatory and fibrinolytic markers, insulin resistance, and metabolic syndrome in middle-aged and older Chinese. Eur J Endocrinol. 2008;159:585–593. doi: 10.1530/EJE-08-0427. [DOI] [PubMed] [Google Scholar]
- 70.Fargnoli JL, et al. Resistin is associated with biomarkers of inflammation while total and high-molecular weight adiponectin are associated with biomarkers of inflammation, insulin resistance, and endothelial function. Eur J Endocrinol. 2010;162:281–288. doi: 10.1530/EJE-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Filkova M, et al. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin Immunol. 2009;133:157–170. doi: 10.1016/j.clim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 72.Anderson PD, et al. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab. 2007;92:2272–2279. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- 73.Sunden-Cullberg J, et al. Pronounced elevation of resistin correlates with severity of disease in severe sepsis and septic shock. Crit Care Med. 2007;35:1536–1542. doi: 10.1097/01.CCM.0000266536.14736.03. [DOI] [PubMed] [Google Scholar]
- 74.Koch A, et al. Serum resistin levels in critically ill patients are associated with inflammation, organ dysfunction and metabolism and may predict survival of non-septic patients. Crit Care. 2009;13:R95. doi: 10.1186/cc7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hillenbrand A, et al. Sepsis induced changes of adipokines and cytokines - septic patients compared to morbidly obese patients. BMC Surg. 2010;10:26. doi: 10.1186/1471-2482-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schaffler A, et al. Admission resistin levels predict peripancreatic necrosis and clinical severity in acute pancreatitis. Am J Gastroenterol. 2010;105:2474–2484. doi: 10.1038/ajg.2010.278. [DOI] [PubMed] [Google Scholar]
- 77.Dick GM, et al. Resistin impairs endothelium-dependent dilation to bradykinin, but not acetylcholine, in the coronary circulation. Am J Physiol Heart Circ Physiol. 2006;291:H2997–3002. doi: 10.1152/ajpheart.01035.2005. [DOI] [PubMed] [Google Scholar]
- 78.Ohmori R, et al. Associations between serum resistin levels and insulin resistance, inflammation, and coronary artery disease. J Am Coll Cardiol. 2005;46:379–380. doi: 10.1016/j.jacc.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 79.Pischon T, et al. Association of plasma resistin levels with coronary heart disease in women. Obes Res. 2005;13:1764–1771. doi: 10.1038/oby.2005.215. [DOI] [PubMed] [Google Scholar]
- 80.Weikert C, et al. Plasma resistin levels and risk of myocardial infarction and ischemic stroke. J Clin Endocrinol Metab. 2008;93:2647–2653. doi: 10.1210/jc.2007-2735. [DOI] [PubMed] [Google Scholar]
- 81.Tang NP, et al. A polymorphism in the resistin gene promoter and the risk of coronary artery disease in a Chinese population. Clin Endocrinol (Oxf) 2008;68:82–87. doi: 10.1111/j.1365-2265.2007.03003.x. [DOI] [PubMed] [Google Scholar]
- 82.Yaturu S, et al. Resistin and adiponectin levels in subjects with coronary artery disease and type 2 diabetes. Cytokine. 2006;34:219–223. doi: 10.1016/j.cyto.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 83.Lim S, et al. Association of adiponectin and resistin with cardiovascular events in Korean patients with type 2 diabetes: the Korean atherosclerosis study (KAS): a 42-month prospective study. Atherosclerosis. 2008;196:398–404. doi: 10.1016/j.atherosclerosis.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 84.Luc G, et al. Adipocytokines and the risk of coronary heart disease in healthy middle aged men: the PRIME Study. Int J Obes (Lond) 2010;34:118–126. doi: 10.1038/ijo.2009.204. [DOI] [PubMed] [Google Scholar]
- 85.Wang H, et al. High serum resistin level may be an indicator of the severity of coronary disease in acute coronary syndrome. Chin Med Sci J. 2009;24:161–166. doi: 10.1016/s1001-9294(09)60082-1. [DOI] [PubMed] [Google Scholar]
- 86.Momiyama Y, et al. Serum Resistin Levels and Cardiovascular Events in Patients Undergoing Percutaneous Coronary Intervention. J Atheroscler Thromb. 2010 doi: 10.5551/jat.6023. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 87.Krecki R, et al. Elevated resistin opposed to adiponectin or angiogenin plasma levels as a strong, independent predictive factor for the occurrence of major adverse cardiac and cerebrovascular events in patients with stable multivessel coronary artery disease over 1-year follow-up. Med Sci Monit. 2011;17:CR26–32. doi: 10.12659/MSM.881325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.On YK, et al. Serum resistin as a biological marker for coronary artery disease and restenosis in type 2 diabetic patients. Circ J. 2007;71:868–873. doi: 10.1253/circj.71.868. [DOI] [PubMed] [Google Scholar]
- 89.Zhang MH, et al. Resistin, exercise capacity, and inducible ischemia in patients with stable coronary heart disease: data from the Heart and Soul study. Atherosclerosis. 2010;213:604–610. doi: 10.1016/j.atherosclerosis.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 90.Efstathiou SP, et al. Prognostic significance of plasma resistin levels in patients with atherothrombotic ischemic stroke. Clin Chim Acta. 2007;378:78–85. doi: 10.1016/j.cca.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 91.Liangos O, et al. Whole blood transcriptomics in cardiac surgery identifies a gene regulatory network connecting ischemia reperfusion with systemic inflammation. PLoS One. 2010;5:e13658. doi: 10.1371/journal.pone.0013658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lubos E, et al. Resistin, acute coronary syndrome and prognosis results from the AtheroGene study. Atherosclerosis. 2007;193:121–128. doi: 10.1016/j.atherosclerosis.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 93.Chu S, et al. Plasma resistin associated with myocardium injury in patients with acute coronary syndrome. Circ J. 2008;72:1249–1253. doi: 10.1253/circj.72.1249. [DOI] [PubMed] [Google Scholar]
- 94.Langheim S, et al. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. Am J Physiol Heart Circ Physiol. 2010;298:H746–753. doi: 10.1152/ajpheart.00617.2009. [DOI] [PubMed] [Google Scholar]
- 95.Kim M, et al. Role of resistin in cardiac contractility and hypertrophy. J Mol Cell Cardiol. 2008;45:270–280. doi: 10.1016/j.yjmcc.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang BW, et al. Mechanical stretch enhances the expression of resistin gene in cultured cardiomyocytes via tumor necrosis factor-alpha. Am J Physiol Heart Circ Physiol. 2007;293:H2305–2312. doi: 10.1152/ajpheart.00361.2007. [DOI] [PubMed] [Google Scholar]
- 97.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 2010;11:31–39. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takeishi Y, et al. Serum resistin is associated with high risk in patients with congestive heart failure--a novel link between metabolic signals and heart failure. Circ J. 2007;71:460–464. doi: 10.1253/circj.71.460. [DOI] [PubMed] [Google Scholar]
- 99.Ho YL, et al. Prognostic significance of adipocytokines and extracellular matrix activity in heart failure patients with high B-type natriuretic peptide. Clin Biochem. 2009;42:1407–1412. doi: 10.1016/j.clinbiochem.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 100.Butler J, et al. Serum resistin concentrations and risk of new onset heart failure in older persons: the health, aging, and body composition (Health ABC) study. Arterioscler Thromb Vasc Biol. 2009;29:1144–1149. doi: 10.1161/ATVBAHA.109.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Frankel DS, et al. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol. 2009;53:754–762. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang MH, et al. Association of resistin with heart failure and mortality in patients with stable coronary heart disease: data from the heart and soul study. J Card Fail. 2011;17:24–30. doi: 10.1016/j.cardfail.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 103.Conneely KN, et al. Variation in the resistin gene is associated with obesity and insulin-related phenotypes in Finnish subjects. Diabetologia. 2004;47:1782–1788. doi: 10.1007/s00125-004-1537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Malo E, et al. Resistin Is an Indicator of the Metabolic Syndrome According to Five Different Definitions in the Finnish Health 2000 Survey. Metab Syndr Relat Disord. 2011 doi: 10.1089/met.2010.0106. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]