Abstract
Among non-Hodgkin lymphoma subtypes, T-cell phenotype confers a poor clinical prognosis. For more aggressive histologies, patients frequently present with advanced disease that is inherently chemoresistant. For cutaneous histologies, disease progresses less rapidly, but is debilitating and often incurable long term. Here we report the retrospective analysis of data from 27 patients with mature T-cell lymphoma treated with salvage allo-HCT at the City of Hope using a reduced-intensity fludarabine/melphalan conditioning regimen between the years 2001 and 2008. Eleven of the twenty-seven patients had cutaneous T-cell lymphoma (CTCL). The majority of patients had advanced disease at the time of transplant (17/27 or 63%). Median followup was 36 months. We observed 2-year overall survival (OS) of 55%, progression-free survival (PFS) of 47%, and cumulative incidence of relapse/progression and non-relapse mortality (NRM) of 30% and 22%, respectively. For CTCL, patients had a 2-yr PFS of 45% and NRM of 27% compared to patients with other histologies, who had PFS of 62% and NRM of 19%. Overall, our results suggest that meaningful long term survival rates and disease control can be achieved with acceptable non-relapse mortality in patients with mature T-cell lymphomas, including CTCL using reduced intensity conditioning with melphalan and fludarabine.
Keywords: T-cell lymphoma, reduced intensity conditioning, allogeneic transplant
INTRODUCTION
For patients with aggressive lymphomas, the clinical prognosis for T-cell phenotype is poor in comparison to B-cell phenotype, with the notable exception of ALK+ ALCL.1 Mature T-cell lymphomas comprise only ~7% of all NHL worldwide and include peripheral T-cell lymphoma not-otherwise specified (PTCL-NOS), cutaneous T-cell lymphoma (CTCL), angioimmunoblastic lymphoma (AILT), anaplastic large cell lymphoma (ALCL) and NK/T-cell neoplasms among others.2, 3 Advanced disease stage, poor prognostic factors at presentation4, and inherent chemoresistance play a part in the dismal outcomes.5 The treatment of CTCL represents additional challenges due to the often protracted, debilitating disease course and the absence of a standard of care.6 Current therapeutic strategies for mature T-cell lymphomas are poorly defined and are associated with a high rate of chemorefractory disease and relapse following chemotherapy, both with and without autologous stem cell rescue. As a result, disease progression remains a significant clinical dilemma in the care of these patients.
In response to this often incurable disease course, we have offered allogeneic (related or matched unrelated) allo-HCT to mature T-cell lymphoma patients as salvage therapy, in an attempt to harness a potential graft-versus-lymphoma (GVL) effect.7–13 Allo-HCT is a potentially curative option, but is associated with high non-relapse mortality (NRM). It is now well established that reduced intensity transplant conditioning (RIC) regimens confer the benefits of the graft-versus-malignancy effect, with efficacies similar to myeloablative conditioning for the treatment of some hematological malignancies,14, 15 including NHL.13, 16, 17 Since RIC regimens have lower intensity and toxicity, they are available to older and more heavily pre-treated patients without increasing NRM; however, their effectiveness varies for different diseases.
Here we report the retrospective analysis of patients with mature T-cell lymphomas who received salvage therapy using allo-HCT with a standardized reduced intensity fludarabine/melphalan conditioning regimen at the City of Hope between 2001 and 2008.
PATIENTS AND METHODS
Inclusion Criteria
Following approval by the Institutional Review Board, a query of the COH transplant database identified a consecutive case-series of 27 patients with mature T-cell lymphomas treated with allo-HCT (HLA-matched sibling or matched unrelated donor) between 2001 and 2008 using a standardized reduced intensity fludarabine/melphalan conditioning regimen. Allo-HCT was offered to the patients with refractory or relapsed disease who were not candidates for autologous HCT due to chemo-refractory/progressive disease, extensive bone marrow involvement, or failure to adequately mobilize stem cells.18, 19 Patients with CTCL were not considered candidates for autologous HCT because of disappointing outcomes with this approach.20, 21 Per institutional practice, all patients were evaluated pre-transplant for organ function of heart, lungs and kidneys. Donor selection was based on the availability of an HLA-identical family donor, or alternatively from an HL-matched unrelated donor based on molecular typing of HLA A, B, C, DR, and DQ loci.
Treatment Regimen
All patients underwent RIC with fludarabine 25 mg/m2 IV daily on days -9 through -5 and melphalan 140 mg/m2 IV on day -4 prior to infusion of bone marrow (11%) or peripheral blood stem cells (89%). 56% of patients received stem cells from HLA-matched siblings, and 44% from matched unrelated donors. A total of 18 patients (67%) received GVHD prophylaxis with sirolimus/tacrolimus, while 9 patients (33%) received cyclosporine/mycophenolate mofetil (MMF)-based prophylaxis. GVHD prophylaxis was at the discretion of treating physicians based on institutional protocols, donor type and product source (prophylaxis for individual patients listed in Table 1).
Table 1.
Summary, Patient Characteristics and Outcomes
| Characteristic/Outcome | N (%) or Median (Range) |
|---|---|
| Patient Gender | |
| Male | 20 (74) |
| Female | 7 (26) |
| Age at Transplant (Years) | 50 (19 – 68) |
| Histology | |
| CTCL | 11 (41) |
| Other | 16 (59) |
| Donor Type | |
| Sibling | 15(56%) |
| Matched Unelated Donor | 12 (44) |
| Disease Status at Transplant | |
| 1st CR/ 2nd CR/ PR | 10 (37) |
| Relapse/ Progression/ Induction Failure | 17 (63) |
| Chemotherapy responsiveness | |
| Refractory | 11 (41) |
| Sensitive | 16 (59) |
| Stem Cell Source | |
| Bone Marrow | 3 (11) |
| Peripheral Blood | 24 (89) |
| Total Regimens Prior to HCT | 4 (1 – 9) |
| CTCL | 6 (4 – 9) |
| Other | 3 (1 – 8) |
| GVHD Prophylaxis | |
| CSA/MMF-Based | 9 (33) |
| Sirolimus-Based | 18 (67) |
| Engraftment: Days to ANC > 500 | 14 (12 – 23) |
| Acute GVHD (yes) | 13 (48) |
| Grade I | 4 |
| Grade II | 5 |
| Grade III | 0 |
| Grade IV | 4 |
| None | 14 (52) |
| Chronic GVHD | 18 (67) |
| Limited | 3 |
| Extensive | 15 |
| None | 3 (11) |
| Not Evaluable (expired<100 days) | 6 (22) |
| Relapse/Progression Post-HCT | 8 (30) |
| Cause of Death | |
| Disease Progression | 5 |
| Infection/GVHD | 9 |
| Day 100 Mortality | |
| Disease Progression | 1 |
| Infection/GVHD | 5 |
| Follow-up (Months) | |
| All patients | 15.6 (1.0 – 104.0) |
| Alive | 35.7 (12.6 – 103.3) |
| Dead | 6.0 (1.9 – 12.2) |
CTCL = cutaneous T-cell lymphoma, CR = complete remission, PR = partial remission, GVHD = graft vs host disease, CSA = cyclosporine A, MMF = mycophen-olate mofetil,,
Patient characteristics
Summary patient characteristics are listed in Table 1, with individual patient details displayed in Table 2. Mature T-cell lymphoma histologies included: periphera l T-cell lymphoma not otherwise specified (PTCL NOS) (n=5); angioimmunoblastic T cell lymphomas (AITL) (n=3); anaplastic large-cell lymphomas (ALCL) (n=2; both ALK+); rare histologies (n=6, NK/T cell nasal and extranasal, subcutaneous panniculitis-like); and cutaneous T-cell lymphoma (CTCL) (n=11, mycosis fungoides and Sezary syndrome). Most patients (n=17, 63%) had advanced disease at the time of transplant: relapse, induction failure or primary refractory disease. The rest of the patients were in complete remission (CR1=1, CR2=5) and partial response (PR=4). Among CTCL patients, only one was in CR following 6 different treatment regimens, and another was in PR after 4 prior regimens. The median age was 50 years (range: 19–68) and 74% of the patients were male (n=20). The median time from diagnosis to transplant for the majority of the patients was greater than one year: for CTCL it was 28.8 months (range 17.2 – 84.2); for other subtypes it was 12.6 months (range 6.2 –112.3). The median number of prior regimens was 4 (range: 1–9) for all patients, 6 (range 4–9) for patients with CTCL, and 3 (range 1–8) for other patients. Only one patient had undergone prior autologous transplant.
Table 2.
Individual Patient Characteristics and Outcomes
| Pt # |
Histology | Age | KPS | Prior Regimens |
Status at HCT |
Donor Type |
GVHD Prophylaxis | aGVHD Grade |
cGVHD Grade |
Relapse/Prog Day post-HCT |
Cause of Death Day post-HCT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CTCL | 32 | 80 | 7 | IF | MUD | Siro/Tacro/MTX | Grade II | Extensive | Relapse: d 72 | Prog: d 213 |
| 2 | CTCL | 35 | 60 | 8 | IF | MUD | CSA/MMF/MTX | none | NA | Infection: d 34 | |
| 3 | CTCL | 35 | 80 | 4 | IF | MUD | CSA/MMF/ATG | Grade IV | NA | Infection: d 91 | |
| 4 | CTCL | 50 | 80 | 4 | IF | MUD | CSA/MMF | Grade II | Extensive | ||
| 5 | CTCL | 50 | 90 | 6 | CR1 | MUD | CSA/MMF/MTX | none | None | ||
| 6 | CTCL | 50 | 90 | 4 | PR1 | SIB | Siro/Tacro | none | None | ||
| 7 | CTCL | 53 | 70 | 4 | IF | SIB | Siro/Tacro | none | Extensive | ||
| 8 | CTCL | 55 | 80 | 9 | IF | SIB | Siro/Tacro | Grade IV | NA | Prog: d 48 | Infection: d 67 |
| 9 | CTCL | 56 | 90 | 8 | R1 | SIB | Siro/Tacro | none | Extensive | Relapse: d 147 | Prog: d 238 |
| 10 | CTCL | 59 | 80 | 6 | IF | SIB | CSA/MMF | none. | Extensive | Infection d 3165 | |
| 11 | CTCL | 61 | 80 | 4 | IF | SIB | Siro/Tacro | Grade IV | NA | aGVHD: d 71 | |
| 12 | PTCL-NOS | 32 | 80 | 4 | R1 | SIB | Siro/Tacro | none | None | Prog: d 129 | Prog: d 370 |
| 13 | PTCL-NOS | 52 | 95 | 3/auto* | CR2 | SIB | CSA/MMF | none | Extensive | ||
| 14 | PTCL-NOS | 53 | 90 | 2 | CR2 | SIB | Siro/Tacro | none | Limited | ||
| 15 | PTCL-NOS | 57 | 100 | 4 | R1 | MUD | Siro/Tacro/MTX | Grade II | Extensive | ||
| 16 | PTCL/CD8+ | 61 | 100 | 5 | IF | MUD | Siro/Tacro/MTX | Grade II | Limited | ||
| 17 | NK-T cell | 19 | 100 | 2 | R1 | MUD | CSA/MMF/MTX | Grade I | Limited | Infection: d 655 | |
| 18 | NK/T cell | 23 | 70 | 3 | CR2 | MUD | Siro/Tacro | none. | Extensive | Relapse: d 62 | Prog: d 293 |
| 19 | NK-T cell | 24 | 90 | 3 | IF | SIB | Siro/Tacro | none | NA | Prog: d 27 | Prog: d 30 |
| 20 | NK-T cell | 32 | 80 | 1 | PR1 | SIB | Siro/Tacro | Grade 2 | Extensive | Relapse: d 235 | Alive – 5 yrs |
| 21 | NK-T cell | 38 | 100 | 3 | CR2 | SIB | Siro/Tacro | none | Extensive | Infection: d 832 | |
| 22 | AILD | 60 | 80 | 2 | PR1 | MUD | Siro/Tacro/MTX | Grade I | Extensive | ||
| 23 | AILD | 61 | 100 | 4 | IF | MUD | Siro/Tacro/MTX/ATG | Grade I | Extensive | ||
| 24 | AILD | 68 | 95 | 6 | IF | SIB | Siro/Tacro | none | Extensive | ||
| 25 | ALCL/ALK+ | 20 | 80 | 2 | CR2 | MUD | CSA/MMF/MTX | none | Extensive | cGVHD: d 152 | |
| 26 | ALCL/ALK+ | 57 | 100 | 3 | PR1 | SIB | CSA/MMF | Grade I | Extensive | Relapse: d 279 | Alive - 7 yrs |
| 27 | sub panniculitis | 40 | 60 | 8 | IF | SIB | Siro/MMF | Grade IV | NA | Infection: d 57 |
includes autologous transplant, KPS – Karnofsky Performance Status score (defined Schag. 1984),CR - complete remission, PR – partial remission, R – relapse, IF – induction failure, PP – primary progressive, MUD – matched unrelated donor, SIB – sibling donor, CSA – cyclosporine, MMF – mycophenylate mofetil,, MTX – methotrexate, SIR – sirolimus, TAC – tacrolimus, ATG – anti-thymocyte globulin, rd = relapse day, dd = death day
Statistics
Survival estimates were calculated based on the product-limit method, and 95% confidence intervals were calculated using the logit transformation and the Greenwood variance estimate.22 Differences between survival curves were assessed by the log rank test. Overall survival (OS) was measured from stem cell infusion to death from any cause. Progression-free survival (PFS) was defined as time from stem cell infusion to recurrence, progression or death from any cause, whichever occurred first. The cumulative incidence of relapse/progression rate was defined as time from stem cell infusion to recurrence or progression. Non-relapse mortality (NRM) (cumulative incidence) was measured from stem cell infusion to death from any cause other than disease relapse or disease progression. Cumulative relapse/progression incidence was calculated controlling for non-relapse related death as a competing risk and conversely, NRM was calculated controlling for relapse/progression as a competing risk.23 Differences between survival curves were assessed by log-rank or Gray’s test24 as appropriate. The significance of demographic/treatment features collected on HCT recipients was assessed by the log rank test, and univariate/multivariable Cox regression analysis25 or the corresponding hazard analysis for competing risks26. Generally statistical significance was defined at the p≤.05 level. As part of the multivariable Cox regression analysis, factors shown to be significant at the 0.10 level in the univariate analysis were eligible for inclusion. Analysis of factors known to be associated with survival and disease outcomes included: histopathological subtype (CTCL vs other histologies), patient age (younger or older 50 years), disease status at the time of allo-HCT (CR/PR vs relapse/progression/induction failure), donor type (sibling vs matched unrelated), acute GVHD grade (0–II vs III–IV), chronic GVHD (none vs present and none/limited vs extensive), and GVHD prophylaxis (sirolimus-based vs other). All analyses were performed using SAS® version 9.2 and/or R 2.11.1.
RESULTS
The median follow-up for the 13 (48%) surviving patients was 35.7 months (range: 12.6–103.3), with median follow-up of 15.6 months for all patients (range: 1.0–104). All patients engrafted. Thirteen patients (48%) experienced acute GVHD: grade I = 4, grade II = 5, grade IV = 4. Among the 21 patients who survived past day 100 and were evaluable for chronic GVHD, 15 (71 %) patients developed extensive cGVHD, 3 (14%) developed limited disease and 3 (14%) had no cGVHD. Table 1 lists summary outcomes for the patient population and Table 2 details outcomes for individual patients.
Survival Outcomes
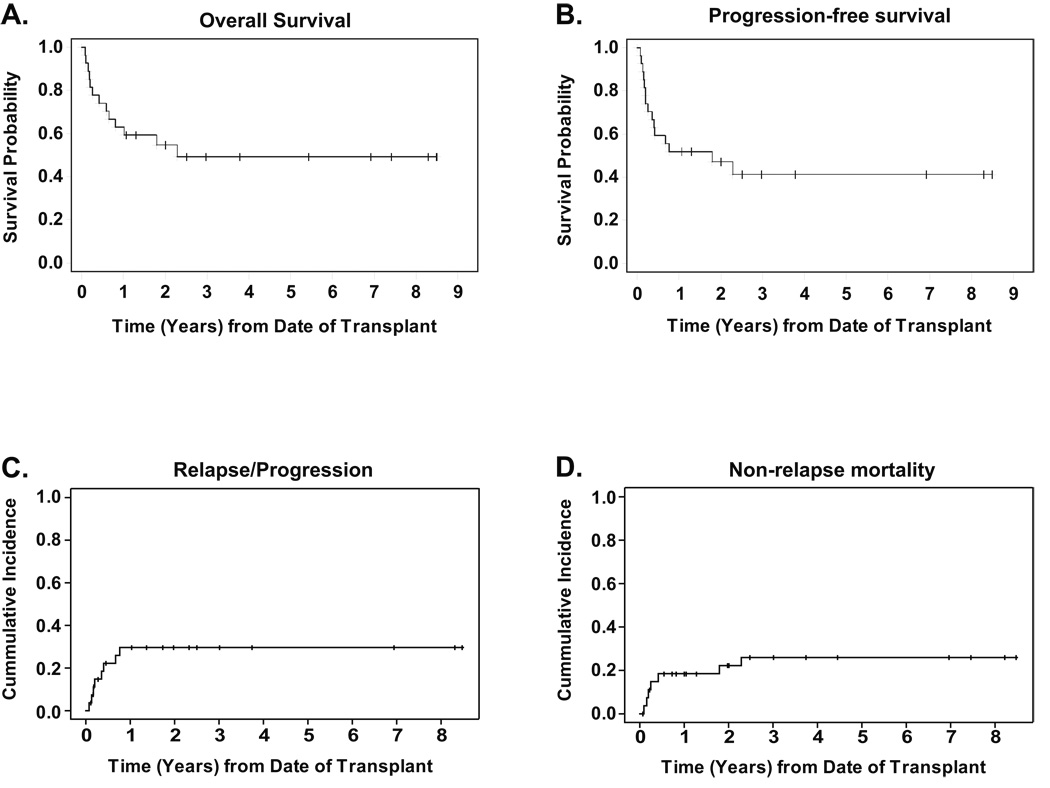
There were a total of 14 deaths; 5 from disease progression/relapse and 9 from non-relapse causes. Non-relapse causes (n=9) included infections (bacterial sepsis, pneumonias due to bacteria, RSV and influenza A, CMV infections) and GVHD related complications. Day 100 mortality was 22% (n=6). The 2-year probability of OS was 55% (95%CI: 44–64%) and PFS was 47% (95%CI: 38–56%), as seen in Figure 1A and 1B. The cumulative incidence of relapse/progression and NRM at 2 years was 30% (95%CI: 15–50%) and 22% (95%CI: 10–42%) respectively, curves shown in Figure 1C and 1D.
Figure 1. Outcomes.
All curves are for the 27-patient population with a median follow-up of 35.7 months for survivors. Panel A shows the Kaplan-Meier estimate of survival probability. Panel B shows progression-free survival, defined as time from stem cell infusion to recurrence, progression, or death from any cause, whichever occurred first. Panel C shows cummulative incidence of relapse/progression, defined as time from stem cell infusion to recurrence or progression. Panel D shows non-relapse mortality, measured as time from stem cell infusion to death from any cause other than disease relapse or disease progression. In panels C and D, relapse/progression and non-relapse mortality were treated as competing risks.
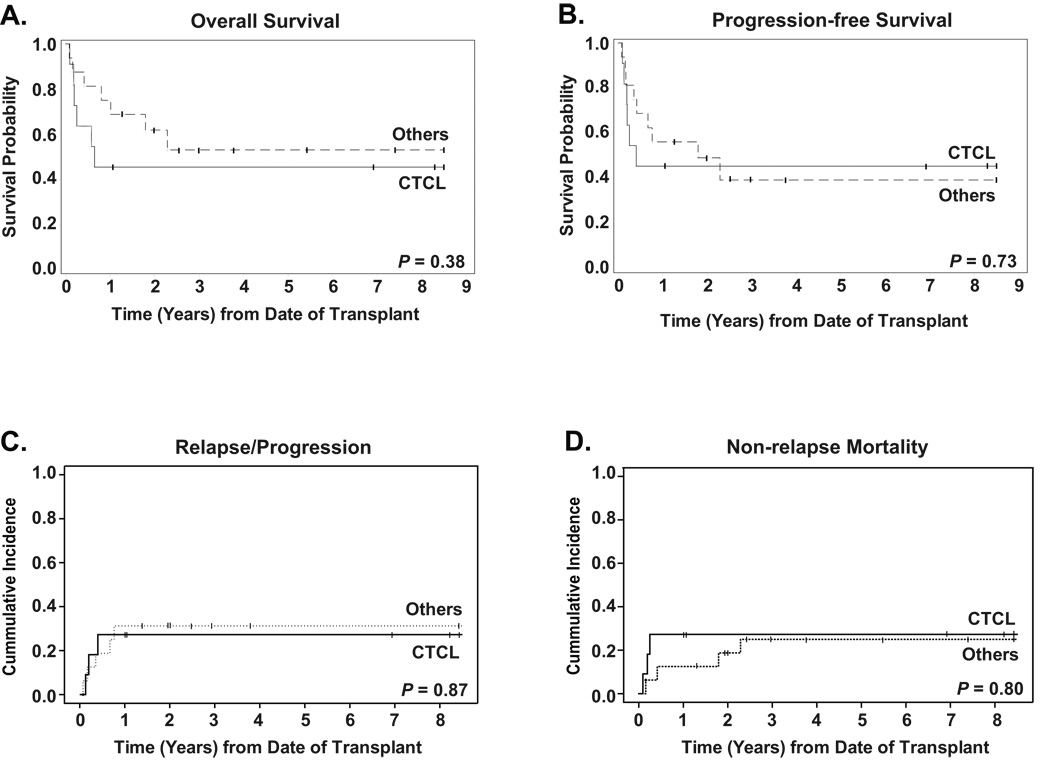
When patients were stratified by histology, as seen in Figure 2, 2-yr OS for CTCL was 45% (95%CI: 32–58%) compared to 62% (95%CI: 45–75%) for the other patients (P=0.38), with PFS of 45% (95%CI: 32–58%) for CTCL and 49% (95%CI: 36–61%) for others (P=0.73). Relapse/progression was similar for both groups with CTCL showing a 2-yr cumulative incidence of 27% (95%CI: 8–61%) versus 31 (95%CI: 13–58%) for non-cutaneous histologies. Non-relapse mortality at 2-years was 27% (95%CI: 8–60%) for the CTCL patients, compared to 19% (95%CI: 6–46%) for the other patients.
Figure 2. Outcomes stratified by histology.
All curves are stratified for histology: CTCL (n=11) versus Others (n=16), with a median follow-up of 83 months for the 5 CTCL surviving patients and 36 months for the 9 Others. P-values for long-rank comparisonof curves is given in the bottom right hand corner of each graph. Panel A shows the Kaplan-Meier estimate of survival probability. Panel B shows progression-free survival, defined as time from stem cell infusion to recurrence, progression, or death from any cause, whichever occurred first. Panel C shows cumulative incidence of relapse/progression, defined as time from stem cell infusion to recurrence or progression. Panel D shows non-relapse mortality, measured as time from stem cell infusion to death from any cause other than disease relapse or disease progression. For data in panels C and D, relapse/progression and non-relapse mortality were treated as competing risks.
Among this high risk patient population, relapse/progression and progression-related death events occurred in the first 1.5 years post transplant; after that no events have been observed thus far. Two of eight patients who progressed shortly after transplant received additional treatment and remain alive 5 and 7 years after transplant; both patients had modification of immunosupression that resulted in extensive chronic GVHD. None of the patients received donor lymphocyte infusion during the post-transplant course.
Univariate/Multivariable analyses
Univariate and multivariable results for PFS are shown in Table 3. Log-rank and Cox regression univariate analyses assessed factors known to be associated with survival and disease outcomes: age at transplant, histopathological subtype, disease status at transplant, donor type, GVHD prophylaxis, presence and grade of GVHD. Patients who were older (≥ 50 years) at the time of transplant, and also those who developed little to no acute GVHD (grade 0–II) had significantly improved overall and progression-free survival. There was no statistically significant impact on PFS, OS, RPR or NRM based on histopathological subtype (CTCL vs. other subtypes), donor type (MUD versus sibling donor), type of GHVD prophylaxis (CSA/MMF versus sirolimus/tacrolimus combinations), presence of cGVHD (none or none/limited vs. extensive), or disease status at the time of transplant. All factors found to be significant at the P ≤ 0.1 level in the univariate analysis were analyzed using multivariable Cox regression analysis. The results of this analysis showed that both younger age and the presence of acute GVHD (grade III/IV) were significantly and independently associated with an increase in PFS hazard risk.
Table 3.
Univariate and Multivariable Analyses of Progression-Free Survival
| Univariate | Reference Group |
Total (N) |
Events/ Censored |
p-Value | Hazard Ratio |
95% Confidence Limits |
|---|---|---|---|---|---|---|
| Age at HCT | Age < 50 | 27 | 16/11 | **0.0005 | **0.13 | 0.04 – 0.41 |
| Diagnosis at HCT | non-CTCL | 27 | 16/11 | 0.74 | 1.20 | 0.42 – 3.38 |
| Disease Status at HCT | 1CR/2CR/PR | 27 | 16/11 | 0.37 | 1.63 | 0.55 – 4.79 |
| Donor Type | Sibling | 27 | 16/11 | 0.62 | 0.77 | 0.27 – 2.17 |
| GVHD Prophylaxis | Siro-Based | 27 | 16/11 | 0.70 | 0.81 | 0.28 – 2.38 |
| aGVHD Max Grade | Grades 0–II | 27 | 16/11 | **0.02 | **7.99 | 1.44 – 44.41 |
| cGVHD Max Grade | Limited | 18 | 9/9 | 0.23 | 2.76 | 0.54 – 14.27 |
| Multivariable |
Reference Group |
Total (N) |
Events/ Censored |
p-Value |
Hazard Ratio |
95% Confidence Limits |
| Age at HCT | Age < 50 | 27 | 16/11 | 0.002 | 0.14 | 0.43 – 0.48 |
| aGVHD Max Grade | Grades 0–II | 27 | 16/11 | 0.02 | 5.68 | 1.39 – 23.3 |
significant to P = 0.01, CTCL = cutaneous T-cell lymphoma, T-NHL =, mature T-cell non-Hodgkin lymphoma, CR = complete remission, PR = partial remission, GVHD = graft vs host disease, a GVHD = acute graft vs host disease, cGVHD = chronic graft vs host disease,
DISCUSSION
It has been difficult to rigorously determine whether RIC allo-HCT is the optimal treatment for advanced hematological diseases, due to a lack of large randomized trials. Assessing RIC for mature T-cell lymphomas, and CTCL in particular, has the additional impediment of the rarity of the diagnosis and the limited data on allo-HCT for these diseases in general. Two European multi-center retrospective studies of allo-HCT (both myeloablative and RIC) for aggressive T-cell lymphoma7 and for angioimmunoblastic T-cell lymphoma (AILT)27 respectively reported 3-yr OS of 57% and 64% and 1-yr NRM of 30% and 25%. Neither study reported a significant difference between RIC and myeloablative regimens in outcomes. Rodriguez et al.16 directly compares conventional myeloablative conditioning to a fludarabine/melphalan RIC regimen for mixed NHL histologies, and sees similar overall survivals between the two regimens, even in the small T-NHL subgroup. In that study the RIC group was at higher-risk due to older median age, a higher proportion of previous autologous transplants and a higher percentage of unrelated donors in that group. Corradini et al. in a prospective phase II trial treated 17 PTCL patients with a fludarabine, thiotepa, and cyclophosphamide RIC regimen, and report an impressive 3 yr OS of 81%, PFS of 60% and 1 yr NRM of 6%10; it is notable that 15 of these 17 patients were chemosensitive. In an expansion and update of this Corradini dataset, reported in a review by Gutierrez et al., for a population of 35 that included chemorefractory patients, the OS/PFS declined to 54%/49%, with chemorefractory patient PFS at 25% compared to 72% for those transplanted in CR13. There is some evidence that the prognostic differences between B-cell and T-cell histologies may be abrogated following treatment by allogeneic transplant; studies by both Rodriguez and Corradini see similar survival and relapse rates for the B- and T-NHL phenotypes after RIC allo-HCT 9, 16.
The literature on allogeneic transplant for CTCL is sparse; until recently, only case reports and small series have been published, including an 8-patient series from City of Hope.28 A recent retrospective analysis of 60 CTCL patients from the EBMT registry29 provides new insight into the role of allo-HCT for this rare disease. Duarte et al. estimate an OS of 54% at 3 years, and show that use of RIC decreases the relative risk for NRM without increasing the relapse rate, resulting in a significantly higher OS.29 The major caveat of this report is the extensive use of T-cell depletion (42%) associated with a reduced PFS.
Here we report the results of a retrospective analysis of 27 patients treated with fludarabine/melphalan RIC alloHCT. The choice of fludarabine/melphalan regimen was based on the hypothesis that these agents have a substantial anti-lymphoma effect and can provide sufficient disease control while the GVL becomes established. Our data showed a 2-year OS of 55%, PFS of 47%, relapse/progression rate of 29% and NRM of 25%. Survival curves in this population appear to plateau after 24 months. Taking into consideration that 13 of 27 patients were induction failures, these results are encouraging.
As 41% of patients in our report had the diagnosis of CTCL, representing a distinctive patient subgroup, we sought to evaluate potential difference between this and other mature T-cell lymphoma histological subtypes. CTCL is characterized by a protracted disease course marked by transient responses to systemic treatment, followed by inevitable recurrence. Patients are in an immunosuppressed state for years, due both to disease persistence and multiple lines of treatment, leading in many cases to death from infections and complications. CTCL remains an incurable disease and patients with advanced stage disease have a median survival of between 1.5 to 4 years from the time of diagnosis.30, 31. This pattern is reflected in our patient group with 9/11 patients with advanced disease at the time of transplant after a median of 6 prior treatment regimens. The 2-year OS and PFS for our CTCL patients after reduced-intensity allo-HCT was 45%, despite a median of 6 prior regimens per patient. Interestingly enough, the diagnosis of CTCL in our analysis was not associated with a statistically significant difference in OS, PFS, NRM or RR compared to other subtypes of mature T-cell NHL (Figure 2). Our observations reiterate the conclusion by Duarte et al. that use of the RIC protocol makes allo-HCT a feasible option, even for these high-risk patients. Performing allo-HCT earlier in the course of the disease, especially for advanced stage CTCL, might well improve the outcome and quality of life for these patients.
While the number of patients is insufficient to draw solid conclusions from the univariate and multivariable analyses, the following observations were made. Age ≥50years appeared to be associated with improved survival, which may be attributed to confounding factors in this dataset, specifically a high proportion of aggressive type lymphoma in the younger patient group; 5 of 11 patients under 50 years of age had high-grade NK-T lymphoma and one patient had subcutaneous panniculitis type lymphoma. In contrast, half the patients older than 50 years had CTCL and only one had high-grade lymphoma. The superior survival for older patients (80% at 2 yrs, n=16) underscores that the melphalan/fludarabine regimen is well tolerated by older patients if the disease can be controlled.
The only other factor to have a significant increase in hazard ratio was the presence of grades III–IV aGVHD compared to Grades 0–II. Five of thirteen cases of aGVHD were grouped as grade III–IV, and all five of them happened to be grade IV. It is therefore not surprising that these patients with very severe aGVHD should be at higher risk for fatal complications.
The day 100 mortality of 22% (6/27) and NRM rate of 25% at 2 years highlight the need for improved control of infections, GVHD and other complications of allo-HCT. When examined on a case by case basis, early NRM appears to correspond to low KPS at the time of transplant corroborating the role of patient selection in transplant outcomes.
Overall, our results demonstrate that meaningful long term survival rates and disease control can be achieved even in heavily pretreated mature T-cell lymphoma patients using reduced intensity conditioning with melphalan and fludarabine. This retrospective single-institution study is an important addition to the literature supporting the potential for cure of mature T-cell lymphoma patients using RIC allo-HCT. While the patient population in our report is heterogeneous – reflecting the scarcity and diversity of the disease – the standardized conditioning regimen allows realistic assessment of efficacy and toxicity of this treatment modality. Due to the rarity of the disease, large multi-center prospective trials would be required to accurately define treatment parameters.
ACKNOWLEDGMENTS
This study was supported in part by grants from the National Institute of Health, CA30206 and CA33572 and CA107399. We would like to acknowledge the dedicated nurses of the City of Hope Bone Marrow Unit for excellent care of our patients and support of medical research.
REFERENCES
- 1.Gisselbrecht C, Gaulard P, Lepage E, Coiffier B, Briere J, Haioun C, et al. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin's lymphomas. Groupe d'Etudes des Lymphomes de l'Adulte (GELA) Blood. 1998;92(1):76–82. [PubMed] [Google Scholar]
- 2.Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110(2):695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 4.Lopez-Guillermo A, Cid J, Salar A, Lopez A, Montalban C, Castrillo JM, et al. Peripheral T-cell lymphomas: initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. Classification. Ann Oncol. 1998;9(8):849–855. doi: 10.1023/a:1008418727472. [DOI] [PubMed] [Google Scholar]
- 5.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4121–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 6.Gardner JM, Evans KG, Musiek A, Rook AH, Kim EJ. Update on treatment of cutaneous T-cell lymphoma. Curr Opin Oncol. 2009;21(2):131–137. doi: 10.1097/CCO.0b013e3283253190. [DOI] [PubMed] [Google Scholar]
- 7.Le Gouill S, Milpied N, Buzyn A, De Latour RP, Vernant JP, Mohty M, et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J Clin Oncol. 2008;26(14):2264–2271. doi: 10.1200/JCO.2007.14.1366. [DOI] [PubMed] [Google Scholar]
- 8.Hamadani M, Awan FT, Elder P, Lin TS, Porcu P, Blum KA, et al. Allogeneic hematopoietic stem cell transplantation for peripheral T cell lymphomas; evidence of graft-versus-T cell lymphoma effect. Biol Blood Marrow Transplant. 2008;14(4):480–483. doi: 10.1016/j.bbmt.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Corradini P, Dodero A, Farina L, Fanin R, Patriarca F, Miceli R, et al. Allogeneic stem cell transplantation following reduced-intensity conditioning can induce durable clinical and molecular remissions in relapsed lymphomas: pre-transplant disease status and histotype heavily influence outcome. Leukemia. 2007;21(11):2316–2323. doi: 10.1038/sj.leu.2404822. [DOI] [PubMed] [Google Scholar]
- 10.Corradini P, Dodero A, Zallio F, Caracciolo D, Casini M, Bregni M, et al. Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin's lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J Clin Oncol. 2004;22(11):2172–2176. doi: 10.1200/JCO.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 11.Wulf GG, Hasenkamp J, Jung W, Chapuy B, Truemper L, Glass B. Reduced intensity conditioning and allogeneic stem cell transplantation after salvage therapy integrating alemtuzumab for patients with relapsed peripheral T-cell non-Hodgkin's lymphoma. Bone Marrow Transplant. 2005;36(3):271–273. doi: 10.1038/sj.bmt.1705036. [DOI] [PubMed] [Google Scholar]
- 12.de Lavallade H, Cassier PA, Bouabdallah R, El-Cheikh J, Faucher C, Furst S, et al. Sustained response after reduced-intensity conditioning allogeneic stem cell transplantation for patients with relapsed peripheral T-cell non-Hodgkin lymphoma. Br J Haematol. 2008;142(5):848–850. doi: 10.1111/j.1365-2141.2008.07212.x. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez A, Caballero MD, Perez-Manga G, Rodriguez J. Hematopoietic SCT for peripheral T-cell lymphoma. Bone Marrow Transplant. 2008;42(12):773–781. doi: 10.1038/bmt.2008.332. [DOI] [PubMed] [Google Scholar]
- 14.Khouri IF, Keating M, Korbling M, Przepiorka D, Anderlini P, O'Brien S, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16(8):2817–2824. doi: 10.1200/JCO.1998.16.8.2817. [DOI] [PubMed] [Google Scholar]
- 15.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez R, Nademanee A, Ruel N, Smith E, Krishnan A, Popplewell L, et al. Comparison of reduced-intensity and conventional myeloablative regimens for allogeneic transplantation in non-Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12(12):1326–1334. doi: 10.1016/j.bbmt.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Vigouroux S, Michallet M, Porcher R, Attal M, Ades L, Bernard M, et al. Long-term outcomes after reduced-intensity conditioning allogeneic stem cell transplantation for low-grade lymphoma: a survey by the French Society of Bone Marrow Graft Transplantation and Cellular Therapy (SFGM-TC) Haematologica. 2007;92(5):627–634. doi: 10.3324/haematol.10924. [DOI] [PubMed] [Google Scholar]
- 18.Jagasia M, Morgan D, Goodman S, Hamilton K, Kinney M, Shyr Y, et al. Histology impacts the outcome of peripheral T-cell lymphomas after high dose chemotherapy and stem cell transplant. Leuk Lymphoma. 2004;45(11):2261–2267. doi: 10.1080/10428190412331272749. [DOI] [PubMed] [Google Scholar]
- 19.Zamkoff KW, Matulis MD, Mehta AC, Beaty MW, Hutchison RE, Gentile TC. High-dose therapy and autologous stem cell transplant does not result in long-term disease-free survival in patients with recurrent chemotherapy-sensitive ALK-negative anaplastic large-cell lymphoma. Bone Marrow Transplant. 2004;33(6):635–638. doi: 10.1038/sj.bmt.1704392. [DOI] [PubMed] [Google Scholar]
- 20.Duarte RF, Schmitz N, Servitje O, Sureda A. Haematopoietic stem cell transplantation for patients with primary cutaneous T-cell lymphoma. Bone Marrow Transplant. 2008;41(7):597–604. doi: 10.1038/sj.bmt.1705968. [DOI] [PubMed] [Google Scholar]
- 21.Wu PA, Kim YH, Lavori PW, Hoppe RT, Stockerl-Goldstein KE. A meta-analysis of patients receiving allogeneic or autologous hematopoietic stem cell transplant in mycosis fungoides and Sezary syndrome. Biol Blood Marrow Transplant. 2009;15(8):982–990. doi: 10.1016/j.bbmt.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breslow NE, Day NE. Statistical methods in cancer research: volume II, the design and analysis of cohort studies. IARC Sci Publ. 1987;82:1–406. [PubMed] [Google Scholar]
- 23.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1140–1154. [Google Scholar]
- 25.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society. 1972;B34:187–220. [Google Scholar]
- 26.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 27.Kyriakou C, Canals C, Finke J, Kobbe G, Harousseau JL, Kolb HJ, et al. Allogeneic stem cell transplantation is able to induce long-term remissions in angioimmunoblastic T-cell lymphoma: a retrospective study from the lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27(24):3951–3958. doi: 10.1200/JCO.2008.20.4628. [DOI] [PubMed] [Google Scholar]
- 28.Molina A, Zain J, Arber DA, Angelopolou M, O'Donnell M, Murata-Collins J, et al. Durable clinical, cytogenetic, and molecular remissions after allogeneic hematopoietic cell transplantation for refractory Sezary syndrome and mycosis fungoides. J Clin Oncol. 2005;23(25):6163–6171. doi: 10.1200/JCO.2005.02.774. [DOI] [PubMed] [Google Scholar]
- 29.Duarte RF, Canals C, Onida F, Gabriel IH, Arranz R, Arcese W, et al. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sezary syndrome: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2010;28(29):4492–4499. doi: 10.1200/JCO.2010.29.3241. [DOI] [PubMed] [Google Scholar]
- 30.Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term Outcome of 525 Patients With Mycosis Fungoides and Sezary Syndrome: Clinical Prognostic Factors and Risk for Disease Progression. Arch Dermatol. 2003;139(7):857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- 31.Arulogun SO, Prince HM, Ng J, Lade S, Ryan GF, Blewitt O, et al. Long-term outcomes of patients with advanced-stage cutaneous T-cell lymphoma and large cell transformation. Blood. 2008;112(8):3082–3087. doi: 10.1182/blood-2008-05-154609. [DOI] [PubMed] [Google Scholar]