Abstract
Steroid-induced changes in dopaminergic activity underlie many correlations between gonadal hormones and social behaviors. However, the effects of steroid hormones on the various behaviorally relevant dopamine cell groups remain unclear, and ecologically relevant species differences remain virtually unexplored. We examined the effects of estradiol (E2) manipulations on dopamine (DA) neurons of male and female zebra finches (Taeniopygia guttata), focusing on numbers of tyrosine hydroxylase-immunoreactive (TH-ir) cells in the A8–A15 cell groups, and on TH colocalization with Fos, conducted in the early A.M., in order to quantify basal transcriptional activity. TH is the rate-limiting enzyme for catecholamine synthesis, and specifically DA in the A8–A15 cell groups. In contrast to other examined birds and mammals, reducing E2 levels with the aromatase-inhibitor Letrozole failed to alter TH-ir neuron numbers within the ventral tegmental area (VTA; A10), while increasing neuron numbers in the central gray (CG; A11) and caudal midbrain A8 populations. Consistent with findings in other birds, but not mammals, we also found no effects of E2 manipulations (Letrozole or Letrozole plus E2 replacement) on TH-Fos colocalization in any location. In accordance with previous observations in both mammals and birds, E2 treatment decreased the number of TH-ir neurons in the A12 population of the tuberal hypothalamus, a cell group that inhibits the release of prolactin. In general, males and females exhibited similar TH-ir neuron numbers, although males exhibited significantly more TH-ir neurons in the A11 CG population than did females. These results suggest partial variability in E2 regulation of DA across species.
Keywords: dopamine, estradiol, zebra finch, bird, Fos, opportunistic breeder
1. Introduction
In all vertebrate species, the catecholaminergic neurotransmitter dopamine (DA)1 modulates motivated, goal-directed social behaviors, such as those associated with reproduction and aggression [e.g., 9,15,24,35,59,68,80,83,84]. In many species, such behaviors are also regulated by levels of circulating steroid hormones, and thus vary with reproductive state and season [e.g., 18,30,78,82]. Because a number of catecholaminergic populations express steroid hormone receptors across a variety of species [e.g., 12,20,39,49,70], it has been hypothesized that steroid hormones directly or indirectly regulate social behaviors via modification of DA circuits [reviewed in 7].
Sex steroids, especially estradiol (E2), have now been shown to regulate DA activity and synthesis via a wide variety of mechanisms, including the phosphorylation of tyrosine hydroxylase (TH; the rate-limiting enzyme in DA production) [58,67], and effects on other factors such as TH mRNA and protein expression [34,40,41,45,72], DA release [26,33,61,62], DA content and turnover in tissues [11,26,54,63,66,but see 75], and concentrations of certain DA receptors and transporter proteins [19,81]. Estrogens have also been shown to affect catecholaminergic neurons directly via nongenomic mechanisms, at least during specific developmental periods [reviewed in 41].
Ample evidence now exists to support the hypothesis that steroidal effects on DA circuits also results in behavioral changes. For example, in humans being treated for disorders of the basal ganglia, dopaminergic drug effectiveness is known to depend upon the patient's sex and gonadal hormone levels [81]. In other species, steroid-dependent DA effects have been demonstrated in the regulation of courtship [31,45,67], reproductive behaviors [18,33,61,62,71], and parental behaviors [reviewed in 56]. Other DA-dependent behavioral processes such as pair bond formation [reviewed in: 5,85], and aggression [reviewed in 53] may also prove to be modulated by steroid-DA interactions. What remains unclear, however, is which DA populations are regulated by steroid hormones to achieve these behavioral effects, and whether there exist species differences in this regulation. The brain distributions of the A8–15 (caudal to rostral) DA populations are strongly conserved across amniotes [27,64,65], and given that these populations extensively overlap the known distributions of androgen and estrogen receptors, steroid hormones may influence DA neurons on an anatomically widespread level.
Based on the colocalization of TH with inducible transcription factors (Fos and/or egr-1), it appears that almost all TH-immunoreactive (-ir) cell groups are involved in the processing of social information. In male zebra finches, Taeniopygia guttata, sociosexual interactions such as copulation and courtship singing (with and without aggressive competition) produce increases in TH-Fos colocalization within the A8, A9, A10, A11, and preoptic A14 populations, whereas these stimuli actually reduce colocalization in the A12 population (using a survival time of 90 min) [15]. Furthermore, TH-Fos colocalization in the caudal ventral tegmental area (VTA, A10) and midbrain central gray (CG, A11) correlates positively with directed singing to females [29], as does the colocalization of TH and egr-1 in the CG [48]. Additionally, TH-ir neuron numbers in the CG correlate positively with constitutive levels of sexual motivation [29]. Copulation similarly induces Fos (though not egr-1) within TH-ir neurons of the VTA and CG in male Japanese quail, Coturnix japonica [18]. In male rats, TH-Fos colocalization increases following both copulation and the anticipation of copulation [6], and finally, in male praire voles, Microtus ochrogaster, copulation increases Fos expression within a large, species-specific TH-ir cell group of the medial extended amygdala [55], which may represent an expanded A15 population.
Most studies of hormonal effects on TH-ir numbers or activity levels focus on just a few DA cell groups, and to our knowledge, none have comprehensively examined the A8–15 cell groups and few have provided extensive data on both males and females. The present study therefore examines E2 regulation of all the major DA populations, A8–A15, in both male and female zebra finches. The A8–A15 cell groups have been shown to be immunonegative for DA β-hydoxylase and are thus presumably dopaminergic and not noradrenergic [52,64]. Zebra finches are an interesting species for this work because they are opportunistic breeders that do not exhibit regular breeding and nonbreeding seasons, but rather initiate reproduction after the unpredictable onset of rainfall, regardless of current gonadal steroid hormone levels [21,60,87]. Opportunistic species may possess neural adaptations that allow them to exhibit and respond to behaviors related to reproduction year-round. Such is the case with socially relevant, extrahypothalamic vasotocin circuitry, which is maintained year-round in zebra finches and several other opportunistically breeding species [36], but fluctuates seasonally and with steroid hormone levels in most seasonally breeding species [22,28,36,37]. Because DA is also involved in the regulation of courtship and copulation, levels of the DA synthesizing enzyme TH may therefore need to be maintained at appropriate levels throughout the year, regardless of hormonal state – at least within certain behaviorally and physiologically relevant DA populations. Therefore, we may expect to see more limited regulation of TH by estrogens in zebra finches than has been reported in other species. However, E2 may still regulate these neurons more subtly by affecting their constitutive activity levels, as recently demonstrated for vasotocin neurons in the bed nucleus of the stria terminalis of zebra finches [36].
Using both male and female zebra finches, we examined the effects of aromatase blockade (preventing conversion of testosterone, T, to E2), with and without E2 replacement, on the numbers of TH-ir neurons in the A8–A15 cell groups, and on their early A.M. (basal) colocalization with Fos protein, which provides a measure of treatment effects on transcriptional activity. To our knowledge, this represents the most comprehensive analysis of this type to be conducted in any vertebrate.
2. Methods
2.1 Subjects
Domestic zebra finches, 18 males and 18 females, were obtained from commercial suppliers and housed on a 14L:10D photoperiod with ad libitum access to finch mix and vitamin-enriched water. Subjects were treated humanely and all procedures were carried out in accordance with federal guidelines and approved by the Institutional Animal Care and Use Committee. The subjects in this experiment are the same as those in another report that examines hormonal effects on vasotocin circuitry [36].
2.2 Treatments
The subjects were divided into three treatment groups, each consisting of six males and six females: 1. control group (CTRL) subjects were given an empty subcutaneous silastic implant and 20 μl daily of 0.9 % saline vehicle; 2. Letrozole (LET) group subjects were given an empty implant and 20 μl daily of 10 mg/ml LET (Fisher Scientific, Pittsburgh, PA); 3. LET plus E2 replacement group (LET+E2) were given an implant containing E2 (i.d. 1.47 mm, o.d. 1.96 mm, total length 0.9 cm, steroid packing length of 0.5 cm) and 20 μl daily of 10 mg/ml LET. All treatments were administered for three weeks. The subjects were housed in six cages (60 cm W × 40 cm H × 36 cm D) containing plastic nests and shredded burlap nesting material. One male and one female of each treatment group were assigned per cage. Any laid eggs were removed within 24 hours. Because several birds became sick, we cut the LET dose in half for the remainder of the treatment period. This change occurred approximately mid-way through the experiment, although the exact date per subject varied since the initiation of treatment was staggered across cages. One LET male and three LET+E2 females died, and another LET female was removed due to sickness (final sample sizes are shown in Table 1). Despite these initial problems, the modified dose was well tolerated and all subjects appeared in good health at the end of the experiment. In addition, there were no significant treatment group differences or sex*treatment group interactions in final body weight (p = 0.65 and p = 0.48, respectively; two-way analysis of variance, ANOVA).
Table 1.
Mass of either the testes or oviduct, plasma testosterone (T) concentration, and plasma estradiol (E2) concentration (± S.E.M.) for male and female zebra finches from mixed-sex housing. Subjects were treated with vehicle or Letrozole on a daily basis for three weeks. Subjects were also given subcutaneous implants that were either empty or filled with crystalline E2 for the duration of the study. Abbreviations: CTRL, vehicle plus empty implant; LET, Letrozole plus empty implant; LET+E2, Letrozole plus E2; N, group size. See text for statistical differences among groups. Adapted from Kabelik et al. [35].
| Females | Males | |||||
|---|---|---|---|---|---|---|
| CTRL | LET | LET+E2 | CTRL | LET | LET+E2 | |
| N | 6 | 5 | 3 | 6 | 5 | 6 |
| testes or oviduct mass (g)* | 0.073 (0.024) | 0.023 (0.006) | 0.43 (0.08) | 0.048 (0.006) | 0.042 (0.005) | 0.003 (0.001) |
|
| ||||||
| T (ng/ml)* | 0.033 (0.006) | 0.28 (0.040) | 0.016 (0.007) | 3.47 (0.67) | 3.84 (0.95) | 0.023 (0.004) |
|
| ||||||
| E2 (ng/ml)* | 0.023 (0.003) | 0.030 (0.004) | 1.82 (0.14) | 0.033 (0.005) | 0.052 (0.010) | 0.99 (0.21) |
*Testes mass, p < 0.0001
*Oviduct mass, p < 0.0001
*T level, males vs. females, p < 0.0001
*T level, treatment effect, p < 0.0001; posthoc: LET > CTRL > LET+E2
*T level, sex*treatment, p < 0.0001; posthoc: ♂ LET = ♂ CTRL > ♀ LET > ♀ CTRL = ♂ LET+E2 = ♀ LET+E2
*E2 level, treatment effect, p < 0.0001; posthoc: LET+E2>CTRL=LET
*E2 level, sex*treatment, p = 0.007; posthoc: ♀ LET+E2 > ♂ LET+E2 > ♂ LET ≥ ♂ CTRL ≥ ♀ LET = ♀ CTRL
At the end of the treatment period, subjects were captured prior to lights-on (thus allowing for assessment of baseline TH-Fos colocalization), rapidly bled from the alar wing vein, and euthanized with isoflurane. Subjects were then transcardially perfused with 0.1 M phosphate-buffered saline (PBS; pH 7.4), followed by 0.4 % paraformaldehyde. Testes and ovaries were removed and weighed. Brains were removed, postfixed overnight, and cryoprotected in 30 % sucrose in PBS for 48 h before sectioning on a cryostat.
2.3 Hormone Analyses
Blood samples were centrifuged and plasma stored at −80 °C. Treatment efficacies were verified by radioimmunoassay. Assays were run using T antibody from Wien Laboratories Inc (now Fitzgerald Industries International, Concord, MA) and E2 antibody from Biogenesis Ltd. (Poole, England). Samples were equilibrated overnight with 2000 cpm of radioactive hormone for determination of individual recoveries. Steroids were extracted from plasma using 30% ethyl acetate in isooctane and separated by stepwise elution using celite chromatography. Collected fractions were dried down, resuspended in PBS, and assayed. Based on an average plasma volume of 113 μl, the minimum detectable hormone values were 24 pg/ml for T and 26 pg/ml for E2. These values were calculated using an average recovery of 89.1% for T and 90.4% for E2, as well as a minimum binding curve value 2 S.D. beyond the means for water blanks.
2.4 Immunocytochemistry
Brains were cut on a cryostat into three 40-μm series. Immunocytochemistry was performed using free-floating sections. Tissues were blocked using 10 % normal donkey serum + 0.3 % Triton-X-100 in PBS. Rinse steps were in PBS, while all antibody steps were in 5 % normal donkey serum + 0.3 % Triton-X-100 + 0.05 % sodium azide in PBS. Primary antibodies were run at 2 μg/ml for sheep anti-tyrosine hydroxylase (Novus Biologicals, Littleton, CO) and 1 μg/ml for rabbit anti-Fos (Santa Cruz Biotechnology, Santa Cruz, CA). Following a two-day primary incubation, sections were rinsed and incubated with donkey anti-rabbit secondary conjugated to Alexa Fluor 594 at 5 μl/ml, and donkey anti-sheep secondary conjugated to Alexa Fluor 680 at 7 μl/ml (all from Invitrogen, Eugene, OR). Sections were post-rinsed, mounted onto slides subbed with gelatin and chrome alum and coverslipped with ProLong Gold mounting medium containing DAPI nuclear stain (Invitrogen). The brains in this study were labeled in three runs, each containing a balance of groups and sexes. The K-25 Fos antibody has been extensively used and validated in songbirds [e.g., 1], and we likewise found that preadsorption of the primary antibody with 100 μM blocking peptide (SC-253P, Santa Cruz) eliminates labeling. Immunizing peptide is not available for the TH antibody; however the specificity of that antibody has been extensively addressed by the manufacturer (manufacturer's data sheet), and both the distribution of labeling and morphology of labeled neurons closely matches previous descriptions of TH labeling in birds [17,64,65].
2.5 Image Acquisition and Quantification
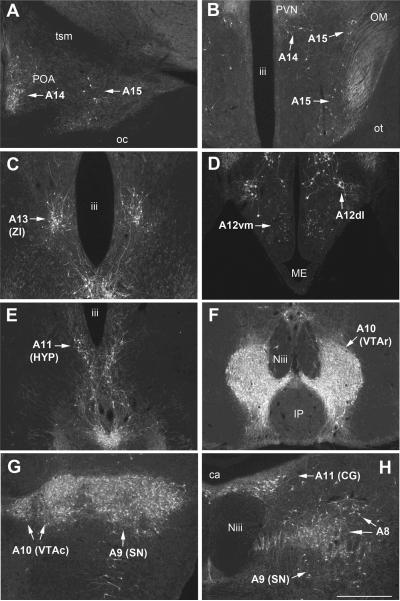
Bilateral photomicrographs (Fig. 1) of the A8–A15 cell groups were captured according to various published sources [3,10,15,17,64]. A series of line drawings showing the distribution of these cell groups is provided in a previous publication [15] and the drawings can be directly and freely accessed via the following web address: (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2570784/figure/F1/). Monochrome images were captured using standardized acquisition parameters on a Zeiss AxioImager microscope (Carl Zeiss Inc., Göttingen, Germany) outfitted with a Zeiss optical dissector (Apotome), a Zeiss Axiocam HRm camera and a high intensity X-Cite fluorescence illuminator (EXFO Photonic Solutions Inc., Mississauga, Canada). Images were captured in Zeiss AxioVision 4.6.3.0 and exported into Photoshop CS3 (Adobe Systems Inc., San Jose, CA). Counts of TH-immunoreactive (TH-ir) cells and TH-ir cells colocalized with Fos (supplementary Fig. 1) were conducted blind to treatment and by a single observer per brain region. The A12–A15 cell groups were each captured at both a rostral and a caudal level, and these counts were summed. The tuberal A12 population was further subdivided into dorsolateral and ventromedial cell groups, which are spatially segregated and contain relatively large and small neurons, respectively. The rostral hypothalamic (HYP) group of A11 neurons was also captured across two levels, but analyzed separately from two levels of the caudal CG A11 cell group. The A10 VTA cell group was analyzed separately at rostral and caudal levels, as these levels have been shown to be somewhat functionally discrete [29,42]. The A9 (substantia nigra, SN) and caudal midbrain A8 cell groups were each captured at a single level. The A8 neurons are contiguous with scattered cells and remnants of the A9 group ventrally, and thus, consistent with Reiner et al. [64], we restricted our analyses to the cells that lie above an imaginary line extending laterally from the ventral margin of the medial longitudinal fasciculus, excluding the A11 population, which is readily distinguished from the A8 population. These counts are intended to provide a reasonable estimate of the relative number of cells across treatment groups, not an estimate of the absolute number of TH-ir cells throughout the forebrain and midbrain, and for such measures of relative density, the optical dissector approach yields comparable accuracy to stereology [79].
Figure 1.
Tyrosine hydroxylase immunoreactive (TH-ir) cell groups in the zebra finch brain, shown from rostral (A15, panel A) to caudal (A8, panel H). Abbreviations: iii, 3rd ventricle; A12dl, A12 dorsolateral subdivision; A12vm, A12 ventromedial subdivision; ca, cerebral aqueduct; CG, central gray; HYP, hypothalamic; IP, interpeduncular nucleus; ME, median eminence; Niii, 3rd nerve; oc, optic chiasm; OM, occipitomesencephalic tract; ot, optic tract; POA, preoptic area; PVN, paraventricular nucleus; SN, substantia nigra; tsm, septomesencephalic tract; VTAc, caudal ventral tegmental area; VTAr, rostral ventral tegmental area; ZI, zona incerta. Scale bar denotes 500 μm.
2.6 Statistical Analysis
Hormone levels, TH-ir cell numbers, and the percent of TH-ir neurons containing Fos-ir nuclei were analyzed using two-way (sex × treatment) ANOVA. Testes and oviduct mass were analyzed separately for males and females, using one-way ANOVA. Testes and oviduct mass, hormone levels, and ventrolateral A12 TH-ir cell counts were positively skewed and thus log10 transformed for analyses. Percent colocalization data were arcsin square-root transformed where necessary. Student's t post hoc analyses were conducted at α=0.05.
3. Results
3.1 Treatment effects on hormones, testes and oviducts
Testes mass was affected by experimental treatment (F2,14 = 41.11, p < 0.0001), with LET+E2 causing testicular regression relative to CTRL and LET males. Oviduct mass was also affected by treatment (F2,11 = 30.01, p < 0.0001), with LET+E2 treatment increasing oviduct size relative to both LET and CTRL females. The oviduct mass of LET females was less than a third that of CTRL females, although these groups did not differ significantly, likely due to a floor effect as both groups had extremely small oviducts (see Table 1 for a summary of testes and oviduct mass).
Levels of T were higher in males than in females (F1,25 = 202.79, p < 0.0001), and they were affected by hormonal treatment (F2,25 = 162.40, p < 0.0001), with LET subjects having higher T levels than CTRL subjects, and CTRL subjects having higher T levels than LET+E2 individuals. A significant sex*treatment effect (F2,25 = 52.93, p < 0.0001) was also present, with LET females having higher T levels than CTRL and LET+E2 females, while both CTRL and LET males had higher T levels than LET+E2 males.
Levels of E2 did not differ between sexes (F1,25 = 0.001, p = 0.97), but did vary with treatment (F2,25 = 242.63, p < 0.0001), with LET+E2 subjects having higher E2 levels than both CTRL and LET subjects. A significant sex*treatment effect was also present for E2 (F2,25 = 6.03, p = 0.007), although it merely reflects a sex difference in the magnitude of treatment effects, such that LET+E2 produced a greater elevation of E2 in females than in males.
Overall, these results suggest that the LET treatment was highly effective, particularly given the strong effects on T levels, with similar trends for oviducts. As described in the following sections, LET treatments also produced significant effects on TH-ir cell counts, some of which were reversible by E2. However, final E2 levels did not differ between LET and CTRL subjects (although both were fairly low; Table 1), which may reflect a temporal variation in hormonal effects, with early E2 reductions in LET subjects slowly disappearing as T levels began to rise (see Discussion).
3.2 Treatment Effects on relative TH-ir cell number and TH-Fos colocalization
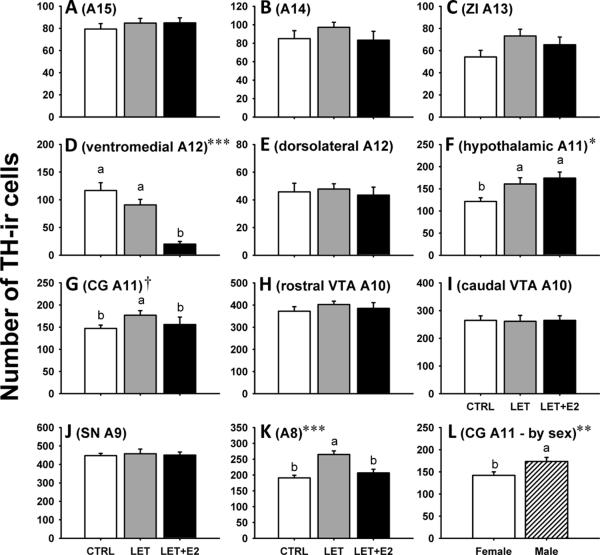
Figure 2 (A–K) depicts results of experimental treatment on relative TH-ir cell counts. Table 2 presents TH-Fos colocalization levels throughout the brain. Table 3 presents results of statistical analyses.
Figure 2.
(A–K) Relative numbers of tyrosine hydroxylase immunoreactive (TH-ir) neurons in the A15-A8 cell groups of male and zebra finches that were administered vehicle control (CTRL; white), Letrozole (LET; gray), and Letrozole plus estradiol (LET+E2; black). There were no sex differences (see Results) and sexes are shown pooled. (L) A sex difference in the number of A11 neurons in the midbrain central gray. Abbreviations: CG, central gray; SN, substantia nigra; VTA, ventral tegmental area; ZI, zona incerta. Symbols: *p < 0.05, **p < 0.01, ***p = 0.0001, †p = 0.066. Different letters above bars denote significant posthoc group differences following significant ANOVA. See text for full ANOVA results.
Table 2.
Baseline TH-Fos colocalization rates (percent of TH-ir neurons containing Fos-ir nuclei) for the A8–A15 cell groups in male and female zebra finches (sexes pooled). No significant effects of treatments were observed. Subjects were orally treated with vehicle or Letrozole on a daily basis for three weeks. Subjects were also given subcutaneous implants that were either empty or filled with crystalline E2 for the duration of the study. Abbreviations: CTRL, vehicle plus empty implant; LET, Letrozole plus empty implant; LET+E2, Letrozole plus E2.
| CTRL | LET | LET+E2 | ||||
|---|---|---|---|---|---|---|
| Mean | S.E.M. | Mean | S.E.M. | Mean | S.E.M. | |
| A15 | 15.5 | 1.9 | 15.3 | 1.3 | 15.2 | 1.5 |
| A14 | 21.1 | 1.6 | 18.5 | 1.9 | 17.3 | 1.4 |
| A13 | 19.3 | 3.1 | 14.8 | 2.3 | 15.1 | 3.5 |
| A12dl | 25.1 | 4.3 | 26.8 | 5.7 | 23.1 | 5.0 |
| A12vm | 10.8 | 1.6 | 9.6 | 2.7 | 14.9 | 3.4 |
| A11-HYP | 19.5 | 3.1 | 17.1 | 2.0 | 11.2 | 2.2 |
| A11-CG | 29.2 | 4.9 | 40.8 | 5.3 | 22.9 | 4.5 |
| A10r VTA | 12.8 | 3.2 | 10.2 | 3.0 | 10.5 | 4.0 |
| A10c VTA | 14.9 | 2.7 | 12.3 | 3.3 | 13.2 | 4.3 |
| A9-SN | 11.9 | 2.4 | 11.1 | 3.1 | 6.1 | 7.4 |
| A8 | 15.3 | 3.5 | 13.2 | 2.5 | 10.6 | 2.0 |
Table 3.
Results from 2-way ANOVAs on both TH-ir cell number and TH-Fos colocalization within the A8–A15 cell groups, and subgroups. Cell groups within the A12, A11, and A8 populations exhibited a change in cell number with estradiol manipulation treatment, the hypothalamic A11 group exhibited a sex difference, and no groups exhibited changes in TH-Fos colocalization. Significant values are depicted in bold. Abbreviations: -ir, immunoreactive; TH, tyrosine hydroxylase; VTA, ventral tegmental area.
| Cell Group | TH-ir Cell Number | TH-Fos Colocalization | ||||
|---|---|---|---|---|---|---|
| Sex | Treatment | Sex by Treatment | Sex | Treatment | Sex by Treatment | |
| A15 (preoptic/hypothalamic) |
F1,25 = 0.60 p = 0.25 |
F2,25 = 1.06 p = 0.36 |
F2,25 = 2.98 p = 0.069 |
F1,25 = 0.35 p = 0.56 |
F2,25 = 0.04 p = 0.96 |
F2,25 = 2.19 p = 0.13 |
| A14 (preoptic/hypothalamic) |
F1,25 = 0.34 p = 0.56 |
F2,25 = 0.76 p = 0.48 |
F2,25 = 0.13 p = 0.88 |
F1,25 = 0.45 p = 0.51 |
F2,25 = 1.05 p = 0.36 |
F2,25 = 2.07 p = 0.15 |
| A13 (zona incerta) |
F1,25 = 0.05 p = 0.82 |
F2,25 = 2.37 p = 0.11 |
F2,25 = 1.13 p = 0.34 |
F1,25 = 0.007 p = 0.93 |
F2,25 = 0.68 p = 0.52 |
F2,25 = 0.07 p = 0.93 |
| A12 (ventromedial) |
F1,25 = 1.09 p = 0.30 |
F1,25 = 45.41
p < 0.0001 |
F2,25 = 0.98 p = 0.39 |
F1,25 = 1.30 p = 0.27 |
F2,25 = 0.18 p = 0.83 |
F2,25 = 1.01 p = 0.38 |
| A12 (dorsolateral) |
F1,25 = 1.16 p = 0.29 |
F2,25 = 0.21 p = 0.81 |
F2,25 = 0.02 p = 0.98 |
F1,25 = 0.26 p = 0.62 |
F2,25 = 0.75 p = 0.48 |
F2,25 = 0.28 p = 0.76 |
| A11 (hypothalamic) |
F1,25 = 0.17 p = 0.69 |
F2,25 = 4.81
p = 0.02 |
F2,25 = 0.20 p = 0.82 |
F1,25 = 0.46 p = 0.50 |
F2,25 = 2.68 p = 0.09 |
F2,25 = 0.21 p = 0.81 |
| A11 (central gray) |
F1,25 = 9.85
p = 0.004 |
F2,25 = 3.04 p = 0.066 |
F2,25 = 2.13 p = 0.14 |
F1,25 = 0.05 p = 0.83 |
F2,25 = 2.89 p = 0.074 |
F2,25 = 0.75 p = 0.48 |
| A10 (rostral VTA) |
F1,25 = 1.02 p = 0.32 |
F2,25 = 0.83 p = 0.45 |
F2,25 = 2.54 p = 0.10 |
F1,25 = 0.27 p = 0.61 |
F2,25 = 0.32 p = 0.73 |
F2,25 = 0.19 p = 0.83 |
| A10 (caudal VTA) |
F1,25 = 1.21 p = 0.28 |
F2,25 = 0.11 p = 0.89 |
F2,25 = 2.51 p = 0.10 |
F1,25 = 0.14 p = 0.71 |
F2,25 = 0.36 p = 0.70 |
F2,25 = 0.16 p = 0.85 |
| A9 (substantia nigra) |
F1,25 = 0.97 p = 0.33 |
F2,25 = 0.17 p = 0.84 |
F2,25 = 0.51 p = 0.61 |
F1,25 = 0.15 p = 0.70 |
F2,25 = 1.19 p = 0.32 |
F2,25 = 0.07 p = 0.93 |
| A8 (caudal midbrain) |
F1,25 = 0.18 p = 0.68 |
F2,25 = 13.53
p = 0.0001 |
F2,25 = 0.69 p = 0.51 |
F1,25 = 0.18 p = 0.68 |
F2,25 = 0.71 p = 0.50 |
F2,25 = 0.21 p = 0.81 |
A large effect of treatment was seen on the number of small ventromedial TH-ir neurons of the A12 cell group (p < 0.0001; Fig. 2D), with posthoc analyses revealing that LET+E2 individuals had fewer cells than did either the CTRL or LET subjects. An effect of treatment was also seen on the number of A11 TH-ir cells in the HYP (p = 0.02; Fig. 2F), with both LET and LET+E2 individuals having more cells than CTRL subjects. There was also a trend toward a treatment effect in the A11 CG population (p = 0.066; Fig. 2G), with posthoc analyses determining that LET subjects possessed significantly more TH-ir cells than did either CTRL or LET+E2 subjects. A strong effect of treatment was also seen on the number of caudal midbrain A8 TH-ir cells (F2,25 = 13.53, p = 0.0001; Fig. 2K), with posthoc analyses revealing that LET subjects have approximately one third more cells than do either CTRL or LET+E2 individuals. No treatment effects, or interactions between treatment and sex, were observed in TH-Fos colocalalization in any cell group.
The only significant sex difference in TH-ir number was within the A11 subpopulation of the CG (p = 0.004; Fig. 2L), with males having more TH-ir cells than females. No sex differences existed in TH-Fos colocalization.
4. Discussion
4.1. Overview
This study demonstrates that estradiol levels can alter the abundance of TH-producing neurons within a limited number of regions of the zebra finch brain, namely within the A8 cell group of the caudal midbrain, the A11 subpopulations of the caudal hypothalamus and midbrain CG, and the ventromedial subpopulation of tuberal A12 neurons. Relative cell numbers in both the midbrain A8 and CG A11 groups were increased by LET treatment in a manner that was clearly reversed by concomitant E2 treatment, whereas the hypothalamic effects were more complex. These results are not entirely consistent with previous findings, suggesting potential species differences in hormonal regulation of TH expression, some of which may reflect variation between opportunistic and seasonal breeders. The lack of E2 effects on the VTA cell group, which differs from previously reported findings in mammals and birds [45,88], is particularly notable in this regard. However, it is important to keep in mind that subtle effects of estradiol manipulation on dopaminergic neurons may not have been detected because we did not examine TH mRNA within this study. Differences were generally not observed between male and female brains, both in terms of responses to hormonal manipulations, and in constitutive numbers of TH neurons. The only exception was the CG A11 population, where males possessed relatively more TH neurons than did females. The CG A11 is one of the brain regions that innervate song control nuclei [2,4], which are themselves sexually dimorphic [3,17]. Surprisingly, no significant effects of hormonal manipulations were observed on constitutive dopaminergic activity, assessed as TH-Fos colocalization, whereas significant effects on vasotocin-Fos colocalization were observed in these same animals [36].
Relative to other aromatase inhibitors, LET has been employed to only a limited extent in songbirds, and although we found clear evidence of its efficacy, the drug was not tolerated well in zebra finches at doses comparable to an orally administered aromatase inhibitor in a previous study [46]. However, LET appears to be more effective than another popular aromatase inhibitor, ATD, at least under certain experimental conditions [36,74]. LET is also known to cross the blood brain barrier and thus likely reduces central aromatase activity [38]. The efficacy of LET in the present study was demonstrated in that, as noted above, some LET effects were reversed with E2 treatment, which returned TH-ir cell numbers to those of controls. Additionally, we observed the expected feedback effects of LET and E2 treatment on T levels. A significant effect of E2, and a trend with LET treatment, were also observed for oviduct mass. Nonetheless, final E2 levels did not differ between LET and CTRL subjects, although both were low. We hypothesize that this paradoxical pattern of effects reflects temporal variation in E2 levels across the course of the study, such that initial reductions in the bioavailability of E2 by LET treatment produced the observed effects on TH-ir cell numbers, while compensatory upregulation of T production returned E2 to control levels by the end of the study. Apart from our study, the efficacy of LET has been also demonstrated in chickens, another avian species [23].
4.2. Hormonal Effects on DA Neuron Numbers
A13–A15 Multiple hypothalamic and midbrain DA populations project to the preoptic area to regulate hormonal release and reproductive behaviors [24]. In Japanese quail [8], and likely other bird species, DA projections to the preoptic area arise primarily from the A9, A10 and A14 populations, with minimal contribution from the A13 and A15 cell groups, all of which were not altered by E2 manipulations in the present study (although given that zebra finches are strongly opportunistic, our negative findings for certain cell groups may not be predictive for other species). However, moderate projections to the preoptic area also arise from the A8 and A11 CG neurons [8], which were indeed affected by E2 in our study, suggesting that E2 regulation of preoptic DA release may still be influenced, at least partially, by E2 effects on the A8 and A11 cell groups.
The lack of treatment effects on relative TH-ir neuron numbers within the A13–15 populations in this study is generally consistent with previous findings. Du and Hull [25] demonstrated that while castration of male rats lowers medial preoptic DA release in response to the presence of a receptive female, presumably via regulation of nitric oxide synthesis, it has no effect on the actual number of A14 TH-ir neurons in the region. Similarly, Lehman et al. [47] observed no effect of E2 manipulations on numbers of A12–A15 TH-ir neurons in ewes. However, the latter authors did observe differences in numbers of A13 and A15 TH-ir neurons across ewes differing in reproductive state, suggesting that some hormonal regulation of TH in these cell populations may still occur. Circulating androgens may regulate rostral A14 neurons in the anteroventral periventricular preoptic area (AVPV) of male prairie voles, as castration reduced TH-ir cell numbers in this region, while ovariectomy had no effect on cell counts in females [43]. However, both androgens and estrogens have been linked to suppression of TH-ir or mRNA within this subpopulation of A14 neurons in male and female rats [73]. These results suggest large species differences in steroidal regulation of A14 neurons, even among rodents. Finally, Lansing and Lonstein [43] also observed no effects of gonadectomy on TH-ir cell counts within other hypothalamic A11–A14 cell groups of male and female prairie voles.
A12 E2 has been shown to inhibit DA release from the A12 tuberal neurons in rats, and in so doing, disinhibits prolactin release from the adenohypophysis [reviewed in 81]. Similar findings have also been obtained in turkeys, where DA has been shown to inhibit prolactin release, though via the intermediary signaling molecule vasoactive intestinal peptide (VIP) [86]. Thus, E2 seems to exert effects on reproductive physiology via its inhibitory actions on TH in A12 tuberal neurons. Our finding that E2 downregulates TH-ir in ventromedial A12 neurons is in line with these findings in other species, while a lack of LET treatment alone may indicate a ceiling effect on TH-ir neuron numbers in this cell group.
A11 and A10 The HYP subpopulation of the A11 cell group increases immediate early gene activity during the performance of reproductive behaviors, whereas the CG subpopulation, along with the A10 VTA, are activated by both reproductive and aggressive interactions in male zebra finches [15,29,48], and by interactions with a female in male Japanese quail [18]. In the present study, suppression of E2 levels by LET tended to increase TH-ir cell numbers in both of these A11 subpopulations. Although E2 replacement (LET+E2) reduced TH-ir neuron numbers to control levels in the CG, cell numbers were increased in both the LET and LET+E2 groups relative to CON subjects within the HYP population. Because A11 neuron numbers and their activity levels are correlated with singing and calling in zebra finches [15,29,48], an increase in the number of these cells in the presence of low E2 levels may help maintain a high level of social vocalization during times of low circulating hormone levels when other behavioral and motivational systems may be downregulated. This differs from female white-throated sparrows, a seasonally breeding songbird species, in which no effect of E2 manipulation on A11 TH-ir cell numbers was detected [45]. However, vocalizations were not monitored in the present study.
The A10 VTA neurons may modulate selectivity for male song in females [50,51] and courtship production in males [32]. Whereas we observed no hormonal effects on these neurons, LeBlanc et al. [45] found that in female white-throated sparrows, E2 increases the number of A10 neurons. Furthermore, Goodson et al. [29] found that the number of caudal A10 TH-ir neurons in male zebra finches correlates positively with gonadal state, which may reflect either a transsynaptic effect or a direct sensitivity to androgen levels. However, consistent with our results, Goodson et al. [29] found no effect of reproductive state on the rostral A10 neurons. These findings are in line with studies demonstrating that canaries possess androgen receptors primarily in the caudal VTA, whereas estrogen receptor α is present primarily in the rostral VTA [49]. However, LeBlanc et al. [45] found E2-induced changes at a more caudal level of the VTA of female white-throated sparrows than where estrogen receptor α presence was reported in the canary brain [49], suggesting that receptor distributions in the VTA exhibit at least a modest amount of species-specificity.
Other findings in zebra finches also suggest that levels of DA and DA metabolites may be less strongly altered by changes in E2 in this species than are DA neurons in other species. For instance, Svec et al. [75] have shown that in female zebra finches, while E2 treatment reduces vocalization and visual scanning in the direction of a speaker playing male song, E2 does not influence DA release or turnover in either the striatum or nucleus accumbens, which are major target sites for DA action. This is in contrast to findings in mammalian species where E2 has been shown to affect DA release within these regions [e.g., 13,14,69,77], or to reduce numbers of TH-ir neurons within certain subdivisions of the VTA [88]. However, we must also consider other alternative explanations for our observed E2 effects, such as dose and experimental duration. For instance, Serova et al. [72] found that whereas short term E2 treatment of ovariectomized female rats can increase TH mRNA in the VTA, this effect disappears following long term, high dose treatment.
A9 The A9 neurons of the SN project heavily to the striatum, thus comprising the nigrostriatal system, but also contribute substantially to mesolimbic projections, and are thus involved in both behavioral motivation and execution [16]. Bharati et al. [15] observed significant, but very modest, elevations of TH-Fos colocalization in these neurons (from 0 % to about 2 %) following both copulatory behaviors and aggressive encounters in male zebra finches, relative to control subjects, whereas Charlier et al. [18] found no changes in TH-Fos or TH-ZENK (egr-1) colocalization in the A9 group following copulation in male Japanese quail. In the present study, we did not observe any effects of E2 manipulation on the numbers of TH-ir neurons in the SN, although E2-induced reductions in TH-ir neuron numbers in this area have been observed in female rats [88].
A8 Like the A9 cell group, the caudal midbrain A8 also exhibits mixed projections to striatal, cortical, and limbic areas [16]. However, a much higher percentage of the A8 neurons respond to various social stimuli than do those in the A9 cells, suggesting a strong behavioral role for the A8 cell group in zebra finches [15]. As in the A11 CG population, LET treatments decreased the number of TH-ir neurons within the A8 cell group and this effect was reversed by simultaneous administration of E2. In contrast, LeBlanc et al. [45] observed no effect of E2 manipulation on the number of A8 TH-ir neurons within female white-throated sparrows. Thus, consistent with our interpretions for the A11 TH population, this may be an adaptation to support year-round expression of behavior.
4.3. Sex Differences
The only dopaminergic region in our experiment to exhibit sex differences was the A11 CG population. Dimorphism of relative TH-ir cell numbers in this region are consistent with previous studies that show innervations of song control nuclei by dopaminergic neurons from this region [2,4], as well as a sexual dimorphism of TH-ir fiber presence within song control regions of the canary brain [3,17]. Lumbar-projecting A11 neurons also exhibit a male-biased sexual dimorphism in mice [57], suggesting that this region may exhibit sexual dimorphism across species. While parts of the A10 VTA also project to song control regions [2,4], we found no evidence for sex differences at either the rostral or caudal levels of this cell group. The lack of sexual dimorphism in other TH-ir containing regions was also consistent with previous findings [17,43,44].
4.4 Conclusions
Comparison of the present results with those from other studies suggests that numerous species differences exist in the regulation of TH-ir neurons by E2. Hence, in contrast to findings for other bird species, E2 manipulations in zebra finches did not alter the number of TH-ir neurons in the A10 cell group of the VTA, while decreasing E2 levels via LET treatment caused an increase in the numbers of TH-ir neurons within the A11 and A8 populations. We hypothesize that these changes are adaptations for the year-round maintenance of reproduction-related neural circuitry in both male and female zebra finches, an opportunistically breeding species that can breed throughout the year. We also found no effects of E2 manipulation on immediate early gene expression within TH-ir neurons, as was also found following steroid hormone manipulations in other bird species [18,45,76], although this lack of effects differs from what has been observed in some DA regions of mammals [47], and may therefore represent a phylogenetic phenomenon. Finally, we observed E2-mediated suppression of A12 TH-ir neurons, and a general lack of sex differences in TH-ir cell counts throughout the brain, except for the A11 subpopulation of the midbrain CG. These findings are consistent with previous findings in both birds and mammals [2,4,17,43,44,57,81,86].
Supplementary Material
Supplementary Figure 1. A11 central gray (CG) neurons expressing fluorescent immunoreactivity for cytoplasmic tyrosine hydroxylase (magenta; the rate limiting enzyme in dopamine production) and the nuclear immediate early gene product Fos (green).
Abbreviations: EW, Edinger-Westphal nucleus; ca, cerebral aqueduct. Scale bar denotes 100 μm.
Acknowledgements
We would like to thank J.A. Morrison for help with animal handling and tissue processing, and ED Ketterson and DJ Whittaker for radioimmunoassay assistance. This research was funded by NIMH grant R01 MH062656 to J.L.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used: iii, 3rd ventricle; A12dl, A12 dorsolateral subdivision; A12vm, A12 ventromedial subdivision; ANOVA, analysis of variance; ca, cerebral aqueduct; CG, central gray; CTRL, control; DA, dopamine; E2, estradiol; EW, Edinger-Westphal nucleus; HYP, hypothalamic; IP, interpeduncular nucleus; ir, immunoreactive; LET, Letrozole; LET+E2, Letrozole plus estradiol replacement; ME, median eminence; Niii, 3rd nerve; oc, optic chiasm; OM, occipitomesencephalic tract; ot, optic tract; POA, preoptic area; PVN, paraventricular nucleus; SN, substantia nigra; T, testosterone; TH, tyrosine hydroxylase; tsm, septomesencephalic tract; VIP, vasoactive intestinal peptide; VTA, ventral tegmental area; VTAc, caudal ventral tegmental area; VTAr, rostral ventral tegmental area; ZI, zona incerta
References
- [1].Alger SJ, Maasch SN, Riters LV. Lesions to the medial preoptic nucleus affect immediate early gene immunolabeling in brain regions involved in song control and social behavior in male European starlings. European Journal of Neuroscience. 2009;29:970–982. doi: 10.1111/j.1460-9568.2009.06637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–33. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- [3].Appeltants D, Ball GF, Balthazart J. The distribution of tyrosine hydroxylase in the canary brain: demonstration of a specific and sexually dimorphic catecholaminergic innervation of the telencephalic song control nuclei. Cell Tissue Res. 2001;304:237–259. doi: 10.1007/s004410100360. [DOI] [PubMed] [Google Scholar]
- [4].Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002;13:649–653. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- [5].Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Front Behav Neurosci. 2009;3:15. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Balfour ME, Yu L, Coolen LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacology. 2004;29:718–30. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- [7].Ball GF, Balthazart J. Seasonal and hormonal modulation of neurotransmitter systems in the song control circuit. J Chem Neuroanat. 2010;39:82–95. doi: 10.1016/j.jchemneu.2009.08.005. [DOI] [PubMed] [Google Scholar]
- [8].Balthazart J, Absil P. Identification of catecholaminergic inputs to and outputs from aromatase-containing brain areas of the Japanese quail by tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Comp Neurol. 1997;382:401–428. [PubMed] [Google Scholar]
- [9].Balthazart J, Castagna C, Ball GF. Differential effects of D1 and D2 dopamine-receptor agonists and antagonists on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Physiol Behav. 1997;62:571–580. doi: 10.1016/s0031-9384(97)00163-7. [DOI] [PubMed] [Google Scholar]
- [10].Balthazart J, Foidart A, Baillien M, Harada N, Ball GF. Anatomical relationships between aromatase and tyrosine hydroxylase in the quail brain: double-label immunocytochemical studies. J Comp Neurol. 1998;391:214–26. [PubMed] [Google Scholar]
- [11].Barclay SR, Harding CF. Differential modulation of monoamine levels and turnover rates by estrogen and/or androgen in hypothalamic and vocal control nuclei of male zebra finches. Brain Res. 1990;523:251–262. doi: 10.1016/0006-8993(90)91494-2. [DOI] [PubMed] [Google Scholar]
- [12].Batailler M, Blache D, Thibault J, Tillet Y. Immunohistochemical colocalization of tyrosine hydroxylase and estradiol receptors in the sheep arcuate nucleus. Neurosci Lett. 1992;146:125–30. doi: 10.1016/0304-3940(92)90059-g. [DOI] [PubMed] [Google Scholar]
- [13].Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19:27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- [14].Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–25. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- [15].Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–70. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Björklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- [17].Bottjer SW. The distribution of tyrosine hydroxylase immunoreactivity in the brains of male and female zebra finches. J Neurobiol. 1993;24:51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- [18].Charlier TD, Ball GF, Balthazart J. Sexual behavior activates the expression of the immediate early genes c-fos and Zenk (egr-1) in catecholaminergic neurons of male Japanese quail. Neuroscience. 2005;131:13–30. doi: 10.1016/j.neuroscience.2004.09.068. [DOI] [PubMed] [Google Scholar]
- [19].Chavez C, Hollaus M, Scarr E, Pavey G, Gogos A, van den Buuse M. The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: an autoradiography study. Brain Res. 2010;1321:51–9. doi: 10.1016/j.brainres.2009.12.093. [DOI] [PubMed] [Google Scholar]
- [20].Creutz LM, Kritzer MF. Estrogen receptor-β immunoreactivity in the midbrain of adult rats: Regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. The Journal of Comparative Neurology. 2002;446:288–300. doi: 10.1002/cne.10207. [DOI] [PubMed] [Google Scholar]
- [21].Cynx J. Effects of humidity on reproductive behavior in male and female zebra finches (Taeniopygia guttata) J Comp Psychol. 2001;115:196–200. doi: 10.1037/0735-7036.115.2.196. [DOI] [PubMed] [Google Scholar]
- [22].De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–55. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Deng YF, Chen XX, Zhou ZL, Hou JF. Letrozole inhibits the osteogenesis of medullary bone in prelay pullets. Poult Sci. 89:917–23. doi: 10.3382/ps.2010-00632. [DOI] [PubMed] [Google Scholar]
- [24].Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- [25].Du J, Hull EM. Effects of testosterone on neuronal nitric oxide synthase and tyrosine hydroxylase. Brain Res. 1999;836:90–8. doi: 10.1016/s0006-8993(99)01618-2. [DOI] [PubMed] [Google Scholar]
- [26].Du J, Lorrain DS, Hull EM. Castration decreases extracellular, but increases intracellular, dopamine in medial preoptic area of male rats. Brain Res. 1998;782:11–7. doi: 10.1016/s0006-8993(97)01144-x. [DOI] [PubMed] [Google Scholar]
- [27].Gale SD, Perkel DJ. Physiological properties of zebra finch ventral tegmental area and substantia nigra pars compacta neurons. J Neurophysiol. 2006;96:2295–2306. doi: 10.1152/jn.01040.2005. [DOI] [PubMed] [Google Scholar]
- [28].Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Brain Res Rev. 2001;35:246–65. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- [29].Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc Natl Acad Sci U S A. 2009;106:8737–42. doi: 10.1073/pnas.0811821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hahn TP, Watts HE, Cornelius JM, Brazeal KR, MacDougall-Shackleton SA. Evolution of environmental cue response mechanisms: Adaptive variation in photorefractoriness. Gen Comp Endocrinol. 2009;163:193–200. doi: 10.1016/j.ygcen.2009.04.012. [DOI] [PubMed] [Google Scholar]
- [31].Heimovics SA, Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiol Behav. 2008;95:258–266. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huang YC, Hessler NA. Social modulation during songbird courtship potentiates midbrain dopaminergic neurons. PLoS ONE. 2008;3:e3281. doi: 10.1371/journal.pone.0003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–71. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ivanova T, Beyer C. Estrogen regulates tyrosine hydroxylase expression in the neonate mouse midbrain. J Neurobiol. 2003;54:638–47. doi: 10.1002/neu.10193. [DOI] [PubMed] [Google Scholar]
- [35].Kabelik D, Kelly AM, Goodson JL. Dopaminergic regulation of mate competition aggression and aromatase-Fos colocalization in vasotocin neurons. Neuropharmacology. 2010;58:862–6. doi: 10.1016/j.neuropharm.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kabelik D, Morrison JA, Goodson JL. Cryptic regulation of vasotocin neuronal activity but not anatomy by sex steroids and social stimuli in opportunistic desert finches. Brain Behav Evol. 2010;75:71–84. doi: 10.1159/000297522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kabelik D, Weiss SL, Moore MC. Arginine vasotocin (AVT) immunoreactivity relates to testosterone but not territorial aggression in the tree lizard, Urosaurus ornatus. Brain Behav Evol. 2008;72:283–94. doi: 10.1159/000174248. [DOI] [PubMed] [Google Scholar]
- [38].Kil KE, Biegon A, Ding YS, Fischer A, Ferrieri RA, Kim SW, Pareto D, Schueller MJ, Fowler JS. Synthesis and PET studies of [(11)C-cyano]letrozole (Femara), an aromatase inhibitor drug. Nucl Med Biol. 2009;36:215–23. doi: 10.1016/j.nucmedbio.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kritzer MF. Selective colocalization of immunoreactivity for intracellular gonadal hormone receptors and tyrosine hydroxylase in the ventral tegmental area, substantia nigra, and retrorubral fields in the rat. J Comp Neurol. 1997;379:247–60. doi: 10.1002/(sici)1096-9861(19970310)379:2<247::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [40].Kritzer MF, Kohama SG. Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1998;395:1–17. [PubMed] [Google Scholar]
- [41].Kuppers E, Ivanova T, Karolczak M, Beyer C. Estrogen: a multifunctional messenger to nigrostriatal dopaminergic neurons. J Neurocytol. 2000;29:375–85. doi: 10.1023/a:1007165307652. [DOI] [PubMed] [Google Scholar]
- [42].Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–73. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- [43].Lansing SW, Lonstein JS. Tyrosine hydroxylase-synthesizing cells in the hypothalamus of prairie voles (Microtus ochrogaster): Sex differences in the anteroventral periventricular preoptic area and effects of adult gonadectomy or neonatal gonadal hormones. J Neurobiol. 2006;66:197–204. doi: 10.1002/neu.20212. [DOI] [PubMed] [Google Scholar]
- [44].Lea RW, Clark JA, Tsutsui K. Changes in central steroid receptor expression, steroid synthesis, and dopaminergic activity related to the reproductive cycle of the ring dove. Microsc Res Tech. 2001;55:12–26. doi: 10.1002/jemt.1152. [DOI] [PubMed] [Google Scholar]
- [45].LeBlanc MM, Goode CT, MacDougall-Shackleton EA, Maney DL. Estradiol modulates brainstem catecholaminergic cell groups and projections to the auditory forebrain in a female songbird. Brain Res. 2007;1171:93–103. doi: 10.1016/j.brainres.2007.06.086. [DOI] [PubMed] [Google Scholar]
- [46].Lee DW, Fernando G, Peterson RS, Allen TA, Schlinger BA. Estrogen mediation of injury-induced cell birth in neuroproliferative regions of the adult zebra finch brain. Dev Neurobiol. 2007;67:1107–17. doi: 10.1002/dneu.20399. [DOI] [PubMed] [Google Scholar]
- [47].Lehman MN, Durham DM, Jansen HT, Adrian B, Goodman RL. Dopaminergic A14/A15 neurons are activated during estradiol negative feedback in anestrous, but not breeding season, ewes. Endocrinology. 1996;137:4443–50. doi: 10.1210/endo.137.10.8828506. [DOI] [PubMed] [Google Scholar]
- [48].Lynch KS, Diekamp B, Ball GF. Catecholaminergic cell groups and vocal communication in male songbirds. Physiol Behav. 2008;93:870–6. doi: 10.1016/j.physbeh.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Maney DL, Bernard DJ, Ball GF. Gonadal steroid receptor mRNA in catecholaminergic nuclei of the canary brainstem. Neurosci Lett. 2001;311:189–192. doi: 10.1016/s0304-3940(01)02157-7. [DOI] [PubMed] [Google Scholar]
- [50].Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23:1523–9. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- [51].Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511:173–86. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- [52].Mello CV, Pinaud R, Ribeiro S. Noradrenergic system of the zebra finch brain: immunocytochemical study of dopamine-beta-hydroxylase. J Comp Neurol. 1998;400:207–28. [PubMed] [Google Scholar]
- [53].Miczek KA, Fish EW, Bold J. F. De, De Almeida RM. Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology (Berl) 2002;163:434–58. doi: 10.1007/s00213-002-1139-6. [DOI] [PubMed] [Google Scholar]
- [54].Mitchell JB, Stewart J. Effects of castration, steroid replacement, and sexual experience on mesolimbic dopamine and sexual behaviors in the male rat. Brain Research. 1989;491:116–127. doi: 10.1016/0006-8993(89)90093-0. [DOI] [PubMed] [Google Scholar]
- [55].Northcutt KV, Lonstein JS. Social contact elicits immediate-early gene expression in dopaminergic cells of the male prairie vole extended olfactory amygdala. Neuroscience. 2009;163:9–22. doi: 10.1016/j.neuroscience.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- [57].Pappas SS, Tiernan CT, Behrouz B, Jordan CL, Breedlove SM, Goudreau JL, Lookingland KJ. Neonatal androgen-dependent sex differences in lumbar spinal cord dopamine concentrations and the number of A11 diencephalospinal dopamine neurons. J Comp Neurol. 2010;518:2423–36. doi: 10.1002/cne.22340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. Acute stimulatory effect of estradiol on striatal dopamine synthesis. J Neurochem. 1995;65:1651–7. doi: 10.1046/j.1471-4159.1995.65041651.x. [DOI] [PubMed] [Google Scholar]
- [59].Pawlisch BA, Riters LV. Selective behavioral responses to male song are affected by the dopamine agonist GBR-12909 in female European starlings (Sturnus vulgaris) Brain Res. doi: 10.1016/j.brainres.2010.07.003. in press. [DOI] [PubMed] [Google Scholar]
- [60].Perfito N, Zann RA, Bentley GE, Hau M. Opportunism at work: habitat predictability affects reproductive readiness in free-living zebra finches. Funct. Ecol. 2007;21:291–301. [Google Scholar]
- [61].Putnam SK, Du J, Sato S, Hull EM. Testosterone restoration of copulatory behavior correlates with medial preoptic dopamine release in castrated male rats. Horm Behav. 2001;39:216–24. doi: 10.1006/hbeh.2001.1648. [DOI] [PubMed] [Google Scholar]
- [62].Putnam SK, Sato S, Hull EM. Effects of testosterone metabolites on copulation and medial preoptic dopamine release in castrated male rats. Horm Behav. 2003;44:419–26. doi: 10.1016/j.yhbeh.2003.06.006. [DOI] [PubMed] [Google Scholar]
- [63].Putnam SK, Sato S, Riolo JV, Hull EM. Effects of testosterone metabolites on copulation, medial preoptic dopamine, and NOS-immunoreactivity in castrated male rats. Horm Behav. 2005;47:513–522. doi: 10.1016/j.yhbeh.2005.01.007. [DOI] [PubMed] [Google Scholar]
- [64].Reiner A, Karle EJ, Anderson KD, Medina L. Catecholaminergic perikarya and fibers in the avian nervous system. In: Smeets WJ, Reiner A, editors. Phylogeny and development of catecholamine systems in the CNS of vertebrates. Cambridge University Press; Cambridge: 1994. pp. 135–181. [Google Scholar]
- [65].Reiner A, Medina L, Veenman CL. Structural and functional evolution of the basal ganglia in vertebrates. Brain Res Brain Res Rev. 1998;28:235–85. doi: 10.1016/s0165-0173(98)00016-2. [DOI] [PubMed] [Google Scholar]
- [66].Renner K, Luine V. Analysis of temporal and dose-dependent effects of estrogen on monoamines in brain nuclei. Brain Res. 1986;366:64–71. doi: 10.1016/0006-8993(86)91281-3. [DOI] [PubMed] [Google Scholar]
- [67].Riters LV, Olesen KM, Auger CJ. Evidence that female endocrine state influences catecholamine responses to male courtship song in European starlings. Gen Comp Endocrinol. 2007;154:137–149. doi: 10.1016/j.ygcen.2007.05.029. [DOI] [PubMed] [Google Scholar]
- [68].Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Aberrant responses in social interaction of dopamine transporter knockout mice. Behav Brain Res. 2004;148:185–198. doi: 10.1016/s0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
- [69].Saigusa T, Takada K, Baker SC, Kumar R, Stephenson JD. Dopamine efflux in the rat nucleus accumbens evoked by dopamine receptor stimulation in the entorhinal cortex is modulated by oestradiol and progesterone. Synapse. 1997;25:37–43. doi: 10.1002/(SICI)1098-2396(199701)25:1<37::AID-SYN5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- [70].Sar M. Estradiol is concentrated in tyrosine hydroxylase-containing neurons of the hypothalamus. Science. 1984;223:938–40. doi: 10.1126/science.6141639. [DOI] [PubMed] [Google Scholar]
- [71].Sato Y, Shibuya A, Adachi H, Kato R, Horita H, Tsukamoto T. Restoration of sexual behavior and dopaminergic neurotransmission by long term exogenous testosterone replacement in aged male rats. J Urol. 1998;160:1572–5. [PubMed] [Google Scholar]
- [72].Serova LI, Maharjan S, Huang A, Sun D, Kaley G, Sabban EL. Response of tyrosine hydroxylase and GTP cyclohydrolase I gene expression to estrogen in brain catecholaminergic regions varies with mode of administration. Brain Res. 2004;1015:1–8. doi: 10.1016/j.brainres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- [73].Simerly RB. Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Brain Res Mol Brain Res. 1989;6:297–310. doi: 10.1016/0169-328x(89)90075-2. [DOI] [PubMed] [Google Scholar]
- [74].Soma KK, Sullivan K, Wingfield J. Combined aromatase inhibitor and antiandrogen treatment decreases territorial aggression in a wild songbird during the nonbreeding season. Gen Comp Endocrinol. 1999;115:442–53. doi: 10.1006/gcen.1999.7334. [DOI] [PubMed] [Google Scholar]
- [75].Svec LA, Lookingland KJ, Wade J. Estradiol and song affect female zebra finch behavior independent of dopamine in the striatum. Physiol Behav. 2009 doi: 10.1016/j.physbeh.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Svec LA, Wade J. Estradiol induces region-specific inhibition of ZENK but does not affect the behavioral preference for tutored song in adult female zebra finches. Behav Brain Res. 2009;199:298–306. doi: 10.1016/j.bbr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Thompson TL, Moss RL. Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neurosci Lett. 1997;229:145–148. doi: 10.1016/s0304-3940(97)00450-3. [DOI] [PubMed] [Google Scholar]
- [78].Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: How interactions between aromatase and the environment modulate aggression. Front Neuroendocrinol. 2006;27:170–179. doi: 10.1016/j.yfrne.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Tramontin AD, Smith GT, Breuner CW, Brenowitz EA. Seasonal plasticity and sexual dimorphism in the avian song control system: stereological measurement of neuron density and number. J Comp Neurol. 1998;396:186–92. doi: 10.1002/(sici)1096-9861(19980629)396:2<186::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- [80].Triemstra JL, Nagatani S, Wood RI. Chemosensory cues are essential for mating-induced dopamine release in MPOA of male Syrian hamsters. Neuropsychopharmacology. 2005;30:1436–42. doi: 10.1038/sj.npp.1300685. [DOI] [PubMed] [Google Scholar]
- [81].Van Hartesveldt C, Joyce JN. Effects of estrogen on the basal ganglia. Neurosci Biobehav Rev. 1986;10:1–14. doi: 10.1016/0149-7634(86)90029-1. [DOI] [PubMed] [Google Scholar]
- [82].Walters MJ, Harding CF. The effects of an aromatization inhibitor on the reproductive behavior of male zebra finches. Horm Behav. 1988;22:207–18. doi: 10.1016/0018-506x(88)90067-0. [DOI] [PubMed] [Google Scholar]
- [83].Watt MJ, Forster GL, Korzan WJ, Renner KJ, Summers CH. Rapid neuroendocrine responses evoked at the onset of social challenge. Physiol Behav. 2007;90:567–75. doi: 10.1016/j.physbeh.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Woolley SC, Sakata JT, Gupta A, Crews D. Evolutionary changes in dopaminergic modulation of courtship behavior in Cnemidophorus whiptail lizards. Horm Behav. 2001;40:483–9. doi: 10.1006/hbeh.2001.1713. [DOI] [PubMed] [Google Scholar]
- [85].Young KA, Liu Y, Wang Z. The neurobiology of social attachment: A comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Physiol C Toxicol Pharmacol. 2008 doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Youngren OM, Chaiseha Y, El Halawani ME. Regulation of prolactin secretion by dopamine and vasoactive intestinal peptide at the level of the pituitary in the turkey. Neuroendocrinology. 1998;68:319–325. doi: 10.1159/000054380. [DOI] [PubMed] [Google Scholar]
- [87].Zann RA. The zebra finch: a synthesis of field and laboratory studies. Oxford University Press; Oxford: 1996. [Google Scholar]
- [88].Zsarnovszky A, Scalise TJ, Horvath TL, Naftolin F. Estrogen effects on tyrosine hydroxylase-immunoreactive cells in the ventral mesencephalon of the female rat: further evidence for the two cell hypothesis of dopamine function. Brain Research. 2000;868:363–366. doi: 10.1016/s0006-8993(00)02323-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. A11 central gray (CG) neurons expressing fluorescent immunoreactivity for cytoplasmic tyrosine hydroxylase (magenta; the rate limiting enzyme in dopamine production) and the nuclear immediate early gene product Fos (green).
Abbreviations: EW, Edinger-Westphal nucleus; ca, cerebral aqueduct. Scale bar denotes 100 μm.