Abstract
Many patients respond positively to treatments for crouch gait, yet surgical outcomes are inconsistent and unpredictable. In this study, we developed a multivariable regression model to determine if biomechanical variables and other subject characteristics measured during a physical exam and gait analysis can predict which subjects with crouch gait will demonstrate improved knee kinematics on a follow-up gait analysis. We formulated the model and tested its performance by retrospectively analyzing 353 limbs of subjects who walked with crouch gait. The regression model was able to predict which subjects would demonstrate ‘improved’ and ‘unimproved’ knee kinematics with over 70% accuracy, and was able to explain approximately 49% of the variance in subjects’ change in knee flexion between gait analyses. We found that improvement in stance phase knee flexion was positively associated with three variables that were drawn from knowledge about the biomechanical contributors to crouch gait: i) adequate hamstrings lengths and velocities, possibly achieved via hamstrings lengthening surgery, ii) normal tibial torsion, possibly achieved via tibial derotation osteotomy, and iii) sufficient muscle strength.
Keywords: Surgical Outcomes, Cerebral Palsy, Crouch Gait, Modeling, Biomechanics
Introduction
Crouch gait, a walking pattern defined by excessive flexion of the knee during stance phase, is a debilitating problem that affects the population of children with spastic cerebral palsy. Many patients benefit from treatments for crouch gait, including hamstrings lengthening surgeries [1–4], tibial derotation osteotomies [5–7], and multi-level surgery [8, 9]; however, treatment outcomes are unpredictable.
Clinical decision-making is challenging, in part because there are no standardized protocols for determining which surgeries a patient should receive. Three-dimensional gait analysis helps clinicians identify which gait abnormalities should be targeted with treatment [10–12], but there are no uniform guidelines for interpreting the wealth of information provided by gait analysis. For example, two clinical teams might examine the same set of patient data and develop two different treatment plans [13].
Several studies have utilized biomechanical modeling and simulation of the musculoskeletal system to objectively identify the contributors to an individual’s crouch gait. For example, the work of Arnold and colleagues [14, 15] suggests that a subject’s hamstrings lengths and velocities during gait may provide information to more effectively prescribe hamstrings surgery. Several investigations have demonstrated that excessive tibial torsion reduces the capacity of muscles to extend the knee [16–18], suggesting that subjects with crouch gait and excess tibial torsion may benefit from a tibial derotation osteotomy. Sufficient strength of the extensor muscles may also be a key component in achieving normal knee motion. Analyzing the dynamics of normal and crouch gait has demonstrated that the gluteal muscles, plantarflexors, and vasti all play a crucial role in extending the knee during stance [18–20].
These biomechanical modeling studies have examined individual mechanical contributors to excess knee flexion for small, specialized groups of subjects, yet a typical patient has many possible contributors to his or her crouch gait. Further, there may be other variables, such as the severity of a subject’s gait pathology or the presence of concomitant gait abnormalities, that affect how crouch gait progresses over time.
To improve clinical decision-making and address the limitations of past biomechanics research, we developed and tested a multivariable linear regression model that used biomechanical variables to predict subjects’ improvement in crouch gait. We retrospectively analyzed subjects with moderate to severe crouch gait to determine if data from a subject’s initial visit to the gait analysis laboratory could predict the change in the subject’s knee kinematics measured on a follow-up gait analysis. We hypothesized that i) adequate hamstrings lengths and velocities, ii) good torsional alignment of the tibia, and iii) sufficient muscle strength would all help predict whether subject’s knee flexion in stance would improve on the follow-up gait analysis.
Methods
We retrospectively analyzed a group of subjects with a primary diagnosis of cerebral palsy, aged 5 to 18, who had at least two visits to a gait analysis laboratory, with or without intervening treatment, as part of routine care. We studied subjects who walked with a crouch gait [14] at their first gait analysis and had a mean knee flexion angle during the first half of the gait cycle larger than 25°. We examined only subjects who walked barefoot and without assistive devices during gait analysis.
We allowed any combination of bony surgery, soft tissue surgery, botox injections, or no treatment between gait analyses, but excluded subjects who received a selective dorsal rhizotomy or intrathecal baclofen pump between gait analyses, since this study focused on the biomechanical contributors to excess knee flexion. A histogram of intervening surgeries for all subjects is included in the supplementary material. Any type of prior surgery was allowed, but we excluded subjects who had botox injections less than 6 months prior or lower extremity surgery or other neurological treatment less than 12 months prior to the first gait analysis. We required that pairs of gait analyses for a subject were between 9 and 36 months apart. If a subject received surgery between gait analyses, we required that the second analysis be at least 9 months after the surgery to ensure sufficient recovery time.
The Gillette database contained 2300 subjects with a diagnosis of cerebral palsy and available gait kinematics. Our subject selection criteria resulted in a group comprised of 212 subjects and 353 pairs of gait analyses. We analyzed a subject’s right and left sides if both met our selection criteria. We also included more than one pair of gait analyses for a subject if all analyses occurred on different days. In other words, a second visit gait analysis for a subject could not also be used as a first visit analysis for the same subject. The individual data points we used to build the regression model were pairs of gait analyses for a subject-side, which we refer to as “limbs”.
Using this set of data, we built a multivariable linear regression model. The general form of the model is:
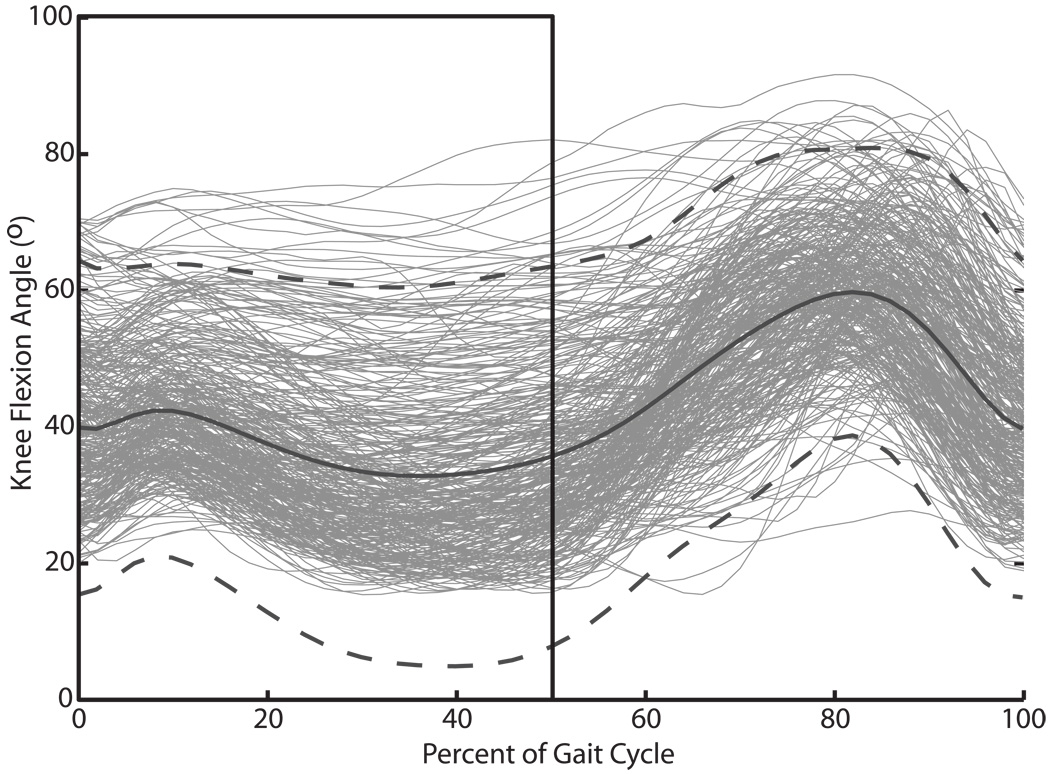
To assess crouch severity, we calculated the mean stance knee flexion for each limb, between 0 and 50% of the gait cycle (Figure 1). The outcome variable, Y, was then the change in mean stance knee flexion between the first and second gait analyses, such that a positive value corresponds with improvement in crouch gait. The xi are the predictive variables, derived from the first gait analysis, intervening treatment, and prior treatment, used to estimate the expected improvement in crouch gait on the second gait analysis. The βi are the linear weighting coefficients for the predictive variables. The interaction terms (β4) allow for the causal effect of one predictor (x2) on the outcome Y to vary according to another predictor (x3). The regression model was formulated using Stata (StataCorp, College Station, TX).
Figure 1.
Knee flexion kinematics at the initial gait analysis. The black solid curve and dashed curves show the mean knee flexion angle over the gait cycle +/− 2 SD for the entire group of limbs. The black box highlights the portion of the knee flexion curve used to derive the mean stance knee flexion measure, our metric to assess improvement in crouch gait.
We started with a set of candidate predictive variables and interaction terms drawn from research about the biomechanical contributors to excess knee flexion and the other factors that may affect improvement in crouch gait (Table 1). We determined a reduced set of predictive variables to include in the model by using the stepwise function in Stata to conduct backward selection. The variable with the least significance was successively dropped from the model until all variables are significant at the p = 0.2 level based on the Wald Test [21].
Table 1.
Candidate Predictive Variables
| GoodHams (1/0) | Mean Stance Dorsiflexion (°) |
| GoodTibia (1/0) | Hip Flexion Range of Motion During Gait (°) |
| GoodTibia and Good Hams (1/0) | Patellar Tendon Advance? (1/0) |
| Strength Score [24] | Gastrocnemius Lengthening? (1/0) |
| Interactions between GoodTibia, GoodHams and Strength Score (two-way) | Femoral Derotation Osteotomy? (1/0) |
| Foot Stabilization Surgery? (1/0) | |
| Gait Deviation Index [30] | Prior Tendo-Achilles Lengthening? (1/0) |
| Normalized Walking Speed | Prior Selective Dorsal Rhizotomy? (1/0) |
| Diagnosis Subtype (Hemi-, Di-, Tri-, or Quadriplegic) | Date of First Gait Analysis |
| Time to Follow-Up Gait Analysis Visit (months) | |
| Ipsilateral Mean Stance Knee Flexion (°) | Age (years) |
| Contralateral Mean Stance Knee Flexion (°) | Body Mass Index (kg/m2) |
| Mean Pelvic Tilt (°) | Multiple Qualifying Pairs of Gait Analyses? (1/0) |
| Knee Flexion Velocity at Toe-Off (°/% Gait Cycle) |
All variables are drawn from the subject’s first gait analysis visit, treatment plan, and surgical history.
The first biomechanical variable we considered was a binary variable denoting whether the subject had an appropriate hamstring length and velocity profile during gait (GoodHams). We calculated each limb’s peak hamstrings lengths and velocities using a model of the lower extremity and classified a limb’s hamstrings as short and/or slow if the peak values were more than 2.5 SDs shorter and/or slower than the value for unaffected children [22]. A limb’s hamstrings function was then classified as ‘Good’ (GoodHams = 1) if the hamstrings were neither short nor slow on the first gait analysis or the hamstrings were short and/or slow and the limb received a hamstrings lengthening surgery between gait analyses. A limb’s hamstrings were classified as ‘Poor’ (GoodHams = 0) if the limb had short and/or slow hamstrings and did not receive hamstrings lengthening surgery.
We similarly created a variable denoting alignment of the knee axis and foot, related to tibial torsion (GoodTibia). We examined the thigh foot angle (TFA) from the physical exam at the first gait analysis and classified the angle as abnormal if it was 10° or more degrees internal or 20° or more external. The standard deviation of the TFA in typically developing children was not available, so we selected this range based on 1 SD from the mean of subjects analyzed in this study. A limb’s tibial torsion was then classified as ‘Good’ (GoodTibia = 1) if the TFA was normal on the first gait analysis or the TFA was abnormal and the limb received a tibial derotation osteotomy. A limb’s tibial torsion was classified as ‘Poor’ (GoodTibia = 0) if the limb had an abnormal TFA and did not receive an osteotomy. A tabulation of the GoodHams and GoodTibia variables is included in the supplementary material.
The third biomechanical variable was a limb’s muscle strength. Manual muscle strength [23] of the muscles crossing the hip, knee, and ankle, as measured on the initial physical exam, was combined into a single muscle strength score using Principal Component Analysis [24].
We examined the overall fit of the model using the R2 statistic. We calculated the standard errors of each of the coefficients using the cluster adjustment in Stata to account for the correlation between limb data points that corresponded to the same subject [25]. We assessed the significance and relative importance of each of the coefficients in the model with the t-test and normalized regression coefficients (z-scores), respectively, for the continuous variables.
After predicting the expected change in mean stance knee flexion, we tested the ability of the regression model to correctly classify each limb as ‘Improved’ or ‘Unimproved’ on the second gait analysis. We defined a limb’s knee flexion as ‘Improved’ if the mean stance angle decreased by at least 10° or if the limb’s mean stance knee flexion was less than 18° on the second analysis, which is within 2 SD of the value for unaffected children. We selected a 10° threshold as this represents a meaningful clinical change and is larger than errors associated with measuring knee flexion kinematics. A summary of characteristics for ‘Improved’ vs. ‘Unimproved’ groups is included in the supplementary material. We estimated the predictive ability of the model for a new group of subjects using 10-fold cross-validation [26].
Results
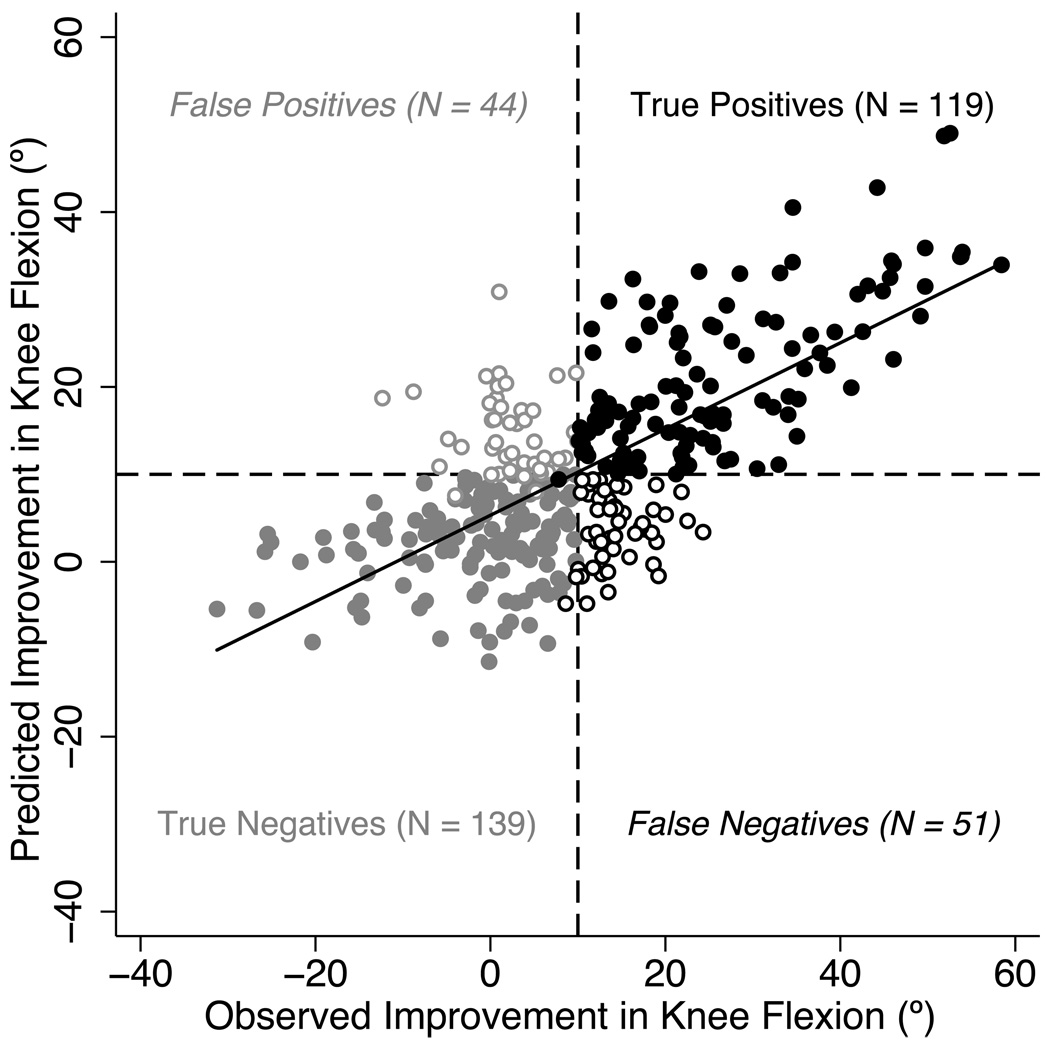
The multivariable linear regression model was able to predict whether a limb’s mean stance knee flexion was ‘Improved’ or ‘Unimproved’ on the second gait analysis with 73% accuracy. The overall performance of the model is displayed in Figure 2, which plots the change in knee flexion predicted by the regression model for each limb vs. the observed change between gait analyses. The regression model correctly classified 119 limbs as ‘Improved’ and 139 limbs as ‘Unimproved’. There were 44 limbs incorrectly classified as ‘Improved’ and 51 limbs incorrectly classified as ‘Unimproved’. The R2 value of the fit was 0.49, which indicates that the model was able to explain 49% of the variance in improvement in knee flexion.
Figure 2.
Performance of the linear regression model. This figure shows the measured improvement in mean stance knee flexion (MSKF) between gait analyses vs. the amount of improvement predicted by the regression model for each limb. The solid black line is the least squares fit to this data (R2 = 0.49). The horizontal and vertical dashed lines indicate the 10° threshold for predicted and measured improvement. Note that limbs were also classified as ‘Improved’ if they had a predicted or measured mean stance knee flexion angle less than 18° on their second gait analysis. Solid black circles indicate limbs that the regression model correctly classified as ‘Improved’. Open black circles indicate limbs whose measured improvement was not detected by the regression model. Solid gray circles indicate limbs correctly classified as ‘Unimproved’, while open gray circles indicate limbs that were incorrectly classified as ‘Improved’.
When we used 10-fold cross-validation to estimate the ability of the regression model to make predictions for a new group of subjects, there was a slight decrease in accuracy, with 71% of limbs correctly classified on average across the 10 cross-validation folds. The average R2 value computed with cross-validation was 0.44.
Examining the coefficients of the variables in the regression model allows us to assess their individual impact on improvement in knee flexion, when holding the other variables in the model constant (Table 2). There was a significant positive relationship between muscle strength and improvement in stance phase knee flexion, with an expected 1.3° more improvement in knee flexion for each one point increase in strength score. Further, limbs with ‘Good’ hamstrings function and ‘Good’ tibial torsion we re expected to have 7.5° more improvement in knee flexion than limbs with ‘Poor’ hamstrings function and tibial torsion.
Table 2.
Specification of the Linear Regression Model Used to Predict Improvement in Mean Stance Knee Flexion.
| First-Visit Variable | Coefficient | Normalized Coefficient |
Robust Standard Error* |
p-value† |
|---|---|---|---|---|
| GoodHams (1/0) | 6.62 | 3.28 | 0.045 | |
| GoodTibia (1/0) | 6.18 | 3.03 | 0.043 | |
| GoodHams and GoodTibia (1/0) | 7.52 | 3.39 | 0.016 | |
| Strength Score [24] | 1.28 | 1.88 | 0.604 | 0.035 |
| Ipsilateral Mean Stance Knee Flexion (°) | 0.898 | 10.0 | 0.108 | 0.001 |
| Contralateral Mean Stance Knee Flexion (°) | −0.235 | −3.35 | 0.0639 | 0.001 |
| Gait Deviation Index [29] | −0.136 | −1.43 | 0.105 | 0.194 |
| Mean Pelvic Tilt (°) | −0.213 | −1.57 | 0.127 | 0.094 |
| Knee Flex Vel at Toe-Off (°/% Gait Cycle) | 2.54 | 2.48 | 0.923 | 0.006 |
| Mean Stance Ankle Dorsiflexion (°) | 0.137 | 1.88 | 0.0615 | 0.027 |
| Prior Tendo-Achilles Lengthening (1/0) | −3.04 | 1.67 | 0.069 | |
| Intervening Patellar Advance/FEO (1/0) | 9.36 | 2.18 | 0.001 | |
| Intervening FDO (1/0) | 3.87 | 1.62 | 0.018 | |
| Triplegic Subtype (1/0) | 5.36 | 2.05 | 0.010 | |
| Quadriplegic Subtype (1/0) | 6.11 | 2.20 | 0.006 | |
| Multiple Qualifying Visit Pairs (1/0) | −5.45 | 1.81 | 0.003 | |
| Constant | −18.7 | −7.4 | 10.1 | 0.066 |
Robust standard errors, adjusted for the correlation between limbs from the same subject
p-value from a student’s t-test.
For each 1° increase in mean stance knee flexion on the initial gait analysis, there was an increase in expected improvement of 0.9°. In contrast, having more severe crouch on the contralateral side was associated with less improvement. More severe involvement, as indicated by cerebral palsy sub-type (triplegic or quadriplegic), was associated with more improvement, while a slower knee flexion velocity at toe-off, a prior tendo-achilles lengthening, and multiple qualifying pairs of gait analyses were associated with less expected improvement. Limbs with a smaller Gait Deviation Index, more equinus, and more anterior pelvic tilt tended to show less improvement in knee flexion, but these relationships were not significant. The receipt of a patellar tendon advancement/distal femoral extension osteotomy or a femoral derotation osteotomy was also associated with significantly more improvement in knee flexion.
Discussion
The multivariable linear regression model was able to predict, with 73% accuracy, whether subjects with crouch gait would be ‘Improved’ or ‘Unimproved’ on their second gait analysis using biomechanical variables and other subject characteristics. In contrast, only 48% of the limbs had ‘Improved’ knee flexion on their second gait analysis. This suggests that there are some patients not receiving surgeries that might improve their crouch gait, and other patients receiving treatment despite a low probability for improvement. Use of the regression model may avoid this in some cases.
Each of the biomechanical variables—adequate hamstrings lengths and velocities, normal tibial torsion, and muscle strength—was associated with improvement in knee flexion, which lends support to using gait analysis and biomechanical modeling to understand gait pathology and plan treatment. We did not see a large difference between having both ‘Good’ hamstrings function and tibial torsion and having a ‘Good’ value for only one of these variables. Subjects may be able to compensate for one of their biomechanical contributors to crouch gait or there may be diminishing returns for surgically correcting both contributors. Given the positive relationship between muscle strength and improvement in crouch gait, we suggest future research on the benefits of pre-operative strength training. Our finding that prior tendo-achilles surgery is associated with less improvement in crouch gait supports earlier investigations that indicate a tendo-achilles lengthening increases the risk of developing crouch gait [27, 28].
Receiving a femoral derotation osteotomy or patellar tendon advancement/distal femoral extension osteotomy was associated with significant improvement in crouch gait. The observed effect corresponds to the amount of improvement expected when these surgeries are prescribed using standard practice at Gillette. Future research should elucidate and test biomechanical guidelines for these procedures. Patients with crouch gait also frequently receive foot stabilization surgeries and gastrocnemius lengthenings, but the variables associated with these surgeries were not retained in the backwards selection process, and thus did not have an independent, significant impact on improvement in knee kinematics.
Crouch severity and overall level of involvement were both associated with more expected improvement, since severe subjects may have received more treatment or due to a “ceiling effect” for milder subjects. In contrast, a lower Gait Deviation Index [29], more severe crouch on the contralateral side, and the presence of other gait abnormalities was associated with less improvement. These concomitant problems may make it more difficult for subjects to recover from their crouch gait. Finally, subjects with multiple qualifying gait analyses tended to show less improvement, which was expected because this group of subjects, by definition, had a recurring crouch gait.
With further validation, the regression model could be used to aid clinical practice. Table 3 describes the characteristics of a hypothetical subject with crouch gait. The subject has excess tibial torsion and short and slow hamstrings. For the other predictive variables, this subject has values close to the mean of the present study’s subject pool. Based on this information, we would recommend that the subject receive a tibial derotation osteotomy, hamstrings lengthening, or both as a part of his treatment plan, to achieve an expected 13° improvement in knee flexion. Without these two additional surgeries, we expect only 6.5° improevement based on the subject’s strength, crouch severity, the planned femoral derotation osteotomy, and other characteristics, although we caution that further, prospective testing is needed to validate our regression model.
Table 3. Pre-Operative Data and Predictions for a Hypothetical Subject with Crouch Gait.
Subject has excess tibial torsion and short and slow hamstrings at his first gait visit
| First-Visit Variable | Coefficient | Subject Value |
Δ Expected Improvement |
|---|---|---|---|
| Strength Score [24] | 1.28 | 0.26 | 0.333 |
| Ipsilateral Mean Stance Knee Flexion (°) | 0.898 | 38.0 | 34.1 |
| Contralateral Mean Stance Knee Flexion (°) | −0.235 | 34.6 | −8.13 |
| Gait Deviation Index [29] | −0.136 | 64.9 | −8.83 |
| Mean Pelvic Tilt (°) | −0.213 | 15.7 | −3.34 |
| Knee Flex Vel at Toe-Off (°/% Gait Cycle) | 2.54 | 1.05 | 2.67 |
| Mean Stance Ankle Dorsiflexion (°) | 0.137 | 10.7 | 1.47 |
| Prior Tendo-Achilles Lengthening (1/0) | −3.04 | YES | −3.04 |
| Planned Patellar Advance/FEO (1/0)* | 9.36 | NO | 0 |
| Planned FDO (1/0)* | 3.87 | YES | 3.87 |
| Triplegic Subtype (1/0) | 5.36 | NO | 0 |
| Quadriplegic Subtype (1/0) | 6.11 | NO | 6.11 |
| Multiple Qualifying Visit Pairs (1/0) † | −5.45 | NO | 0 |
| Constant | −18.7 | −18.7 | |
| Expected Improvement without Hamstrings Lengthening or TDO | ~6.5° | ||
| Expected Improvement with Hamstrings Lengthening and/or TDO | ~13° | ||
The clinical team has decided to perform a femoral derotation osteotomy for this hypothetical subject, but not a patellar advance.
This is the first time the patient has visited the gait lab exhibiting a moderate crouch gait pattern
The regression model, which estimates the expected amount of change in knee flexion, could also be used by clinicians to set realistic expectations for the outcome of multi-level surgery given a subject’s treatment plan, muscle strength, and other gait problems. If the regression model predicts little or no improvement for a patient, extensive surgical treatment may be contraindicated.
Interpretation of the regression model must be undertaken with caution. For new subjects, cross-validation estimated that the model would explain only 44% of the variance in our outcome measure. Additionally, while we adjusted the standard errors to account for the correlation between limbs from the same subject, this approach may not fully correct for the lack of independence in our subject pool, inflating the cross-validation prediction accuracy. The p-values reported for the regression coefficients may be too small, since backwards selection was used to formulate the reduced model and no standard technique is available to correct for the multiple statistical comparisons used in backwards selection.
We did not penalize subjects for receiving an ‘unnecessary’ hamstrings lengthening or tibial derotation osteotomy. Receiving an ‘unnecessary’ hamstrings lengthening can have a detrimental effect on pelvic motion, but a similar negative effect on knee kinematics has not been demonstrated [15]. Since surgeons adjust the amount of correction to the severity of a subject’s tibial deformity, we did not expect a tibial derotation osteotomy to have a detrimental effect on knee kinematics in subjects whose pre-operative tibial torsion was within our defined “normal” range.
We were only able to explain 49% of the variance in subjects’ improvement in knee flexion because there were many factors that we could not measure accurately or at all. For example, manual strength measures are an approximate assessment of a subject’s muscle strength and the thigh foot angle is an approximation of a subject’s tibial torsion [30]. There may have also been errors in the other gait and physical exam measures. In addition, we were unable to account for motor control, patient motivation, variability in surgical technique, compliance with post-operative rehabilitation, and potential recurrence of hamstrings tightness or tibial mal-alignment.
In this study, we built and tested the first statistical model of its kind to predict improvement in crouch gait using knowledge about the biomechanical contributors to excess knee flexion. This statistical model can help improve treatment outcomes by identifying good candidates for common surgeries performed on subjects with crouch gait, in particular hamstrings lengthenings and tibial derotation osteotomies. The methods used to build the regression model in this study could be used to develop similar predictive models for other patient populations and gait pathologies. For example, we are currently investigating a linear regression model to predict improvement in overall gait kinematics after multi-level surgery. As demonstrated here, retrospective statistical analysis of patient data and biomechanical modeling are powerful and complementary tools to improve the treatment of movement disorders and make outcomes more predictable.
Supplementary Material
Acknowledgements
The authors thank the staff of the Center for Gait and Motion Analysis at Gillette Children’s Specialty Healthcare for collection of the subject data and Trevor Hastie for providing technical guidance on the statistical analysis techniques. This work was funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54GM072970 and through NIH Grants HD33929, R24 HD065690, and HD046814. Support was also provided by Stanford’s Bio-X program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon AB, Baird GO, McMulkin ML, Caskey PM, Ferguson RL. Gait analysis outcomes of percutaneous medial hamstring tenotomies in children with cerebral palsy. Journal of Pediatric Orthopaedics. 2008;28(3):324–329. doi: 10.1097/BPO.0b013e318168d1c0. [DOI] [PubMed] [Google Scholar]
- 2.DeLuca PA, Ounpuu S, Davis RB, Walsh JH. Effect of hamstring and psoas lengthening on pelvic tilt in patients with spastic diplegic cerebral palsy. Journal of Pediatric Orthopaedics. 1998;18:712–718. [PubMed] [Google Scholar]
- 3.Abel MF, Damiano DL, Pannunzio M, Bush J. Muscle-tendon surgery in diplegic cerebral palsy: functional and mechanical changes. Journal of Pediatric Orthopaedics. 1999;19(3):366–375. [PubMed] [Google Scholar]
- 4.Kay RM, Rethlefsen SA, Skaggs D, Leet A. Outcome of medial versus combined medial and lateral hamstring lengthening surgery in cerebral palsy. Journal of Pediatric Orthopaedics. 2002;22(2):169–172. [PubMed] [Google Scholar]
- 5.Stefko RM, de Swart RJ, Dodgin DA, Wyatt MP, Kaufman KR, Sutherland DH, Chambers HG. Kinematic and kinetic analysis of distal derotational osteotomy of the leg in children with cerebral palsy. Journal of Pediatric Orthopaedics. 1998;18(1):81–87. [PubMed] [Google Scholar]
- 6.Selber P, Filho ER, Dallalana R, Pirpiris M, Nattrass GR, Graham HK. Supramalleolar derotation osteotomy of the tibia, with T plate fixation. Technique and results in patients with neuromuscular disease. Journal of Bone and Joint Surgery [Br] 2004;86(8):1170–1175. doi: 10.1302/0301-620x.86b8.14479. [DOI] [PubMed] [Google Scholar]
- 7.Ryan DD, Rethlefsen SA, Skaggs DL, Kay RM. Results of tibial rotational osteotomy without concomitant fibular osteotomy in children with cerebral palsy. Journal of Pediatric Orthopaedics. 2005;25(1):84–88. doi: 10.1097/00004694-200501000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Adolfsen SE, Ounpuu S, Bell KJ, DeLuca PA. Kinematic and kinetic outcomes after identical multilevel soft tissue surgery in children with cerebral palsy. Journal of Pediatric Orthopaedics. 2007;27(6):658–667. doi: 10.1097/BPO.0b013e3180dca114. [DOI] [PubMed] [Google Scholar]
- 9.Rodda JM, Graham HK, Nattrass GR, Galea MP, Baker R, Wolfe R. Correction of severe crouch gait in patients with spastic diplegia with use of multilevel orthopaedic surgery. Journal of Bone and Joint Surgery [Am] 2006;88(12):2653–2664. doi: 10.2106/JBJS.E.00993. [DOI] [PubMed] [Google Scholar]
- 10.Filho MC, Yoshida R, Carvalho Wda S, Stein HE, Novo NF. Are the recommendations from three-dimensional gait analysis associated with better postoperative outcomes in patients with cerebral palsy? Gait & Posture. 2008;28(2):316–322. doi: 10.1016/j.gaitpost.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Gough M, Shortland AP. Can clinical gait analysis guide the management of ambulant children with bilateral spastic cerebral palsy? Journal of Pediatric Orthopaedics. 2008;28(8):879–883. doi: 10.1097/BPO.0b013e31818e197c. [DOI] [PubMed] [Google Scholar]
- 12.Lofterod B, Terjesen T, Skaaret I, Huse AB, Jahnsen R. Preoperative gait analysis has a substantial effect on orthopedic decision making in children with cerebral palsy: comparison between clinical evaluation and gait analysis in 60 patients. Acta Orthopaedica. 2007;78(1):74–80. doi: 10.1080/17453670610013448. [DOI] [PubMed] [Google Scholar]
- 13.Skaggs DL, Rethlefsen SA, Kay RM, Dennis SW, Reynolds RA, Tolo VT. Variability in gait analysis interpretation. Journal of Pediatric Orthopaedics. 2000;20(6):759–764. doi: 10.1097/00004694-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Arnold AS, Liu MQ, Schwartz MH, Ounpuu S, Dias LS, Delp SL. Do the hamstrings operate at increased muscle-tendon lengths and velocities after surgical lengthening? Journal of Biomechanics. 2006;39(8):1498–1506. doi: 10.1016/j.jbiomech.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Arnold AS, Liu MQ, Schwartz MH, Ounpuu S, Delp SL. The role of estimating muscle-tendon lengths and velocities of the hamstrings in the evaluation and treatment of crouch gait. Gait & Posture. 2006;23(3):273–281. doi: 10.1016/j.gaitpost.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz M, Lakin G. The effect of tibial torsion on the dynamic function of the soleus during gait. Gait & Posture. 2003;17(2):113–118. doi: 10.1016/s0966-6362(02)00058-9. [DOI] [PubMed] [Google Scholar]
- 17.Hicks J, Arnold A, Anderson F, Schwartz M, Delp S. The effect of excessive tibial torsion on the capacity of muscles to extend the hip and knee during single-limb stance. Gait & Posture. 2007;26(4):546–552. doi: 10.1016/j.gaitpost.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hicks JL, Schwartz MH, Arnold AS, Delp SL. Crouched postures reduce the capacity of muscles to extend the hip and knee during the single-limb stance phase of gait. Journal of Biomechanics. 2008;41(5):960–967. doi: 10.1016/j.jbiomech.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold AS, Anderson FC, Pandy MG, Delp SL. Muscular contributions to hip and knee extension during the single limb stance phase of normal gait: a framework for investigating the causes of crouch gait. Journal of Biomechanics. 2005;38(11):2181–2189. doi: 10.1016/j.jbiomech.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 20.Steele KM, Seth A, Hicks JL, Schwartz MS, Delp SL. Muscle contributions to support and progression during single-limb stance in crouch gait. Journal of Biomechanics. 2010;43(11):2099–2105. doi: 10.1016/j.jbiomech.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maldonado G, Greenland S. Estimating causal effects. International Journal of Epidemiology. 2002;31(2):422–429. [PubMed] [Google Scholar]
- 22.Arnold AS, Blemker SS, Delp SL. Evaluation of a deformable musculoskeletal model for estimating muscle-tendon lengths during crouch gait. Annals of Biomedical Engineering. 2001;29(3):263–274. doi: 10.1114/1.1355277. [DOI] [PubMed] [Google Scholar]
- 23.Kendall H, Kendall F, Wadsworth G. Muscle Testing and Function. 2nd ed. London: Williams and Wilkins; 1971. [Google Scholar]
- 24.Rozumalski A, Schwartz MH. Crouch gait patterns defined using k-means cluster analysis are related to underlying clinical pathology. Gait & Posture. 2009;30(2):155–160. doi: 10.1016/j.gaitpost.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. New York: Oxford University Press, Inc.; 2003. [Google Scholar]
- 26.Kohavi R. A study of cross-validation and bootstrap for accuracy estimation and model selection; Proceedings of the 14th International Joint Conference on Artificial Intelligence; Montreal, Quebec, Canada: Morgan Kaufmann Publishers Inc.; 1995. [Google Scholar]
- 27.Delp SL, Statler K, Carroll NC. Preserving plantar flexion strength after surgical treatment for contracture of the triceps surae: A computer simulation study. Journal of Orthopaedic Research. 1995;13(1):96–104. doi: 10.1002/jor.1100130115. [DOI] [PubMed] [Google Scholar]
- 28.Dietz FR, Albright JC, Dolan L. Medium-term follow-up of Achilles tendon lengthening in the treatment of ankle equinus in cerebral palsy. Iowa Orthopaedic Journal. 2006;26:27–32. [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz MH, Rozumalski A. The Gait Deviation Index: a new comprehensive index of gait pathology. Gait & Posture. 2008;28(3):351–357. doi: 10.1016/j.gaitpost.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 30.McDowell BC, Hewitt V, Nurse A, Weston T, Baker R. The variability of goniometric measurements in ambulatory children with spastic cerebral palsy. Gait & Posture. 2000;12(2):114–121. doi: 10.1016/s0966-6362(00)00068-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.