Abstract
Background
Immunosuppressive medication non-adherence is one of the most prevalent but preventable causes of poor outcomes in adult renal transplant recipients, yet there is a paucity of studies testing interventions in this area.
Methods
Using a randomized controlled trial design, 30 adult renal transplant recipients were screened for medication non-adherence using electronic monitoring. Fifteen non-adherent participants were randomized to receive either a continuous self-improvement intervention or attention control management. The six-month continuous self-improvement intervention involved the participant and clinical nurse specialist collaboratively identifying the person’s life routines, important people, and possible solutions to enhance medication taking. The participant then received individual monthly medication taking feedback delivered via a graphic printout of daily medication taking generated from electronic monitoring.
Results
The mean medication adherence score for the continuous self-improvement intervention group (n = 8) was statistically significantly higher than the attention control group’s (n = 5) mean medication adherence score (p = 0.03). The continuous self-improvement intervention effect size (Cohen’s d) was large at 1.4. Participants’ perceptions of the intervention were highly favorable.
Conclusions
The continuous self-improvement intervention shows promise as an effective and feasible approach to improve medication adherence in adult renal transplant recipients. A fully-powered study with a diverse sample is needed to confirm these preliminary findings.
Keywords: electronic monitoring, kidney, medication adherence, randomized controlled trial, transplantation
Renal transplantation is the treatment of choice for those with chronic kidney disease resulting in superior psychological, social, and physiologic outcomes (1–3). Although medical and surgical advancements have improved care for those receiving a kidney transplant, morbidity and mortality have remained relatively unchanged, with one-and three-yr graft survival rates at 91.0 and 69.3 for deceased donor recipients and 96.3 and 81.4 for living donors (4). Medication non-adherence has been targeted as a key factor for improvement in graft survival. Adherence, as defined by the World Health Organization, is “the extent to which a person’s behavior (taking medications, following a recommended diet and/or executing life-style changes) corresponds with the agreed recommendations of a health care provider” (5). Of all transplant types, kidney transplant recipients appear to have the highest medication non-adherence rate at 35.6 per 100 patients per yr (6). Medication non-adherence may include problems such as non-acceptance (not initiating the medication), poor execution (not taking the correct number of doses per day or not taking doses at the correct time), and non-persistence (not taking the medication for the prescribed period of time) (7). When both dosing and timing adherence are considered, medication non-adherence escalates (8). Medication non-adherent transplant recipients have poorer outcomes including increased acute and chronic rejection, kidney loss, and death (9–11). Traditional interventions have focused on improving patient knowledge (cognitive strategies) and/or changing patient attitudes (affective strategies) (12) with marginally effective improvements in medication adherence for those with acute and chronic illnesses (13–16) and adult transplant recipients (12). One non-traditional intervention focused on the patient’s habits has shown promise in changing difficult-to-change behaviors. Called continuous self-improvement, this intervention has decreased asthma attacks (17), improved hypertension care (18), enhanced stress management (19), and improved eating behaviors (20). Transplant patients urgently need effective interventions for non-adherence. This pilot study sought to examine the feasibility and effectiveness of a continuous self-improvement intervention to enhance immunosuppressive medication adherence in non-adherent adult kidney transplant patients.
Patients and methods
Design
A randomized, controlled pilot trial was carried out to determine the feasibility and efficacy of a six-month continuous self-improvement intervention versus an attention control management in medication non-adherent adult kidney transplant patients. Prior to the intervention, a three-month screening phase was conducted after which those who were medication non-adherent were randomized into either the continuous self-improvement intervention versus an attention control intervention. The screening phase for documenting medication non-adherence provided a more homogenous group and prevented the “ceiling effect” seen in studies that include medication adherent participants.
Study participants
Thirty kidney transplant patients from a tertiary care transplant center located in the Midwestern United States meeting the following criteria were included: (i) 21 yr of age or older, (ii) prescribed at least one, twice daily prescribed, immunosuppressive medication, (iii) non-adherent with immunosuppressive medication as evidenced by a medication non-adherence score of <0.85, (iv) functioning kidney transplant, (v) transplant physician and nephrologists’ assent that recipient is able to participate in the study, (vi) ability to speak, hear, and understand English, (vii) able to open an electronic medication cap, (viii) administers immunosuppressive medications to self, (ix) has a telephone or has access to a telephone, (x) no cognitive impairment, (xi) no other diagnoses that may shorten life span, such as metastatic cancer, as determined by the transplant physician or nephrologists’ statement that life will be shortened by the diagnosis.
Intervention
Independent variables: continuous self-improvement intervention
The continuous self-improvement intervention, which focuses on changing the systems in which the person lives using the plan-do-check-act process, was initiated by the Primary Investigator (C.R.) during the initial home visit and reviewed each month during the six-month intervention with the treatment group. Table 1 delineates the continuous self-improvement intervention which is described in detail elsewhere (21).
Table 1.
Continuous self-improvement intervention using the plan-do-check-act steps delivered to intervention participants
| “Plan” step |
| Identify life routines |
| Identify optimal steps for medication taking and how life routines impact these steps |
| Identify important people in medication-taking process |
| Select system level solutions that foster routines that enhance medication taking rather than focusing on personal effort and “remembering” |
| “Do” step |
| Incorporate change into existing routines |
| Implement more than one system-wide solution |
| “Check” step |
| Track data using the mailed MEMS report |
| “Act” step |
| Evaluate MEMS report data to see whether outcome was met |
MEMS, Medication Event Monitoring System®.
Attention control intervention
Each month during the six-month intervention phase, attention control participants were provided with educational brochures developed by the International Transplant Nurses Society (ITNS) addressing healthy post-transplant behaviors (healthy lifestyle, skin cancer risk, dental care, gastrointestinal upset, risk for developing or worsening post-transplant diabetes) (22). The first brochure was delivered to the participants via a home visit with subsequent brochures mailed. Monthly telephone calls were made to review the information in the brochures and to ask participants whether they have any questions about the information. The home visit, monthly telephone calls, and mailed transplant educational materials were delivered to provide equal attention time and perceived benefits to the control group.
Intervention evaluation measures
Dependent variable: medication non-adherence
The primary outcome of interest was medication non-adherence measured by the Medication Event Monitoring System® ([MEMS], MEMS Track Cap™; Aprex Corp., Union City, CA, USA) electronic medication cap and bottle. Each MEMS cap contains a battery and microelectronic circuitry that record a date and time with each removal of the cap from the medication vial. The batteries for the MEMS have a 36-month battery-life, can store up to 3800 medication events, and have been more than satisfactory for capturing 12 months of medication-taking activity (23). Reliability has been established in temperatures ranging from −20 to 70°C and up to 95% humidity, is accurate to within two min per month, and has a reported 0.04 to 2% malfunction rate (24–26). To enhance data validity, participants used a MEMS diary to document any accidental cap openings, openings when no medication was ingested, e.g., when refilling MEMS bottle, and early openings when a medication was removed early to take later, but on time, e.g., clinic appointments. The diary successfully corrects any invalid data from MEMS opening when medications were not ingested (26). After these corrections were made, each cap removal represented a presumed participant ingestion of one dose of the prescribed immunosuppressant. A cumulative record of cap openings was compiled for each participant. Using the time of the cap openings and the scheduled time for the medications to be taken, a medication adherence score was calculated (8).
Although calculation of medication adherence scores is described in detail in our prior studies, a brief review will be provided here (8, 23). We established a three-h window in the morning and evening in which the twice daily prescribed immunosuppressive medication should have been self-administered to increase immunosuppressive medication bioavailability and effectiveness. Each morning and evening a participant received a medication score of 0.5 if the dose of the immunosuppressive medication was taken within a three-h window of the prescribed 12-h time frame; 0.25 if the dose of the immunosuppressive medication was not within the three-h window but was taken within a 12-h window; and 0 if the dose of the immunosuppressive medication was not taken within a 12-h window. An individual could be assigned a score of 0, 0.25, 0.50, 0.75, or 1 point each day. We believe calculating medication adherence scores in this manner represents the dynamic and complex nature of daily medication taking and is superior to dichotomizing medication taking into binary data (took medication or did not take medication).
Procedures
The study was approved by the Institutional Review Board at the institution where the study was conducted. The transplant program’s Clinical Nurse Specialist and Social Worker identified patients and asked whether they were willing to have the research assistant (RA) contact them to discuss possible participation in a study. Those who agreed to participate were contacted by the RA, and study details were discussed. Informed consent was obtained from all study participants. Those meeting inclusion criteria continued in the study.
Screening phase
During the screening phase, participants used MEMS for three months. At the end of three months, a medication non-adherence score was calculated for each participant (8, 27). Participants with a medication score <0.85 were considered non-adherent and were eligible for the next phase of the study. The medication adherence “cut-o.” score of 0.85 has been validated in our prior work with both adult and older adult kidney transplant recipients (8, 23). The first month of data was deleted because MEMS has a weak intervention effect during the first month of use (26, 28).
Randomization
Using a block randomization approach (29), non-adherent participants were randomly assigned after the screening phase, by a person independent of the research team to either the continuous self-improvement intervention group or the attention control group.
Study completion
At the end of the study, the MEMS caps were collected from both groups by the RA. A monetary gift was provided to thank participants for their time.
Analysis
SAS v9.1 (SAS Institute, Inc., Cary, NC, USA) was used by the project biostatistician to conduct all data analyses. Descriptive statistics were used to characterize the sample. Between-group differences at baseline were evaluated using the two-sample t-test for continuous variables to determine whether the attention control group and treatment group were equivalent. The effect (or changes) of medication adherence (calculated on a monthly basis) within and between groups was explored using a two-factor analysis of variance. The Mixed procedure in SAS was used to examine the time by group interaction effect. Finally, between-group differences after completion of the intervention were analyzed using the two-sample t-test for continuous variables to evaluate whether the treatment had an effect. Alpha level was set at p < 0.05. Effect size was calculated to allow the comparison of results with other studies.
Results
Intervention effectiveness
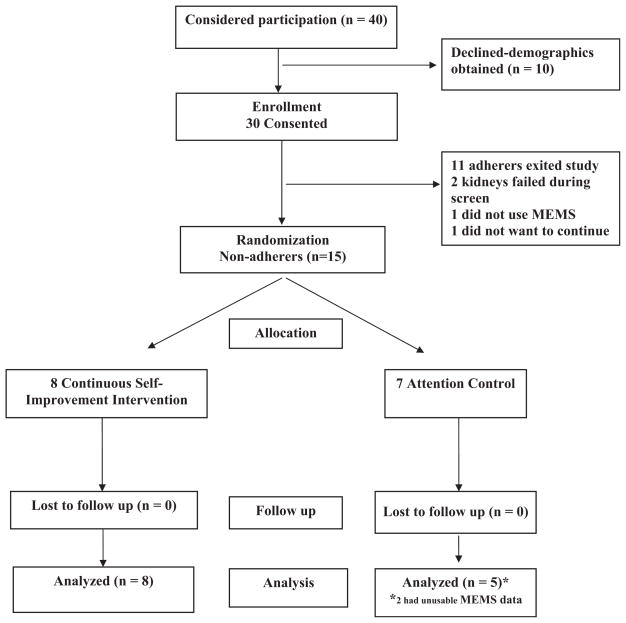
A convenience sample of 30 adult renal transplant recipients was recruited for the study. Participants were recruited from a Midwestern transplant center’s list of patients who had received a transplant and had a functioning kidney. Ten individuals declined to be in the study but provided demographic information. Demographics for this group were not statistically significantly different from those who agreed to be in the study with respect to age, gender, educational level, and ethnicity. However, decliners were more likely to be employed full time (df = 3; p = 0.026). Fig. 1 delineates the flow of participants through the study including number of participants randomly assigned, receiving intended treatment, completing the study protocol, and analyzed for the primary outcome of medication adherence (30). Of the 30 that agreed to be in the study, during the threemonth screening period, two participants’ kidneys failed, one did not use the MEMS, and one did not desire to continue. Eleven adherers exited the study after the three-month screening period. Consequently, 15 participants were randomly assigned to either the continuous self-improvement intervention or the attention control intervention.
Fig. 1.
Flow of participants through the study.
Table 2 describes the demographic characteristics of the groups at time of random assignment and compares the continuous self-improvement group with the attention control group. As expected, in view of randomization of participants to groups, the groups did not differ with respect to the medication adherence scores determined at the screening stage (Wilcoxon rank sum test, p = 0.75). Baseline medication score for the treatment group was 0.72 and for the attention control group 0.75.
Table 2.
Characteristics of the sample at baseline
| Characteristics | Total sample (n = 15) | Control (n = 7) | Treatment (n = 8) | p-Value |
|---|---|---|---|---|
| Age M (SE) | 51.5 (7.2) | 44 (15.7) | 55 (12.1) | 0.2362 |
| Gender, n (%) (female) | 8 (53) | 4 (57) | 4 (50) | 0.5649 |
| Ethnicity, n (%) (Caucasian) | 12 (80) | 4 (57) | 8 (100) | 0.3846 |
| Education level, n (%)(some high school/high school) | 6 (40) | 1 (14) | 5 (63) | 0.9161 |
| Marital status (married) | 9 (60) | 3 (43) | 6 (75) | 0.5105 |
| Employment (disabled) | 8 (53) | 3 (43) | 5 (63) | 0.8368 |
| No. of transplants (1) | 8 (53) | 2 (29) | 6 (75) | 0.3473 |
| Type of transplant (deceased donor) | 11 (73) | 4 (57) | 7 (88) | 1.0000 |
| Pillbox use (yes) | 9 (60) | 2 (29) | 7 (88) | 0.2168 |
All randomized participants completed the study. Two participants in the attention control did not use the MEMS protocol (one “triggered” the MEMS [e.g., opened the MEMS cap to indicate taking a medication, but did not store medications in bottle]; one removed the medication too quickly for the cap to capture the opening) so these data were excluded.
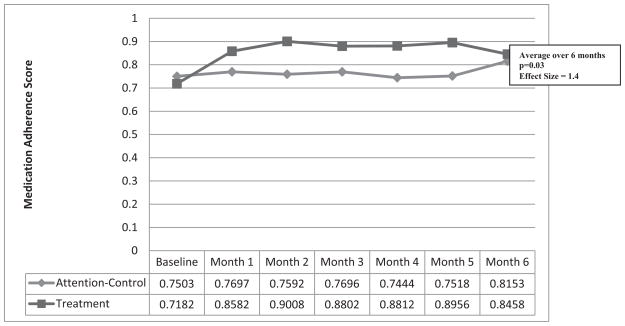
Fig. 2 presents medication adherence scores by month for both groups. There was a statistically significant difference between groups over the entire six-month period (t11 = 2.33, p = 0.0396) with the continuous self-improvement group having significantly higher medication adherence scores (M = 0.88, SD = 0.09) than the attention control group (M = 0.77, SD = 0.06). The continuous self-improvement intervention effect size, which is a standardized mean difference between groups, was large (Cohen’s d = 1.4; r = 0.6).
Fig. 2.
Comparison of medication adherence scores between groups.
The most prevalent solutions implemented by the continuous self-improvement group for improving medication adherence included placing medications in “plain view” next to other routines such as making coffee, brushing teeth, traveling in the car to/from work, working on the computer, watching the nightly news or favorite television program (8/8), and using cell phone and pillbox alarms (2/8). Only one participant used an important support person to improve medication adherence.
Feasibility of interventions
At the completion of the intervention, both groups were asked for their perceptions of their respective intervention’s burden. All of the participants responded that their involvement in the study took “very little” or the “right amount” of time. Only one attention control participant perceived that the monthly telephone calls took too much time. No participants expressed a desire to move between groups.
Discussion
Immunosuppressive medication adherence is paramount for optimal kidney transplant outcomes in adult recipients yet intervention testing has been scant. This pilot study is the first to examine the effectiveness and feasibility of a continuous self-improvement intervention to enhance immunosuppressive medication adherence in non-adherent adult kidney transplant patients. The continuous self-improvement intervention is unique from other interventions in that its systems approach removes motivation and remembering from medication adherence behavior. Forgetting to take medications is a frequent barrier to medication adherence (31). Instead, daily habits and routines are linked with medication taking, and medication-taking reports from electronic monitoring provide feedback in a continuous self-improvement process. Participant acceptability and the large effect size of the continuous self-improvement intervention found in this randomized controlled trial pilot study provide early promising evidence that this approach is significantly more effective than the attention control intervention. Particularly, impressive was our finding that the intervention improved medication adherence immediately which shows its potential for one-time delivery in the hospital or outpatient setting. Our findings are consistent with other studies using a personal systems approach to change health behavior (17–20). While traditional medication adherence interventions have shown modest effect sizes even when combined (educational 0.11–0.13, affective 0.18, behavioral 0.07–0.20, and combination 0.08– 0.24) (14, 15), this systems-focused intervention provides a promising new approach that removes blame from the patient for medication-taking failures (5).
The diminishing differences between the two groups’ medication adherence score between months 5 and 6 were primarily because of an increase in the attention control group’s scores this last month (from 0.75 to 0.81). This finding may be attributed to the attention control participants’ attentiveness to medication adherence in anticipation of the return of the MEMS cap to the research team for evaluation at study end. The persistence of the intervention effect must be evaluated in a fully powered study.
Our findings show promise for possible clinical significance as well. The medication adherence score difference between the groups of 0.11 could correspond, for example, to an attention control participant taking half of the medications on time and half late (an average score of 0.75), and a continuous self-improvement participant taking 70% of the medications on time, and only 30% late (an average score of 0.85 – a difference of about 0.11). Previous studies have shown that even minor deviations in medication taking result in poor transplant outcomes (32–34).
Future medication adherence intervention research should evaluate the potential financial benefits of the continuous self-improvement intervention once efficacy is established in a fully powered study. The economic costs of immunosuppressive medication non-adherence are high. Non-adherers experience $33 000 more in healthcare costs at the end of three yr when compared to adherers, with all but the highest medication adherers at risk for poorer health outcomes (34). The continuous self-improvement intervention’s early impact and potential for delivery in a hospital or clinic setting make the findings of this study of interest to all transplant providers. Future research could also evaluate the efficacy of the continuous self-improvement intervention in other transplant populations.
Study conclusions must be tempered with consideration of study limitations. This study sample size was small, and the study was not powered to detect small effects, although small effects may not be clinically significant. The study was conducted with participants from a single center, so results may not be able to be generalized broadly.
In conclusion, transplant patients urgently need effective interventions for medication non-adherence. Although renal transplantation recently reached its 50-yr anniversary, clinicians have few empirically tested interventions available to assist patients with the challenging behavior of immunosuppressive adherence. This continuous self-improvement intervention shows promise as an effective and feasible approach to improve medication adherence in adult renal transplant recipients. A fully powered study with a diverse sample is needed to confirm these findings.
Acknowledgments
This study was supported by grants from American Nephrology Nurses Association, National Kidney Foundation, Interdisciplinary Center on Aging at the University of Missouri, University of Missouri Research Council, and Iowa Gerontological Nursing Intervention Research Center.
Footnotes
Conflict of interest: None.
References
- 1.Crom DB, Hathaway DK, Tolley E. The use of stepwise multiple linear regression to predict quality of life after kidney transplantation. J Transpl Coord. 1995;5:72. [Google Scholar]
- 2.Hauser ML, Williams J, Strong M, Ganza M, Hathaway D. Predicted and actual quality of life changes following renal transplantation. ANNA J. 1991;18:295. [PubMed] [Google Scholar]
- 3.Manninen DL, Evans RW, Dugan MK. Work disability, functional limitations, and the health status of kidney transplantation recipients posttransplant. In: Terasaki P, editor. Clin Transplant. Los Angeles, CA: UCLA Tissue Typing Laboratory; 1991. p. 193. [PubMed] [Google Scholar]
- 4.Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(Pt 2):961. doi: 10.1111/j.1600-6143.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 5.Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 6.Dew MA, DeMartini AF, De Vito Dabbs A, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83:858. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 7.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically complied dosing histories. BMJ. 2008 doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell CL, Conn V, Ashbaugh C, Madsen R, Hayes K, Ross G. Medication adherence patterns in adult renal transplant recipients. Res Nurs Health. 2006;29:521. doi: 10.1002/nur.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Geest S, Borgermans L, Gemoets H, et al. Incidence, determinants, and consequences of subclinical noncompliance with immunosuppressive therapy in renal transplant recipients. Transplantation. 1995;59:340. [PubMed] [Google Scholar]
- 10.Nevins TE, Kruse L, Skeans MA, Thomas W. The natural history of azathioprine compliance after renal transplantation. Kidney Int. 2001;60:1565. doi: 10.1046/j.1523-1755.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- 11.Vlaminck H, Maes B, Evers G, et al. Prospective study on late consequences of subclinical non-compliance with immunosuppressive therapy in renal transplant patients. Am J Transplant. 2004;4:1509. doi: 10.1111/j.1600-6143.2004.00537.x. [DOI] [PubMed] [Google Scholar]
- 12.De Bleser L, Matteson M, Dobbels F, Russell C, De Geest S. Interventions to improve medication-adherence after transplantation: a systematic review. Transpl Int. 2009 doi: 10.1111/j.1432-2277.2009.00881.x. [DOI] [PubMed] [Google Scholar]
- 13.Haynes RB, Yao X, Degani A, Kripalani S, Garg A, McDonald HP. Interventions for enhancing medication adherence [Systematic Review] Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD000011.pub2. Art.No.: CD000011.pub2. [DOI] [Google Scholar]
- 14.Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60:657. doi: 10.1093/ajhp/60.7.657. [DOI] [PubMed] [Google Scholar]
- 15.Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care. 1998;36:1138. doi: 10.1097/00005650-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Russell CL, Conn V, Jantarakupt P. Older adult medication compliance: integrated review of randomized controlled trials. Am J Health Behav. 2006;30:636. doi: 10.5555/ajhb.2006.30.6.636. [DOI] [PubMed] [Google Scholar]
- 17.Alemi F, Neuhauser D. Time-between control charts for monitoring asthma attacks. Jt Comm J Qual Patient Saf. 2004;30:95. doi: 10.1016/s1549-3741(04)30011-0. [DOI] [PubMed] [Google Scholar]
- 18.Hebert C, Neuhauser D. Improving hypertension care with patient-generated run charts: physician, patient, and management perspectives. Qual Manag Health Care. 2004;13:174. doi: 10.1097/00019514-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Lundeen E, Pai-Fisher E, Neuhauser D. Continuous self improvement to reduce stress: developing an individualized model of health. Qual Manag Health Care. 2001;9:47. doi: 10.1097/00019514-200109030-00006. [DOI] [PubMed] [Google Scholar]
- 20.Alemi F, Pawloski L, Fallon WF., Jr System thinking in a personal context to improve eating behaviors. J Healthc Qual. 2003;25:20. doi: 10.1111/j.1945-1474.2003.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 21.Russell CL. A clinical nurse specialist-led intervention to enhance medication adherence using the plan-do-check-act cycle for continuous self-improvement. Clin Nurs Spec. 2010;24:69. doi: 10.1097/NUR.0b013e3181cf554d. [DOI] [PubMed] [Google Scholar]
- 22. [Accessed March 1, 2010];International Transplant Nurses Society Patient Education Books. Available at: http://www.itns.org/ITNS_transplant_educational_materials.php.
- 23.Russell CL, Cetingok M, Hamburger K, et al. Medication non-adherence in older renal transplant recipients. Clin Nurs Res. 2010 doi: 10.1177/1054773810362039. [DOI] [PubMed] [Google Scholar]
- 24.Dunbar-Jacob J, Sereika SM, Foley SM, Bass DC, Ness RB. Adherence to oral therapies in pelvic inflammatory disease. J Womens Health. 2004;13:285. doi: 10.1089/154099904323016446. [DOI] [PubMed] [Google Scholar]
- 25.Russell CL, Conn V, Ashbaugh C, Madsen R, Hayes K, Ross G. Intra-subject medication adherence patterns. Clin Nurs Res. 2007;16:153. doi: 10.1177/1054773806296429. [DOI] [PubMed] [Google Scholar]
- 26.Denhaerynck K, Schaefer-Keller P, Young J, Steiger J, Bock A, De Geest S. Examining assumptions regarding valid electronic monitoring of medication therapy: development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med Res Methodol. 2008;8:5. doi: 10.1186/1471-2288-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell CL, Owens S, Hamburger K, et al. Medication adherence and older renal transplant patients’ perceptions of electronic medication event monitoring. J Gerontol Nurs. 2009;35:17. doi: 10.3928/00989134-20090903-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Geest S, Schäfer-Keller P, Denhaerynck K, Thannberger N, Köfer S, Bock A, Surber C, Steiger J. Supporting medication adherence in renal transplantation (SMART): a pilot RCT to improve adherence to immunosuppressive regimens. Clin Transplant. 2006;20:359. doi: 10.1111/j.1399-0012.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 29.Altman D, Bland JM. Statistics notes. Treatment allocation in controlled trials: why randomise? Br Med J. 1999;319:853. doi: 10.1136/bmj.318.7192.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 31.Gordon EJ, Gallant M, Sehgal AR, Conti D, Siminoff LA. Medication-taking among adult renal transplant recipients: barriers and strategies. Transpl Int. 2009;22:534. doi: 10.1111/j.1432-2277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Geest S, Abraham I, Moons P, et al. Late acute rejection and subclinical noncompliance with cyclosporine therapy in heart transplant recipients. J Heart Lung Transplant. 1998;17:854. [PubMed] [Google Scholar]
- 33.Desmyttere A, Dobbels F, Cleemput I, De Geest S. Noncompliance with immunosuppressive regimen in organ transplantation: is it worth worrying about? Acta Gastroenterol Belg. 2005;68:347. [PubMed] [Google Scholar]
- 34.Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant. 2009;9:2597. doi: 10.1111/j.1600-6143.2009.02798.x. [DOI] [PubMed] [Google Scholar]