Abstract
Recent animal and human studies have suggested that the cuprizone (CPZ, a copper chelator)-fed C57BL/6 mouse may be used as an animal model of schizophrenia. The goals of this study were to see the recovery processes of CPZ-induced behavioral changes and damaged white matter and to examine possible effects of antipsychotic drugs on the recovery processes. Mice were fed a CPZ-containing diet for 5 weeks then returned to normal food for 3 weeks, during which period mice were treated with different antipsychotic drugs. Various behaviors were measured at the end of CPZ-feeding phase as well as on the 14th and 21st days after CPZ withdrawal. The damage to and recovery status of white matter in the brains of mice were examined. Dietary CPZ resulted in white matter damage and behavioral abnormalities in the elevated plus-maze (EPM), social interaction (SI), and Y-maze test. EPM performance recovered to normal range within 2 weeks after CPZ withdrawal. Alterations in SI showed no recovery. Antipsychotics did not alter animals’ behavior in either of these tests during the recovery period. Altered performance in the Y-maze showed some recovery in the vehicle group; atypical antipsychotics, but not haloperidol, significantly promoted this recovery process. The recovery of damaged white matter was incomplete during the recovery period. None of the drugs significantly promoted the recovery of damaged white matter. These results suggest that CPZ-induced white matter damage and SI deficit may be resistant to the antipsychotic treatment employed in this study. They are in good accordance with the clinical observations that positive symptoms in schizophrenic patients respond well to antipsychotic drugs while social dysfunction is usually intractable.
Keywords: schizophrenia, antipsychotic drugs, oligodendrocytes, myelin, behavior, cuprizone
Introduction
Schizophrenia is a severe and chronic mental illness affecting 0.5–1% of the worldwide population. Patients with schizophrenia show abnormal mental functions that have been categorized into positive (hallucinations, delusions, and thought or speech disorganization) and negative symptoms (affect flattening, apathy, and social withdrawal). In addition, disturbances in basic cognitive functions, such as attention, executive function, and specific forms of memory (particularly working memory), are also consistently observed in sufferers of this disorder (Andreasen et al., 1994; Andreasen, 1995). Some patients even show concomitant mood symptoms including depression and anxiety (Carpenter and Buchanan, 1994). These diverse symptoms and disturbances have not been attributable to abnormalities in any single brain area or neurotransmitter system.
One explanation proposed to account for this broad array of schizophrenia symptoms is a reduction in connectivity between brain regions (Selemon and Goldman-Rakic, 1999; Hoffman and McGlashan, 2001), particularly affecting the prefrontal cortex (PFC) and its connections with the limbic system, the striatum, and the thalamus. In support of this theory, functional magnetic resonance imaging (fMRI) studies have documented altered fronto-temporal (Lawrie et al., 2002), frontal–parietal (Schlösser et al., 2003a, 2005), frontal–cerebellar, cerebellar–thalamic, and thalamo-cortical (Schlösser et al., 2003b) connectivity in patients with schizophrenia. In addition, studies using the diffusion tensor imaging (DTI) technique have shown reduced fractional anisotropy (FA) – evidence of impaired white matter integrity, in several brain regions, including prefrontal, temporal, parietal, and occipital lobes (Heimer, 2000; Middleton and Strick, 2000; Kanaan et al., 2005; Kubicki et al., 2007), as well as some of the fiber bundles connecting these regions, such as uncinate fasciculus (Andreasen et al., 1997), cingulum bundle (Benes, 2000; Grace, 2000; Carlsson et al., 2001), and arcuate fasciculus (Andreasen et al., 1997). Such changes have also been seen in the brains of never-medicated, first-episode schizophrenia patients (Chua et al., 2007), and in neuroleptic-naïve schizophrenics (Zou et al., 2008). Interestingly, patients with white matter lesions, such as leukodystrophies, leukoencephalopathies, and multiple sclerosis, often present with psychotic symptoms that are sometimes indistinguishable from those of schizophrenia (Davis et al., 2003; Walterfang et al., 2005; Denier et al., 2007). Taken together, the existing evidence from human studies suggests a primary role for compromised white matter in the pathophysiology of schizophrenia.
In spite of the aforementioned work, there are few studies that correlate the disease progression with changes in white matter and examine effects of existing effective antipsychotic drugs on compromised white matter. To accomplish these goals, animal studies are indispensable. In recent studies (Xiao et al., 2008; Makinodan et al., 2009; Xu et al., 2009), C57BL/6 mice with demyelination and oligodendrocyte loss caused by cuprizone (CPZ) showed a number of abnormal behaviors, including deficits in prepulse inhibition (PPI), reduced social interaction (SI), and impaired spatial working memory. CPZ is a copper chelator with neurotoxic effects for mouse and rat. Studies have demonstrated that mice, after consuming CPZ-containing diet for several weeks, show widespread demyelination, myelin breakdown, and oligodendrocyte loss (Matsushima and Morell, 2001; Remington et al., 2007). However, other cell types in the central nervous system were not affected (Selvaraju et al., 2004). In another study, CPZ-treated rats displayed a specific deficit in the ability to shift between perceptual dimensions in the attention set-shifting task, a PFC-mediated behavioral paradigm (Gregg et al., 2009) which parallels to the Wisconsin card sorting test. Therefore, the CPZ-fed rodents appear to be a useful animal model for exploring the role of white matter abnormalities in the pathophysiology of schizophrenia (Herring and Konradi, 2011).
With the above-mentioned long-term goal in mind, in a recent animal study we examined effects of the antipsychotic drugs haloperidol (HAL), clozapine (CLZ), and quetiapine (QUE) on the CPZ-induced behavioral changes and white matter damage in C57BL/6 mice (Xu et al., 2010). Administration of HAL (1 mg/kg/day, i.p.) significantly ameliorated the PPI deficit and spatial working memory impairment in mice consuming a CPZ-containing diet; HAL showed no effect on the CPZ-induced deficit in SI of mice. CLZ (10 mg/kg/day, i.p.) and QUE (10 mg/kg/day, i.p.) not only prevented mice from all the above mentioned CPZ-induced behavioral changes, but also protected against the CPZ-induced white matter damage (Xu et al., 2010). These different effects of HAL, CLZ, and QUE on CPZ-induced behavioral changes and white matter damage in mice encouraged us to perform the current study.
In the present study, young adult male C57BL/6 mice were fed the CPZ-containing diet for 5 weeks then returned to normal food without CPZ for 3 weeks as the recovery period, during which the antipsychotic drugs HAL, CLZ, olanzapine (OLZ), or QUE were administered. HAL is a prototype of the first-generation or typical antipsychotic drugs. The therapeutic effects of typical antipsychotic drugs are predominantly limited to positive symptoms of schizophrenia (Kane et al., 1998; Weiss et al., 2002). This action is most likely due to their ability to block dopamine D2 receptors in mesolimbic areas (Davis et al., 1991). These drugs are also associated with a variety of extrapyramidal side-effects (EPS; Gerlach, 2002), which, in turn, may exacerbate negative symptoms and cognitive impairment of patients with schizophrenia (Jibson and Tandon, 1998). CLZ, OLZ, and QUE are second-generation or atypical antipsychotic drugs. In addition to their efficacy on positive symptoms, atypical antipsychotic drugs have been reported to ameliorate negative symptoms of schizophrenia compared to placebo (King, 1998; Muller, 1999). Furthermore, these drugs have some beneficial effects on cognitive function of schizophrenic patients (Weiss et al., 2002; Woodward et al., 2005). Atypical antipsychotic drugs affect the dopaminergic system to a lesser extent than typical antipsychotic drugs and, in addition, inhibit serotonergic, muscarinic, histaminergic, and α1 adrenergic receptors (see the review by Konradi and Heckers, 2001).
The goals of this study were to see the recovery processes of CPZ-induced behavioral changes and damaged white matter and to examine possible effects of these antipsychotic drugs on the recovery processes. The behavioral tests applied in this study include elevated plus-maze (EPM), SI, and Y-maze tests. EPM test is used to measure anxiety levels of mice, which has relevance to the anxiety symptoms of schizophrenic patients; low levels of SI in mice is the analogous behavior of social withdrawal of patients with schizophrenia; Y-maze test is used to measure the short-term spatial memory of rodents, which is relevant to schizophrenia as patients of this disease have cognitive impairment including memory deficits. Spontaneous recovery was expected since significant remyelination occurs following withdrawal of CPZ (Stidworthy et al., 2003). Also, we expected that HAL might have different effects on the recovery of the CPZ-induced changes than the atypical antipsychotic drugs used in this study.
Materials and Methods
Animals
All animal procedures in this study were in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Southern Illinois University at Carbondale. Young adult male C57BL/6 mice (6 weeks old) were obtained from Charles River Laboratories (Wilmington, MA, USA) and housed in the vivarium of Southern Illinois University at Carbondale with a 12 h dark/12 h light cycle, at a constant temperature of 22 ± 1°C and a relative humidity of 60%. Young adult mice were chosen in this study as they have been demonstrated to be more sensitive to CPZ treatment and have been commonly used in previous studies (Xiao et al., 2008; Zhang et al., 2008; Makinodan et al., 2009; Xu et al., 2009, 2010). Although male and female C57BL/6 mice showed the same demyelination after consuming CPZ-containing diet in previous study (Stidworthy et al., 2003), only male mice were used in this study to minimize behavioral variance.
Experimental protocols
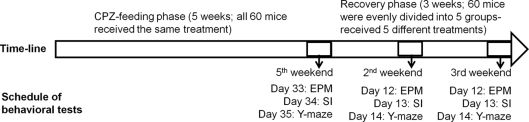
Two experiments were performed in this study. In experiment 1 (Figure 1), mice (n = 60) ate the rodent chow mixed with CPZ (Sigma-Aldrich, St. Louis, MO, USA) at 0.2% w/w for 5 weeks. During the last 3 days of the fifth week, the mice were evaluated in EPM, SI, and Y-maze tests in three groups of 20 each. Then, mice were fed the normal rodent chow without CPZ for 3 weeks to allow them to recover. During the recovery period, mice were subjected to the following post-CPZ treatments in groups of 12 each: vehicle (VEH) in which mice ate normal diet and received intraperitoneal (i.p.) injection of the vehicle (0.4% acetic acid in saline, 10 ml/kg) once a day for 21 days, CLZ in which mice ate normal diet and received CLZ i.p. once a day for 21 days (10 mg/kg; Sigma-Aldrich), HAL in which mice ate normal diet and received HAL (1 mg/kg; Sigma-Aldrich), OLZ in which mice ate normal diet and received OLZ (3 mg/kg; Lilly Research Laboratories, Indianapolis, IN, USA), QUE in which mice ate normal diet and received QUE (10 mg/kg; AstraZeneca, Wilmington, DE, USA). These dosages were chosen on the basis of previous studies (Chlan-Fourney et al., 2002; Bai et al., 2004; Zhang et al., 2008). In the last 3 days of the second and third week of the recovery period, all mice were subjected to the same behavioral tests as those used in the end of CPZ-feeding phase. On day 22, all mice were sacrificed under deep anesthesia and their brains were processed for morphological analyses. Choosing 5 weeks for the CPZ-feeding phase and 3 weeks for recovery was based on previous studies, which showed that after 5 or 6 weeks of continuous CPZ administration demyelination is maximal and that CPZ withdrawal at this stage results in significant remyelination over the course of about 4 weeks (Matsushima and Morell, 2001).
Figure 1.
The time-line and schedule for the behavioral tests in experiment. CPZ, cuprizone; EPM, elevated plus-maze; SI, social interaction.
In the second experiment, mice (n = 60) were randomly divided into five groups of 12 each and subjected to the same treatments of VEH, CLZ, HAL, OLZ, and QUE for 21 days as in the recovery phase of the first experiment. During this period mice were fed a normal diet without CPZ. In the last 3 days of the third week mice were assessed in the same behavioral tests as in the first experiment. These groups were used to determine effects of the antipsychotic drugs on behaviors of normal mice. In addition, behaviors from normal mice (n = 12) that had never eaten the CPZ-containing diet nor received antipsychotic drugs in our previous study (Xu et al., 2009) were used as normal controls (CNT); five mice that ate CPZ-containing diet for 5 weeks in the same study were used as a comparator for morphological analyses.
Behavioral tests
Elevated plus-maze, SI, and Y-maze tests were performed as described previously (Xu et al., 2009). They were performed before the injection of the vehicle or antipsychotic drugs on the same day. EPM was to measure anxiety levels of mice as they avoid the open arms of the plus-maze (Lister, 1990). Briefly, a mouse was initially placed in the center area facing an open arm. The mouse's entry into any of the four arms was counted when all four paws crossed from the central region into an arm. The number of total arm entries and the amount of time spent on the open arms during a 10-min testing period were recorded.
The SI test was to evaluate social withdrawal, a key component of the negative symptoms of patients with schizophrenia (Weinberger, 1987; Abi-Dargham et al., 2000). Pairs of unfamiliar mice (housed in separate cages) of the same experimental group were initially placed in the center of an open-field box (56 cm × 56 cm × 31 cm) about 10 cm apart. A video camera was placed above the open-field box to monitor the animals’ movements. During a 10-min test period, SI was defined as when the subjects were closer than 5 cm (between the center points of the back of one mouse and the other one) for at least 0.2 s. Data were recorded and analyzed automatically using the SMART video tracking program (San Diego Instruments, San Diego, CA, USA). Although this method only measures subject proximity, it has been used in previous studies to evaluate SIs (Shi et al., 2003; Egashira et al., 2007).
Y-maze test is a simple recognition test for measuring spatial working memory (Maurice et al., 1994; Dellu et al., 2000). Each mouse was placed at the end of one arm of a Y-maze (two shorter arms of 15 cm and a longer arm of 20 cm) and allowed to move freely through the maze for an 8-min test period. Alternation was defined as successive entries into the three arms on overlapping triplet sets. The maximum number of possible spontaneous alternations was determined as the total number of arms entered – 2, and the percentage correct was calculated as the ratio of actual to possible alternations × 100.
Morphological analyses
Mice were deeply anaesthetized with chloral hydrate (400 mg/kg, i.p.) and perfused through the ascending aorta with 0.1 M phosphate buffered saline (PBS, pH = 7.4), followed by 4% paraformaldehyde in PBS. Their brains were then removed and post-fixed overnight in the same fixative, followed by cryoprotection in 25% sucrose at 4°C for 24–48 h. Serial coronal sections (25 μm thick) were cut using a sliding microtome (Lipshaw, Detroit, MI, USA) and collected in six-well plates containing 0.01 M PBS.
One set of the sections was used to evaluate myelinated fibers using luxol fast blue–periodic acid Schiff (LFB–PAS) staining. The other two sets of sections were used for immunohistochemical staining. Briefly, free-floating sections were pre-treated with 0.5% hydrogen peroxide in methanol for 20 min, then washed with PBS and incubated for 1 h at 22°C in a blocking solution composed of 1% Triton X-100 and 5% normal (goat/rabbit) serum in PBS. The sections were subsequently incubated in the blocking solution for 48 h at 4°C with the primary antibody to MBP (1:6000; Chemicon, Temecula, CA, USA) or to GST-pi (the pi form of glutathione-transferase; 1:500; Stressgen, Victoria, BC, Canada). Anti-MBP detects myelin tracts while anti-GST-pi labels mature oligodendrocytes (Mason et al., 2004). After rinsing in PBS, brain sections were incubated in the biotinylated secondary antisera (1:200) of the Vectastain (Elite) ABC kit (Burlingame, CA, USA) for 2 h at 22°C. Following PBS rinses, the sections were then incubated in an avidin–biotin–horseradish peroxidase complex (ABC) for 1 h at 22°C. Finally, the antigen–antibody complexes were visualized using 0.025% 3, 3′-diaminobenzidine (DAB, Sigma-Aldrich, St. Louis, MO, USA) as the chromogen. Immunohistochemical controls were done as above except that the primary antibodies were omitted.
The processed brain sections were examined using a Leica DMI 4000 B microscope (Leica Microsystems, Wetzlar, Germany). In immunohistochemically stained sections, no positive immunostaining was found in the control sections. The normal myelin sheaths (in experiment 1) appeared blue in LFB–PAS staining and in brown in MBP-immunohistochemistry. The area of blue myelin sheaths in the central part of corpus callosum (CC) and MBP-positive staining (in brown) in the medial dorsal cerebral cortex (CTX) were measured using the Image-Pro Plus software (version 4.1, Media Cybernetics, Inc., Silver Spring, MD, USA) and values of normal control group (CNT) were used as comparators for all other groups. That is, values of all other groups were normalized as percentages of CNT group. The numbers of GST-pi positive cells in CTX of mice were counted (five sections/mouse) and expressed as number per square millimeter.
Data analysis
The behavioral data from the CNT group (in which mice ate rodent chow without CPZ) and that obtained at the end of CPZ-feeding phase were analyzed with the Tukey quick test. For the data obtained at the three time points of the recovery phase, two-way ANOVA was performed considering the recovery time and antipsychotics as two factors. The data of the second experiment were analyzed by one-way ANOVA followed by a post hoc (Scheffe's) test. When a p-value was less than 0.05, the difference was considered significant.
Results
CPZ-induced behavioral changes and white matter damage
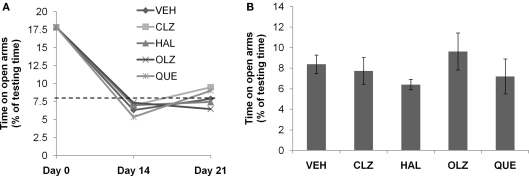
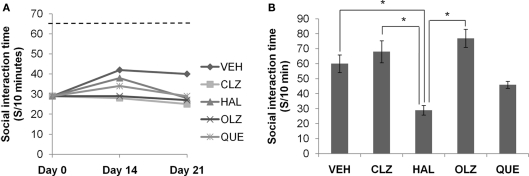
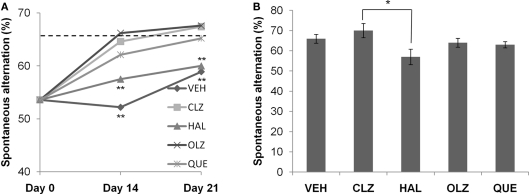
Mice fed CPZ-containing diet for 5 weeks spent longer periods (total end count ≥ 10, p < 0.001) on open arms of EPM (Figure 2A), but less time (total end count ≥ 7, p < 0.05) in SI (Figure 3A), and showed lower spontaneous alternation (total end count ≥ 10, p < 0.001) in the Y-maze (Figure 4A), as compared to the CNT group. However, the CPZ-induced increase in the number of arm entries in Y-maze test did not reach a significant level (data are not shown), suggesting the treatment did not affect the locomotor activity of mice. The CPZ-fed mice showed complete demyelination (Figure 5B), widespread myelin break-down (Figure 6B), and oligodendrocyte loss (Figure 7B) in their brains.
Figure 2.
Complete recovery of abnormal elevated plus-maze behavior after CPZ withdrawal. (A) The data from the recovery experiment, in which the dotted line indicates the value of the controls (CNT) never exposed to CPZ or antipsychotic drugs. (B) The data from mice exposed to antipsychotics, but not to CPZ. Data are mean ± SEM (n = 6–12/group). VEH, vehicle; CLZ, clozapine; HAL, haloperidol; OLZ, olanzapine; QUE, quetiapine.
Figure 3.
Social interaction deficit did not recover after CPZ withdrawal. (A) The data from the recovery experiment, in which the dotted line indicates the value of the controls (CNT), which was significantly higher than all other groups. (B) The data from mice exposed to antipsychotics, but not to CPZ. Data are mean ± SEM (n = 6/group). *p < 0.05.
Figure 4.
Atypical antipsychotic drugs, but not haloperidol, promoted the recovery of spontaneous alternation after CPZ withdrawal. (A) The data from the recovery experiment, in which the dotted line indicates the value of the controls (CNT). (B) The data from mice exposed to antipsychotics, but not to CPZ. Data are mean ± SEM (n = 6–12/group). **p < 0.01, compared to CNT.
Figure 5.
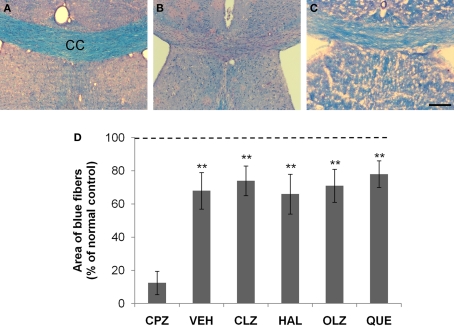
Incomplete remyelination of the corpus callosum (CC) after CPZ withdrawal. (A) An image from an animal of the control (CNT) group showing normal myelinated fibers (in blue) in CC. (B) An image from a mouse exposed to CPZ for 5 weeks showing no normal myelinated fibers in CC. (C) An image from a mouse treated with VEH during the 3-week recovery period after CPZ withdrawal. (D) The quantitative data (mean ± SEM; n = 5/group). The dotted line indicates the value of the CNT group, which was significantly higher than all other groups. **p < 0.01, compared to the CPZ group. The scale bar in the image (C) represents about 250 μm.
Figure 6.
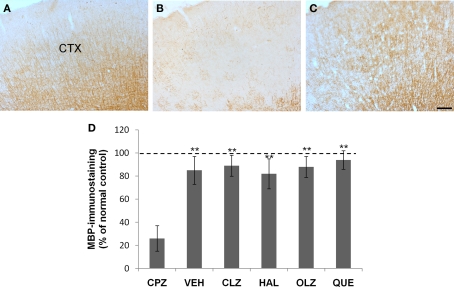
Incomplete recovery of myelin breakdown in the neocortex (CTX) after CPZ withdrawal. (A) An image from a mouse of the control (CNT) group showing normal MBP-labeled neural fibers which traverse the CTX from deep to superficial layers in parallels. (B) An image from a mouse of the CPZ group showing widespread myelin breakdown in CTX. (C) An image from a mouse treated with VEH during the 3-week recovery period after CPZ withdrawal. (D) The quantitative data (mean ± SEM; n = 5/group). The dotted line indicates the value of the CNT group, which was significantly higher than the CPZ group. **p < 0.01, compared to the CPZ group. The scale bar in the image (C) represents about 300 μm.
Figure 7.
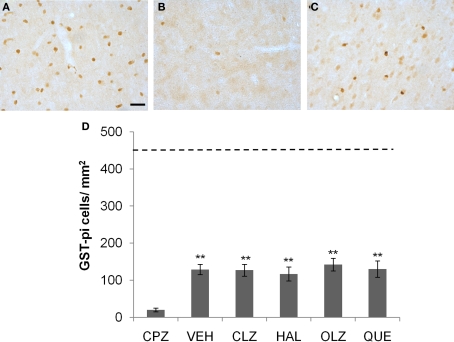
Incomplete recovery of the loss of mature OLs in CTX after CPZ withdrawal. (A) An image showing GST-pi positive cells in CTX of a mouse of the control (CNT) group. (B) An image from a mouse of the CPZ group in which only a few GST-pi positive cells left in CTX after CPZ treatment. (C) An image showing GST-pi positive cells in CTX of a mouse treated with VEH during the 3-week recovery period after CPZ withdrawal. (D) The quantitative data (mean ± SEM; n = 5/group). The dotted line indicates the value of the CNT group, which was significantly higher than all other groups. **p < 0.01, compared to the CPZ group. The scale bar in image (C) represents about 25 μm.
The CPZ-induced abnormal behavior in EPM spontaneously recovered to normal by 14th day after CPZ withdrawal
The aforementioned disrupted behaviors showed different recovery processes during the 3-week recovery period. On day 14 after CPZ withdrawal, the increased time spent on open arms of the EPM returned to the level of CNT group (see the dotted line in Figure 2A). The same normalized performance was also seen on day 21 after CPZ withdrawal. Two-way ANOVA analysis indicated a significant effect of recovery time (F2, 135 = 66.80; p < 0.0001) but no significant effect of treatment (F4, 135 = 0.15; p = NS). Post hoc comparisons showed significant differences between the data obtained on day 14 after CPZ withdrawal and those on day 0 (the end of CPZ-feeding phase). Significant differences also existed between the data obtained on day 21 and those on day 0. When comparisons were made between data obtained on days 14 and 21, no significant differences were found between the VEH group and any antipsychotic group. In the second experiment (Figure 2B), all groups showed comparable time on open arms of EPM. One-way ANOVA indicated no effect (F4, 41 = 0.73; p = NS) of the antipsychotics on the time spent on open arms of the EPM.
The CPZ-induced SI deficit remained at low levels during the recovery phase and was not affected by antipsychotic treatment
The decreased SI time measured on day 0 remained at low levels during the 3-week recovery phase in all CPZ-withdrawn mice. Two-way ANOVA analysis indicated a significant effect of recovery time (F2, 89 = 6.73; p = 0.002), but no effect of treatment (F4, 89 = 1.69; p = NS). Post hoc comparisons showed no significant differences between any two antipsychotic groups (Figure 3A). In the second experiment, all animal groups except for HAL showed comparable SI time (Figure 3B). One-way ANOVA indicated a significant effect of the antipsychotics (F4, 37 = 8.88; p < 0.0001) on SI time of normal mice. Post hoc comparisons showed significant differences between the HAL group and the VEH, CLZ, and OLZ groups (p < 0.05).
Atypical antipsychotic drugs, but not haloperidol, promoted the recovery of spontaneous alternation after CPZ withdrawal
All CPZ-withdrawn mice showed some improvement in spontaneous alternation during the 3-week recovery phase. Two-way ANOVA analysis indicated significant effects of both recovery time (F2, 149 = 30.4; p < 0.0001) and antipsychotic treatment (F4, 149 = 6.97, p < 0.0001), with an interaction that was almost significant (F8, 149 = 1.97; p = 0.055). For CLZ, OLZ, and QUE groups, spontaneous alternation measured on day 14 was significantly higher than those measured on day 0. For HAL and VEH groups, significant increases in spontaneous alternation were recorded on day 21, although their values were still lower compared to CNT (see the dotted line in Figure 4A). For effects of antipsychotic drugs on the spontaneous alternation of normal mice, one-way ANOVA showed a significant effect of the antipsychotics (F4, 49 = 2.58, p = 0.05; Figure 4B). Post hoc comparisons found a significant difference between HAL and CLZ groups (57 ± 4 over 70 ± 4, p = 0.03), but no group was significantly different from VEH group.
The recovery of compromised white matter was not complete in the 3-week recovery phase
Normal myelinated neural fibers were stained blue in LFB–PAS-stained brain sections, exemplified by a microphotograph of the CC (Figure 5A). Blue staining was rarely seen in the CC of mice exposed to CPZ for 5 weeks (Figure 5B), suggesting widespread demyelination. After recovery for 3 weeks blue staining in the CC reappeared and occupied the most part of CC, although a few residual demyelinated fibers (in red) were still visible (Figure 5C). This pattern was observed in the CC of all CPZ-withdrawn mice (images from antipsychotic-treated mice are not shown). The differences between the CNT group and the VEH- or antipsychotics-treated groups were significant (Figure 5D).
Fine MBP-positive fibers were well illustrated in brain sections from CNT mice (Figure 6A). In contrast, the mice exposed to CPZ for 5 weeks showed widespread myelin break-down, which was most obvious in CTX (Figure 6B). After 3 weeks recovery following CPZ withdrawal, the MBP immunostaining in the CTX seemed to be similar to that of normal mice under a lower magnification (Figure 6C). One-way ANOVA performed for the quantitative data of groups VEH, CLZ, HAL, OLA, and QUE in experiment 1 showed no significant effect (F4, 24 = 1.43; p = NS) of treatment on the MBP immunostaining in CTX. And post hoc comparisons showed no differences between VEH and any antipsychotic group as well as between any two antipsychotic groups. However, when comparisons (Student's t-test) were made between the CPZ group and anyone of the above-mentioned groups, differences were significant. The differences between the CNT group and the VEH- or antipsychotics-treated groups were not significant (Figure 6D). At higher magnifications (20× or 40×) MBP-positive stained myelin sheaths of CPZ-withdrawn mice were short and seemed to be disconnected (not shown), suggesting the existence of incomplete myelin sheaths.
Mature oligodendrocytes labeled with GST-pi are distinctly detectable in brain sections (Figure 7). Exposure to CPZ for 5 weeks almost completely depleted the GST-pi cells in CTX (Figure 7B). After 3 weeks recovery, GST-pi cells increased in the same brain region (Figure 7C). One-way ANOVA performed for the quantitative data in experiment 1 showed no significant effect (F4, 24 = 1.29; p = NS) of treatment on the number of GST-pi cells in CTX. Post hoc comparisons showed no differences between VEH and any antipsychotic group as well as between any two antipsychotic groups. All CPZ-withdrawn mice had many more GST-pi cells compared to the CPZ-fed mice, but the numbers were still much lower than that of the CNT group (see the dotted line in Figure 7D).
Discussion
The abnormal behaviors and obvious demyelination, myelin break-down, and oligodendrocyte loss seen in C57BL/6 mice given CPZ-containing diet for 5 weeks confirmed the results reported in recent studies (Xiao et al., 2008; Koutsoudaki et al., 2009; Makinodan et al., 2009; Norkute et al., 2009; Xu et al., 2009). Therefore, a correlation between white matter damage and certain behavioral abnormalities in the CPZ-treated mice is suggested. Moreover, this animal model allowed us to correlate the behavioral dynamics in CPZ-withdrawn mice with the status of white matter anomalies during a recovery period, since remyelination happens automatically following CPZ withdrawal (Matsushima and Morell, 2001; Skripuletz et al., 2008; Makinodan et al., 2009; Werner et al., 2010).
Behavioral recovery in EPM was complete at the end of second week after CPZ withdrawal in VEH group (Figure 2A). This rapid and complete recovery did not parallel the remyelination process, which was not yet finished on day 21 (Figures 5–7). These results suggest that the white matter damage seen in CPZ-treated mice was not a main contributor to this abnormal behavior. In support of this suggestion, in a recent study mice showed increased time on open arms of EPM beginning on day 14 following CPZ-feeding when no obvious demyelination was observed (Xu et al., 2009). We speculate that this abnormal behavior could be attributed to increased concentrations of inflammatory cytokines as mRNAs coding for many cytokines increase, with peak expression in fourth week following CPZ-feeding (Jurevics et al., 2002). Further supporting evidence to this speculation includes: the demyelinating effect of CPZ was shown to be initiated through secretion of tumor necrosis factor-α and interferon-γ (Cammer, 1999; Pasquiini et al., 2007); intracerebral injection of interleukin-1β resulted in acute white matter and neuronal injury in the neonatal rat (Cai et al., 2004; Fan et al., 2009); and mice with white matter damage, including loss of mature oligodendrocytes and impaired myelination induced by interleukin-1β exposure, spent longer periods on open arms of EPM (Fan et al., 2010).
Mice treated with antipsychotic drugs showed the same recovery course of this anxiety behavior as the VEH group. This phenomenon may be explained by either one of the following possibilities: (1) the treatment paradigms tested in this study had no effect on recovery; (2) the doses chosen for these drugs in this study were not optimal for these mice and measures; (3) their effects were masked by the rapid spontaneous recovery. The first possibility is in line with the result that all the drugs did not influence EPM behavior in mice that had never been exposed to CPZ (Figure 2B). The second possibility warrants further studies using different doses of these antipsychotic drugs or using different antipsychotic drugs. In support of the third possibility, the dramatic increases in microglia and astrocytes at demyelinated sites were almost completely prevented by QUE concurrently administered to CPZ-fed mice (Zhang et al., 2008), and the same treatment effectively prevented the CPZ-fed mice from increases in time spent on open arms of EPM (Xu et al., 2010). Future studies should test different doses of the drugs and schedule earlier EPM tests before day 14 after CPZ withdrawal so that possible beneficial effects of the antipsychotic drugs on the recovery process in CPZ-withdrawn mice might be detected.
The decreased SI time observed in CPZ-fed mice remained at low levels during the 3-week recovery phase in the VEH group (Figure 3A). This lack of behavioral improvement did not correspond with the significant recovery of damaged white matter (Figures 5–7). However, this may only reflect the need for nearly intact myelination for normal SI to occur because the remyelination was incomplete by 21st day after CPZ withdrawal (Figure 5) and the number of mature oligodendrocytes in CTX was still low at the same time (Figure 7). Further, work of others showed that SI deficits in CPZ-fed mice disappeared after a longer recovery period (6 weeks) which allowed a complete remyelination (Makinodan et al., 2009). Finally, concurrent administration of CLZ or QUE with CPZ effectively protected mice against the CPZ-induced white matter damage (Zhang et al., 2008; Xu et al., 2010) and SI deficits (Xu et al., 2010). Taken together, the data strongly suggest that intact brain myelin is essential for mice to perform normal SI.
None of the antipsychotic drugs tested in this study promoted the recovery of either SI decrease (Figure 3A) and or white matter remyelination (Figure 5) in CPZ-withdrawn mice. These results are in contrast to the effective prophylactic effects of CLZ/QUE as shown in our recent studies (Zhang et al., 2008; Xu et al., 2010). Extrapolation of these findings suggests that (atypical) antipsychotic drugs should be given to schizophrenia patients as soon as possible to achieve better outcomes.
In normal mice that had never been exposed to CPZ, HAL, but not atypical antipsychotic drugs (CLZ, OLZ, and QUE), reduced SI time (Figure 3B). These results are similar to previous reports that HAL decreased social behavior of marmosets (Scraggs et al., 1979) and of rats (Corbett et al., 1993). Since intact myelin sheath in the brain is essential to normal SI as discussed above, it is plausible to speculate that chronic administration of HAL might, by itself, have adverse effects on white matter. In support of this speculation, it has been shown that chronic HAL treatment decreased the expression of myelin/oligodendrocyte-related genes in the mouse brain (Narayan et al., 2007). It is unknown if HAL has similar adverse effects on white matter in humans. Nevertheless, the detrimental effect of HAL on SI may help explain why conventional antipsychotics have only a modest or no effect on negative symptoms of patients with schizophrenia, as illustrated in a recent human study in which HAL was inferior to aripiprazole, an atypical antipsychotic drug, in improving PANSS items related to social functioning (Docherty et al., 2010).
Spontaneous alternation showed significant, but not complete, recovery in VEH group after CPZ withdrawal (Figure 4A). This paralleled the remyelination process seen in CPZ-withdrawn mice, suggesting a causal relationship between white matter damage and this behavioral abnormality. However, it should be noted that, in an earlier study, decreased spontaneous alternation was also seen at the end of second week after CPZ-feeding when no obvious demyelination was found (Xu et al., 2009). Therefore, compromised white matter is just one of contributors to the decreased spontaneous alternation in CPZ-treated mice. Among the other contributors are likely dysfunctional neurotransmitter systems, especially the DA and 5-HT systems. Supporting evidence for dysfunctional DA system in CPZ-treated mice includes findings of increased DA in the frontal cortex of CPZ-treated mice (Xu et al., 2009) and that co-administration of HAL and CPZ prevented mice from spontaneous alternation decreases (Xu et al., 2010). Although the 5-HT system has not been examined in brains of CPZ-treated mice, the beneficial effect of all antipsychotic drugs on the recovery process of CPZ-induced spontaneous alternation impairments provide indirect supporting evidence for the presence of a dysfunctional 5-HT system following white matter damage. In accordance with this explanation, ketanserin (a 5-HT2 receptor antagonist) and risperidone (which has affinities for both 5-HT2 and D2 receptors) significantly ameliorated a seizure-induced impairment of spontaneous alternation behavior in rats (Hidaka et al., 2010). HAL, however, did not promote functional recovery (Figure 4A). This inefficacy is not surprising since that HAL has adverse effects on myelin and oligodendrocytes, as discussed earlier, and that it only binds to D2 receptors. Therefore, the CPZ-exposed mouse may be used as a new animal model to explore the neurobiological mechanisms of impaired spatial working memory in schizophrenia (Gold and Harvey, 1993; Xu et al., 2011).
In summary, the abnormal performance of mice in EPM spontaneously recovered to normal levels within 2 weeks after CPZ withdrawal, suggesting that white matter abnormality is not a main contributor to this abnormal anxiety behavior in CPZ-treated mice. Reduced SI remained at low levels in all CPZ-withdrawn mice during the 3-weeks recovery period, suggesting a resistance of the CPZ-induced SI deficit to the antipsychotic drugs tested in this study. Impaired spontaneous alternation in CPZ-treated mice significantly recovered after CPZ withdrawal. CLZ, OLZ, and QUE, but not HAL, significantly promoted this recovery process. The CPZ-induced white matter damage in mice did not completely recover within 3 weeks after CPZ withdrawal and none of the antipsychotic drugs tested seemed to accelerate the remyelination process. Although the antipsychotic drugs tested in this study neither improved the performance of CPZ-withdrawn mice in SIs nor promoted the recovery of damaged white matter, these results may be due to inappropriate doses of tested drugs in this study and/or reflect intractable features of these abnormalities. In support of this explanation, previous studies by our team (Xiao et al., 2008; Zhang et al., 2008; Xu et al., 2009, 2010) and others (Gregg et al., 2009; Makinodan et al., 2009) have demonstrated that white matter damage in CPZ-fed mice and rats are accompanied with schizophrenia-like behaviors. More significantly, these CPZ-induced morphological and behavioral changes responded well to some antipsychotic drugs co-administered to mice while they consumed a CPZ-containing diet (Xu et al., 2010). With these previous results, the data presented in this study may be considered to be in good accordance with the clinical observations that positive symptoms in schizophrenic patients respond well to antipsychotic drugs while social dysfunction is difficult to treat (Lublin et al., 2005).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from Southern Illinois University Carbondale (Haiyun Xu) and the University of Manitoba (Haiyun Xu and Xin-Min Li).
References
- Abi-Dargham A., Rodenhiser J., Printz D., Zea-Ponce Y., Gil R., Kegeles L. S., Weiss R., Cooper T. B., Mann J. J., Van Heertum R. L., Gorman J. M., Laruelle M. (2000). Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 97, 8104–8109 10.1073/pnas.97.14.8104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N. C. (1995). Symptoms, signs, and diagnosis of schizophrenia. Lancet 346, 477–481 10.1016/S0140-6736(95)91325-4 [DOI] [PubMed] [Google Scholar]
- Andreasen N. C., Nopoulos P., Schultz S., Miller D., Gupta S., Swayze V., Flaum M. (1994). Positive and negative symptoms of schizophrenia: past, present, and future. Acta Psychiatr. Scand. 384(Suppl.), 51–59 10.1111/j.1600-0447.1994.tb05891.x [DOI] [PubMed] [Google Scholar]
- Andreasen N. C., O'Leary D. S., Flaum M., Nopoulos P., Watkins G. L., Boles Ponto L. L., Hichwa R. D. (1997). Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet 349, 1730–1734 10.1016/S0140-6736(96)08258-X [DOI] [PubMed] [Google Scholar]
- Bai O., Zhang H., Li X. M. (2004). Antipsychotic drugs clozapine and olanzapine upregulate bcl-2 mRNA and protein in rat frontal cortex and hippocampus. Brain Res. 1010, 81–86 10.1016/j.brainres.2004.02.064 [DOI] [PubMed] [Google Scholar]
- Benes F. M. (2000). Emerging principles of altered neural circuitry in schizophrenia. Brain Res. Brain Res. Rev. 31, 251–269 10.1016/S0165-0173(99)00041-7 [DOI] [PubMed] [Google Scholar]
- Cai Z., Lin S., Pang Y., Rhodes P. G. (2004). Brain injury induced by intracerebral injection of interleukin-1 beta and tumor necrosis factor-alpha in the neonatal rat. Pediatr. Res. 56, 377–384 10.1203/01.PDR.0000134249.92944.14 [DOI] [PubMed] [Google Scholar]
- Cammer W. (1999). The neurotoxicant cuprizone retards the differentiation of oligodendrocytes in vitro. J. Neurol. Sci. 168, 116–120 10.1016/S0022-510X(99)00181-1 [DOI] [PubMed] [Google Scholar]
- Carlsson A., Waters N., Holm-Waters S., Tedroff J., Nilsson M., Carlsson M. L. (2001). Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu. Rev. Pharmacol. Toxicol. 41, 237–260 10.1146/annurev.pharmtox.41.1.237 [DOI] [PubMed] [Google Scholar]
- Carpenter W. T., Buchanan R. W. (1994). Schizophrenia. N. Engl. J. Med. 330, 681–690 10.1056/NEJM199403103301006 [DOI] [PubMed] [Google Scholar]
- Chlan-Fourney J., Ashe P., Nylen K., Juorio A. V., Li X. M. (2002). Differential regulation of hippocampal BDNF mRNA by typical and atypical antipsychotic administration. Brain Res. 954, 11–20 10.1016/S0006-8993(02)03215-8 [DOI] [PubMed] [Google Scholar]
- Chua S. E., Cheung C., Cheung V., Tsang J. T., Chen E. Y., Wong J. C., Cheung J. P., Yip L., Tai K. S., Suckling J., McAlonan G. M. (2007). Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr. Res. 89, 12–21 10.1016/j.schres.2006.09.009 [DOI] [PubMed] [Google Scholar]
- Corbett R., Hartman H., Kerman L. L., Woods A. T., Strupczewski J. T., Helsley G. C., Conway P. C., Dunn R. W. (1993). Effects of atypical antipsychotic agents on social behavior in rodents. Pharmacol. Biochem. Behav. 45, 9–17 10.1016/0091-3057(93)90079-9 [DOI] [PubMed] [Google Scholar]
- Davis K. L., Kahn R. S., Ko G., Davidson M. (1991). Dopamine in schizophrenia: a review and reconceptualization. Am. J. Psychiatry 148, 1474–1486 [DOI] [PubMed] [Google Scholar]
- Dellu F., Contarino A., Simon H., Koob G. F., Gold L. H. (2000). Genetic differences in response to novelty and spatial memory using a two-trial recognition task in mice. Neurobiol. Learn. Mem. 73, 31–48 10.1006/nlme.1999.3919 [DOI] [PubMed] [Google Scholar]
- Davis K. L., Stewart D. G., Friedman J. I., Buchsbaum M., Harvey P. D., Hof P. R., Buxbaum J., Haroutunian V. (2003). White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch. Gen. Psychiatry 60, 443–456 10.1001/archpsyc.60.5.443 [DOI] [PubMed] [Google Scholar]
- Denier C., Orgibet A., Roffi F., Jouvent E., Buhl C., Niel F., Boespflug-Tanguy O., Said G., Ducreux D. (2007). Adult-onset vanishing white matter leukoencephalopathy presenting as psychosis. Neurology 68, 1538–1539 10.1212/01.wnl.0000260701.76868.44 [DOI] [PubMed] [Google Scholar]
- Docherty J. P., Baker R. A., Eudicone J., Mathew S., Marcus R. N., McQuade R. D., Mankoski R. (2010). Effect of aripiprazole versus haloperidol on PANSS prosocial items in early-episode patients with schizophrenia. Schizophr. Res. 120, 199–203 10.1016/j.schres.2010.03.040 [DOI] [PubMed] [Google Scholar]
- Egashira N., Tanoue A., Matsuda T., Koushi E., Harada S., Takano Y., Tsujimoto G., Mishima K., Iwasaki K., Fujiwara M. (2007). Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav. Brain Res. 178, 123–127 10.1016/j.bbr.2006.12.009 [DOI] [PubMed] [Google Scholar]
- Fan L. W., Mirchell H. J., Tien L. T., Rhodes P. G., Cai Z. (2009). Interleukin-1β-induced brain injury in the neonatal rat can be ameliorated by α-phenyl-n-tert-butyl-nitrone. Exp. Neurol. 220, 143–153 10.1016/j.expneurol.2009.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L. W., Tien L. T., Zheng B., Pang Y., Rhodes P. G., Cai Z. (2010). Interleukin-1β-induced brain injury and neurobehavioral dysfunctions in juvenile rats can be attenuated by α-phenyl-n-tert-butyl-nitrone. Neuroscience 168, 240–252 10.1016/j.neuroscience.2010.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach J. (2002). Improving outcome in schizophrenia: the potential importance of EPS and neuroleptic dysphoria. Ann. Clin. Psychiatry 14, 47–57 10.1023/A:1015276028425 [DOI] [PubMed] [Google Scholar]
- Gold J. M., Harvey P. D. (1993). Cognitive deficits in schizophrenia. Psychiatr. Clin. North Am. 16, 295–312 [PubMed] [Google Scholar]
- Grace A. A. (2000). Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res. Brain Res. Rev. 31, 330–341 10.1016/S0165-0173(99)00049-1 [DOI] [PubMed] [Google Scholar]
- Gregg J. R., Herring N. R., Naydenov A. V., Hanlin R. P., Konradi C. (2009). Downregulation of oligodendrocyte transcripts is associated with impaired prefrontal cortex function in rats. Schizophr. Res. 113, 277–287 10.1016/j.schres.2009.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L. (2000). Basal forebrain in the context of schizophrenia. Brain Res. Brain Res. Rev. 31, 205–235 10.1016/S0165-0173(99)00039-9 [DOI] [PubMed] [Google Scholar]
- Herring N. R., Konradi C. (2011). Myelin, copper and the cuprizone model of schizophrenia. Front. Biosci. 3, 23–40 10.2741/s129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka N., Suemaru K., Araki H. (2010). Serotonin-dopamine antagonism ameliorates impairments of spontaneous alternation and locomotor hyperactivity induced by repeated electroconvulsive seizures in rats. Epilepsy Res. 90, 221–227 10.1016/j.eplepsyres.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Hoffman R. E., McGlashan T. H. (2001). Neural network models of schizophrenia. Neuroscientist. 7, 441–454 10.1177/107385840100700513 [DOI] [PubMed] [Google Scholar]
- Jibson M. D., Tandon R. (1998). New atypical antipsychotic medications. J. Psychiatr. Res. 32, 215–228 10.1016/S0022-3956(98)00023-5 [DOI] [PubMed] [Google Scholar]
- Jurevics H., Largent C., Hostettler J., Sammond D. W., Matsushima G. K., Kleindienst A., Toews A. D., Morell P. (2002). Alterations in metabolism and gene expression in brain regions during cuprizone-induced demyelination and remyelination. J. Neurochem. 82, 126–136 10.1046/j.1471-4159.2002.00954.x [DOI] [PubMed] [Google Scholar]
- Kanaan R. A., Kim J. S., Kaufmann W. E., Pearlson G. D., Barker G. J., McGuire P. K. (2005). Diffusion tensor imaging in schizophrenia. Biol. Psychiatry 58, 921–929 10.1016/j.biopsych.2005.05.015 [DOI] [PubMed] [Google Scholar]
- Kane J. M., Honigfeld G., Singer J., Meltzer H. (1998). Clozapine for the treatment-resistant schizophrenia: a double-blind comparisons versus chlorpromazine/benztropine. Arch. Gen. Psychiatry 45, 789–796 [DOI] [PubMed] [Google Scholar]
- King D. J. (1998). Drug treatment of the negative symptoms of schizophrenia. Eur. Neuropsychopharmacol. 8, 33–42 10.1016/S0924-977X(98)90108-0 [DOI] [PubMed] [Google Scholar]
- Konradi C., Heckers S. (2001). Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biol. Psychiatry 50, 729–742 10.1016/S0006-3223(01)01267-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsoudaki P. N., Skipuletz T., Gudi V., Moharregh-Khiabani D., Hildebrandt H., Trebst C., Stangel M. (2009). Demyelination of the hippocampus is prominent in the cuprizone model. Neurosci. Lett. 451, 83–88 10.1016/j.neulet.2008.11.058 [DOI] [PubMed] [Google Scholar]
- Kubicki M., McCarley R., Westin C. F., Park H. J., Maier S., Kikinis R., Jolesz F. A., Shenton M. E. (2007). A review of diffusion tensor imaging studies in schizophrenia. J. Psychiatric. Res. 41, 15–30 10.1016/j.jpsychires.2005.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie S. M., Buechel C., Whalley H. C., Frith C. D., Friston K. J., Johnstone E. C. (2002). Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol. Psychiatry 51, 1008–1011 10.1016/S0006-3223(02)01316-1 [DOI] [PubMed] [Google Scholar]
- Lister R. G. (1990). Ethologically-based animal models of anxiety disorders. Pharmacol. Ther. 46, 321–340 10.1016/0163-7258(90)90021-S [DOI] [PubMed] [Google Scholar]
- Lublin H., Eberhard J., Levander S. (2005). Current therapy issues and unmet clinical needs in the treatment of schizophrenia: a review of the new generation antipsychotics. Int. Clin. Psychopharmacol. 20, 183–198 10.1097/00004850-200507000-00001 [DOI] [PubMed] [Google Scholar]
- Makinodan M., Yamauchi T., Tatsumi K., Okuda H., Takeda T., Kiuchi K., Sadamatsu M., Wanaka A., Kishimoto T. (2009). Demyelination in the juvenile period, but not in adulthood, leads to long-lasting cognitive impairment and deficient social interaction in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 978–985 10.1016/j.pnpbp.2009.05.006 [DOI] [PubMed] [Google Scholar]
- Mason J. L., Towes A., Hostettler J. D., Morell P., Suzuki K., Goldman J. E., Matsushima G. K. (2004). Oligodendrocytes and progenitors become progressively depleted within chronically demyelinated lesions. Am. J. Pathol. 164, 1673–1682 10.1016/S0002-9440(10)63726-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima G. K., Morell P. (2001). The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 11, 107–116 10.1111/j.1750-3639.2001.tb00385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T., Hiramatsu M., Itoh J., Kameyama T., Hasegawa T., Nabeshima T. (1994). Behavioral evidence for a modulating role of σ ligands in memory processes. I. Attenuation of dizocilpine (MK-801)-induced amnesia. Brain Res. 647, 44–56 10.1016/0006-8993(94)91397-8 [DOI] [PubMed] [Google Scholar]
- Middleton F. A., Strick P. L. (2000). Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Brain Res. Rev. 31, 236–250 10.1016/S0165-0173(99)00040-5 [DOI] [PubMed] [Google Scholar]
- Muller J. J. (1999). Atypical neuroleptics: a new approach in the treatment of negative symptoms. Eur. Arch. Psychiatry Clin. Neurosci. 249(Suppl. 4), 99–107 [DOI] [PubMed] [Google Scholar]
- Narayan S., Kass K. E., Thomas E. A. (2007). Chronic haloperidol treatment results in a decrease in the expression of myelin/oligodendrocyte-related genes in the mouse brain. J. Neurosci. Res. 85, 757–765 10.1002/jnr.21161 [DOI] [PubMed] [Google Scholar]
- Norkute A., Hieble A., Braun A., Johann S., Clarner T., Baumgartner W., Beyer C., Kipp M. (2009). Cuprizone treatment induces demyelination and astrocytosis in the mouse hippocampus. J. Neurosci. Res. 87, 1343–1355 10.1002/jnr.21946 [DOI] [PubMed] [Google Scholar]
- Pasquiini L. A., Calatayud C. A., Bertone Una A. L., Millet V., Pasquini J. M., Soto E. F. (2007). The neurotoxic effects of cuprizone on oligodendrocytes depends on the presence of pro-inflammatory cytokines secreted by microglia. Neurochem. Res. 32, 279–292 10.1007/s11064-006-9165-0 [DOI] [PubMed] [Google Scholar]
- Remington L. T., Babcock A. A., Zehntner S. P., Owens T. (2007). Microglial recruitment, activation, and proliferation in response to primary demyelination. Am. J. Pathol. 170, 1713–1724 10.2353/ajpath.2007.060783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlösser R., Gesierich T., Kaufmann B., Vucurevic G., Hunsche S., Gawehn J., Stoeter P. (2003a). Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage 19, 751–763 10.1016/S1053-8119(03)00106-X [DOI] [PubMed] [Google Scholar]
- Schlösser R., Gesierich T., Kaufmann B., Vucurevic G., Stoeter P. (2003b). Altered effective connectivity in drug free schizophrenic patients. Neuroreport 14, 2233–2237 10.1097/00001756-200312020-00020 [DOI] [PubMed] [Google Scholar]
- Schlösser R., Wagner G., Köhler S., Sauer H. (2005). Schizophrenia as a disconnection syndrome. Studies with functional magnetic resonance imaging and structural equation modeling. Radiologe 45, 137–140; 142–143 10.1007/s00117-004-1160-3 [DOI] [PubMed] [Google Scholar]
- Scraggs P. R., Baker H. F., Ridley R. M. (1979). Interaction of apomorphine and haloperidol: effects on locomotion and others behavior in the marmoset. Psychopharmacology (Berl.) 66, 41–43 10.1007/BF00426033 [DOI] [PubMed] [Google Scholar]
- Selemon L. D., Goldman-Rakic P. S. (1999). The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol. Psychiatry 45, 17–25 10.1016/S0006-3223(98)00281-9 [DOI] [PubMed] [Google Scholar]
- Selvaraju R., Bernasconi L., Losberger C., Graber P., Kadi L., Avellana-Adalid V., Picard-Riera N., Van Evercooren A. B., Cirillo R., Kosco-Vilbois M., Feger G., Papoian R., Boschert U. (2004). Osteopontin is upregulated during in vivo demyelination and remyelination and enhances myelin formation in vitro. Mol. Cell. Neurosci. 25, 707–721 10.1016/j.mcn.2003.12.014 [DOI] [PubMed] [Google Scholar]
- Shi L., Fatemi S. H., Sidwell R. W., Patterson P. H. (2003). Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 23, 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skripuletz T., Lindner M., Kotsiari A., Garde N., Fokuhl J., Linsmeier F., Trebst C., Stangel M. (2008). Cortical demyelination in prominent in the murine cuprizone model and is strain-dependent. Am. J. Pathol. 172, 1053–1061 10.2353/ajpath.2008.070850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stidworthy M. F., Genoud S., Suter U., Mantei N., Franklin R. J. M. (2003). Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathol. 13, 329–339 10.1111/j.1750-3639.2003.tb00032.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterfang M., Wood S. J., Velakoulis D., Copolov D., Pantelis C. (2005). Diseases of white matter and schizophrenia-like psychosis. Aust. N. Z. J. Psychiatry 39, 746–756 10.1111/j.1440-1614.2005.01678.x [DOI] [PubMed] [Google Scholar]
- Weinberger D. R. (1987). Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry 44, 660–669 [DOI] [PubMed] [Google Scholar]
- Weiss E. M., Bilder R. M., Fleischhacker W. W. (2002). The effects of second-generation antipsychotics on cognitive functioning and psychosocial outcome in schizophrenia. Psychopharmacology (Berl.) 162, 11–17 10.1007/s00213-002-1053-y [DOI] [PubMed] [Google Scholar]
- Werner S. R., Saha J. K., Broderick C. L., Zhen E. Y., Higgs R. E., Duffin K. L., Smith R. C. (2010). Proteomic analysis of demyelinated and remyelinating brain tissue following dietary cuprizone administration. J. Mol. Neurosci. 42, 210–225 10.1007/s12031-010-9354-9 [DOI] [PubMed] [Google Scholar]
- Woodward N. D., Purdon S. E., Meltzer H. Y., Zald D. H. (2005). A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int. J. Neuropsychopharmacol. 8, 1–16 10.1017/S146114570500516X [DOI] [PubMed] [Google Scholar]
- Xiao L., Xu H., Zhang Y., Wei Z., He J., Jiang W., Li X., Dyck L. E., Devon R. M., Deng Y., Li X. M. (2008). Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol. Psychiatry 13, 697–708 10.1038/sj.mp.4002064 [DOI] [PubMed] [Google Scholar]
- Xu H., Yang H. J., McConomy B., Browning R., Li X. M. (2010). Behavioral and neurobiological changes in C57BL/6 mouse exposed to cuprizone: effects of antipsychotics. Front. Behav. Neurosci. 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Yang H. J., Rose G. (2011). “Working memory deficits in schizophrenia: neurobiological correlates and treatment,” in Working Memory: Capacity, Developments and Improvement Techniques, ed. Levin E. S. (New York: Nova Science Publishers, Inc.), 313–344 [Google Scholar]
- Xu H., Yang H. J., Zhang Y., Clough R., Browning R., Li X. M. (2009). Behavioral and neurobiological changes in C57BL/6 mice exposed to cuprizone. Behav. Neurosci. 123, 418–429 10.1037/a0015243 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xu H., Jiang W., Xiao L., Yan B., He J., Wang Y., Bi X., Li X., Kong J., Li X. M. (2008). Quetiapine alleviates the cuprizoneinduced white matter pathology in the brain of C57BL/6 mouse. Schizophr. Res. 106, 182–191 10.1016/j.schres.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Zou L. Q., Xie J. X., Yuan H. S., Pei X. L., Dong W. T., Liu P. C. (2008). Diffusion tensor imaging study of the anterior limb of internal capsules in neuroleptic-naive schizophrenia. Acad. Radiol. 15, 285–289 10.1016/j.acra.2007.09.026 [DOI] [PubMed] [Google Scholar]