Abstract
Progenitor cells expressing proteoglycan NG2 (also known as oligodendrocyte precursor cells or polydendrocytes) are widespread in the grey and white matter of the CNS; they comprise 8–9% of the total cell population in adult white matter, and 2–3% of total cells in adult grey matter. NG2 cells have a complex stellate morphology, with highly branched processes that may extend more than 100 μm around the cell body. NG2 cells express a complex set of voltage-gated channels, AMPA/kainate and/or γ-aminobutyric acid (GABA)A receptors, and receive glutamatergic and/or GABAergic synaptic input from neurons. In every region of the brain NG2 cells are found as proliferative cells, and the fraction of actively cycling NG2 cells is quite high in young as well as in adult animals. During development NG2 cells either differentiate into myelinating oligodendrocytes (and possibly also few astrocytes and neurons) or persist in the brain parenchyma as NG2 cells. This review highlights new findings related to the morphological and electrophysiological changes of NG2 cells, and the fate of synaptic input between neurons and NG2 cells during proliferation and differentiation of these cells in the neonatal and adult nervous system of rodents.
Keywords: cell cycle, glutamate, NG2 glia, oligodendrocytes, proliferation, synaptic transmission
Introduction
Progenitor cells expressing proteoglycan NG2 [also known as oligodendrocyte precursor cells (OPCs) or polydendrocytes] are widespread in the grey and white matter of the CNS; they comprise 8–9% of the total cell population in adult white matter, and 2–3% of total cells in adult grey matter (Dawson et al. 2003). The fact that a certain population of glial cells in the brain expresses proteoglycan NG2 has been known for more than 20 years (Levine & Card, 1987; Stallcup & Beasley, 1987). NG2-expressing cells rapidly came to light following the discovery of synapse-like associations between these glial cells and neurons in multiple regions of the developing and adult CNS (Bergles et al. 2000; Lin & Bergles, 2004a; Lin et al. 2005). NG2 cells have attracted greater attention, and steady progress in our understanding of their development and lineage progression has been made to date; yet, we still know surprisingly little about physiological properties and function of these cells in normal and diseased brain.
NG2 cells can be identified by the expression of the NG2 chondroitin sulphate proteoglycan and alpha receptor for platelet-derived growth factor (PDGF-Rα). Microvascular pericytes also express the NG2 proteoglycan (Ozerdem et al. 2001; Zhu et al. 2008a; Hamilton et al. 2010), but the two cell types are clearly distinguished by their morphology: NG2-glia are stellate cells with multiple processes, and pericytes are perivascular cells with two or more primary processes extending along blood vessels (Wigley & Butt, 2009).
Electrophysiological studies indicate that NG2 cells express a complex set of voltage-gated channels, including tetrodotoxin (TTX)-sensitive sodium channels and several types of potassium channels (Bergles et al. 2000; Chittajallu et al. 2004; Karadottir et al. 2008; Kukley et al. 2008, 2010; De Biase et al. 2010). Furthermore, NG2 cells in grey and white matter areas of the brain express α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/kainate and/or γ-aminobutyric acid (GABAA) receptors, and receive glutamatergic and/or GABAergic synaptic input from neurons (Bergles et al. 2000; Lin & Bergles, 2004a; Karadottir et al. 2005; Lin et al. 2005; Kukley et al. 2007; Ziskin et al. 2007). It is not known why neurons have developed dedicated release machinery at specific sites of contact with NG2 glial cells or if neuron–glia synapses are dynamic; although some recent studies have started to probe possible changes that neuron–glia synapses undergo when NG2 cells divide or differentiate (Kukley et al. 2008, 2010; Ge et al. 2009; De Biase et al. 2010).
This review highlights new findings related to the morphological and electrophysiological changes of NG2 cells, and the fate of synaptic input between neurons and NG2 cells during proliferation and differentiation of these cells in the neonatal and adult nervous system of rodents. First, we summarize current knowledge of the morphological and electrophysiological properties of NG2 cells and discuss evidence that NG2 cells receive synaptic input from neurons. Next, we talk about recent discoveries that suggest that NG2 cells maintain synapses with neurons during cell division and pass them onto their progeny. Finally, we emphasize the latest findings related to the fate of neuron–glia synapses upon differentiation of NG2 cells into myelinating oligodendrocytes.
Appearance, distribution and progeny of NG2 cells
To date it is not entirely clear at what point during development the first oligodendroglial progenitors expressing NG2 appear in the CNS. Several reports indicate that the earliest NG2-positive parenchymal cells appear after E14.5 in the mouse or E15–E17 in the rat (Nishiyama et al. 1996; Zhu et al. 2011). Before that time NG2 labelling within the CNS is detected in the developing capillaries, and no areas derived from the neuroepithelium appear to be stained (Nishiyama et al. 1996; Trotter et al. 2010). All the non-vascular NG2-positive cells also express PDGF-Rα. Other studies demonstrate that the earliest oligodendrocyte progenitors are generated in the ventral telencephalon at about E11.5–E12.5 in the mouse, from the ventricular zone of the medial ganglionic eminence (Tekki-Kessaris et al. 2001; Kessaris et al. 2006). These oligodendrocyte progenitors express PDGF-Rα, but it is not clear whether they are also positive for NG2.
The peak in the density of NG2 cells in the rat is reached during the first postnatal week. By this time regional variations in the density of the NG2 cells appear to be less apparent because the entire CNS becomes populated more uniformly by these cells (Nishiyama et al. 1996). Starting from the second postnatal week the density of NG2-immunoreactive cells begins to decrease, although both NG2 and PDGF-Rα molecules continue to be expressed into adulthood.
In the developing and also in the adult brain many NG2 cells develop into myelinating oligodendrocytes. A proportion of NG2 cells fail to differentiate past the stage at which they express NG2 and the lipid antigen O4 and persist in the CNS in a presumably immature form (Dawson et al. 2003; Rivers et al. 2008; Zhu et al. 2008a). Whether NG2 cells are oligodendrocyte precursors with restricted lineage potential or whether they are multipotent progenitors is not well understood. Earlier studies attempting to address the question of the NG2 cells’ fate report that NG2 cells (OPC or O-2A progenitors) generate oligodendrocytes, but in addition they also give rise to a number of neurons and/or astrocytes (Belachew et al. 2003; Aguirre & Gallo, 2004; Aguirre et al. 2004; Tamura et al. 2007). Recent advances in gene technology have allowed further evaluation of NG2 cells‘ fate in the mouse CNS in vivo using Cre-loxP fate mapping in different transgenic mouse lines (Dimou et al. 2008; Rivers et al. 2008; Zhu et al. 2008a,b, 2011; Guo et al. 2009; Kang et al. 2010). These studies agree that NG2 cells are capable of generating oligodendrocytes. In addition, some studies reported that NG2 cells are the precursors of astrocytes in ventral areas of the brain and spinal cord (Zhu et al. 2008a,b; Guo et al. 2009). Other findings suggested that NG2 cells can differentiate into principal neurons in the ventral forebrain, dorsal cerebral cortex and hippocampus in the postnatal and adult animals (Rivers et al. 2008; Guo et al. 2009, 2010). At the same time, some investigators point out that NG2 cells remain committed to the oligodendrocyte lineage in postnatal life (Kang et al. 2010) and even following neurodegeneration (Kang et al. 2010). Interestingly, the fate of NG2 cells is likely to be age dependent, because a new study showed that NG2 cells in the postnatal brain generate only NG2 cells or oligodendrocytes, whereas NG2 cells in the embryonic brain generate protoplasmic astrocytes in addition to oligodendrocytes and NG2 cells (Zhu et al. 2011). Thus, it is clear that NG2 cells are the precursors of oligodendrocytes, but conclusions about the alternative fate of these cells remain controversial. This issue is difficult to investigate because of several reasons. First of all, NG2 proteoglycan is a surface marker that is lost before the terminal differentiation of the cells. Therefore, it is not possible to define the lineage potential of NG2-expressing cells based solely on NG2 expression, and the use of multiple markers is necessary to identify what types of progeny NG2 cells can generate. Second, although Cre-loxP technology brought many advantages, caution is needed in interpreting the results of Cre-loxP-mediated fate-mapping experiments (Nishiyama et al. 2009). Even in transgenic animals designed to express Cre recombinase under a specific promoter, transient expression of Cre recombinase in cells distinct from the lineage of interest is possible (Nishiyama et al. 2009). Therefore, confirmation of the fate-mapping results with other lineage-tracing methods is always desirable. The research on fate mapping of NG2 cells is further complicated by the fact that pericytes also express NG2 proteoglycan and, therefore, they and their progeny may be labelled by reporter genes in NG2 transgenic strains. This may bring confusion to the interpretation of data obtained from transgenic strains, especially when taking into account possible neurogenic potential of pericytes recently reported in vitro (Dore-Duffy et al. 2006).
Morphological features of NG2 cells based on single-cell fluorescent dye labelling
NG2 glial cells are characterized by a small (10–15 μm) polygonal soma and a multipolar tree of fine processes (Bergles et al. 2000; Chittajallu et al. 2004; Kukley et al. 2007, 2008, 2010; Gallo et al. 2008). The morphology of the NG2 cells differs slightly depending on their location in the brain. In grey matter the cells have a centrally located soma, from which extend several long, slender primary processes, which bifurcate two or more times, to form a symmetrical process field (Fig. 1; Bergles et al. 2000; Chittajallu et al. 2004). In white matter areas of the CNS, for example in the corpus callosum and optic nerve, NG2 cells often have a more polarized appearance, extending processes along the axonal axis (Berry et al. 2002; Butt et al. 2004; Chittajallu et al. 2004; Kukley et al. 2007). Some authors show that NG2 cells in white matter have classic bipolar morphology of neural precursor cells: they possess only few processes that are short in length and are emanating from the opposing poles of the cell body (Chittajallu et al. 2004). However, other studies demonstrate that the process fields of white matter NG2 cells may ramify through the neuropil for distances up to 160–200 μm (Berry et al. 2002). Possible reasons for these discrepancies are not known, but may include different age of experimental animals and/or variations in cell-labelling techniques.
Fig. 1.
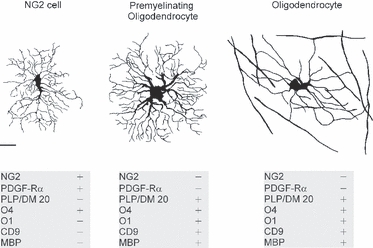
Morphological comparison of the oligodendrocyte lineage cells. Drawings of an NG2 cell, a premyelinating oligodendrocyte and a myelinating oligodendrocyte. The drawings are traced from Lucifer-Yellow-filled cells in brain slices, and represent the typical morphology of oligodendrocyte lineage cells at different stages of differentiation. The grey panels beneath the drawings list some of the important molecular markers that are present (+) or absent (−) in the oligodendrocytes lineage cells. Scale bar: 10 μm (applies to all three drawings). Upper panel is modified with permission from Kukley et al. (2010).
An interesting morphological feature of NG2 cells in grey and white matter is the occurrence of multiple small varicosities, also called nodules, throughout the length of their processes (Fig. 2a; Butt et al. 1999; Berry et al. 2002; Jabs et al. 2005; Kukley et al. 2010). Functional meaning of those structures has not been identified, but it is possible that these regions indicate points where branching of NG2 cell processes has just started (Chatterjee et al. 2008). Alternatively, varicosities may represent sites of contacts between NG2 cells and other types of cells in the brain. Notably, in primary oligodendrocyte cultures, an intense co-localization of NG2 and a PDZ (postsynaptic density-95/discs large/zona occludens-1) domain protein syntenin-1 is seen at varicosities in NG2 cell processes (Chatterjee et al. 2008). The majority of the PDZ domain-containing proteins are associated with plasma membrane proteins, and they are generally restricted to specific sub-cellular domains such as synapses or cell–cell contact points (Sheng & Sala, 2001; Chatterjee et al. 2008). In neurons, syntenin binds to kainate receptor subunits and to all forms of AMPA receptor subunits, GluR1–4 (Hirbec et al. 2002, 2005). NG2 cells also express different subunits of AMPA receptors (see below). Therefore, it is possible that by interaction with ionotropic glutamate receptors syntenin plays a role in determining the formation and maturation of neuron–glia synapses. It might be of future interest to investigate whether varicosities along NG2 cell processes represent sites of functional input from neurons (‘glial spines’), and whether different PDZ domain proteins and receptors for neurotransmitters are mainly targeted to the varicosities in situ and in vivo.
Fig. 2.
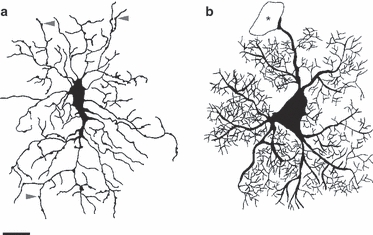
Morphological comparison between an NG2 cell and a grey matter astrocyte. Drawings of an NG2 cell (a) and an astrocyte (b) represent typical morphological appearance of the two cell types in brain slices. The drawing of the NG2 cell is traced from a Lucifer-Yellow-filled cell. Arrowheads point to varicosities present along the processes of the NG2 cell. Drawing of the grey matter astrocyte is a collage based on anti-GFP staining of astrocytes in brain slices from GFP/GFAP transgenic mouse. The asterisk indicates a blood vessel. Note the difference in the size of the soma, the thickness of the main and finer processes, as well as in the branching of fine processes. Scale bar: 10 μm (applies to both drawings). Part (a) is modified with permission from Kukley et al. (2010).
Are NG2 cells morphologically different from other types of glial cells in the brain, i.e. from astrocytes, microglia and oligodendrocytes? Our observations based on immunohistochemical labelling in hippocampal slices as well as on labelling individual astrocytes and NG2 cells with fluorescent dye Lucifer Yellow during patch-clamp recordings indicate that in grey matter those two cell types are morphologically distinct. Hippocampal astrocytes show large soma and asymmetrically radiating processes that consist of several primary thick processes, from which emanate multiple smaller collateral branching secondary processes, giving them a bushy, spongiform appearance (Fig. 2b; Nishiyama et al. 2005). NG2 cells, in contrast, have smaller soma and numerous irregular fine processes, but the smallest processes of NG2 cells are not as thin as those of the astrocytes, and thus NG2 cells clearly lack sponge-like appearance (Fig. 2a; Nishiyama et al. 2005). Astrocyte processes often end in bulbous swellings, or terminal end-feet, which form the vascular and pial glia limitans. In contrast, NG2-glial processes taper to an end and do not appear to contribute to the glia limitans (Nishiyama et al. 2005). It is so far not clear whether those morphological differences hold true for NG2 cells and fibrous astrocytes in white matter. NG2 cells and astrocytes are also diverse in respect with molecular markers: NG2 cells do not express glial fibrillary acidic protein (GFAP) or the glial glutamate–aspartate transporter, which are expressed by astrocytes; while astrocytes in turn do not express NG2, PDGF-Rα and O4 (Levison et al. 1999; Zhou et al. 2006; Nishiyama et al. 2009; Kukley et al. 2010).
Clear differences in the number, length and appearance of the processes as well as in the size of cell soma have been observed between NG2 cells and more mature cells of the oligodendrocyte lineage, and in striking contrast to mature oligodendrocytes NG2 cells do not bear myelin sheathes (Fig. 1; Trapp et al. 1997; De Biase et al. 2010; Kukley et al. 2010). NG2 cells appear negative for the molecular markers of the more mature cells of the oligodendroglial lineage, such as proteolipid protein (PLP) and/or its splice variant DM20, myelin basic protein, 2,3-cyclic nucleotide 3-phosphodiesterase, galactocerebroside O1 and tetraspanin CD9 (Polito & Reynolds, 2005; Nishiyama et al. 2009; Kukley et al. 2010). Notably, some premyelinating oligodendrocytes may still carry residual amounts of NG2 proteoglycan, indicating that they are indeed the descendants of NG2 cells.
NG2 cells are likely to hold some morphological similarities with ramified microglial cells. To date, no direct comparison of morphological properties of these two cell types has been performed based on single-cell dye labelling under identical experimental conditions. Some insights into the morphological structure of ramified microglia come from reconstructions of Lucifer Yellow-filled cells in brain slices of the adult mice (Boucsein et al. 2003), and from the in vivo 2-photon imaging of microglial cells in adult EGFP-CX3CR1 transgenic mice (Davalos et al. 2005; Nimmerjahn et al. 2005). Similar to NG2 cells, microglial cells have small cell soma from which numerous thin and highly ramified processes extend symmetrically (Boucsein et al. 2003; Davalos et al. 2005; Nimmerjahn et al. 2005). In contrast to NG2 cells, microglia cell processes lack small nodules but display highly motile filopodia-like protrusions of variable shape, typically forming bulbous endings under normal conditions and/or during injury (Davalos et al. 2005; Nimmerjahn et al. 2005). The double-labelling immunohistochemical studies have established that NG2 cells are clearly distinct from resting or activated microglia: NG2 cells are negatively stained with the markers of the microglial cells, for example 4H1, CD68, F4/80 or OX42 (Nishiyama et al. 1997, 2002; Horner et al. 2002; Kukley et al. 2010).
Thus, NG2 cells possess some unique morphological features that could be their individual hallmarks; in brain slices NG2 cells can be reliably distinguished from other cells on the basis of their morphology and antigenic expression.
NG2 cells express voltage-gated potassium and sodium channels
NG2 cells in grey and white matter express different types of voltage-gated channels. Depolarizing voltage steps from the resting potential elicit both rapidly inactivating and sustained outward K+ currents (Knutson et al. 1997; Chittajallu et al. 2002, 2004; Kukley et al. 2010). These currents consist of conventional ‘A-type’ and ‘delayed rectifier’ type K+ currents that are antagonized by 4-aminopyridine and tetraethylammonium, respectively (Chittajallu et al. 2004). The functional role of voltage-activated K+ channels in NG2 cells is not entirely clear. Cell proliferation studies and electrophysiological analysis in cultured cells and in brain slices have suggested that potassium channels may have a prominent role in the oligodendrocyte lineage cell proliferation (Gallo et al. 1996; Knutson et al. 1997; Yuan et al. 1998; Ghiani et al. 1999). It has been observed that over-expression of Kv1.3 or Kv1.4 subunits of potassium channel increases oligodendrocyte lineage cell proliferation in the absence of mitogens, whereas Kv1.6 over-expression inhibits mitogen-induced cell cycle progression (Vautier et al. 2004). Thus, it is possible that K+ channels in NG2 cells are in some way involved in the regulation and/or modulation of the cell cycle. Interestingly, the amplitude of voltage-gated outward potassium currents begins to decrease as NG2 cells stop dividing and start to differentiate (Kukley et al. 2010).
In addition to the outward K+ currents, NG2 cells also exhibit inward K+ currents to a variable degree (Lin & Bergles, 2002; Chittajallu et al. 2004). These currents are likely to result from the activity of both ATP-sensitive K+ channels and inwardly rectifying K+ channels (Kir). Kir channels in glial cells have been implicated in several functions, which include setting and/or stabilizing the resting membrane potential near the K+ equilibrium potential, as well as accumulation, buffering or siphoning of K+ released during neuronal activity (Reimann & Ashcroft, 1999; Lin & Bergles, 2002).
Most authors agree on the fact that NG2 cells carry voltage-gated sodium channels (NaV channels), yet there is some disagreement concerning the proportion of NG2 cells that express NaV (Bergles et al. 2000; Chittajallu et al. 2004; Jabs et al. 2005; Karadottir et al. 2008; De Biase et al. 2010; Kukley et al. 2010; Maldonado et al. 2011). A recent study aimed to define the prevalence of NaV channel expression and excitability within the NG2 cell population in grey and white matter throughout development (De Biase et al. 2010). Whole-cell voltage-clamp recordings in acute brain slices prepared from NG2-DsRed mice of different ages (P5–P8, P12–P15, P20–P26, P40–P45) revealed that all NG2 cells in corpus callosum, hippocampus, molecular layer of the cerebellum and cerebellar white matter exhibit TTX-sensitive NaV channel-mediated currents (De Biase et al. 2010). These data suggest that NaV channel expression is a universal property of NG2 cells in both grey and white matter regions of the developing and mature brain. At the same time, other studies point towards heterogeneity of the NG2 cell population in the brain with respect to expression of NaV channels and define two classes of NG2 glial cells: one type lacks voltage-gated Na+ channels completely (∼ 30% of all NG2 cells); whereas the other type expresses a sufficient number of NaV channels to generate Na+ currents upon depolarization of cell membrane (Karadottir et al. 2008). Importantly, relatively high passive membrane conductance of some NG2 cells, for example in the adult brain, may mask Na+ currents in these cells (Maldonado et al. 2011). Using an effective way to increase membrane resistance (e.g. bath application of barium) may help in uncovering Na+ currents in NG2 cells (Maldonado et al. 2011). Maldonado et al. (2011) have recently applied this approach to demonstrate that the large majority, if not all NG2 cells, of the somatosensory cortex express voltage-gated Na+ channels regardless of the age of the animal.
Expression of NaV channels by NG2 glial cells has raised the question whether these cells are able to generate action potentials. Apparently the peak amplitude of the Na+ current in NG2 cells is > 10 times smaller than that observed in mature neurons under similar conditions (Chittajallu et al. 2004; De Biase et al. 2010; Kukley et al. 2010). The small number of these channels and the comparatively large K+ conductances present prevents the initiation of regenerative spikes in NG2 cells (De Biase et al. 2010; Kukley et al. 2010). But it should be noted that some NG2 cells in the cortex and corpus callosum may generate a single spike upon depolarization (Chittajallu et al. 2004; Ge et al. 2009; De Biase et al. 2010; Maldonado et al. 2011). However, these spikes do not meet the criteria normally used to define action potentials in neurons. Action potentials are regenerative, all-or-none events, with a discrete threshold of activation (normally of about −45 to −55 mV), constant amplitude, and a peak depolarization that approaches the sodium equilibrium potential (ENa; Kandel et al. 2000; Squire et al. 2003). Single spikes observed in NG2 cells exhibit a more depolarized threshold for activation (−22 ± 1 mV), their amplitudes increase with larger current injections, and they do not substantially overshoot 0 mV (Chittajallu et al. 2004; Ge et al. 2009; De Biase et al. 2010). In addition, NG2 cells that exhibit spikes are unable to sustain repetitive firing with continued depolarization and they do not exhibit spontaneous firing (De Biase et al. 2010; Maldonado et al. 2011; but see Karadottir et al. 2008). If NG2 cells do not use NaV channels for firing typical action potentials, what could be the functional meaning of these channels in NG2 glia? The influx of positive charge through the Na+ channels could boost other depolarizing stimuli, allowing activation of other voltage-dependent channels (Lin & Bergles, 2004b). Alternatively, in the absence of excitability, the Na+ influx through these channels could regulate the efficiency of the Na+/K+ ATPase or Na+-dependent transporters (Lin & Bergles, 2004b). Recent data demonstrate that Na+ channels mediate Ca2+ influx via Na+/Ca2+ exchangers in NG2 cells upon GABA induction of membrane depolarization (Tong et al. 2009). The authors go on to show that this unique Ca2+-signalling pathway is involved in the migration of NG2 cells (Tong et al. 2009). They further speculate that any ligand that induces sufficient membrane depolarization in NG2 cells may elevate intracellular Ca2+ by activating the Na+ channel–Na+/Ca2+ exchanger pathway. This induces NG2 cell migration by triggering the Ca2+-dependent mechanisms required for cell migration, including cytoskeletal reorganization, cell mobility, membrane traffic, and cell adhesion and de-adhesion (Tong et al. 2009).
The lack of ability to fire bona fide action potentials does not necessarily imply that NG2 cells do not possess any output signalling and can not be involved in the information processing. Action potentials may be absent from the responses of NG2 cells because these cells have relatively short processes (up to 100–200 μm) and do not convey signals over long distances, analogous to, for example, bipolar cells in distal retina (Dowling, 1987). Therefore, electrotonic spread of the potential changes might be sufficient for information to reach from one end of the cell to the other. NG2 cells may use graded potentials rather than action potentials to distinguish a wide range of signal intensities, for example in analogy to various types of cells in the retina that use graded potentials rather than action potentials for fine intensity discriminations (Dowling, 1987).
NG2 cells express AMPA/kainate and GABAA receptors, and receive synaptic input from neurons
The first demonstration of glutamate-activated channels in glia goes back to 1989, when multiple conductance channels activated by l-glutamate, quisqualate or kainate have been reported in type-2 astrocytes in culture (Usowicz et al. 1989). At that time it has been suggested that glial cells possess glutamate receptors, which could be activated by glutamate released from axons and may, therefore, be important for formation and maintenance of the nodes of Ranvier (Usowicz et al. 1989). Follow-up studies revealed that glutamate or kainate, agonists at AMPA/kainate receptors, elicit inward currents in O-2A cells acutely isolated from the optic nerve (Barres et al. 1990), in ‘complex cells’ from hippocampal slices (Steinhauser et al. 1994) and in glial precursor cells in the corpus callosum (Berger, 1995). Glial GABAA receptors were first discovered in cultured astrocytes and cells of oligodendrocyte lineage (Gilbert et al. 1984; Kettenmann et al. 1984), and subsequently in in situ preparations, for example in ‘complex cells’ from slices of the rat hippocampus (Steinhauser et al. 1994) and in glioblasts from the corpus callosum (Berger et al. 1992). Although no immunohistochemical staining for NG2 was performed at that time, it is likely that the large proportion of O-2A cells, ‘complex cells’, and possibly also callosal precursor cells, investigated in those studies were cells now known as NG2 glia. This assumption is based on two major facts. Firstly, a detailed comparison in the developing rodent CNS between the distribution of the NG2 proteoglycan and the PDGF-Rα shows that these two molecules are co-expressed by glial progenitor cells of the O-2A lineage and can serve as reliable markers for identification of O-2A cells in vivo (Nishiyama et al. 1996). Secondly, there is now an emerging consensus in the field that ‘complex cells’ (also termed GluR cells) exhibit many of the properties that have been described for NG2 cells, and that ‘complex cells’ and NG2 cells represent overlapping populations (Bergles et al. 2010).
The expression of neurotransmitter receptors by NG2 glia inspired thinking of how these receptors become activated in situ. Bergles et al. (2000) were the first to demonstrate that upon propagation of single action potentials glutamate is released directly onto NG2 cells’ AMPA receptors at neuron–NG2 cell synapses. The authors made whole-cell patch-clamp recordings from NG2 cells in the hippocampus and measured their response to stimulation of afferent excitatory axons. They were able to record inward currents in NG2 cells, which were strongly inhibited by the AMPA/kainate receptor antagonists (Bergles et al. 2000). The quantal nature of these responses and their rapid kinetics indicated that they are produced by the exocytosis of vesicles filled with glutamate directly opposite AMPA receptors on NG2 glia (Bergles et al. 2000). A similar type of fast neuron–glia synaptic signalling has been later discovered between GABAergic interneurons and NG2 cells in the hippocampus (Lin & Bergles, 2004a). Additional evidence from electron microscopic analysis revealed accumulations of small vesicles at contact sites between axons and the processes of NG2 cells in both the young and adult rodent hippocampus (Bergles et al. 2000; Lin & Bergles, 2004a).
Since the first discovery of glutamatergic and GABAergic innervation of NG2 cells, several groups demonstrated that NG2 cells receive synaptic input from neurons in different grey matter areas, including the cerebral cortex, hippocampus, cerebellum, brain stem and ventrobasal thalamus (Chittajallu et al. 2004; Lin & Bergles, 2004a; Lin et al. 2005; Karadottir et al. 2008; Kukley et al. 2008; Muller et al. 2009; De Biase et al. 2010; Parri et al. 2010; Velez-Fort et al. 2010). More surprisingly, unmyelinated axons passing through white matter (e.g. corpus callosum, optic nerve) also establish excitatory glutamatergic synapses with local NG2 cells, and neurotransmitter release along axons in white matter is quite similar to vesicle fusion at synapses: it is reliable, it depends on action potential propagation, involves highly localized calcium microdomain signalling and is strongly calcium cooperative (Kukley et al. 2007; Ziskin et al. 2007).
Do only few NG2 cells receive synaptic input from neurons, or is it a universal property of the whole population of NG2 cells throughout the brain? The data obtained by different groups to date remain controversial. For example, Chittajallu et al. (2004) observed functional AMPA receptors in all NG2 cells in the neocortex, but could detect spontaneous AMPA receptor-mediated synaptic currents only in ∼ 6% of these cells. These findings are consistent with a recent report indicating that > 90% of synaptic currents in NG2 cells in the neocortex are GABAergic early in postnatal life (Velez-Fort et al. 2010). In the cerebellum NG2 cells have been shown to fall into two classes: cells expressing Na+ channels and cells lacking Na+ channels. Interestingly, these two populations of NG2 cells appeared to sense their environment in different ways: 81% of NG2 cells expressing Na+ channels showed spontaneous synaptic input; while only 2% of the NG2 cells lacking Na+ channels showed detectable synaptic events (Karadottir et al. 2008). On the contrary, another study reports that in the cerebellum (both in white matter and in the molecular layer) the ability to receive glutamatergic synaptic input from neurons is a general feature of each and every NG2 cell (De Biase et al. 2010). To obtain an unbiased sample of the NG2 cell population, De Biase et al. used transgenic mice expressing DsRed-T1 under the control of the NG2 promoter (Ziskin et al. 2007; Zhu et al. 2008a). They made whole-cell voltage-clamp recordings from NG2 cells, and focally applied hypertonic solution (500 mm sucrose in artificial cerebral spinal fluid) to force fusion of docked, primed synaptic vesicles from presynaptic membranes in contact with these cells (De Biase et al. 2010). At the earliest postnatal periods examined (P5–P10), hypertonic challenge evoked glutamatergic synaptic responses in the majority of NG2 cells. At later developmental periods (P12–P15, P20–P26, P40–45), hypertonic challenge elicited glutamatergic synaptic responses in all NG2 cells (De Biase et al. 2010). The authors report similar findings for NG2 cells in the hippocampus and corpus callosum (De Biase et al. 2010). It remains unclear why the proportion of synaptically innervated cerebellar NG2 cells found in the study of Karadottir et al. (2008) and that of De Biase et al. (2010) is different. One possibility could be species differences, because Karadottir et al. (2008) recorded in the rat cerebellum while De Biase et al. (2010) used a transgenic mouse.
Taken together, the controversy on whether or not all NG2 cells throughout the brain receive synaptic input is not completely resolved. It remains to be ascertained whether the proportion of synaptically innervated NG2 cells depends on the brain region and/or on the age of the animal. It is especially interesting to find out whether glutamatergic and GABAergic synaptic inputs onto NG2 cells are differently regulated during development, and whether those two inputs have different meaning for a given NG2 cell contacted by both glutamatergic and GABAergic synapses (e.g. in the hippocampus).
The process of forming a synapse involves complex coordinated cell morphological and structural changes that are instructed through ligand-receptor interactions, intracellular signalling cascades and complex filamentous actin remodelling (Shen & Cowan, 2010). Why do neurons establish synapses with NG2 glial cell and why do NG2 cells attempt to receive synaptic input from neurons? Discovery of the functional importance of neuron–NG2 synapses remains a challenge for future research. So far a number of studies proposed that neuron–glia communication is essential for regulating development of NG2 cells, and suggested that there is a link between activation of neurotransmitter receptors and the proliferation and/or differentiation of NG2 cells (Karadottir & Attwell, 2007). We will discuss these findings in more detail in the following sections of this review.
NG2 cells represent the major cycling cell type in the brain
From the time of birth, NG2 cells are more or less evenly distributed throughout the brain, both in grey matter and white matter (Nishiyama et al. 1996; Dimou et al. 2008; Rivers et al. 2008). Interestingly, in every region of the brain NG2 cells are found as proliferative cells (Dawson et al. 2000, 2003; Horner et al. 2002; Nishiyama et al. 2002; Aguirre et al. 2004), and the fraction of actively cycling NG2 cells (growth fraction) is quite high in young (1–2 weeks old) as well as adult (∼ 18 months old) animals (Kukley et al. 2008; Psachoulia et al. 2009). In order to estimate the growth fraction of NG2 cells, Kukley et al. (2008) performed a double staining for NG2 and the proliferating cell nuclear antigen (PCNA), which is only detected in cycling but not in resting cells. At P9 and P11 mouse hippocampus, the authors determined the growth fraction (NG2+ and PCNA+) of NG2 glia to be as large as 48% and 49% (93/194 and 52/106 of NG2 cells), respectively (Kukley et al. 2008). These growth fraction measurements are in keeping with a follow-up report indicating that the growth fraction [defined by cumulative bromodeoxyuridine (BrdU) labeling] of P6 mouse NG2 cells in corpus callosum and cerebral cortex is ∼ 55% (Psachoulia et al. 2009).
As an animal grows into adulthood the density of NG2 cells decreases (Nishiyama et al. 1996; Velez-Fort et al. 2009), and the absolute number of cycling NG2 cells declines (Panagiotakos et al. 2007; Velez-Fort et al. 2009). However, although the actual number of proliferating NG2 cells in the adult brain is largely below that of the first postnatal weeks, the fraction of actively cycling NG2 cells changes only slightly with age, being ∼ 46% in corpus callosum and ∼ 39% in cerebral cortex of 2–18-month-old mice (Psachoulia et al. 2009). Thus, approximately half of all NG2 cells are constantly dividing, independently of the brain area and the age of the animals. Remarkably, recent findings indicate that all NG2+ cells in the mature brain, regardless of region, have the ability to divide (Kang et al. 2010). Therefore, it is possible that the total number of cycling NG2 cells in the brain is even higher than suggested previously (Kukley et al. 2008; Psachoulia et al. 2009).
Are there any differences in proliferation parameters of NG2 cells between the neonatal and adult brain? Cumulative BrdU labelling performed in mice of various ages revealed a striking discrepancy in cell cycle time of NG2 cells (Kukley et al. 2008; Rivers et al. 2008; Psachoulia et al. 2009). During the first two postnatal weeks the cell cycle time of NG2 cells in the hippocampus, cerebral cortex and corpus callosum is about 2–3 days, meaning each cycling NG2 cell divides approximately every 48–72 h (Kukley et al. 2008; Psachoulia et al. 2009). As the animal matures, cell cycle time increases steadily, being > 100 days at P540 (Psachoulia et al. 2009). Interestingly, the rate of oligodendrocyte production from NG2 cells declines in parallel with the increase of the NG2 cells’ cell cycle duration. In the corpus callosum, for example, the cell cycle slows down ∼ 10-fold between P45 and P240, and the rate of oligodendrocyte production slows ∼ 20-fold at the same period (Psachoulia et al. 2009).
NG2 cells represent the major cycling cell population in the rodent brain parenchyma. Thus, studies using BrdU labelling and immunohistochemistry show that NG2 cells account for approximately 70% of BrdU-positive cells in the adult cerebral cortex, hippocampus, corpus callosum and spinal cord after a short BrdU pulse (Horner et al. 2000; Dawson et al. 2003; Polito & Reynolds, 2005; Lasiene et al. 2009). Other studies using BrdU labelling, immunohistochemistry and/or transgenic mice where NG2 or Olig2 cells are labelled suggest that virtually all cells (≥ 90%) incorporating BrdU in the brain parenchyma represent NG2 cells (Alonso, 2000; Dayer et al. 2005; Dimou et al. 2008; Mori et al. 2009). NG2+/Olig2+ cells also represent the major cycling population of the healthy adult human brain (Geha et al. 2010).
NG2 cells maintain complex morphology during cell division
NG2 cells in grey and white matter of the rodent brain have a complex morphology, with highly branched processes that may extend more than 100 μm in radial (grey matter) or bipolar (white matter) shape around the small cell body. At the same time, in the neonatal as well as in the adult brain about 50% of NG2 cells are actively involved in the cell cycle. How does the cell morphology change when an NG2 cell enters the cell cycle? To address this question, patch-clamp recordings from individual hippocampal NG2 cells with mitotic DNA configuration have been carried out, and the cells were filled with a fluorescent dye Lucifer Yellow (Kukley et al. 2008). Subsequent morphological analysis of these dye-filled cells revealed that metaphase and telophase NG2 cells possess a rich tree of branching processes (Kukley et al. 2008). Similar findings have been reported by Ge et al. (2009), who carried out morphological examination of mitotic glial cells positively labelled for NG2 in brain slices (Fig. 3A–C). We, therefore, believe that NG2 cells maintain their complex morphology during division. These findings, however, do not completely exclude the possibility that NG2 cells are able to retract and re-grow some of their processes very quickly, for example in the range of minutes, and at the moment of chromosome separation the cells withdraw many of their processes. Time-lapse imaging of zebrafish OPCs in vivo shows that cells can rapidly remove filopodium-like processes, divide and then re-grow their processes again and migrate away from each other (Kirby et al. 2006). In vivo time-lapse imaging of mammalian NG2 cells has not been reported to date, and it is unclear whether a similar sequence of events happens during cell division of mammalian NG2 cells. Ge et al. (2009) attempted to perform time-lapse imaging of dividing NG2 cells in acute mouse brain slices, and observed that the soma of the mother cell was splitting into two, while all of the processes remained un-retracted. This result strongly supports the idea that NG2 cells can indeed retain their complex morphology during mitosis. However, the observation of Ge et al. is so far based only on a single example. Future experiments involving time-lapse imaging in transgenic mice where soma and processes of NG2 cells are specifically labelled would be necessary to further investigate this issue.
Fig. 3.
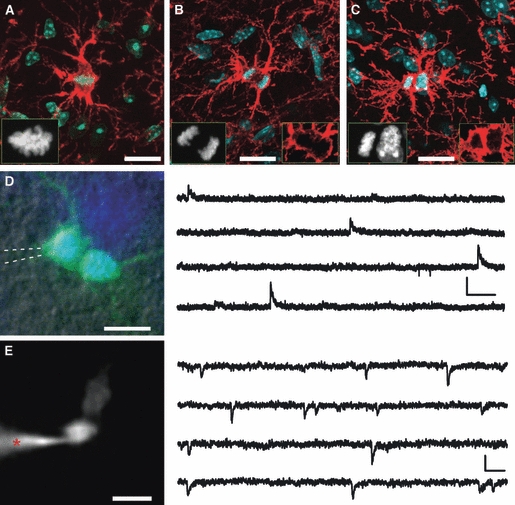
NG2 cells maintain complex morphology and functional synapses during mitosis. (A–C) Three NG2 cells at different stages of cell division labelled by DAPI (blue) and NG2 antibodies (red) in brain slices prepared from P25 mouse: metaphase (A), anaphase (B) and telophase (C). Note that mitotic cells possess a rich tree of processes, and that NG2 expression is upregulated during mitosis. Scale bars: 20 μm (A–C). Insets show blue and red channels separately. Note the difference between the appearance of the DAPI signal in NG2 cells at different stages of mitosis. (D) Left: dividing NG2 cell showing mitotic chromatin configuration in a live brain slice. The cell is filled with Lucifer Yellow (green) during patch-clamp recordings. Chromatin is labelled with Hoechst 33342 (blue). Patch-pipette is indicated by the dashed white lines. Scale bar: 10 μm. Right: GABAergic synaptic currents recorded from the cell shown on the left (Vh = +30 mV), in the presence of 30 μm CNQX and 100 μm ruthenium red. Scale bar: 400 ms, 20 pA. (E) Left: mitotic NG2 cell filled with Cy5-conjugated dextran (10 kDa) during patch-clamp recordings. Dextran (10 kDa) does not spread through gap junctions, so filling of two cells indicates that the division process is not completed and cells still share the cytoplasm. Asterisk indicates the patch-pipette. Scale bar: 10 μm. Right: glutamatergic synaptic currents recorded from the cell shown on the left (Vh = −80 mV), in the presence of 10 μm bicuculline. Scale bar: 20 ms, 10 pA. (A–C) Modified with permission from Ge et al. (2009). (D,E) Modified with permission from Kukley et al. (2008).
NG2 cells keep functional synapses during mitosis and pass them onto their progeny
Do NG2 cells maintain synapses with neurons during cell division? To investigate synaptic responses directly in proliferating cells, patch-clamp recordings have been performed from mitotic NG2 cells (metaphase or telophase of mitosis) in acute hippocampal and cortical slices from neonatal (7–12 days old) mice (Kukley et al. 2008). In the presence of AMPA/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μm), clear GABAergic synaptic currents were detected in NG2 cells with mitotic chromosomes configurations (Fig. 3D; Kukley et al. 2008). On removal of AMPA/kainate receptor antagonists from the extracellular solution and adding GABAA receptor antagonist bicuculline (10 μm), fast rising and decaying excitatory postsynaptic currents could be recorded in NG2 cells during metaphase and telophase of mitosis (Fig. 3E; Kukley et al. 2008). In line with these findings are the recent data of Ge et al. (2009), who demonstrate that grey and white matter NG2 cells in metaphase and telophase of mitosis receive glutamatergic synaptic input from neurons not only in young but also in older animals (up to 20 weeks old). Furthermore, electron microscopy images show that synaptic terminals contact mitotic NG2 cells, suggesting that during cell division at least some synaptic contacts are maintained (Kukley et al. 2008).
Based on the described evidence it seems likely that NG2 cells in the rodent brain can enter cell cycle and undergo cell division without losing functional GABAergic and glutamatergic synapses. The evidence so far is indirect, because we and other groups have not tracked single synapses on NG2 cells during mitosis in native brain slices or in vivo. However, the following facts speak in favour of the inheritance. First, the frequency of synaptic currents is comparable between metaphase and telophase NG2 cells (Kukley et al. 2008). Synaptic currents recorded in telophase NG2 cells are likely to stem from both daughter cells, because in telophase two daughter cells are not completely separated yet and share cell membrane and cytoplasm. Therefore, the invariant frequency of synaptic currents between metaphase and telophase cells most likely indicates that the number of functional synaptic contacts of the parent cell is comparable with the number found in two daughter cells. In addition, the number of glutamate decarboxylase-positive (GAD65+) terminals (putative GABAergic boutons) in each daughter NG2 cell is roughly half of the synaptic terminals found in the parent cell (Kukley et al. 2008). Furthermore, if we assume that the time necessary for the assembly and disassembly of neuron–glia synapses is the same as of neuronal synapses, then AMPA receptor-containing synapses can be eliminated as quickly as 90 min and assembled within 1–2 h of initial axo-dendritic contact (Friedman et al. 2000; Eaton & Davis, 2003; Goda & Davis, 2003). If NG2 cells have to disassemble all synapses before cell division and reform all of them from scratch after cytokinesis, there should be a period of time equal to at least 1 h when synaptic currents are absent in NG2 cells. However, synaptic currents could be recorded in NG2 cells presumably 30 min before and after chromosome segregation (Kukley et al. 2008).
Taken together, we believe that newly generated NG2 cells can inherit synapses from their parent cell in the postnatal rodent brain. What could be the benefit of inheriting a synapse? Keeping synaptic contacts with dividing NG2 cells may give neurons the opportunity to directly or indirectly regulate proliferation of NG2 cells, for example by influencing the expression of potassium channels during the cell cycle and/or by controlling other intracellular signalling cascades. On the other hand, inheritance of synapses may allow for the direct transfer of environmental interactions to clonal descendants of NG2 cells, which might be important for effective colonization and perhaps future myelination of the developing brain.
Discovery of glutamatergic innervation of dividing NG2 cells (Kukley et al. 2008; Ge et al. 2009) re-opened an essential question regarding the role of glutamate and its receptors for proliferation of the oligodendrocyte lineage cells, which was first addressed 15 years ago (Gallo et al. 1996; Yuan et al. 1998). At that time work performed in dissociated cell cultures and organotypic slice cultures showed that glutamate acts through AMPA/kainate receptors to inhibit O-2A progenitor (presumably NG2 cells) proliferation (Gallo et al. 1996; Yuan et al. 1998). It has been suggested that this effect is mediated by the increase in intracellular Na+ concentration triggered by the opening of the AMPA channels in the O-2A membrane, and subsequent block of voltage-gated K+ channels (Gallo et al. 1996; Yuan et al. 1998). Now it turned out that glutamate released from neurons activates AMPA/kainate receptors on dividing NG2 cells in acute brain slices (Kukley et al. 2008; Ge et al. 2009). How can NG2 cells receiving glutamatergic synaptic input divide if glutamate inhibits proliferation? In the earlier studies agonists and antagonists of glutamate receptors have been added to the culture medium for 1–48 h, and therefore cells were constantly exposed to these substances for a prolonged period of time (Gallo et al. 1996; Yuan et al. 1998). Glutamatergic synaptic-like signalling between neurons and NG2 cells under physiological conditions is a much finer ‘tool’ for exposing glial cells to glutamate and activating their glutamate receptors. Importantly, glutamatergic synaptic signalling can be tuned in time and space both pre- and postsynaptically, and it can perhaps be tuned during the cell cycle of NG2 cells. For example, AMPA receptors expression on NG2 cells and/or properties of the axonal release machinery could be higher in G1 phase and decreasing in G2/M phase, thereby allowing NG2 cell division. Blocking AMPA/kainate receptors and subsequent increase in cell proliferation described by Yuan et al. (1998) would be an extreme of this situation. It is not known today whether and how surface expression of neurotransmitter receptors on NG2 cells, the number and properties of synapses, the location of synapses along NG2 cell processes and the strength of synaptic input change during the cell cycle of an NG2 cell. It remains to be discovered whether and how glutamatergic synaptic signalling between neurons and glia regulates proliferation of NG2 cells.
Synaptic signalling and glutamate receptor expression are rapidly decreased during differentiation of NG2 cells
During animal development a large proportion of NG2 cells evolve into premyelinating oligodendroglial cells, which then differentiate further into myelinating oligodendrocytes (Trapp et al. 1997; Zhu et al. 2008a,b; De Biase et al. 2010; Kukley et al. 2010). Differentiation of NG2 cells is accompanied by dramatic alterations of the cell morphology and by definite changes of the expression pattern of specific molecular markers (Fig. 1). Detailed morphometric analysis based on single cell dye-filling reveals that premyelinating oligodendrocytes in grey matter display a significantly larger total processes length (2891 ± 177 vs. 1198 ± 190 μm), area covered by the processes (6169 ± 235 vs. 3328 ± 215 μm2) and diameter of the soma (9.7 ± 0.9 vs. 6.5 ± 0.7 μm) when compared with NG2 cells (Kukley et al. 2010). In contrast to NG2 cells, premyelinating oligodendrocytes are largely negative for NG2 and PDGF-Rα, but are positively stained by the PLP/DM20 antibodies and by O1 antibodies showing labelling of the entire cell surface (Sommer & Schachner, 1981; Trapp et al. 1997; Kukley et al. 2010). Premyelinating oligodendrocytes do not display myelin sheathes (Fig. 1). Mature myelinating oligodendrocytes in the grey matter possess fewer processes than NG2 cells or premyelinating cells, but they clearly show numerous myelin sheathes. Myelin sheathes of hippocampal oligodendrocytes in CA1 stratum radiatum rarely appear as parallel, linear structures like those frequently observed in the white matter. They rather show random orientation, which reflects the orientation of axons in the stratum radiatum (Fig. 1).
Do neurons keep synapses that they have established with NG2 cells when NG2 cells advance through the process of differentiation? Two recent studies used whole-cell patch-clamp recordings from distinct developmental stages of oligodendroglial cells in brain slices to address the question what is the fate of synaptic input upon differentiation of NG2 cells (De Biase et al. 2010; Kukley et al. 2010). The authors employed two separate approaches to identify oligodendroglial cells at distinct stages of differentiation: while De Biase et al. (2010) used NG2-CreER;Z/EG transgenic mice where NG2 cells and their progeny show green fluorescence, Kukley et al. (2010) recorded from C57black/N mice and PLP-GFP mice and applied post-recording immunohistochemistry to prove the identity of the cells. Neuron–glial synapses exhibit a low rate of spontaneous release (Bergles et al. 2000; Chittajallu et al. 2004; Kukley et al. 2008), therefore it might be challenging to measure the innervation of the oligodendroglial cells in distinct stages of differentiation. It would be necessary to employ an approach that reliably brings about high exocytotic activity in the presynaptic nerve terminals contacting glial cells. To date, several manipulations have been extensively used to study vesicular release at neuronal synapses (Trudeau et al. 1996). One method used is to locally apply hypertonic solution (e.g. sucrose), which rapidly increases the quantal release rate to a relatively high level (Rosenmund & Stevens, 1996). Importantly, the pool of quanta defined with stimulation by hypertonic solution is the same as that employed when release is evoked by action potentials (Rosenmund & Stevens, 1996; De Biase et al. 2010). Another tool is application of polyvalent cations, such as lanthanum and ruthenium red (Raastad et al. 1992; Trudeau et al. 1996). The mechanism of action of polyvalent cations is not completely clear, but it has been suggested that they effectively induce quantal neurotransmitter release by binding to an external site on the presynaptic plasma membrane (Trudeau et al. 1996). In two recent studies the authors employed those approaches to study vesicular neurotransmitter release at neuron–glia synapses in different regions of the brain in situ (De Biase et al. 2010; Kukley et al. 2010). Whereas bath perfusion of 100 μm ruthenium red or local application of 500 mm sucrose elicited spontaneous synaptic responses in all NG2 cells tested, only a few premyelinating cells showed synaptic currents upon those manipulations, and no currents could be induced in myelinating oligodendrocytes (De Biase et al. 2010; Kukley et al. 2010).
A different way to test for the presence of synaptic input onto a given cell is to record this cell and use extracellular stimulation to synchronously induce action potentials in a large number of nearby axons. After an action potential arrives at a presynaptic terminal membrane depolarization causes rapid and brief opening of voltage-gated Ca2+ channels. The localization of Ca2+ channels at active zones provides a high, local rise in the Ca2+ concentration more than a thousand-fold (to ∼ 100 μm) within a few hundred microseconds at the site of transmitter release. This large and rapid local increase is required for the rapid synchronous release of neurotransmitter. When axons were stimulated electrically during recording of an NG2 cell, a robust synchronous compound response to the release of hundreds of synaptic vesicles could be observed in the NG2 cell (Fig. 4a, c; Kukley et al. 2010; see also Bergles et al. 2000). Synaptic response in the NG2 cell appeared with a brief latency (< 5 ms) following electrical stimulation, steeply increased to a sharp peak (20–80% rise time is < 1 ms) and then rapidly decayed back to baseline (decay time constant is about 2–4 ms; Fig. 4a,c). In contrast to NG2 cells, it was not possible to elicit any synchronous synaptic responses in the mature myelinating oligodendrocytes as well as in the majority of premyelinating oligodendrocytes (Fig. 4b,d). However, in ∼ 30% of premyelinating oligodendrocytes small asynchronous responses could be recorded upon electrical stimulation (Fig. 4b,d). Those individual release events typically started to appear as late as 20 ms after the presynaptic stimulation and continued for hundreds of milliseconds (Fig. 4b,d; Kukley et al. 2010). Taken together, during electrical stimulation of axons the dominant component of the synaptic response in an NG2 cell is attributable to phasic neurotransmitter release. In a premyelinating cell only late release events caused by delayed asynchronous release are detected. No synaptic events mediated by release of single neurotransmitter-filled vesicles can be identified in myelinating oligodendrocytes. At neuronal synapses, and probably at neuron–glia synapses as well, phasic neurotransmitter release is driven by a brief strong increase in presynaptic calcium concentration (10–100 μm) local to the calcium channel pore (Heidelberger et al. 1994; Xu-Friedman & Regehr, 2000). Experimentally slowing and reducing the calcium signal seen by the calcium sensor or removing important components of the presynaptic release machinery leads to the appearance of asynchronous synaptic currents and dramatically reduces synchronous transmitter release (Schneggenburger & Neher, 2000; Xu-Friedman & Regehr, 2000; Sun et al. 2007). It is plausible that the asynchronous synaptic currents encountered in some premyelinating oligodendrocytes represent the residual and transient function of the presynaptic release machinery that is in the process of being disassembled.
Fig. 4.
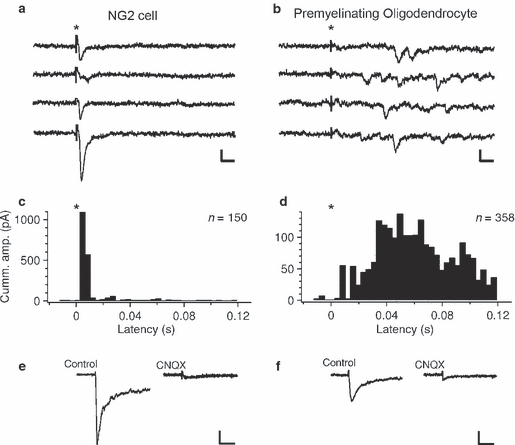
Synaptic signalling and AMPA/kainate receptor expression is downregulated upon differentiation of NG2 cells. (a,b) Four successive sweeps recorded in an NG2 cell (a) and in a premyelinating oligodendrocyte (b) in response to extracellular electrical stimulation of nearby axons in the presence of 25 μm APV and 10 μm bicuculline (the time point of stimulation is indicated by an asterisk). The holding potential is −80 mV. Synaptic responses in the NG2 cell appear with a brief latency following electrical stimulation, steeply increase to a sharp peak and then rapidly decay back to baseline (a). In a premyelinating oligodendrocyte, synchronous responses briefly after the stimulus are absent; however, there are individual release events appearing late after the stimulation and continuing for hundreds of milliseconds (b). Scale bars: 10 ms, 10 pA. (c,d) Histograms summarize the latency of synaptic currents recorded in three NG2 cells (150 events) and in five premyelinating oligodendrocytes (358 events). The cumulative peak amplitude was calculated for each latency by summing current amplitudes of all events falling into the same latency bin (bin width is 3.75 ms). The asterisks mark the time point of stimulation. (e,f) Whole-cell current responses of an NG2 cell (e) and a premyelinating oligodendrocyte (f) to glutamate un-caging, in the absence (left) or presence (right) of AMPA/kainate receptor antagonist CNQX (30 μm). The holding potential is −80 mV. The current amplitude is smaller in the premyelinating cells compared with NG2 cells. Pre-application of CNQX strongly reduces current response. Scale bar: 20 ms, 20 pA. (A–F) Modified with permission from Kukley et al. (2010).
Notably, recent experimental evidence suggests that also during the re-myelination process in mouse, corpus callosum axonal glutamatergic synapses contact NG2-positive cells (Etxeberria et al. 2010). Similar to the situation in the healthy brain, synaptic connectivity appears to be lost during differentiation of NG2 cells into mature oligodendrocytes (Etxeberria et al. 2010). The authors suggest that reduction in glutamatergic activity may be necessary for NG2 cells to differentiate into oligodendrocytes, as predicted by the inhibitory influence of glutamate on the differentiation of OPCs (Gallo et al. 1996; Yuan et al. 1998).
Is the loss of synaptic signalling accompanied by changes in surface glutamate receptors during differentiation of NG2 cells? One way to address this question is to investigate the current response of oligodendrocyte lineage cells to the application of glutamate. Recent evidence demonstrates that upon fast application of glutamate by mean of fast photolysis of glutamate (un-caging), NG2 cells and premyelinating oligodendrocytes show clear responses that are prevented by AMPA/kainate receptor antagonists, however, the amplitude of those responses is ∼ two–three times smaller in the premyelinating cells compared with NG2 cells (Fig. 4e,f; De Biase et al. 2010; Kukley et al. 2010). Interestingly, the amplitude of AMPA/kainate receptor-mediated current decreases further as premyelinating cells differentiate into mature oligodendrocytes (De Biase et al. 2010; Kukley et al. 2010).
AMPA receptors are not expressed uniformly over the surface of NG2 cells, but rather are clustered into discrete patches along their processes (Bergles et al. 2010). Recently freeze-fracture immunolabelling of brain tissue from transgenic mice in which NG2 cells can be clearly identified through labelling with anti-GFP antibodies has been performed (Bergles et al. 2010). Preliminary results of these experiments showed clusters of AMPA receptor-immunoreactive intra-membrane particles along NG2 cells processes (Bergles et al. 2010). Also, functional mapping of AMPA receptors using two-photon un-caging of MNI-L-glutamate has shown the presence of ‘hot spots’ along NG2 cell processes where AMPA receptors are enriched (Bergles et al. 2010). These ‘hot spots’ of AMPA receptors may represent receptors that are accumulated to form functional microdomains opposite the presynaptic glutamatergic terminal boutons that release neurotransmitters. Synaptic clustering of glial neurotransmitter receptors into microdomains could, in analogy to neurons, be crucial for efficient signal transduction. The NG2 proteoglycan has been suggested to play an important role in clustering the glial AMPA receptors towards the site of neuronal glutamate release (Stegmuller et al. 2003; Trotter et al. 2010). NG2 proteoglycan is indirectly associated with the AMPA receptors of NG2-expressing cells as a result of molecular linkage with glutamate receptor interaction protein via a PDZ domain-mediated interaction (Stegmuller et al. 2003). Interestingly, the NG2 proteoglycan is expressed by OPCs but downregulated upon differentiation of the cells into premyelinating oligodendrocytes, which also show lower surface expression of AMPA receptors (Levine et al. 1993; Dawson et al. 2000). Thus, it is possible that downregulation of NG2 proteoglycan contributes to a disruption of postsynaptic AMPA receptor clusters and subsequent removal of AMPA receptors from the postsynaptic glial membrane.
Loss of synaptic signalling and decrease in the number of AMPA receptors upon differentiation of NG2 cells is accompanied by a downregulation of voltage-gated sodium and potassium channels, as demonstrated by electrophysiological experiments (De Biase et al. 2010; Kukley et al. 2010). In accordance with the physiological studies, mRNAs encoding most principal CNS NaV channel subunits (Scn1a, Scn2a1, Scn3a) are significantly lower in mature oligodendrocytes compared with NG2 cells (De Biase et al. 2010).
In summary, as NG2 cells differentiate down an oligodendrocyte lineage dramatic changes in synaptic input occur with the altered expression pattern of neurotransmitter receptors and voltage-gated ion channels on NG2 cells; those changes reflect a rapid decrease in the expression of ion channel and AMPA receptor genes.
Conclusions
NG2 cells are widespread in the grey and white matter of the CNS and, depending on the region they comprise, 2–9% of the total cell population (Dawson et al. 2003). These cells are intriguing because, on one hand they exhibit properties of immature progenitor cells, while on the other hand they show features of differentiated cells that can perhaps perform some essential physiological role on their own. NG2 cells are considered precursor cells because they are able to divide and migrate; during development many NG2 cells evolve into the myelinating oligodendrocytes. Unlike precursor cells, however, NG2 cells show complex ramified morphology, express different types of voltage-gated ion channels, neurotransmitter receptors and receive synaptic input from neurons. The fact that NG2 cells keep cellular processes and synaptic junctions with neurons during cytokinesis and pass them onto the daughter cells indicates that directly from birth NG2 cells are integrated into the complex neuronal circuitry of the CNS. Upon differentiation NG2 cells loose synapses with neurons, so neuronal input in the form of a functional synapse is most likely important exclusively for NG2 cells and not for more mature cells of the oligodendrocyte lineage. The functional role of synaptic input onto NG2 cells remains to be discovered. This is especially exciting if we consider that the total number of NG2 cells within the entire cell population (Dawson et al. 2003) roughly equals the entire number of neurons in the adult brain (2–10% of all cells), and that the total length of processes of an NG2 cell (about 1250 μm) is not much smaller than the total length of the dendritic tree of some neurons (Benavides-Piccione et al. 2006). Those facts indicate that, if the probability of establishing a neuron–neuron or a neuron–glia synapse is the same, the overall number of synaptic contacts that a given neuron receives from other neurons might be only slightly larger than the amount of synapses that a given NG2 glial cell receives from neurons (at least in some brain areas) and, thus, point to the importance of neuron–glia synapses in the brain. Synaptic input from neurons onto NG2 cells also raises the question whether NG2 cells possess any output signalling, for example release neurotransmitters and/or other molecules that may influence behaviour of neighbouring cells or intercellular communication.
Acknowledgments
We thank Vittorio Gallo (Center for Neuroscience Research, Children's National Medical Center, Washington, USA), Akiko Nishiyama (Department of Physiology and Neurobiology, University of Connecticut, Storrs, USA) and Charles J. Gilbride (Centre for Integrative Neuroscience, University of Tübingen, Germany) for their helpful discussions during the writing of this manuscript. The authors are supported by the Centre for Integrative Neuroscience (Deutsche Forschungsgemeinschaft, EXC 307). NF is supported by the Christiane Nüsslein-Volhard Foundation.
References
- Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10 530–10 541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre AA, Chittajallu R, Belachew S, et al. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Barres BA, Koroshetz WJ, Swartz KJ, et al. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron. 1990;4:507–524. doi: 10.1016/0896-6273(90)90109-s. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, et al. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides-Piccione R, Hamzei-Sichani F, Ballesteros-Yanez I, et al. Dendritic size of pyramidal neurons differs among mouse cortical regions. Cereb Cortex. 2006;16:990–1001. doi: 10.1093/cercor/bhj041. [DOI] [PubMed] [Google Scholar]
- Berger T. AMPA-type glutamate receptors in glial precursor cells of the rat corpus callosum: ionic and pharmacological properties. Glia. 1995;14:101–114. doi: 10.1002/glia.440140205. [DOI] [PubMed] [Google Scholar]
- Berger T, Walz W, Schnitzer J, et al. GABA- and glutamate-activated currents in glial cells of the mouse corpus callosum slice. J Neurosci Res. 1992;31:21–27. doi: 10.1002/jnr.490310104. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, et al. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jabs R, Steinhauser C. Neuron–glia synapses in the brain. Brain Res Rev. 2010;63:130–137. doi: 10.1016/j.brainresrev.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M, Hubbard P, Butt AM. Cytology and lineage of NG2-positive glia. J Neurocytol. 2002;31:457–467. doi: 10.1023/a:1025735513560. [DOI] [PubMed] [Google Scholar]
- Boucsein C, Zacharias R, Farber K, et al. Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur J Neurosci. 2003;17:2267–2276. doi: 10.1046/j.1460-9568.2003.02663.x. [DOI] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Hornby MF, et al. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- Butt AM, Pugh M, Hubbard P, et al. Functions of optic nerve glia: axoglial signalling in physiology and pathology. Eye. 2004;18:1110–1121. doi: 10.1038/sj.eye.6701595. [DOI] [PubMed] [Google Scholar]
- Chatterjee N, Stegmuller J, Schatzle P, et al. Interaction of syntenin-1 and the NG2 proteoglycan in migratory oligodendrocyte precursor cells. J Biol Chem. 2008;283:8310–8317. doi: 10.1074/jbc.M706074200. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Chen Y, Wang H, et al. Regulation of Kv1 subunit expression in oligodendrocyte progenitor cells and their role in G1/S phase progression of the cell cycle. Proc Natl Acad Sci USA. 2002;99:2350–2355. doi: 10.1073/pnas.042698399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, et al. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, et al. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase LM, Nishiyama A, Bergles DE. Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci. 2010;30:3600–3611. doi: 10.1523/JNEUROSCI.6000-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, et al. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10 434–10 442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, et al. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Dowling JE. The Retina: an Approachable Part of the Brain. Cambridge, Mass: Belknap Press of Harvard University Press; 1987. [Google Scholar]
- Eaton BA, Davis GW. Synapse disassembly. Genes Dev. 2003;17:2075–2082. doi: 10.1101/gad.1113703. [DOI] [PubMed] [Google Scholar]
- Etxeberria A, Mangin JM, Aguirre A, et al. Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat Neurosci. 2010;13:287–289. doi: 10.1038/nn.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, et al. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, et al. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Mangin JM, Kukley M, et al. Synapses on NG2-expressing progenitors in the brain: multiple functions? J Physiol. 2008;586:3767–3781. doi: 10.1113/jphysiol.2008.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WP, Zhou W, Luo Q, et al. Dividing glial cells maintain differentiated properties including complex morphology and functional synapses. Proc Natl Acad Sci USA. 2009;106:328–333. doi: 10.1073/pnas.0811353106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha S, Pallud J, Junier MP, et al. NG2+/Olig2+ cells are the major cycle-related cell population of the adult human normal brain. Brain Pathol. 2010;20:399–411. doi: 10.1111/j.1750-3639.2009.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Yuan X, Eisen AM, et al. Voltage-activated K+ channels and membrane depolarization regulate accumulation of the cyclin-dependent kinase inhibitors p27(Kip1) and p21(CIP1) in glial progenitor cells. J Neurosci. 1999;19:5380–5392. doi: 10.1523/JNEUROSCI.19-13-05380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P, Kettenmann H, Schachner M. Gamma-aminobutyric acid directly depolarizes cultured oligodendrocytes. J Neurosci. 1984;4:561–569. doi: 10.1523/JNEUROSCI.04-02-00561.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y, Davis GW. Mechanisms of synapse assembly and disassembly. Neuron. 2003;40:243–264. doi: 10.1016/s0896-6273(03)00608-1. [DOI] [PubMed] [Google Scholar]
- Guo F, Ma J, McCauley E, et al. Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci. 2009;29:7256–7270. doi: 10.1523/JNEUROSCI.5653-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ma J, et al. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci. 2010;30:12 036–12 049. doi: 10.1523/JNEUROSCI.1360-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton N, Vayro S, Wigley R, et al. Axons and astrocytes release ATP and glutamate to evoke calcium signals in NG2-glia. Glia. 2010;58:66–79. doi: 10.1002/glia.20902. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Heinemann C, Neher E, et al. Calcium dependence of the rate of exocytosis in a synaptic terminal. Nature. 1994;371:513–515. doi: 10.1038/371513a0. [DOI] [PubMed] [Google Scholar]
- Hirbec H, Perestenko O, Nishimune A, et al. The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes: analysis of PDZ binding motifs. J Biol Chem. 2002;277:15 221–15 224. doi: 10.1074/jbc.C200112200. [DOI] [PubMed] [Google Scholar]
- Hirbec H, Martin S, Henley JM. Syntenin is involved in the developmental regulation of neuronal membrane architecture. Mol Cell Neurosci. 2005;28:737–746. doi: 10.1016/j.mcn.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Thallmair M, Gage FH. Defining the NG2-expressing cell of the adult CNS. J Neurocytol. 2002;31:469–480. doi: 10.1023/a:1025739630398. [DOI] [PubMed] [Google Scholar]
- Jabs R, Pivneva T, Huttmann K, et al. Synaptic transmission onto hippocampal glial cells with hGFAP promoter activity. J Cell Sci. 2005;118:3791–3803. doi: 10.1242/jcs.02515. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. New York: McGraw-Hill; 2000. [Google Scholar]
- Kang SH, Fukaya M, Yang JK, et al. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Attwell D. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience. 2007;145:1426–1438. doi: 10.1016/j.neuroscience.2006.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, et al. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, et al. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, et al. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Gilbert P, Schachner M. Depolarization of cultured oligodendrocytes by glutamate and GABA. Neurosci Lett. 1984;47:271–276. doi: 10.1016/0304-3940(84)90525-1. [DOI] [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, et al. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci. 2006;9:1506–1511. doi: 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- Knutson P, Ghiani CA, Zhou JM, et al. K+ channel expression and cell proliferation are regulated by intracellular sodium and membrane depolarization in oligodendrocyte progenitor cells. J Neurosci. 1997;17:2669–2682. doi: 10.1523/JNEUROSCI.17-08-02669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Kukley M, Kiladze M, Tognatta R, et al. Glial cells are born with synapses. FASEB J. 2008;22:2957–2969. doi: 10.1096/fj.07-090985. [DOI] [PubMed] [Google Scholar]
- Kukley M, Nishiyama A, Dietrich D. The fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J Neurosci. 2010;30:8320–8331. doi: 10.1523/JNEUROSCI.0854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiene J, Matsui A, Sawa Y, et al. Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell. 2009;8:201–213. doi: 10.1111/j.1474-9726.2009.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Card JP. Light and electron microscopic localization of a cell surface antigen (NG2) in the rat cerebellum: association with smooth protoplasmic astrocytes. J Neurosci. 1987;7:2711–2720. doi: 10.1523/JNEUROSCI.07-09-02711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stincone F, Lee YS. Development and differentiation of glial precursor cells in the rat cerebellum. Glia. 1993;7:307–321. doi: 10.1002/glia.440070406. [DOI] [PubMed] [Google Scholar]
- Levison SW, Young GM, Goldman JE. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57:435–446. [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Physiological characteristics of NG2-expressing glial cells. J Neurocytol. 2002;31:537–549. doi: 10.1023/a:1025799816285. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004a;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between neurons and glia. Glia. 2004b;47:290–298. doi: 10.1002/glia.20060. [DOI] [PubMed] [Google Scholar]
- Lin SC, Huck JH, Roberts JD, et al. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46:773–785. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Maldonado PP, Velez-Fort M, Angulo MC. Is neuronal communication with NG2 cells synaptic or extrasynaptic? J Anat. 2011 doi: 10.1111/j.1469-7580.2011.01350.x. doi: 10.1111/j.1469-7580.2011.01350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Wakabayashi T, Takamori Y, et al. Phenotype analysis and quantification of proliferating cells in the cortical gray matter of the adult rat. Acta Histochem Cytochem. 2009;42:1–8. doi: 10.1267/ahc.08037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Reyes-Haro D, Pivneva T, et al. The principal neurons of the medial nucleus of the trapezoid body and NG2(+) glial cells receive coordinated excitatory synaptic input. J Gen Physiol. 2009;134:115–127. doi: 10.1085/jgp.200910194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, et al. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yu M, Drazba JA, et al. Normal and reactive NG2+ glial cells are distinct from resting and activated microglia. J Neurosci Res. 1997;48:299–312. doi: 10.1002/(sici)1097-4547(19970515)48:4<299::aid-jnr2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Watanabe M, Yang Z, et al. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Yang Z, Butt A. Astrocytes and NG2-glia: what's in a name? J Anat. 2005;207:687–693. doi: 10.1111/j.1469-7580.2005.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, et al. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, et al. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Panagiotakos G, Alshamy G, Chan B, et al. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS ONE. 2007;2:e588. doi: 10.1371/journal.pone.0000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Sensory and cortical activation of distinct glial cell subtypes in the somatosensory thalamus of young rats. Eur J Neurosci. 2010;32:29–40. doi: 10.1111/j.1460-9568.2010.07281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005;207:707–716. doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psachoulia K, Jamen F, Young KM, et al. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5:57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raastad M, Storm JF, Andersen P. Putative single quantum and single fibre excitatory postsynaptic currents show similar amplitude range and variability in rat hippocampal slices. Eur J Neurosci. 1992;4:113–117. doi: 10.1111/j.1460-9568.1992.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Reimann F, Ashcroft FM. Inwardly rectifying potassium channels. Curr Opin Cell Biol. 1999;11:503–508. doi: 10.1016/S0955-0674(99)80073-8. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol. 2010;2:a001842. doi: 10.1101/cshperspect.a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- Squire LR, Bloom FE, McConnell SK, et al. Fundamental Neuroscience. USA: Academic Press; 2003. [Google Scholar]
- Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmuller J, Werner H, Nave KA, et al. The proteoglycan NG2 is complexed with alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by the PDZ glutamate receptor interaction protein (GRIP) in glial progenitor cells: implications for glial-neuronal signaling. J Biol Chem. 2003;278:3590–3598. doi: 10.1074/jbc.M210010200. [DOI] [PubMed] [Google Scholar]
- Steinhauser C, Jabs R, Kettenmann H. Properties of GABA and glutamate responses in identified glial cells of the mouse hippocampal slice. Hippocampus. 1994;4:19–35. doi: 10.1002/hipo.450040105. [DOI] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, et al. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Kataoka Y, Cui Y, et al. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur J Neurosci. 2007;25:3489–3498. doi: 10.1111/j.1460-9568.2007.05617.x. [DOI] [PubMed] [Google Scholar]
- Tekki-Kessaris N, Woodruff R, Hall AC, et al. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 2001;128:2545–2554. doi: 10.1242/dev.128.13.2545. [DOI] [PubMed] [Google Scholar]
- Tong XP, Li XY, Zhou B, et al. Ca(2+) signaling evoked by activation of Na(+) channels and Na(+)/Ca(2+) exchangers is required for GABA-induced NG2 cell migration. J Cell Biol. 2009;186:113–128. doi: 10.1083/jcb.200811071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Nishiyama A, Cheng D, et al. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137:459–468. doi: 10.1083/jcb.137.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J, Karram K, Nishiyama A. NG2 cells: properties, progeny and origin. Brain Res Rev. 2010;63:72–82. doi: 10.1016/j.brainresrev.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau LE, Doyle RT, Emery DG, et al. Calcium-independent activation of the secretory apparatus by ruthenium red in hippocampal neurons: a new tool to assess modulation of presynaptic function. J Neurosci. 1996;16:46–54. doi: 10.1523/JNEUROSCI.16-01-00046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usowicz MM, Gallo V, Cull-Candy SG. Multiple conductance channels in type-2 cerebellar astrocytes activated by excitatory amino acids. Nature. 1989;339:380–383. doi: 10.1038/339380a0. [DOI] [PubMed] [Google Scholar]
- Vautier F, Belachew S, Chittajallu R, et al. Shaker-type potassium channel subunits differentially control oligodendrocyte progenitor proliferation. Glia. 2004;48:337–345. doi: 10.1002/glia.20088. [DOI] [PubMed] [Google Scholar]
- Velez-Fort M, Audinat E, Angulo MC. Functional alpha 7-containing nicotinic receptors of NG2-expressing cells in the hippocampus. Glia. 2009;57:1104–1114. doi: 10.1002/glia.20834. [DOI] [PubMed] [Google Scholar]
- Velez-Fort M, Maldonado PP, Butt AM, et al. Postnatal switch from synaptic to extrasynaptic transmission between interneurons and NG2 cells. J Neurosci. 2010;30:6921–6929. doi: 10.1523/JNEUROSCI.0238-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigley R, Butt AM. Integration of NG2-glia (synantocytes) into the neuroglial network. Neuron Glia Biol. 2009;5:21–28. doi: 10.1017/S1740925X09990329. [DOI] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Probing fundamental aspects of synaptic transmission with strontium. J Neurosci. 2000;20:4414–4422. doi: 10.1523/JNEUROSCI.20-12-04414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Eisen AM, McBain CJ, et al. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125:2901–2914. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]
- Zhou M, Schools GP, Kimelberg HK. Development of GLAST(+) astrocytes and NG2(+) glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol. 2006;95:134–143. doi: 10.1152/jn.00570.2005. [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008a;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol. 2008b;4:19–26. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Dietrich D, et al. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, et al. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]


