Abstract
Astrocytes are one of the major glial cell types that maintain homeostasis in the undamaged CNS. After injury and disease, astrocytes become reactive and prevent regeneration; however, it has also been suggested that astrocytes can become activated and promote regeneration. Thus, it is hypothesised that astrocytes have an important role in modulating CNS repair. This review will focus on the variable phenotypic state of astrocytes that range from inactive/quiescent to reactive, and relate these to their ability to influence myelination. Using myelinating cultures plated on astrocytes we propose a possible mechanism for oligodendrocyte precursor cell interaction with the axon, leading to myelination. The phenotypic status of astrocytes is an intriguing and widely discussed issue, which is critical for understanding the mechanisms involved in CNS injury and its subsequent repair.
Keywords: astrocytes, myelination, phenotypes
Introduction
Astrocytes and myelination
Astrocytes are the most abundant glial cells in the CNS defined by their stellate morphology and expression of glial fibrillary acidic protein (GFAP; Eng et al. 1971). They have been termed ‘support cells’, and in the normal adult brain are thought to have a relatively passive role in the CNS. However, astrocytes are emerging as key players in many aspects of CNS diseases and injury. Their diverse roles extend from energy metabolism and neurotransmitter homeostasis, myelination and axonal outgrowth to maintenance of the blood–brain barrier (BBB) and synaptogenesis (Ullian et al. 2001; Liberto et al. 2004; Silver & Miller, 2004; Pellerin, 2005; Nair et al. 2008; Sorensen et al. 2008; Watkins et al. 2008). There have been many reviews that have described the broad range of biological properties attributed to astrocytes (Eddleston & Mucke, 1993; Liberto et al. 2004; Williams et al. 2007; Sofroniew & Vinters, 2010), but for the purpose of this review we will focus on the variable phenotypic ‘state’ of the astrocyte and relate it to its ability to affect myelination. We have used the term phenotype to represent the various properties of astrocytes that change after injury, and includes their morphological, antigenic and physiological characteristics. The phenotypic status of astrocytes has been an intriguing and widely discussed issue as they appear to play an important role in the pathology of neurological diseases.
Myelination is a complex process by which an axon becomes insulated by the continuous wrapping of the proteolipid oligodendroglial membrane known as myelin. The mechanism by which an oligodendrocyte enwraps myelin around an axon has still not been elucidated. It has been suggested that at least four important stages occur during the process of myelination, including: (i) the selection of axons by the oligodendrocyte process and the initiation of cell–cell interaction between them; (ii) the establishment of stable intercellular contacts and assembly of the nodes of Ranvier; (iii) regulation of myelin thickness; and (iv) longitudinal extension of myelin segments in response to the lengthening of axons during postnatal growth (taken from a review by Sherman & Brophy, 2005).
Since the seminal discovery by Geren & Raskind (1953) that PNS myelin is formed by the Schwann cell plasma membrane wrapping around the axon, several theories have been proposed to explain how this membrane wrap evolves into a perfect and, thought to be, almost crystalline structure. Myelination in the CNS and PNS is dissimilar not only because different cell types carry out the process, but also from research that has shown that the regulatory factors are quite diverse. In the CNS, the oligodendrocyte extends multiple processes that contact and wrap around several axons to form the myelin sheath. In contrast, in the PNS Schwann cells contact a single axon, making a one-one relationship with the characteristic signet ring appearance of peripheral myelin (Bunge & Wood, 2006). Recently, it has been shown that the thickness of the myelin sheath generated by a Schwann cell is regulated by neuregulin 1 type III (Michailov et al. 2004; Taveggia et al. 2005), but to date no single molecule has been shown to have such an instructive role in the CNS. For CNS myelination, as one cell wraps several axons it has been assumed that each process wraps around the axons while the cell body remains stationary (Bauer et al. 2009). In fact, two models have been proposed on how an oligodendrocyte wraps an axon with myelin (Pedraza et al. 2001; Bauer et al. 2009). This has been depicted nicely in a review by ffrench-Constant and colleagues (Bauer et al. 2009). One model involves a spiral of the oligodendrocyte process wrapping around the axon, which then extends laterally into overlapping sheets, while the second model involves the myelin wrapping around the axon in a sheet-like manner forming an initial wrap, which then extends by longitudinal movement underneath the sheet that continues to extend.
We have developed cultures in which the many stages of myelination can be followed over time. These myelinating cultures involve plating dissociated rat spinal cord cells onto a confluent monolayer of neurosphere-derived astrocytes (Sorensen et al. 2008). The cultures develop over 26–28 days, and from Day 1 to 12 allow the study of neuronal survival and neurite outgrowth, followed by oligodendrocyte precursor proliferation and extension of their processes to axons (Days 13–18), which progresses to ensheathment of axons and formation of myelin internodes and nodes of Ranvier (Days 22–28). Although this work was carried out using neurosphere-derived astrocytes, myelination was also seen on a monolayer of cortical astrocytes (Sorensen et al. 2008), suggesting there was not a great deal of difference in the biological properties of the astrocytes despite the difference in their origin. However, it is possible that P1 neurosphere-derived astrocytes may have different properties from astrocytes derived from P1 cortices, as neurosphere-derived astrocytes are generated by differentiation from neural stem cells. Images summarising the several stages of myelination in these cultures can be seen in Fig. 1. Our data suggest that oligodendrocytes appear to wrap a fine process around an axon, which gradually extends out along the axon rather like that described for model one (Bauer et al. 2009; Fig. 2).
Fig. 1.
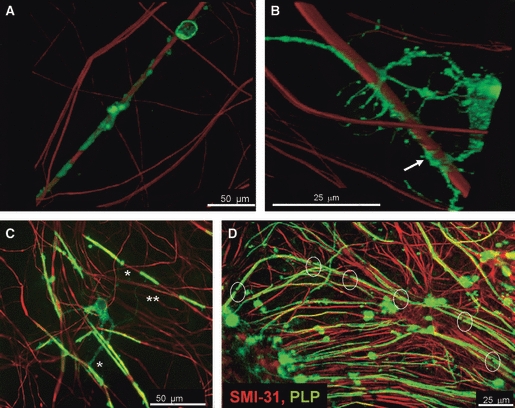
Stages of myelination visualised from the myelinating cultures immunolabelled with SMI-31 to depict neurites (red) and PLP (green) for mature myelin. Stages of myelination in vitro can be followed in the cultures. (A) At early stages of myelination, OPCs wrap a myelin sheath as a spiral around the axons (arrow). Image taken after 18 days in culture. (B) The myelin sheath begins to fill in around from the initial spirals around the axon. Image taken at 18 days in culture. (C) Oligodendrocytes can make many internodes of myelin often from primary processes (*), but sometimes from secondary (**). (D) The myelinating cultures form many nodes of Ranvier. Image taken at 22–28 days in culture.
Fig. 2.
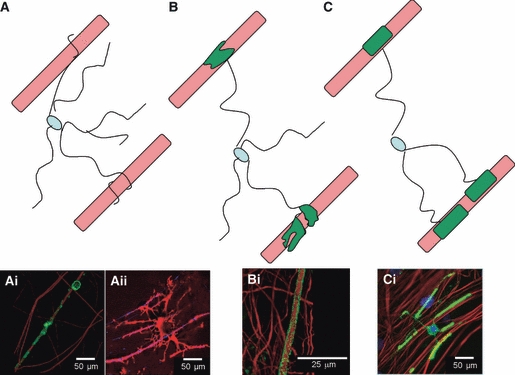
Schematic for a mechanism for myelination based on images obtained from the myelinating cultures. (A) The glial cell puts out many processes and spirals a sheath around the axon. (B) The spiral of myelin fills forming a larger expanse of membrane. (C) Eventually this fills out to form myelin internodes. (Ai) OPCs wrapping a spiral of myelin around the axon. (Aii) An example of an oligodendrocyte extending multiple processes towards axons. (Bi) The myelin from the membrane spiral expands out along the axon. (Ci) Oligodendrocytes form internodes of myelin separated by nodal spacing over several axons. Myelinating cultures were immunolabelled with SMI-31 to depict neurites (red) and PLP (green) for mature myelin.
Using these myelinating cultures, we have shown that astrocytes have a direct role in promoting myelination (Sorensen et al. 2008). In fact, myelination was poor on monolayers of other supporting glia, such as olfactory ensheathing cells or Schwann cells, suggesting that astrocytes promote myelination either by releasing a soluble factor or by cell–cell contact. More recently, we have shown that the astrocyte phenotype can influence the ability of oligodendrocytes to myelinate axons. Astrocytes can exhibit a continuum of phenotypes ranging from quiescent/resting to reactive (Liberto et al. 2004; Williams et al. 2007; Sofroniew & Vinters, 2010). Myelination was poor when the myelinating cultures were plated on quiescent astrocytes, but enhanced when they were plated on a more reactive/activated phenotype (Nash et al. in press). Others have also shown that astrocytes can modulate myelination. Indeed, in 1904 it was proposed that hypertrophic/reactive astrocytes were a pathological feature of multiple sclerosis (MS), and it was hypothesised that they had an important role in the disease (Müller, 1904). A similar hypothesis was raised by Wu & Raine (1992) from studies of human MS lesions. In this study the authors demonstrated a correlation between hypertrophic astrocytes and MS pathology (Wu & Raine, 1992). Several other reviews have discussed the role of astrocytes in MS (Lassmann, 2005; Williams et al. 2007; Nair et al. 2008; Sofroniew & Vinters, 2010), and have detailed the importance of astrocyte-secreted factors that either promote or impede myelination (Moore et al. 2011). It has also been shown from immunohistochemical studies of the developing rat spinal cord that a temporary increase in GFAP-positive cells correlated with an increase in immunoreactivity with the late myelin marker, myelin basic protein, suggesting that this increase of GFAP-positive cells accompanying myelination is necessary for normal myelin development (Dziewulska et al. 1999). Other in vitro studies have confirmed the role of astrocytes in myelination. For example, Barres and colleagues demonstrated that astrocytes secrete factors that influence the rate of myelin ensheathment in vitro (Watkins et al. 2008). This highlights the importance astrocytes have on influencing myelination, and the use of such cultures may provide a way to define the differing phenotypes of astrocytes.
Astrocytes and myelination in the normal brain
In the normal CNS, astrocytes are often described as resting or quiescent (Eddleston & Mucke, 1993; Holley et al. 2005), but it is more likely that they are actively regulating normal brain function. For example, they express a wide range of receptors, making them responsive to many growth factors and neurotransmitters, as well as development and regulation of neuronal synaptic plasticity and function (for review, see Williams et al. 2007; Sofroniew & Vinters, 2010). In response to damage, astrocytes within the CNS tissue acquire a change in phenotype known as astrocytosis, where their morphology, size and secretory profile changes from that of a resting/quiescent astrocyte to one that is reactive, also known as anisomorphic astrocytosis. It has also been suggested that there is an intermediate phenotype in which the astrocytes are activated, known as isomorphic astrocytosis (Liberto et al. 2004). These categories are not completely exclusive, but instead show a graded progression from one to the other, with possible phenotypes in between (Sofroniew & Vinters, 2010; Fig. 3). In this review we aim to further elucidate the various astrocyte phenotypes, with emphasis on those that appear to play a role in influencing myelination.
Fig. 3.
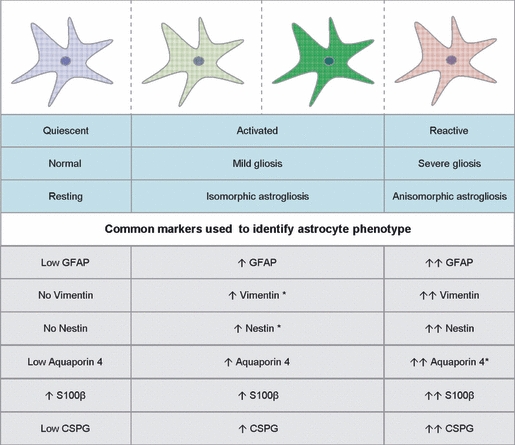
Summary of astrocyte phenotypes and markers used to identify them. The astrocyte can be termed quiescent in normal adult resting CNS tissue. They can become activated by various mechanisms that result in mild/isomorphic astrogliosis, with a mild increase in cytoskeleton markers. Activated astrocytes are also more distal to the injury site. The most severe phenotype is the reactive/anisomorphic astrocyte that forms at the gliotic scar. This phenotype is characterised by a high increase in cytoskeletal molecules (Ridet et al. 1997; Fawcett & Asher, 1999,Liberto et al. 2004; East et al. 2009; Sofroniew & Vinters, 2010). Asterisks represent our predicted information on expression of these markers. Arrows indicate direction of expression and intensity. CSPG, chondroitin sulphate proteoglycans; GFAP, glial fibrillary acidic protein.
A key indication for the role of astrocytes in myelination comes from a series of astrocyte mutations. One such mutation in GFAP, which is upregulated when the astrocyte becomes activated, results in a loss of integrity of CNS white matter architecture and long-term maintenance of myelination (Liedtke et al. 1996). Furthermore, there is a strong association of GFAP mutations with Alexander disease (Brenner et al. 2001; Li et al. 2002), a neurodegenerative disease classified as a leukodystrophy with loss of myelin, oligodendrocytes and neuronal degeneration (Alexander, 1949). Mutations in eukaryotic translation initiation factor 2B, EIF2B5, cause childhood ataxia with CNS hypomyelination, also known as vanishing white matter disease (VWM). This mutation was found in neural progenitor cells, preventing their differentiation into astrocytes, suggesting that a lack of astrocytic development in the brain may contribute to VWM (Dietrich et al. 2005). Additional evidence that astrocytes are important for CNS repair came from elegant transgenic mice experiments in which dividing, and therefore reactive, astrocytes were selectively ablated after induction of spinal cord injury. Resulting injury was far worse in groups where astrocytes were removed with a failure of BBB repair, leukocyte infiltration, local tissue disruption, severe demyelination, neuronal and oligodendrocyte death, and pronounced motor function loss (Faulkner et al. 2004).
Experimental models of demyelination have also been used to demonstrate an important role for astrocytes in the subsequent remyelination. These use the glial toxin ethidium bromide to create a focal area of demyelination in the CNS (Graça & Blakemore, 1986). It was shown that oligodendrocytes favour the remyelination of axons in areas where astrocytes were localised (Blakemore, 1984; Talbot et al. 2005). Likewise, transplantation of astrocytes into these experimentally created demyelinated lesions enhanced remyelination by host oligodendrocytes (Franklin et al. 1991). The observation that endogenous oligodendrocyte precursor cells (OPCs) cannot myelinate demyelinated axons in areas devoid of astrocytes suggests that remyelination failure was due to either: (i) the dominant influence of inhibitory signals in astrocyte-free regions preventing terminal differentiation of OPCs; or (ii) the absence of astrocyte-derived signals enhancing terminal differentiation of OPCs (Talbot et al. 2005). It is possible that both types of signals compete for promotion of myelination.
It has been known for many years that astrocytes secrete promyelinating factors that directly affect the oligodendrocyte lineage. In fact, astrocyte conditioned medium was often used as an OPC mitogen (Noble & Murray, 1984), and recently it was shown that the presence of astrocytes allowed faster and more thorough ensheathment of axons by oligodendrocytes (Watkins et al. 2008). Although the above studies do not specify the mechanisms by which astrocytes affect myelination, it is clear that they do have a major influence on oligodendrocyte function. A link has also been demonstrated between astrocytes, myelination and electrical activity in axons (Ishibashi et al. 2006). In this study it was shown that astrocytes secrete leukaemia inhibitory factor (LIF) in response to ATP being liberated from axons firing, which in turn increased the number of myelinated fibres. Thus, LIF secretion by electrically stimulated astrocytes results in enhanced oligodendrocyte differentiation. Therefore, secreted factors by astrocytes help create an accommodating and permissive environment for oligodendrocytes to mature into successful myelin-producing cells. The evidence above suggests that any change in the secretory profile of astrocytes may subsequently have an effect on the cells within the surrounding environment, including a direct functional effect on oligodendrocytes.
Changes in astrocytic phenotype following homeostatic disturbance and injury
The glial reaction is a hallmark of many CNS pathologies, including MS, spinal cord injury and Alzheimer's disease (Eng, 1985; Ridet et al. 1997; Nair et al. 2008; Verkhratsky & Parpura, 2010). The glial reaction can range from mild to severe, and comprises mainly of an increase in structural molecules such as GFAP and vimentin, an increase in proliferation, a change in secretory and metabolic profile, and at the extreme leads to the formation of a scar. The remyelinating capacity of oligodendrocytes in the vicinity of reactive astrocytes appears to depend on the severity of this astrocytic response. The following sections describe the phenotypic changes that occur in astrocytes after injury.
Mild gliosis
Mild astrogliosis is also often described as isomorphic astrogliosis or astrocyte activation. This phenotype usually refers to astrocytes that have upregulated a relatively low expression of GFAP, show some degree of proliferation, and secrete anti-inflammatory cytokines (John et al. 2003; Liberto et al. 2004). There is currently a general view that mild astrogliosis is beneficial to axonal regeneration and remyelination in injured CNS tissue (White & Jakeman, 2008; Sofroniew & Vinters, 2010). Cytokine-treated astrocytes are thought to be activated and have previously been shown to promote angiogenesis, restore the BBB, neuronal survival and synaptogenesis (Krum & Rosenstein, 1998; Gozes et al. 1999; Marz et al. 1999; Blondel et al. 2000; Herx & Yong, 2001; Mason et al. 2001).
A great deal of research has been undertaken to identify the secretory profile of cytokine-treated astrocytes, with emphasis on their effect on OPC differentiation (John et al. 2003, 2005; Meeuwsen et al. 2003). For example, ciliary neurotrophic factor (CNTF) treatment of mouse spinal cord astrocytes leads to an increase in fibroblast growth factor (FGF)-2 mRNA (Albrecht et al. 2002). FGF-2 is a growth factor known to be secreted by astrocytes, whilst its receptor is on the oligodendrocyte (Woodward et al. 1992; Redwine et al. 1997). In a viral-induced model of spinal cord demyelination, FGF-2 mRNA peaked 30 days post-infection, the early remyelination stage of disease progression showing a correlation of FGF-2 expression and myelination (Messersmith et al. 2000). Other cytokines that have been reported to activate astrocytes include interleukin (IL)-1β, interferon-γ (IFNγ) and IL-11 (Meeuwsen et al. 2003; John et al. 2005). Direct injection of IL-1β into the mammalian brain results in an inflammatory response and reactive astrogliosis (Giulian et al. 1988), and application of exogenous IFNγ activates astrocytes in a corticectomy model of CNS injury (Yong et al. 1991). IL-11 has been shown to influence astrocyte differentiation and GFAP expression (Yanagisawa et al. 2000).
Mild astrogliosis usually occurs in areas where there is a much less disrupted environment or in areas distal from the injury site. The establishment of such astrocytes in these remote areas may be due to: (i) the release and widespread diffusion of cytokines from the injury site; (ii) migration of reactive astrocytes from the injured area to distant sites; (iii) neuronal degeneration at the site of injury leading to anterograde and retrograde fibre degeneration that affects the astrocyte at the projection territory; or (iv) direct astrocyte injury leading to reduced gap junction activity, which then propagated via the astrocyte syncytial network to distant astrocytes (Malhotra & Shintka, 2002). Thus, cytokine-activated distal astrocytes play a role in the re-establishment of the injured CNS. The presence of these astrocytes contributes to the regeneration capacity of the tissue, partly via the secretion of promyelinating factors.
Severe gliosis
Severe astrogliosis, also known as anisomorphic astrogliosis or astrocyte reactivity, results in a much higher increase in GFAP expression, elevated proliferation rates and secretion of pro-inflammatory cytokines (Fedoroff et al. 1983; Eddleston & Mucke, 1993; Daginakatte et al. 2008; Sofroniew & Vinters, 2010). This astrocytic phenotype is regularly associated with the glial scar, where astrocytes usually directly border an area of CNS damage. The environment is generally inhibitory to regeneration due to the presence of inflammatory cytokines, residual tissue damage products and immune cells, which is why the glial scar is often described as an impediment for axonal regeneration (Fawcett & Asher, 1999; Fitch & Silver, 2008). Reactive astrocytes also upregulate chondroitin sulphate proteoglycans, hyaluronic acid and tenascins, extracellular molecules that have been associated and are thought to play a part in the regeneration failure in demyelinated regions (Canning et al. 1996; Back et al. 2005; East et al. 2009; Filous et al. 2010).
The deleterious effects of secreted factors from reactive astrocytes have been well documented. The most striking example is a cytokine of the tumour necrosis factor (TNF) family. TNFα levels increase in experimental autoimmune encephalomyelitis (EAE; Villarroya et al. 1996) as well as in the cerebrospinal fluid of patients with MS (Tsukada et al. 1991). TNFα has been reported to induce myelin and oligodendrocyte damage in vitro (Selmaj & Raine, 1988), and its expression in MS plaques from patients is positively correlated with the extent of demyelination (Bitsch et al. 2000). Furthermore, it has been shown that the major target for TNFα is the maturation of oligodendrocytes (Cammer & Zhang, 1999), and thus supports evidence that OPCs are locally present in lesion sites but are unable to differentiate (Levine & Reynolds, 1999; Redwine & Armstrong, 1998). Reactive astrocytes have also been shown to secrete CXCL10 (IP-10; Ransohoff et al. 1993). This is a small molecule belonging to the CXC chemokine family, and shares its CXCR3 receptor with CXCL9/MIG and CXCL11/I-TAC. Interestingly, CXCL10 has been shown to be expressed by reactive astrocytes surrounding active MS lesions (Omari et al. 2005; Carter et al. 2007). Because oligodendrocytes express the CXCR3 receptor they may be the target for CXCL10. Furthermore, CXCL10 mRNA was shown to be significantly increased during peak disease and reduced during the recovery phases in animal models of MS, including myelin oligodendrocyte glycoprotein-induced EAE (Godiska et al. 1995; Glabinski et al. 1997; Fife et al. 2001). In an in vitro model of myelination, astrocytes with increased expression of CXCL10 failed to promote myelination, an observation that was reversed by the addition of CNTF, astrocyte conditioned media or the neutralisation of CXCL10 using antibodies (Nash et al. in press). Furthermore, it was demonstrated that the addition of CXCL10 protein to normal myelinating cultures leads to the reduction of myelinated axons within the culture. These data suggest that astrocyte-derived CXCL10 acts directly on oligodendrocyte maturation and axonal wrapping. It has been shown, however, that CXCL10−/− or CXCR3−/− mice provoked more severe clinical and histological symptoms and earlier onset of MS disease models compared with wild-type controls (Klein et al. 2004; Liu et al. 2006; Muller et al. 2007). Furthermore, in a viral model of MS, neutralising antibodies to CXCL10 lead to a reduction in disease progression and suppressed ongoing demyelination (Liu et al. 2001). This conflicting evidence for the effects of CXCL10 suggests possible multiple functions in CNS pathologies.
Generally, reactive astrocytes are regarded as a barrier for regeneration, partly via the secretion of factors that halt the survival and maturation of OPCs. Understanding the factors that lead to the creation of reactive astrocytes and its possible reversal may be of vast therapeutic potential for CNS diseases and injuries.
Environment and astrocytosis
The ability of astrocytes to present a graded response to the intensity of injury highlights the versatility and adaptability of these cells. Although severe astrogliosis is correlated with well-documented studies regarding them as an obstacle to regeneration, it must be noted that other cell types, such as microglia (Liberto et al. 2004), are much more responsive and can override any beneficial effects of the astrocytes. Apart from the vast array of growth factors and cytokines secreted by astrocytes, the physical barrier they form may be more protective than inhibitory. The evolutionary conservation of the glial scar (Larner et al. 1995) suggests that it must serve some kind of functional role, and its absence may be more detrimental if it was not present at all (Faulkner et al. 2004). Cytokine activation of astrocytes is clearly favourable for OPC proliferation, maturation and myelination. Understanding how cytokine-activated astrocytes influence oligodendrocyte differentiation could provide important clues for devising a therapy for demyelinating diseases.
The phenotypic state of astrocytes and their ability to support myelination depends on many factors, including the age of the astrocytes, the proximity to injury site and the state of activation (Fig. 4). It is known that the severity of CNS damage in neonatal animals is much less severe than that of adult animals (Barrett et al. 1984; Fujimoto et al. 2006; Filous et al. 2010), an observation that is likely to relate to the phenotype of the astrocyte. For example, astrocytes in neonatal animals may be more activated compared with the quiescent astrocyte in the adult. If astrocyte phenotype is considered as a spectrum of reactivity intensity, it can be argued that the closer the astrocyte is to being in an ‘activated’ state, the better it responds to injury and subsequently (re)myelination. Thus, the neonatal astrocyte is more equipped to deal with insults than that of an adult. This observation highlights why many researchers generate neural cultures from neonatal rather than adult tissue.
Fig. 4.
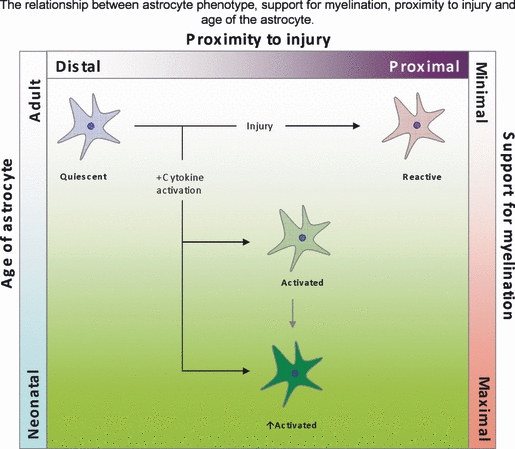
Summary of how an astrocyte phenotype, which is determined by age and location, can influence myelination. Astrocytes in close proximity to the site of injury are typically described as reactive and their support for myelination is poor. Astrocytes more distal to the site of injury receive ‘injury’ signal in the form of cytokines and increase their state of activation, ultimately increasing their support for oligodendrocytes via the secretion of trophic factors. Astrocytes in the neonatal animals respond better to injury possibly due to the reactivation state of the astrocytes, and are therefore more equipped to support myelination than in the adult. Green represents a more positive effect on myelination, while red represents a more negative effect on myelination.
Astrocytes in vitro are usually grown as a monolayer, which is very different to their 3D environment in tissue. In fact, the process of dissection and enzymatic dissociation of tissue for generation of cells in culture induces an activated state. Indeed, expression of the reactivity markers, GFAP, vimentin and aquaporin 4 were reduced in astrocytes grown on a 3D collagen gel system compared with astrocytes grown in a monolayer (East et al. 2009). Furthermore, astrocytes in vitro are usually exposed to foetal bovine serum, whereas those in the normal brain are not. Serum contains many factors, including bone morphogenetic protein-like factors that can induce astrocyte differentiation (Obayashi et al. 2009). Therefore, the artificial culture conditions to grow and maintain astrocytes may lead to a higher state of activation than at their tissue source. As astrocytes in culture have been shown to be supportive for myelination (Ishibashi et al. 2006; Sorensen et al. 2008; Watkins et al. 2008), it can be argued that the greater the level of activation, the more supportive an astrocyte is for myelination (Fig. 4).
It is generally accepted that astrocytes within the glial scar inhibit regeneration and can have negative effects on remyelination (Rudge & Silver, 1990; Silver & Miller, 2004). Conversely the emerging view is that astrocytes can also be essential for repair and subsequently myelination (Williams et al. 2007; White & Jakeman, 2008; Sofroniew & Vinters, 2010). However, it is thought that these astrocytes are generally at more distal sites and probably respond to molecules emerging from the damaged area (Ridet et al. 1997). Furthermore, astrocytes more distal to injury contribute to a great extent to regeneration via the secretion of growth factors and cytokines (Ridet et al. 1997; Williams et al. 2007; Nair et al. 2008; Sofroniew & Vinters, 2010). With the identification of more markers that define the various astrocyte phenotypes, it is hoped that consistency in the terminology and classification of astrocytes will be made and lead to a better understanding of their specific roles in CNS pathology.
Concluding remarks
Research into astrocyte phenotypes looks at a ‘snapshot’ of a very active and constantly changing environment that is highly dependent on environmental cues. It is important for future strategies in CNS repair to identify the molecular and cellular changes in these polarised astrocyte phenotypes. Furthermore, it should be acknowledged that the environment around CNS damage and demyelinated areas contain heterogeneous cell populations, and relatively small changes in this responsive milieu may have an impact on other areas within the CNS. Thus, results observed are very likely to be due to an indirect activation of complex cell–cell interactions. It is probable that the dynamic nature of astrocytes allows them to effectively respond to minute changes in the microenvironment mainly for the benefit of the host.
Acknowledgments
This work was funded by the Multiple Sclerosis Society of Great Britain (BN) and the MRC (KI). We thank Dr Jennifer Higginson and Dr Susan Lindsay for critical reading of the manuscript.
References
- Albrecht PJ, Dahl JP, Stoltzfus OK, et al. Ciliary neurotrophic factor activates spinal cord astrocytes, stimulating their production and release of fibroblast growth factor-2, to increase motor neuron survival. Exp Neurol. 2002;173:46–62. doi: 10.1006/exnr.2001.7834. [DOI] [PubMed] [Google Scholar]
- Alexander WS. Progressive fibrinoid degeneration of fibrillary astrocytes associated with menal retardation in a hydrocephalic infant. Brain. 1949;72:373–381. doi: 10.1093/brain/72.3.373. [DOI] [PubMed] [Google Scholar]
- Back SA, Tuohy TM, Chen H, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- Barrett CP, Donati EG, Guth L. Differences between adult and neonatal rats in their astroglial response to spinal injury. Exp Neurol. 1984;84:374–385. doi: 10.1016/0014-4886(84)90234-6. [DOI] [PubMed] [Google Scholar]
- Bauer NG, Richter-Landsberg C, ffrench-Constant C. Role of the oligodendrocyte cytoskeleton in differentiation and myelination. Glia. 2009;57:1790–1801. doi: 10.1002/glia.20885. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Schuchardt J, Bunkowski S, et al. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 2000;123:1174–1183. doi: 10.1093/brain/123.6.1174. [DOI] [PubMed] [Google Scholar]
- Blakemore WK. The response of oligodendrocytes to chemical injury. Acta Neurol Scand Suppl. 1984;100:33–38. [PubMed] [Google Scholar]
- Blondel O, Collin C, McCarran WJ, et al. A glia-derived signal regulating neuronal differentiation. J Neurosci. 2000;20:8012–8020. doi: 10.1523/JNEUROSCI.20-21-08012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Johnson AB, Boespflug-Tanguy O, et al. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet. 2001;27:117–120. doi: 10.1038/83679. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Wood PM. Transplantation of Schwann cells and olfactory ensheathing cells to promote regeneration in the CNS. In: Selzer ME, Clarke S, Cohen LG, Duncan PW, Gage FH, editors. Textbook of Neural Repair and Rehabilitation. Cambridge: Cambridge University Press; 2006. pp. 513–531. [Google Scholar]
- Cammer W, Zhang H. Maturation of oligodendrocytes is more sensitive to TNF alpha than is survival of precursors and immature oligodendrocytes. J Neuroimmunol. 1999;97:37–42. doi: 10.1016/s0165-5728(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Canning DR, Höke A, Malemud CJ, et al. A potent inhibitor of neurite outgrowth that predominates in the extracellular matrix of reactive astrocytes. Int J Dev Neurosci. 1996;14:153–175. doi: 10.1016/0736-5748(96)00004-4. [DOI] [PubMed] [Google Scholar]
- Carter SL, Müller M, Manders P, et al. Induction of the genes for Cxcl9 and Cxcl10 is dependent on IFNγ but shows differential cellular expression in experimental autoimmune encephalomolyelitis and by astrocytes and microglia in vitro. Glia. 2007;55:1728–1739. doi: 10.1002/glia.20587. [DOI] [PubMed] [Google Scholar]
- Daginakatte GC, Gadzinski A, Emnett RJ, et al. Expression profiling identifies a molecular signature of reactive astrocytes stimulated by cyclic AMP or proinflammatory cytokines. Exp Neurol. 2008;210:261–267. doi: 10.1016/j.expneurol.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Lacagnina M, Gass D, et al. EIF2B5 mutations compromise GFAP+ astrocyte generation in vanishing white matter leukodystrophy. Nat Med. 2005;11:277–283. doi: 10.1038/nm1195. [DOI] [PubMed] [Google Scholar]
- Dziewulska D, Jamrozik Z, Podlecka A, et al. Do astrocytes participate in rat spinal cord myelination? Folia Neuropathol. 1999;37:81–86. [PubMed] [Google Scholar]
- East E, Golding JP, Phillips JB. A versatile 3D culture model facilitates monitoring of astrocytes undergoing reactive gliosis. J Tissue Eng Regen Med. 2009;3:634–646. doi: 10.1002/term.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleston M, Mucke L. Molecular profile of reactive astrocytes - implications for their role in neurological disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng LF. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol. 1985a;8:203–214. doi: 10.1016/s0165-5728(85)80063-1. [DOI] [PubMed] [Google Scholar]
- Eng LF, Vanderhaeghen JJ, Bignami A, et al. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971;28:351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Fedoroff S, White R, Neal J, et al. Astrocyte cell lineage. II. Mouse fibrous astrocytes and reactive astrocytes in cultures have vimentin- and GFP-containing intermediate filaments. Brain Res. 1983;283:303–315. doi: 10.1016/0165-3806(83)90187-6. [DOI] [PubMed] [Google Scholar]
- Fife BT, Kennedy KJ, Paniagua MC, et al. CXCL10 (IFN-gamma-inducible protein-10) control of encephalitogenic CD4+ T cell accumulation in the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2001;166:7617–7624. doi: 10.4049/jimmunol.166.12.7617. [DOI] [PubMed] [Google Scholar]
- Filous AR, Miller JH, Coulson-Thomas YM, et al. Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC. Dev Neurobiol. 2010;70:826–841. doi: 10.1002/dneu.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Crang AJ, Blakemore WF. Transplanted type-1 astrocytes facilitate repair of demyelinating lesions by host oligodendrocytes in adult rat spinal cord. J Neurocytol. 1991;20:420–430. doi: 10.1007/BF01355538. [DOI] [PubMed] [Google Scholar]
- Fujimoto Y, Yamasaki T, Tanaka N, et al. Differential activation of astrocytes and microglia after spinal cord injury in the fetal rat. Eur Spine J. 2006;15:22–33. doi: 10.1007/s00586-005-0933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geren BB, Raskind J. Development of the fine structure of the myelin sheath in sciatic nerves of chick embryos. Proc Natl Acad Sci USA. 1953;39:880–884. doi: 10.1073/pnas.39.8.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Woodward J, Young DG, et al. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci. 1988;8:2485–2490. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabinski AR, Tani M, Strieter RM, et al. Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am J Pathol. 1997;15:617–630. [PMC free article] [PubMed] [Google Scholar]
- Godiska R, Chantry D, Dietsch GN, et al. Chemokine expression in murine experimental allergic encephalomyelitis. J Neuroimmunol. 1995;58:167–176. doi: 10.1016/0165-5728(95)00008-p. [DOI] [PubMed] [Google Scholar]
- Gozes I, Bassan M, Zamostiano R, et al. A novel signaling molecule for neuropeptide action: activity-dependent neuroprotective protein. Ann NY Acad Sci. 1999;897:25–135. doi: 10.1111/j.1749-6632.1999.tb07884.x. [DOI] [PubMed] [Google Scholar]
- Graça DL, Blakemore WF. Delayed remyelination in rat spinal cord following ethidium bromide injection. Neuropathol Appl Neurobiol. 1986;12:595–605. doi: 10.1111/j.1365-2990.1986.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Herx LM, Yong VW. Interleukin-1 beta is required for the early evolution of reactive astrogliosis following CNS lesion. J Neuropathol Exp Neurol. 2001;60:961–971. doi: 10.1093/jnen/60.10.961. [DOI] [PubMed] [Google Scholar]
- Holley JE, Gveric D, Whatmore JL, et al. Tenascin C induces a quiescent phenotype in cultured adult human astrocytes. Glia. 2005;52:53–58. doi: 10.1002/glia.20231. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, et al. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006a;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Lee SC, Brosnan CF. Cytokines: powerful regulators of glial cell activation. Neuroscientist. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- John GR, Lee SC, Song X, et al. IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia. 2005;49:161–176. doi: 10.1002/glia.20109. [DOI] [PubMed] [Google Scholar]
- Klein RS, Izikson L, Means T, et al. IFN-inducible protein 10/CXC chemokine ligand 10-independent induction of experimental autoimmune encephalomyelitis. J Immunol. 2004;172:550–559. doi: 10.4049/jimmunol.172.1.550. [DOI] [PubMed] [Google Scholar]
- Krum JM, Rosenstein JM. VEGF mRNA and its receptor flt-1 are expressed in reactive astrocytes following neural grafting and tumor cell implantation in the adult CNS. Exp Neurol. 1998;154:57–65. doi: 10.1006/exnr.1998.6930. [DOI] [PubMed] [Google Scholar]
- Larner AJ, Johnson AR, Keynes RJ. Regeneration in the vertebrate central nervous system: phylogeny, ontogeny, and mechanism. Biol Rev Camb Philos Soc. 1995;70:597–619. doi: 10.1111/j.1469-185x.1995.tb01653.x. [DOI] [PubMed] [Google Scholar]
- Lassmann H. Multiple sclerosis pathology: evolution of pathogenetic concepts. Brain Pathol. 2005;15:217–222. doi: 10.1111/j.1750-3639.2005.tb00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Reynolds R. Activation and proliferation of endogenous oligodendrocytes precursor cells during ethidium bromide demyelination. Exp Neurol. 1999;160:333–347. doi: 10.1006/exnr.1999.7224. [DOI] [PubMed] [Google Scholar]
- Li R, Messing A, Goldman JE, et al. GFAP mutations in Alexander disease. Int J Dev Neurosci. 2002;20:259–268. doi: 10.1016/s0736-5748(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Liberto CM, Albrecht PJ, Herx LM, et al. Pro-regenerative properties of cytokine-activated astrocytes. J Neurochem. 2004;89:1092–1100. doi: 10.1111/j.1471-4159.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Edelmann W, Bieri PL, et al. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron. 1996;17:607–615. doi: 10.1016/s0896-6273(00)80194-4. [DOI] [PubMed] [Google Scholar]
- Liu MT, Keirstead HS, Lane TE. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J Immunol. 2001;167:4091–4097. doi: 10.4049/jimmunol.167.7.4091. [DOI] [PubMed] [Google Scholar]
- Liu L, Huang D, Matsui M, et al. Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. J Immunol. 2006;176:4399–4409. [Google Scholar]
- Malhotra SK, Shnitka TK. Diversity in reactive astrocytes. In: De Vellis J, editor. Neuroglia in the Aging Brain. New Jersey: Humana Press; 2002. pp. 17–33. [Google Scholar]
- Marz P, Heese K, Dimitriades-Schmutz B, et al. Role of interleukin-6 and soluble IL-6 receptor in region-specific induction of astrocytic differentiation and neurotrophin expression. Glia. 1999;26:191–200. doi: 10.1002/(sici)1098-1136(199905)26:3<191::aid-glia1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, et al. Interleukin-1beta promotes repair of the CNS. J Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeuwsen S, Persoon-Deen C, Bsibsi M, et al. Cytokine, chemokine and growth factor gene profiling of cultured human astrocytes after exposure to proinflammatory stimuli. Glia. 2003;43:243–253. doi: 10.1002/glia.10259. [DOI] [PubMed] [Google Scholar]
- Messersmith DJ, Murtie JC, Le TQ, et al. Fibroblast growth factor 2 (FGF2) and FGF receptor expression in an experimental demyelinating disease with extensive remyelination. J Neurosci Res. 2000;62:241–256. doi: 10.1002/1097-4547(20001015)62:2<241::AID-JNR9>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Moore CS, Abdullah SL, Brown A, et al. How factors secreted from astrocytes impact myelin repair. J Neurosci Res. 2011;89:13–21. doi: 10.1002/jnr.22482. [DOI] [PubMed] [Google Scholar]
- Müller E. Pathologische anatomie und pathogenese. In: Fisher G, editor. Die Multiple. Jena: Skleroze des Gehirns und Rückenmarks; 1904. pp. 300–344. [Google Scholar]
- Muller M, Carer SL, Hofer MJ, et al. CXCR3 signalling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J Immunol. 2007;178:8175–8182. doi: 10.4049/jimmunol.179.5.2774. [DOI] [PubMed] [Google Scholar]
- Nair A, Frederick TJ, Miller SD. Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci. 2008;65:2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash B, Thomson C, Linington C, et al. Differential regulation of myelination by CNTF- and tenascin-dependent effects on astrocytes. J Neurosci. (in press) [submitted] [Google Scholar]
- Noble M, Murray K. Purified astrocytes promote the in vitro division of a bipotential glial progenitor cell. EMBO J. 1984;3:2243–2247. doi: 10.1002/j.1460-2075.1984.tb02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi S, Tabunoki H, Kim SU, et al. Gene expression profiling of human neural progenitor cells following the serum-induced astrocyte differentiation. Cell Mol Neurobiol. 2009;29:423–438. doi: 10.1007/s10571-008-9338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omari KM, John GR, Sealfon SC, et al. CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain. 2005;128:1003–1015. doi: 10.1093/brain/awh479. [DOI] [PubMed] [Google Scholar]
- Pedraza L, Huang JK, Colman DR. Organizing principles of the axonal axoglial apparatus. Neuron. 2001;30:335–344. doi: 10.1016/s0896-6273(01)00306-3. [DOI] [PubMed] [Google Scholar]
- Pellerin L. How astrocytes feed hungry neurons. Mol Neurobiol. 2005;32:59–72. doi: 10.1385/MN:32:1:059. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Hamilton TA, Tani M, et al. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- Redwine JM, Armstrong RC. In vivo proliferation of oligodendrocyte progenitors expressing PDGF-alphaR during early remyelination. J Neurobiol. 1998;37:413–428. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Redwine JM, Blinder KL, Armstrong RC. In situ expression of fibroblast growth factor receptors by oligodendrocyte progenitors and oligodendrocytes in adult mouse central nervous system. J Neurosci Res. 1997;50:229–237. doi: 10.1002/(SICI)1097-4547(19971015)50:2<229::AID-JNR11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Ridet JL, Malhotra SK, Privat A, et al. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Rudge JS, Silver J. Inhibition of neurite outgrowth on astroglial scars in vitro. J Neurosci. 1990;10:3594–3604. doi: 10.1523/JNEUROSCI.10-11-03594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K, Raine CS. Tumor necrosis factor mediates myelin damage in organotypic cultures of nervous tissue. Ann N Y Acad Sci. 1988;540:568–570. doi: 10.1111/j.1749-6632.1988.tb27175.x. [DOI] [PubMed] [Google Scholar]
- Sherman D, Brophy P. Mechanism of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sofroniew M, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen A, Moffat K, Thomson C, et al. Astrocytes, but not olfactory ensheathing cells or Schwann cells, promote myelination of CNS axons in vitro. Glia. 2008;56:750–763. doi: 10.1002/glia.20650. [DOI] [PubMed] [Google Scholar]
- Talbot JF, Loy DN, Liu Y, et al. Endogenous Nkx2.2+/Olig2+ oligodendrocytes precursors cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Exp Neurol. 2005;192:11–24. doi: 10.1016/j.expneurol.2004.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada N, Miyagi K, Matsuda M, et al. Tumor necrosis factor and interleukin-1 in the CSF and sera of patients with multiple sclerosis. J Neurol Sci. 1991;104:230–234. doi: 10.1016/0022-510x(91)90315-x. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, et al. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Parpura V. Recent advances in (patho)physiology of astroglia. Acta Pharmacol Sin. 2010;31:1044–1054. doi: 10.1038/aps.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya H, Violleau K, Ben Younes-Chennoufi A, et al. Myelin-induced experimental allergic encephalomyelitis in Lewis rats: tumor necrosis factor alpha levels in serum and cerebrospinal fluid immunohistochemical expression in glial cells and macrophages of optic nerve and spinal cord. J Neuroimmunol. 1996;64:55–61. doi: 10.1016/0165-5728(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Watkins TA, Emery B, Mulinyawe S, et al. Distinct stages of myelination regulated by g-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron. 2008;60:555–569. doi: 10.1016/j.neuron.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RE, Jakeman LB. Don't fence me in: harnessing the beneficial roles of astrocytes for spinal cord repair. Restor Neurol Neurosci. 2008;26:197–214. [PMC free article] [PubMed] [Google Scholar]
- Williams A, Piaton G, Lubetzki C. Astrocytes – friends or foes in multiple sclerosis? Glia. 2007;55:1300–1312. doi: 10.1002/glia.20546. [DOI] [PubMed] [Google Scholar]
- Woodward WR, Nishi R, Meshul CK, et al. Nuclear and cytoplasmic localization of basic fibroblast growth factor in astrocytes and CA2 hippocampal neurons. J Neurosci. 1992;12:142–152. doi: 10.1523/JNEUROSCI.12-01-00142.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E, Raine CS. Multiple sclerosis. Interactions between oligodendrocytes and hypertrophic astrocytes and their occurrence in other, nondemyelinating conditions. Lab Invest. 1992;67:88–99. [PubMed] [Google Scholar]
- Yanagisawa M, Nakashima K, Arakawa H, et al. Astrocyte differentiation of fetal neuroepithelial cells by interleukin-11 via activation of a common cytokine signal transducer, gp130, and a transcription factor, STAT3. J Neurochem. 2000;74:1498–1504. doi: 10.1046/j.1471-4159.2000.0741498.x. [DOI] [PubMed] [Google Scholar]
- Yong VW, Moumdjian R, Yong FP, et al. Gamma-interferon promotes proliferation of adult human astrocytes in vitro and reactive gliosis in the adult mouse brain in vivo. Proc Natl Acad Sci USA. 1991;88:7016–7020. doi: 10.1073/pnas.88.16.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]


