Abstract
It is widely acknowledged that neural stem cells generate new neurons through the process of neurogenesis in the adult brain. In mammals, adult neurogenesis occurs in two areas of the CNS: the subventricular zone and the subgranular zone of the dentate gyrus of the hippocampus. The newly generated cells display neuronal morphology, generate action potentials and receive functional synaptic inputs, their properties being equivalent to those of mature neurons. Alzheimer's disease (AD) is the widespread cause of dementia, and is an age-related, progressive and irreversible neurodegenerative disease that results in massive neuronal death and deterioration of cognitive functions. Here, we overview the relations between adult neurogenesis and AD, and try to analyse the controversies in the field. We also summarise recent data obtained in the triple transgenic model of AD that show time- and region-specific impairment of neurogenesis, which may account for the early changes in synaptic plasticity and cognitive impairments that develop prior to gross neurodegenerative alterations and that could underlie new rescue therapies.
Keywords: Alzheimer's disease, hippocampus, neurodegeneration, neurogenesis, plasticity, subventricular zone
Neurogenesis in the CNS
In the second half of the 20th century, one of neuroscience's central tenets was the ‘no new neurons’ doctrine, which was subsequently challenged experimentally and overthrown. The ‘no new neurons’ doctrine assumed that all neurons are generated (through the process generally known as neurogenesis) exclusively during prenatal development and early postnatal life; in an adult brain, neurogenesis was considered to be absent. This doctrine was initially formulated by Santiago Ramón y Cajal, who wrote: ‘Once development was ended, the fonts of growth and regeneration of the axons and dendrites dried up irrevocably. In adult centres, the nerve paths are something fixed and immutable: everything may die, nothing may be regenerated. It is for the science of the future to change, if possible, this harsh decree’ (Cajal, 1913; Gross, 2000; Colucci-D'Amato et al. 2006). In the 1960s and1970s, however, this doctrine was reconsidered following the seminal discovery of Joseph Altman (Altman, 1962; Altman & Das, 1965), who found thymidine-H3-labelled neurons and neuroblasts in the adult rat brain. This initial observation was subsequently confirmed at the ultrastructural level in the late 1970s (Kaplan & Hinds, 1977).
Adult neurogenesis is now generally acknowledged. Numerous experiments have demonstrated that, in adulthood, neurogenesis operates mainly in two areas of the mammalian CNS, in the anterior part of the subventricular zone (SVZ) along the lateral ventricles, and in the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus (Cajal, 1913; Taupin & Gage, 2002; Abrous et al. 2005; Colucci-D'Amato et al. 2006). In both areas, neurogenesis progresses as a complex multi-stage process, which starts with the proliferation of neural precursors residing in the SVZ and the SGZ (Taupin & Gage, 2002; Abrous et al. 2005). These areas (also known as neurogenic niches) contain multipotent neural stem cells (NSCs; Seaberg & van der Kooy, 2002; Taupin & Gage, 2002). The NSCs that exhibit slow self-renewal produce neural progenitor cells (NPCs) with a faster dividing cell cycle, which ultimately differentiate into neurons or neuroglia; the differentiation process is regulated by numerous trophic factors (Lois & Alvarez-Buylla, 1993; Seaberg & van der Kooy, 2002; Abrous et al. 2005). Another important pool of neural precursor cells is represented by astrocytes residing in the germinal centres of the adult brain; these astrocytes retain the stem cell properties throughout the life span, and are involved in both neuro- and glio-genesis (see Doetsch et al. 1999; Alvarez-Buylla et al. 2001; Gotz & Huttner, 2005 and Mori et al. 2005 for review). In addition, astrocytes appear to regulate the rate of proliferation of hippocampal stem cells, and favour the generation of new neurons (Song et al. 2002).
Immature neurons generated in the SVZ migrate via the rostral migratory stream to the olfactory bulb, where they differentiate into local interneurons (Lledo & Gheusi, 2003). Although many thousands of new neurons are born in the SVZ and migrate into the olfactory bulb every day, only a minority of them survive and acquire adult neuronal properties (Lois & Alvarez-Buylla, 1993; Seaberg & van der Kooy, 2002). A proportion of SVZ-derived neurons also migrate radially into neocortical areas and the hippocampal formation (Gould et al. 1999; Abrous et al. 2005). Incidentally, those cells may act as neuronal replacements after injury or disease (Brandt & Storch, 2008).
For the hippocampus, it has also been estimated that several thousands of new cells are generated in the SGZ daily (Abrous et al. 2005). However, within several days after their birth, and similarly to what happens in the SVZ, at least half of the newborn cells die (Abrous et al. 2005). The cells that remain alive differentiate mainly into DG granule neurons and survive for months, passing through several differentiation stages, and ultimately becoming immature and mature granule cells within their specific location in the DG granule cell layer (GCL; Abrous et al. 2005; Fig. 1a,b). These newly generated neurons receive synaptic inputs (Fig. 1c), extend axons along the mossy fibres tract that pass through the hilus and exhibit electrophysiological properties similar to those of mature dentate granule cells (Paterson et al. 1973; Levison & Goldman, 1993). In addition, these newly generated neurons express the full complement of ionotropic and metabotropic membrane receptors (Kempermann & Gage, 1999). From a functional point of view, CNS neurogenesis and, more specifically, hippocampal neurogenesis, play an important role in structural plasticity and network maintenance. Therefore, it is likely to contribute to information storage, as well as learning and memory processes (Paterson et al. 1973; Levison & Goldman, 1993; Kempermann & Gage, 1999; Abrous et al. 2005).
Fig. 1.
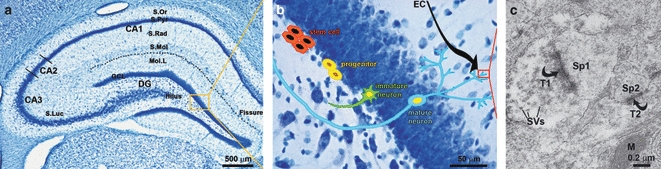
Neurogenesis in the hippocampus: neuronal formation, maturation and connectivity. (a) Brightfield micrograph showing the anatomical divisions of the dorsal hippocampus including the DG, one of the adult brain neurogenic changes. (b) High-magnification detail of the GCL of the hippocampal DG with a schematic cartoon illustrating the different phases from putative hippocampal stem cells (in the SGZ) to mature neurons, passing by the previous stages of progenitors and immature neurons. (c) Electron micrograph illustrating the synaptic contacts established by tow axon terminals (T1–T2) onto the newly-formed neurons within the molecular layer (Mol.L) of the DG onto two neighbouring dendritic spines (SP1–SP2). DG, dentate gyrus; GCL, granule cell layer; M, mitochondria; S.Luc, stratum lucidum; S.Mol, stratum lacunosum molecular; S.Or, stratum oriens; S.Pyr, stratum pyramidale; S.Rad, stratum radiatum; SVs, synaptic vesicles.
Neurogenesis in Alzheimer's disease (AD)
Alzheimer's disease, described by the German neuropathologist Alois Alzheimer as ‘Dementia Praecox’ (Alzheimer, 1907) appears in both genetic (family AD) and sporadic forms. AD, the widespread cause of dementia, is characterised by progressive neurodegeneration and the appearance of specific histopathological markers represented by focal extracellular deposits of fibrillar β-amyloid (also called neuritic or senile plaques) in the brain parenchyma and in the wall of blood vessels, and by the intraneuronal accumulation of neurofibrillary tangles (NFT) formed as a result of the abnormal hyperphosphorylation of cytoskeletal Tau filaments (Selkoe, 2001). The initial neurodegenerative events of AD appear in the transentorhinal cortex, and subsequently spread to the entorhinal cortex and to the hippocampus. At the later stages of the disease the neurodegenerative process disseminates through the temporal, frontal and parietal lobes (Thompson et al. 2003, 2007). At these late stages the grey matter also undergoes severe atrophy manifested by a profound loss of neurons and synaptic contacts (Scheff et al. 2006).
The hippocampus is affected early in AD. As described above, the hippocampus is also one of only two neurogenic niches of the adult brain (Taupin, 2006). Therefore, the pathological process associated with AD is likely to impinge upon neurogenesis. Impaired neurogenesis, in turn, can be relevant for the disease progression arguably being involved in the cognitive impairments linked with neurodegeneration (Haughey et al. 2002a; Seaberg & van der Kooy, 2002; Jin et al. 2004a; Chevallier et al. 2005; Lopez-Toledano & Shelanski, 2007; Verret et al. 2007; Rodríguez et al. 2008, 2009; Zhao et al. 2008).
Neurogenesis in post mortem tissues and in animal models of AD
Alzheimer's disease, like every other form of dementia, is unique to humans; no other creature acquires AD (Toledano & Alvarez, 2004). Therefore, substantial efforts have been invested in generating relevant animal models of AD that reproduce various subsets of neuropathological, behavioural and/or biochemical alterations resembling those seen in human AD (Gotz et al. 2004; Cassel et al. 2008). The absence of a standard model makes it understandable, hence, that the data on neurogenesis arising from analysis of a post mortem AD brain (Haughey et al. 2002b; Jin et al. 2004b) as well as various transgenic mouse models of AD remain controversial.
The majority of studies performed on transgenic animals expressing the mutant amyloid precursor protein (APP) demonstrated decreased neurogenesis either in the DG or in both the DG and the SVZ (Feng et al. 2001; Haughey et al. 2002b; Wen et al. 2002; Wang et al. 2004; Donovan et al. 2006; Wolf et al. 2006; Zhang et al. 2007). However, in another study of APPSwe, Ind mutant transgenic mouse, the enhanced neurogenesis linked to the presence of oligomeric Aβ was described (Jin et al. 2004a; Chevallier et al. 2005; Lopez-Toledano & Shelanski, 2007). In transgenic mice expressing various mutated presenilins, enhanced as well as decreased neurogenesis was observed (Chevallier et al. 2005). Increased neurogenesis was also reported in the SVZ of young APP/presenilin 1 (PS1) mice (these animals expressed mutant APP and PS1). Similarly, an increase in neurogenesis was observed following in vivo and in vitro exposure to Aβ1–42 (Sotthibundhu et al. 2009). Analysis of post mortem brain tissues from humans clinically diagnosed with AD revealed a reduction in progenitors in the SVZ (Ziabreva et al. 2006), but an increase in progenitor cells in the DG (Jin et al. 2004b).
Obviously, the changes in neurogenesis associated with AD are complex and require further clarification, as all the animal models studied exhibit only a reduced profile of AD pathology; thus, it is unclear whether the observed variations in neurogenesis are dependent on genotype in these studies (Wolf et al. 2006; Herring et al. 2009). Regarding the data from post mortem human tissues, these are intrinsically controversial and/or difficult to interpret because the post mortem material as a rule reflects the late stages of the disease. In addition, artefacts and misinterpretations can arise from post mortem delay, stage of the disease and treatments provided (these factors occurring either in isolation or combined). Furthermore, the discrepancies also depend on the methods used for labelling proliferating cells. For example, doublecortin labelling is known to identify both young and immature neurons (Chevallier et al. 2005; Verret et al. 2007). This alone may alter the interpretation of the data because more than 50% of the newborn cells die (Abrous et al. 2005). Labelling with 5-bromo-2′-deoxyuridine (BrdU) also suffers from uncertainties because it may not detect a distinct proliferative state, but instead mark repaired DNA in post-mitotic neurons and/or in cells entering an abortive cell cycle (Cooper-Kuhn & Kuhn, 2002; Rakic, 2002).
Neurogenesis in a triple transgenic mouse model of AD
Recently the triple transgenic mice model (3xTg-AD), which harbours three mutant genes for APPSwe, for PS1M146V and for tauP301L, was developed (Oddo et al. 2003a,b;). These mice show temporal and region-specific Aβ and tau pathology, which closely resembles that seen in the human AD brain (Table 1). In addition, the 3xTg-AD animals show clear functional and cognitive impairments, including reduced LTP, as well as deficient spatial, long-term and contextual memory (Oddo et al. 2003a,b;). The pathological changes start in the neocortex and expand towards the hippocampus (from 6 and 12 months old, respectively, for plaques, whereas NFTs start to be detectable from 12 months old; Rodríguez et al. 2008; Fig. 2). The functional deficits precede the appearance of histological hallmarks (Oddo et al. 2003a,b;) and correlate with the accumulation of intraneuronal Aβ (Carroll et al. 2007; McKee et al. 2008; Fig. 2d,e). Therefore, the 3xTg-AD model is most accurate and relevant for studying the neurogenic and other pathological and physiological changes in AD, as even if there are other double transgenic animal models, such as the Tg2576xJNPL3 (APPSWE) mouse, and even if Tg2576 and VLW mice (Lewis et al. 2001; Ribe et al. 2005) reproduce plaques and NFTs (Table 1), they fail to completely mimic disease evolution by not developing early behavioural alterations and having ectopic (spinal cord) NFTs formation (Lewis et al. 2001), by not having plaques present throughout the whole hippocampus, and by not having long-lasting spatial memory deficits except at a very advanced (over 16 months) age (Ribe et al. 2005).
Table 1.
Neuropathology in the main AD animal models
| Lesion and transgenic mouse, rat and primate models | Neuropathology | Reference |
|---|---|---|
| Ageing | Cholinergic involution and amyloid deposition | Sani et al. (2003) |
| Fischer et al. (1992) | ||
| Michalek et al. (1989) | ||
| Electrolytic lesion | Neuronal death | Lescaudron and Stein (1999) |
| Vale-Martinez et al. (2002) | ||
| Unspecific toxins (NMDA, ibotenic acid, quisalic acid, quinolic acid, colchicine, alkaloids, alcohol) | Neuronal death | Dunnett et al. (1991) |
| Winkler et al. (1998) | ||
| Boegman et al. (1985) | ||
| Shaughnessy et al. (1994) | ||
| Di Patre et al. (1989) | ||
| Arendt (1994) | ||
| Specific toxins (AF64A, 192Ig-G saporin) | Cholinergic neuronal death | Waite et al. (1995) |
| Chrobak et al. (1988) | ||
| Hanin (1996) | ||
| Wiley et al. (1991); Wiley (1992) | ||
| β-Amyloid | Cholinergic dysfunction | Giovannini et al. (2002) |
| Pavia et al. (2000) | ||
| PS1M146L | Diffused plaques | Blanchard et al. (2003) |
| APP751SL | Plaques | Blanchard et al. (2003) |
| APP/Ld/2 | Plaques | Moechars et al. (1996) |
| APPSwe | Plaques | Eriksen and Janus (2007) |
| APP Swedish, 695.K670N M671L | Plaques | Sturchler-Pierrat et al. (1997) |
| APP751SL/ PS1M146L | Plaques | Blanchard et al. (2003) |
| APPSWE/PS1dE9 | Plaques | Savonenko et al. (2005) |
| APPSwedish and PS1M146L | Plaques | Janus et al. (2000) |
| APP695SWE | Plaques | Hsiao et al. (1996) |
| APPV717F | Plaques | Dodart et al. (2000) |
| K670N/M671L and V717F | Plaques | Janus et al. (2000) |
| APP Swedish, 695.K670N-M671L and Indiana V717F | Plaques | Eriksen and Janus (2007) |
| TgAPPsw and PS1 M146L | Plaques | Takeuchi et al. (2000) |
| APPSwedish and V717F | Plaques | Chishti et al. (2001) |
| V337M | Tangles | Tanemura et al. (2002) |
| 4R/2N | Tangles | Tatebayashi et al. (2002) |
| TauP301L (4R,2-,3-) | Tangles | Lewis et al. (2000) |
| P301L | Tangles | Gotz et al. (2001) |
| TauP301L | Tangles | Arendash et al. (2004) |
| P301S/G272V | Tangles | Schindowski et al. (2006) |
| P301S | Tangles | Allen et al. (2002) |
| G272V, P301L, R406W | Tangles | Eriksen and Janus (2007) |
| Endogenous tau knocked out | Tangles | Andorfer et al. (2003) |
| P301L TET-off | Tangles | Ramsden et al. (2005) |
| 7TauTg | Tangles | Ishihara et al. (2001) |
| Tg2576×JNPL3 (APPSWE) | Plaques and tangles | Lewis et al. (2001) |
| Tg2576 and VLW | Plaques and tangles | Ribe et al. (2005) |
| 3xTg-AD | Plaques and tangles | Oddo et al. (2003b) |
| Tg478 | None | Flood et al. (2009) |
| Tg1116 | None | Flood et al. (2009) |
| K670M/N671L | Plaques | Kloskowska et al. (2010) |
| Tg478/Tg1116 | Plaques | Flood et al. (2009) |
NMDA, N-methyl-d-aspartic acid; AF64A, ethylcholine mustard aziridinium ion; 192 IgG Saporin, 12 clone monoclonal antibody to the nerve growth factor receptor; APP, amyloid precursor protein; APP751SL, single transgenic mouse expressing APP751SL mutation; APP/Ld/2, single transgenic mouse expressing APP London V642I mutation; APPSwe, double transgenic mouse expressing APP Swedish 695.K670N-M671L mutations; APP Swedish 695.K670N/M671L, double transgenic mouse expressing APP Swedish 695.K670N-M671L mutations; PS1M146L, single transgenic mouse expressing PS1M146L mutation; APP751SL/ PS1M146L, double transgenic mouse expressing APP751SL and PS1M146L mutations; APPSWE/PS1dE9, double transgenic mouse expressing APPswe (KM 593/594 NL) and PS1dE9 mutations; APPSwedish and PS1M146L, double transgenic mouse expressing APP swe (K595N, M596L) and PS1 (A246E) mutations; APP695SWE, double transgenic mouse expressing Swedish double mutations K670N/M671L; APPV717F, double transgenic mouse expressing Swedish and Indiana (D664A) mutations; K670N/M671L and V717F, double transgenic mouse expressing APP Swedish K670N/M671L and V717F mutations; K670N/M671L and Indiana V717F, double transgenic mouse expressing APP Swedish K670N/M671L and Indiana V717F mutations; TgAPPsw and PS1 M146L, double transgenic mouse expressing APP Swedish K670N/M671L and PS1M146L mutations; APPSwedish and V717F, double transgenic mouse expressing APP Swedish (KM670/671NL) and V717F mutations; V337M, single transgenic mouse expressing human Tau V337M mutation; 4R/2N, single transgenic mouse expressing human Tau R406W mutation; TauP301L (4R,2-,3-), single transgenic mouse expressing tau P301L mutation; P301L, single transgenic mouse expressing tau P301L mutation; G272V, P301L, R406W, triple transgenic mouse expressing G272V, P301L and R406W mutations; Htau, mouse model with endogenous tau knocked out; P301L TET-off, single transgenic mouse model expressing P301L mutation regulated by tetracycline responsive promoters; 7TauTg, single transgenic mouse model overexpressing tau protein without L neurofilament subunit; Tg2576×JNPL3, triple transgenic mouse expressing APP Swedish (KM670/671NL), V717F and tau P301L mutations; Tg2576×VLW, triple transgenic mouse expressing APP Swedish (KM670/671NL), V717F and triple tau (G272V, P301L and R406W) mutations; 3xTg-AD, triple transgenic mouse expressing APPSwedish, PS1M146L and TauP301L; Tg478, single transgenic rat expressing APP Swedish K670N/M671L mutation; Tg1116, single transgenic rat expressing APP V717I mutation; K670M/N671L, single transgenic rat expressing APP Swedish K670N/M671L mutation; Tg478/Tg1116, double transgenic rat expressing APP Swedish K670N/M671L and V717I mutations; Tg478/Tg1116/Tg11587, triple transgenic rat expressing APP Swedish K670N/M671L, V717I and PS-1 M146V mutations.
Fig. 2.
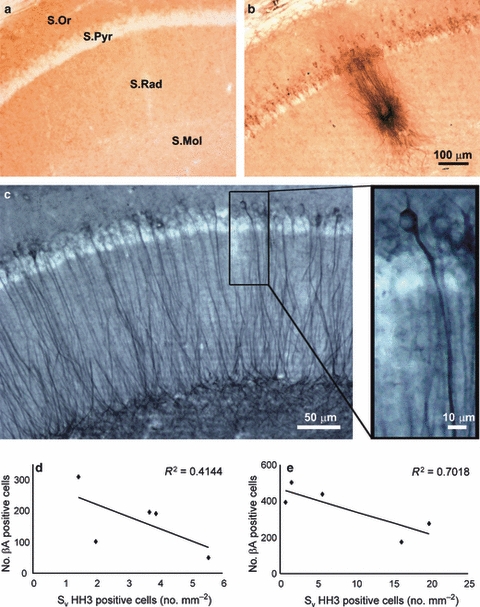
Brightfield micrographs showing the presence of β-amyloid within the pyramidal neurons of CA1 as well as the presence of a plaque in 12 months 3xTg-AD mice (b) compared with a non-Tg control animal (a). (c) We can see the accumulation phosphorylated Tau within the CA1 of 3xTg-AD mice. (d,e) Linear correlations between the mean number of cells containing β-amyloid in the hippocampal CA1 and the mean area density of HH3-positive cells in the GCL of the DG of male (d) and female (e) 3xTg-AD mice. S.Mol, stratum lacunosum molecular; S.Or, stratum oriens; S.Pyr, stratum pyramidale; S.Rad, stratum radiatum. Modified from Rodríguez et al. (2008) with permission.
In experiments on 3xTg-AD animals, we found impaired neurogenic capabilities in both the SVZ and SGZ of the hippocampal DG (Rodríguez et al. 2008, 2009). In both neurogenic niches, the SVZ and SGZ of the 3xTg-AD mice, a fair number of newly generated cells can be detected with different proliferation markers, such as BrdU, Ki67, PCNA and HH3 (Abrous et al. 2005; Rodríguez et al. 2008, 2009; Figs 3a–f and 4a,b,d). These newly-formed cells always show the distinctive characteristics of proliferating cells, being mainly localized in the inferior part of the GCL and demonstrating typical morphology such as irregular shape and small size (Figs 1b, 3a–f and 4a,b,d). Sometimes they tend to appear close together and/or form clusters (Abrous et al. 2005; Rodríguez et al. 2008, 2009), which rarely co-localize with glial fibrillary acidic protein (GFAP; the main glial cytoskeletal component; < 5%; Rodríguez et al. 2008, 2009; Figs 3c–f and 4d).
Fig. 3.

(a–d) Photomicrographs showing phosphorylated HH3 (a proliferating mitotic marker) within the DG of non-Tg mice. (a,b) Single labelling of HH3-positive cells (arrows) in the DG of 2 (a) and 12 months (b) non-Tg mice. (c,d) Dual labelling of HH3-positive cells (arrows) and glial cells (GFAP, blue) in the DG of 2 (c) and 12 months (d) non-Tg mice. Brightfield micrographs showing HH3-labelled cells within the DG of 3xTg-AD. (e,f) Dual labelling of HH3-positive cells (arrows) and glial cells (GFAP, red) in the DG of 2 (e) and 12 months (f) 3xTg-AD mice. (g,h) Bar graphs showing the mean area density HH3-labelled cells within the GCL of the dorsal DG of both 3xTg-AD and control non-Tg-AD mice males (g) and females (h). Asterisks indicate a significant difference in the means. GCL, granule cell layer; Mol.L, molecular layer. Modified from Rodríguez et al. (2008) with permission.
Fig. 4.

Brightfield micrographs showing phosphorylated HH3 (a proliferating mitotic marker) within the SVZ of both non-Tg and 3xTg-AD mice. (a,b) Single labelling of HH3-positive cells in the SVZ of 3 months non-Tg (a) and 3 months 3xTg-AD mice (b). (c) Bar graphs showing the mean area density of HH3-labelled cells within the SVZ of both control non-Tg and 3xTg-AD mice. Asterisks indicate a significant difference in the means. (d) Photomicrograph showing one of the few examples of co-localisation (marked by red circle) of HH3-positive cells (arrows) and glial cells (GFAP, blue/black; asterisks) in the SVZ of a 6 months non-Tg mouse, as the majority of proliferating cells did not have glial phenotype. CC, corpus callosum; CPU, caudate putamen nucleus; LV, lateral ventricles; SVZ, subventricular zone. Modified from Rodríguez et al. (2009) with permission.
In the GCL of the hippocampal DG of 3xTg-AD mice (as in normal aging; Kuhn et al. 1997; Abrous et al. 2005), the rate of neurogenesis starts to decrease from the age of 6 months (over 50% reduction when compared with early ages such as 2 months) and decreases further at later ages (Fig. 3g,h), affecting more females than males (Rodríguez et al. 2008). The age-dependent decrease in neurogenesis, however, is much more prominent in the 3xTg-AD mice when compared with the controls. From 9 to 12 months old, both genders retained very little capacity of forming new cells within the GCL; in non-Tg control animals the neurogenic levels that account approximately for a 20–35% of the young age levels were still preserved (Fig. 3g,h). A significant decrease in neurogenesis was also observed in the SVZ during normal aging, but at a lower level ranging between 19% and 31% (Rodríguez et al. 2009; Fig. 4a–c). The 3xTg-AD when compared with normal animals presented a further 40% decrease (Fig. 4c), that appears as early as 3 months old, and is sustained through later ages (Rodríguez et al. 2009).
Our results are in agreement with the majority of data obtained from APP mutant animals (Feng et al. 2001; Haughey et al. 2002b; Wen et al. 2002; Wang et al. 2004; Donovan et al. 2006; Wolf et al. 2006; Zhang et al. 2007), and have recently been confirmed and expanded by showing a generalised decrease not only in proliferating cells but also in early neuronal progenitors and neuroblasts (Hamilton et al. 2010). All in all, these data suggest that the process of impaired neuronal proliferation and/or its deregulation can be not only a consequence of the disease but may represent a cause for the cognitive alterations associated with AD.
Studies in the 3xTg-AD model also demonstrated that female mice are affected earlier than males (4 vs. 9 months old; Fig. 3g,h; Rodríguez et al. 2008). These findings are not only in line with the recently reported sexual dimorphism observed in cognitive performances (Clinton et al. 2007), but also correlate to the well-known fact that AD affects women earlier and with more severity than men (Baum, 2005; Webber et al. 2005). Several lines of evidence suggest that this difference, even with a potential similar disruption mechanism, might be exacerbated by the circulating levels of oestrogens (Manly et al. 2000; Baum, 2005; Webber et al. 2005) as a result of the endocrine status (Galea et al. 2006).
However, the exact mechanism by which neurogenesis is affected during the progression of AD needs to be fully understood and ultimately discovered. Recent evidence indicates several potential systems as shown in the APP and in the 3xTg-AD models (Crews & Masliah, 2010; Crews et al. 2010; Hamilton et al. 2010). The potential mechanisms include the following. (i) Abnormal activation of the p35 cyclin-dependent kinase-5 (CDK5), which becomes hyperactive during the progression of AD as a result of Aβ accumulation and plaque formation. Abnormal activity of CDK5 may cause physiological alterations in NPCs, impair or even arrest neuroblast migration, and trigger aberrant synaptic plasticity (Ohshima et al. 1996; Chae et al. 1997; Fischer et al. 2005; Johansson et al. 2005; Hirota et al. 2007; Jessberger et al. 2008; Lagace et al. 2008; Crews & Masliah, 2010). (ii) Altered metabolism in NPCs as shown by abnormal accumulation of lipid droplets in the SVZ, which is directly linked with the ApoE4 risk genetic factor (Hamilton et al. 2010). (iii) Parallel abnormal Tau hyperphosphorylation in adjacent SVZ striatal neurons and in DG hilar neurons, which may impair the maturation and network connectivity of newly-formed cells (Kippin et al. 2005; Tozuka et al. 2005; Ge et al. 2006, 2007; Liu et al. 2006; Hamilton et al. 2010).
Neurogenesis modulation: a potential therapeutic approach to AD
The changes in the neurogenesis observed during the initial stages and progression of AD suggest that a potential enhancement and/or potentiation of the new production of neurons in neurogenic sites may represent a valid therapeutic strategy to halt and/or delay the cognitive and mnesic alterations associated with the disease. Therefore, interventions preserving neurogenesis could be helpful. For example, increased physical/cognitive activity is associated with reduced risk of AD (Wang et al. 2002; Benedetti et al. 2008). Longitudinal studies suggest that regular physical activity and a higher educational level may help prevent the development of dementia (Costa et al. 2007; Mohajeri & Leuba, 2009). Voluntary running (RUN) and environmental enrichment (ENR) housing environments in rodents have been used to reproduce the physical and cognitive ‘active’ lifestyle in humans (Cracchiolo et al. 2007; Wood et al. 2010). Exposure to either RUN or ENR enhanced memory in normal rodents (Nilsson et al. 1999; van Praag et al. 1999; Kempermann et al. 2002), and in neuropathologically incomplete transgenic mouse models of AD (Adlard et al. 2005; Lazarov et al. 2005; Costa et al. 2007; Nichol et al. 2007, 2009), as well as increasing hippocampal neurogenesis (Wen et al. 2004; Lazarov et al. 2005; Wolf et al. 2006; Catlow et al. 2009; Herring et al. 2009). Furthermore, we have recently demonstrated that in the 3xTg-AD mice, environmental enrichment (6 month exposure) not only increased the neurogenesis rate, but in fact recovered it to the normal values observed in age-matched controls at 9 months old (J.J. Rodríguez, H.N. Noristani, M. Olabarria, J. Fletcher, T.D.D. Somerville, C.Y. Yeh, A. Verkhratsky, submitted).
Treatments with antidepressants and 5-HT receptor agonists also have been shown to increase neurogenesis rate and brain-derived neurotrophic factor production, which in parallel increased the survival of the newly-formed neurons (Santarelli et al. 2003). This 5-HT facilitation of neurogenesis agrees with our recent findings of serotonin fibre sprouting at late ages (12–18 months) when neurogenesis in the 3xTg-AD model is almost absent (Noristani et al. 2010). Finally, there is preliminary evidence of neurosphere neuroprotection increase in neuronal survival, which can directly affect neurons in the newborn through an immunomodulatory mechanism (Pluchino et al. 2005).
Conclusions
Here we provide compelling evidence for the impairment of cell proliferation and neurogenesis in both neurogenic niches of the adult brain, the SVZ and the SGZ of the hippocampal DG, during the progression of AD. Both neurogenic areas are involved in the supply of new neurons to the neocortex and hippocampus, two of the brain regions primarily affected by AD (Haughey et al. 2002a; Brandt & Storch, 2008; Sotthibundhu et al. 2009). The degree of impaired neurogenesis increases with age, and is more pronounced in females. This latter finding is in agreement with the disease prevalence and appears early in the SVZ suggesting a differential effect on neurogenic regions, which can be a reflection of the different role and final circuit incorporation of the newly-formed cells (neocortex vs. hippocampus). Without doubt, the regulation of endogenous neurogenesis is a major target in the development of future treatments for neurodegenerative disorders, including AD. Although these neurogenic therapies theoretically have the potential to halt or even reverse cognitive decline, further understanding of the factors regulating neurogenesis in AD may prove invaluable for the development of these future treatments.
Acknowledgments
We thank Miss Chia-Yu Yeh, and Mr Markel Olabarria and Harun N. Noristani for their help and assistance in the table and figures preparation. The authors’ research was supported by Alzheimer's Research Trust (UK) Programme Grant (ART/PG2004A/1) to AV and JJR; by National Institute of Health (NIH) grant to AV; by the Grant Agency of the Czech Republic (GACR 309/09/1696 and GACR 304/11/0184) to JJR and (GACR 309/08/1381 and GACR 305/08/1384) to AV, and by the Government of the Basque Country (AE-2010-1-28; AEGV10/16) to JJR.
References
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Pop V, et al. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B, Ingram E, Takao M, et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiat Psych-Gericht Med. 1907;64:146–148. [Google Scholar]
- Andorfer C, Kress Y, Espinoza M, et al. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Lewis J, Leighty RE, et al. Multi-metric behavioral comparison of APPsw and P301L models for Alzheimer's disease: linkage of poorer cognitive performance to tau pathology in forebrain. Brain Res. 2004;1012:29–41. doi: 10.1016/j.brainres.2004.02.081. [DOI] [PubMed] [Google Scholar]
- Arendt T. Impairment in memory function and neurodegenerative changes in the cholinergic basal forebrain system induced by chronic intake of ethanol. J Neural Transm Suppl. 1994;44:173–187. doi: 10.1007/978-3-7091-9350-1_13. [DOI] [PubMed] [Google Scholar]
- Baum LW. Sex, hormones, and Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2005;60:736–743. doi: 10.1093/gerona/60.6.736. [DOI] [PubMed] [Google Scholar]
- Benedetti TR, Borges LJ, Petroski EL, et al. Physical activity and mental health status among elderly people. Rev Saude Publica. 2008;42:302–307. [PubMed] [Google Scholar]
- Blanchard V, Moussaoui S, Czech C, et al. Time sequence of maturation of dystrophic neurites associated with Abeta deposits in APP/PS1 transgenic mice. Exp Neurol. 2003;184:247–263. doi: 10.1016/s0014-4886(03)00252-8. [DOI] [PubMed] [Google Scholar]
- Boegman RJ, el-Defrawy SR, Jhamandas K, et al. Quinolinic acid neurotoxicity in the nucleus basalis antagonized by kynurenic acid. Neurobiol Aging. 1985;6:331–336. doi: 10.1016/0197-4580(85)90012-0. [DOI] [PubMed] [Google Scholar]
- Brandt MD, Storch A. Neurogenesis in the adult brain: from bench to bedside? Fortschr Neurol Psychiatr. 2008;76:517–529. doi: 10.1055/s-2008-1038218. [DOI] [PubMed] [Google Scholar]
- Cajal Ry. Estudios Sobre la Degeneración y Regeneración Del Sistema Nervioso. Madrid: Moya; 1913. [Google Scholar]
- Carroll JC, Rosario ER, Chang L, et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13 357–13 365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel JC, Mathis C, Majchrzak M, et al. Coexisting cholinergic and parahippocampal degeneration: a key to memory loss in dementia and a challenge for transgenic models? Neurodegen Dis. 2008;5:304–317. doi: 10.1159/000135615. [DOI] [PubMed] [Google Scholar]
- Catlow BJ, Rowe AR, Clearwater CR, et al. Effects of environmental enrichment and physical activity on neurogenesis in transgenic PS1/APP mice. Brain Res. 2009;1256:173–179. doi: 10.1016/j.brainres.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, et al. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Chevallier NL, Soriano S, Kang DE, et al. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am J Pathol. 2005;167:151–159. doi: 10.1016/S0002-9440(10)62962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Hanin I, Schmechel DE, et al. AF64A-induced working memory impairment: behavioral, neurochemical and histological correlates. Brain Res. 1988;463:107–117. doi: 10.1016/0006-8993(88)90532-x. [DOI] [PubMed] [Google Scholar]
- Clinton LK, Billings LM, Green KN, et al. Age-dependent sexual dimorphism in cognition and stress response in the 3xTg-AD mice. Neurobiol Dis. 2007;28:76–82. doi: 10.1016/j.nbd.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci-D'Amato L, Bonavita V, di Porzio U. The end of the central dogma of neurobiology: stem cells and neurogenesis in adult CNS. Neurol Sci. 2006;27:266–270. doi: 10.1007/s10072-006-0682-z. [DOI] [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Kuhn HG. Is it all DNA repair? Brain Res Dev Brain Res. 2002;134:13–21. doi: 10.1016/s0165-3806(01)00243-7. [DOI] [PubMed] [Google Scholar]
- Costa DA, Cracchiolo JR, Bachstetter AD, et al. Enrichment improves cognition in AD mice by amyloid-related and unrelated mechanisms. Neurobiol Aging. 2007;28:831–844. doi: 10.1016/j.neurobiolaging.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Cracchiolo JR, Mori T, Nazian SJ, et al. Enhanced cognitive activity - over and above social or physical activity - is required to protect Alzheimer's mice against cognitive impairment, reduce Abeta deposition, and increase synaptic immunoreactivity. Neurobiol Learn Mem. 2007;88:277–294. doi: 10.1016/j.nlm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer's disease. Hum Mol Genet. 2010;19:R12–R20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Rockenstein E, Masliah E. APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis. Brain Struct Funct. 2010;214:111–126. doi: 10.1007/s00429-009-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Patre PL, Abbamondi A, Bartolini L, et al. GM1 ganglioside counteracts cholinergic and behavioral deficits induced in the rat by intracerebral injection of vincristine. Eur J Pharmacol. 1989;162:43–50. doi: 10.1016/0014-2999(89)90602-x. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Mathis C, Saura J, et al. Neuroanatomical abnormalities in behaviorally characterized APP(V717F) transgenic mice. Neurobiol Dis. 2000;7:71–85. doi: 10.1006/nbdi.1999.0278. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Donovan MH, Yazdani U, Norris RD, et al. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Everitt BJ, Robbins TW. The basal forebrain-cortical cholinergic system: interpreting the functional consequences of excitotoxic lesions. Trends Neurosci. 1991;14:494–501. doi: 10.1016/0166-2236(91)90061-x. [DOI] [PubMed] [Google Scholar]
- Eriksen JL, Janus CG. Plaques, tangles, and memory loss in mouse models of neurodegeneration. Behav Genet. 2007;37:79–100. doi: 10.1007/s10519-006-9118-z. [DOI] [PubMed] [Google Scholar]
- Feng R, Rampon C, Tang YP, et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32:911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- Fischer W, Chen KS, Gage FH, et al. Progressive decline in spatial learning and integrity of forebrain cholinergic neurons in rats during aging. Neurobiol Aging. 1992;13:9–23. doi: 10.1016/0197-4580(92)90003-g. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, et al. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Flood DG, Lin YG, Lang DM, et al. A transgenic rat model of Alzheimer's disease with extracellular Abeta deposition. Neurobiol Aging. 2009;30:1078–1090. doi: 10.1016/j.neurobiolaging.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Galea LA, Spritzer MD, Barker JM, et al. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, et al. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Scali C, Prosperi C, et al. Beta-amyloid-induced inflammation and cholinergic hypofunction in the rat brain in vivo: involvement of the p38MAPK pathway. Neurobiol Dis. 2002;11:257–274. doi: 10.1006/nbdi.2002.0538. [DOI] [PubMed] [Google Scholar]
- Gotz J, Chen F, van Dorpe J, et al. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Gotz J, Streffer JR, David D, et al. Transgenic animal models of Alzheimer's disease and related disorders: histopathology, behavior and therapy. Mol Psychiatry. 2004;9:664–683. doi: 10.1038/sj.mp.4001508. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, et al. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- Hamilton LK, Aumont A, Julien C, et al. Widespread deficits in adult neurogenesis precede plaque and tangle formation in the 3xTg mouse model of Alzheimer's disease. Eur J Neurosci. 2010;32:905–920. doi: 10.1111/j.1460-9568.2010.07379.x. [DOI] [PubMed] [Google Scholar]
- Hanin I. The AF64A model of cholinergic hypofunction: an update. Life Sci. 1996;58:1955–1964. doi: 10.1016/0024-3205(96)00185-3. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Liu D, Nath A, et al. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer's disease. Neuromolec Med. 2002a;1:125–135. doi: 10.1385/NMM:1:2:125. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Chan SL, et al. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J Neurochem. 2002b;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- Herring A, Ambree O, Tomm M, et al. Environmental enrichment enhances cellular plasticity in transgenic mice with Alzheimer-like pathology. Exp Neurol. 2009;216:184–192. doi: 10.1016/j.expneurol.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Hirota Y, Ohshima T, Kaneko N, et al. Cyclin-dependent kinase 5 is required for control of neuroblast migration in the postnatal subventricular zone. J Neurosci. 2007;27:12 829–12 838. doi: 10.1523/JNEUROSCI.1014-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Higuchi M, Zhang B, et al. Attenuated neurodegenerative disease phenotype in tau transgenic mouse lacking neurofilaments. J Neurosci. 2001;21:6026–6035. doi: 10.1523/JNEUROSCI.21-16-06026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Aigner S, Clemenson GD, Jr, et al. Cdk5 regulates accurate maturation of newborn granule cells in the adult hippocampus. PLoS Biol. 2008;6:e272. doi: 10.1371/journal.pbio.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Galvan V, Xie L, et al. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw,Ind) mice. Proc Natl Acad Sci U S A. 2004a;101:13 363–13 367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Peel AL, Mao XO, et al. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci U S A. 2004b;101:343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JU, Lilja L, Chen XL, et al. Cyclin-dependent kinase 5 activators p35 and p39 facilitate formation of functional synapses. Brain Res Mol Brain Res. 2005;138:215–227. doi: 10.1016/j.molbrainres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. New nerve cells for the adult brain. Sci Am. 1999;280:48–53. doi: 10.1038/scientificamerican0599-48. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Kapur S, van der Kooy D. Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J Neurosci. 2005;25:5815–5823. doi: 10.1523/JNEUROSCI.1120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloskowska E, Pham TM, Nilsson T, et al. Cognitive impairment in the Tg6590 transgenic rat model of Alzheimer's disease. J Cell Mol Med. 2010;14:1816–1823. doi: 10.1111/j.1582-4934.2009.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, et al. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Benavides DR, Kansy JW, et al. Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2008;105:18 567–18 571. doi: 10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Robinson J, Tang YP, et al. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Lescaudron L, Stein DG. Differences in memory impairment and response to GM1 ganglioside treatment following electrolytic or ibotenic acid lesions of the nucleus basalis magnocellularis. Restor Neurol Neurosci. 1999;15:25–37. [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Gheusi G. Olfactory processing in a changing brain. Neuroreport. 2003;14:1655–1663. doi: 10.1097/00001756-200309150-00001. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Toledano MA, Shelanski ML. Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind) J Alzheimers Dis. 2007;12:229–240. doi: 10.3233/jad-2007-12304. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Merchant CA, Jacobs DM, et al. Endogenous estrogen levels and Alzheimer's disease among postmenopausal women. Neurology. 2000;54:833–837. doi: 10.1212/wnl.54.4.833. [DOI] [PubMed] [Google Scholar]
- McKee AC, Carreras I, Hossain L, et al. Ibuprofen reduces Abeta, hyperphosphorylated tau and memory deficits in Alzheimer mice. Brain Res. 2008;1207:225–236. doi: 10.1016/j.brainres.2008.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek H, Fortuna S, Pintor A. Age-related differences in brain choline acetyltransferase, cholinesterases and muscarinic receptor sites in two strains of rats. Neurobiol Aging. 1989;10:143–148. doi: 10.1016/0197-4580(89)90023-7. [DOI] [PubMed] [Google Scholar]
- Moechars D, Lorent K, De Strooper B, et al. Expression in brain of amyloid precursor protein mutated in the alpha-secretase site causes disturbed behavior, neuronal degeneration and premature death in transgenic mice. EMBO J. 1996;15:1265–1274. [PMC free article] [PubMed] [Google Scholar]
- Mohajeri MH, Leuba G. Prevention of age-associated dementia. Brain Res Bull. 2009;80:315–325. doi: 10.1016/j.brainresbull.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Mori T, Buffo A, Gotz M. The novel roles of glial cells revisited: the contribution of radial glia and astrocytes to neurogenesis. Curr Top Dev Biol. 2005;69:67–99. doi: 10.1016/S0070-2153(05)69004-7. [DOI] [PubMed] [Google Scholar]
- Nichol KE, Parachikova AI, Cotman CW. Three weeks of running wheel exposure improves cognitive performance in the aged Tg2576 mouse. Behav Brain Res. 2007;184:124–132. doi: 10.1016/j.bbr.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol K, Deeny SP, Seif J, et al. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, et al. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Noristani HN, Olabarria M, Verkhratsky A, et al. Serotonin fibre sprouting and increase in serotonin transporter immunoreactivity in the CA1 area of hippocampus in a triple transgenic mouse model of Alzheimer's disease. Eur J Neurosci. 2010;32:71–79. doi: 10.1111/j.1460-9568.2010.07274.x. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, et al. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003a;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003b;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11 173–11 178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson JA, Privat A, Ling EA, et al. Investigation of glial cells in semithin sections. 3 Transformation of subependymal cells into glial cells, as shown by radioautography after 3 H-thymidine injection into the lateral ventricle of the brain of young rats. J Comp Neurol. 1973;149:83–102. doi: 10.1002/cne.901490106. [DOI] [PubMed] [Google Scholar]
- Pavia J, Alberch J, Alvarez I, et al. Repeated intracerebroventricular administration of beta-amyloid(25-35) to rats decreases muscarinic receptors in cerebral cortex. Neurosci Lett. 2000;278:69–72. doi: 10.1016/s0304-3940(99)00900-3. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Rakic P. Adult neurogenesis in mammals: an identity crisis. J Neurosci. 2002;22:614–618. doi: 10.1523/JNEUROSCI.22-03-00614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M, Kotilinek L, Forster C, et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) J Neurosci. 2005;25:10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribe EM, Perez M, Puig B, et al. Accelerated amyloid deposition, neurofibrillary degeneration and neuronal loss in double mutant APP/tau transgenic mice. Neurobiol Dis. 2005;20:814–822. doi: 10.1016/j.nbd.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Rodríguez JJ, Jones VC, Tabuchi M, et al. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS ONE. 2008;3:e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez JJ, Jones VC, Verkhratsky A. Impaired cell proliferation in the subventricular zone in an Alzheimer's disease model. Neuroreport. 2009;20:907–912. doi: 10.1097/WNR.0b013e32832be77d. [DOI] [PubMed] [Google Scholar]
- Sani S, Traul D, Klink A, et al. Distribution, progression and chemical composition of cortical amyloid-beta deposits in aged rhesus monkeys: similarities to the human. Acta Neuropathol. 2003;105:145–156. doi: 10.1007/s00401-002-0626-5. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Savonenko A, Xu GM, Melnikova T, et al. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer's disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005;18:602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, et al. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Schindowski K, Bretteville A, Leroy K, et al. Alzheimer's disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am J Pathol. 2006;169:599–616. doi: 10.2353/ajpath.2006.060002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Shaughnessy LW, Barone S, Jr, Mundy WR, et al. Comparison of intracranial infusions of colchicine and ibotenic acid as models of neurodegeneration in the basal forebrain. Brain Res. 1994;637:15–26. doi: 10.1016/0006-8993(94)91212-2. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Sotthibundhu A, Li QX, Thangnipon W, et al. Abeta(1–42) stimulates adult SVZ neurogenesis through the p75 neurotrophin receptor. Neurobiol Aging. 2009;30:1975–1985. doi: 10.1016/j.neurobiolaging.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A, Irizarry MC, Duff K, et al. Age-related amyloid beta deposition in transgenic mice overexpressing both Alzheimer mutant presenilin 1 and amyloid beta precursor protein Swedish mutant is not associated with global neuronal loss. Am J Pathol. 2000;157:331–339. doi: 10.1016/s0002-9440(10)64544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanemura K, Murayama M, Akagi T, et al. Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J Neurosci. 2002;22:133–141. doi: 10.1523/JNEUROSCI.22-01-00133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi Y, Miyasaka T, Chui DH, et al. Tau filament formation and associative memory deficit in aged mice expressing mutant (R406W) human tau. Proc Natl Acad Sci USA. 2002;99:13896–13901. doi: 10.1073/pnas.202205599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. Neurogenesis in the adult central nervous system. C R Biol. 2006;329:465–475. doi: 10.1016/j.crvi.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res. 2002;69:745–749. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray G, et al. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Dutton RA, et al. Tracking Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:183–214. doi: 10.1196/annals.1379.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano A, Alvarez MI. Lesions and dysfunctions of the nucleus basalis as Alzheimer's disease models: general and critical overview and analysis of the long-term changes in several excitotoxic models. Curr Alzheimer Res. 2004;1:189–214. doi: 10.2174/1567205043332117. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, et al. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Vale-Martinez A, Baxter MG, Eichenbaum H. Selective lesions of basal forebrain cholinergic neurons produce anterograde and retrograde deficits in a social transmission of food preference task in rats. Eur J Neurosci. 2002;16:983–998. doi: 10.1046/j.1460-9568.2002.02153.x. [DOI] [PubMed] [Google Scholar]
- Verret L, Jankowsky JL, Xu GM, et al. Alzheimer's-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis. J Neurosci. 2007;27:6771–6780. doi: 10.1523/JNEUROSCI.5564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite JJ, Chen AD, Wardlow ML, et al. 192 immunoglobulin G-saporin produces graded behavioral and biochemical changes accompanying the loss of cholinergic neurons of the basal forebrain and cerebellar Purkinje cells. Neuroscience. 1995;65:463–476. doi: 10.1016/0306-4522(94)00479-o. [DOI] [PubMed] [Google Scholar]
- Wang HX, Karp A, Winblad B, et al. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155:1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- Wang R, Dineley KT, Sweatt JD, et al. Presenilin 1 familial Alzheimer's disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004;126:305–312. doi: 10.1016/j.neuroscience.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Webber KM, Casadesus G, Perry G, et al. Gender differences in Alzheimer disease: the role of luteinizing hormone in disease pathogenesis. Alzheimer Dis Assoc Disord. 2005;19:95–99. doi: 10.1097/01.wad.0000165512.90864.3f. [DOI] [PubMed] [Google Scholar]
- Wen PH, Shao X, Shao Z, et al. Overexpression of wild type but not an FAD mutant presenilin-1 promotes neurogenesis in the hippocampus of adult mice. Neurobiol Dis. 2002;10:8–19. doi: 10.1006/nbdi.2002.0490. [DOI] [PubMed] [Google Scholar]
- Wen PH, Hof PR, Chen X, et al. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol. 2004;188:224–237. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wiley RG. Neural lesioning with ribosome-inactivating proteins: suicide transport and immunolesioning. Trends Neurosci. 1992;15:285–290. doi: 10.1016/0166-2236(92)90078-m. [DOI] [PubMed] [Google Scholar]
- Wiley RG, Oeltmann TN, Lappi DA. Immunolesioning: selective destruction of neurons using immunotoxin to rat NGF receptor. Brain Res. 1991;562:149–153. doi: 10.1016/0006-8993(91)91199-b. [DOI] [PubMed] [Google Scholar]
- Winkler J, Power AE, Ramirez GA, et al. Short-term and complete reversal of NGF effects in rats with lesions of the nucleus basalis magnocellularis. Brain Res. 1998;788:1–12. doi: 10.1016/s0006-8993(97)01508-4. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Kronenberg G, Lehmann K, et al. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer's disease. Biol Psychiatry. 2006;60:1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Wood NI, Carta V, Milde S, et al. Responses to environmental enrichment differ with sex and genotype in a transgenic mouse model of Huntington's disease. PLoS ONE. 2010;5:e9077. doi: 10.1371/journal.pone.0009077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, McNeil E, Dressler L, et al. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease. Exp Neurol. 2007;204:77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Ziabreva I, Perry E, Perry R, et al. Altered neurogenesis in Alzheimer's disease. J Psychosom Res. 2006;61:311–316. doi: 10.1016/j.jpsychores.2006.07.017. [DOI] [PubMed] [Google Scholar]


