Abstract
Plant primary energy metabolism is profoundly reorganized under biotic stress conditions and there is increasing evidence for a role for the fermentative pathway in biotic interactions. However, the mechanisms regulating metabolic reprogramming are not well understood despite its critical function in the biotic stress response. Here the function of alcohol dehydrogenase (ADH) in the interaction of barley with the parasitic fungus Blumeria graminis f.sp. hordei (Bgh) is addressed. Challenge of susceptible barley leaves with Bgh resulted in transcriptional activation of HvADH1 and an induction of ADH enzyme activity starting 24 h after infection and reaching a clear-cut effect 4 d after infection. This increase in ADH enzyme activity was not observed in the resistant near-isogenic mlo5 line. Moreover, an induction of ADH enzyme activity by Bgh was enhanced in the presence of sucrose in hydroponically grown seedlings. Transient knock-down or overexpression of HvADH1 in barley epidermal cells mediated a decrease or increase in the penetration success of Bgh, respectively. Inhibition of ADH activity by pyrazole resulted in a delay in symptoms. The pyrazole effect could be overcome by adding glucose to the incubation medium, pinpointing a nutritional effect of ADH in the barley–Bgh interaction. Taken together, misexpression of pathogen-inducible HvADH1 or variation of ADH activity modulates the pathogen response of barley to the biotrophic fungal parasite Bgh. In this way, ADH knock-down/inhibition results in reduced fungal success. The possibility is discussed that ADH activity supports biotrophy by maintaining glycolytic metabolism in pathogen-stressed barley.
Keywords: Alcohol dehydrogenase, barley, Blumeria graminis, carbohydrate metabolism, fermentation, pathogen response
Introduction
Plants are obligate aerobic organisms; the vast majority of ATP for cellular metabolism is provided by photosynthesis and oxidative phosphorylation. Under conditions of hypoxia/anoxia, plants mount adaptive mechanisms to compensate for the energy crisis. Thereby, pyruvate decarboxylase and alcohol dehydrogenase (ADH), central enzymes in fermentative metabolism, are induced (Drew, 1997; Fukao and Bailey-Serres, 2004). By the sequential activity of those enzymes pyruvate is eventually converted to ethanol and the concomitant regeneration of NAD+ is essential to maintain glycolysis and ATP production. Due to a lack of an active transport system for molecular oxygen and rather slow diffusion-controlled transport through plant tissues, the oxygen pressure in tissues can vary widely and it is possible that in the presence of sufficiently high external oxygen pressure the internal oxygen availability does not meet the demand. Hence, hypoxia or anoxia is a frequent metabolic status even during normal development, particularly in tissues with a high O2 demand and/or restricted O2 entry (Geigenberger, 2003). ADH has a well established function in protection against hypoxic stress after flooding (Kennedy et al., 1992; Bailey-Serres and Voesenek, 2008), during seed development (Hanson et al., 1984; Macnicol and Jacobsen, 2001), and in aerobic metabolism in pollen (Buchner et al., 1995). Besides this, little is known about the role of ADH in the physiology of the plant.
The barley ADH gene family consists of three members, ADH1, ADH2, and ADH3 (Harberd and Edwards, 1983; Hanson and Brown, 1984; Trick et al., 1988). ADH1 is expressed in different tissues and is responsible for almost all of the activity detected during aerobic growth. Expression of ADH2 and ADH3 requires hypoxic induction (Good and Crosby, 1989; Hanson and Brown, 1984; Hanson et al., 1984). ADH function in barley has been intensively studied with respect to the development of barley grains. The aleurone in the mature barley grain contains only ADH1 homodimers (Hanson et al., 1984) and ADH1 gene expression in the aleurone is regulated by an abscisic acid–gibberellic acid interaction (Macnicol and Jacobsen, 2001). In the course of the establishment of malate/ethanol fermentation in the aleurone layer during seed development there is a sequential change in the relative amount of ADH isozymes, with minor contributions of ADH2 and ADH3 (Macnicol and Jacobsen, 1992).
There is mounting evidence for a critical role for fermentative metabolism in plant–pathogen interactions. In Arabidopsis thaliana, glycolysis, respiration, and fermentation are up-regulated at the site of powdery mildew attack, and it is speculated that fermentation is favoured under cellular conditions associated with parasitic nutrient acquisition (Chandran et al., 2010; Wildermuth, 2010). Thereby, localized DNA endoreduplication may function at the biotroph–plant interaction sites to enhance metabolic capacity (Wildermuth, 2010). This is in line with the observation of an increased sink strength mediated by elevated invertase and hexose transporter expression at the site of pathogen attack (Fotopolous et al., 2003; Swarbrick et al., 2006; Proels and Roitsch, 2009).
In this study, the role of ADH in the interaction of barley with the biotrophic parasitic fungus Blumeria graminis f.sp. hordei (Bgh) is addressed. Based on preliminary microarray data suggesting an up-regulation of HvADH1 in response to Bgh, the changes in ADH activity and expression in the barley–Bgh interaction were analysed and the specific role of HvADH1 in susceptibility of barley to Bgh was investigated. Moreover, the effect of ADH inhibition by pyrazole in the presence and absence of metabolizable sugars was analysed in the course of barley–Bgh interaction.
Materials and methods
Plant material and inoculation
Donor material of the barley (Hordeum vulgare L.) cultivar ‘Ingrid’ and the mlo5 isoline (‘Ingrid backcross mlo5’) was grown in a growth chamber at 18 °C, 60% relative humidity, and a photoperiod of 16 h (150 μmol s−1 m−2 photon flux density) for 7–8 d (for ADH activity, gene expression, and pyrazole experiments). For ADH activity and gene expression experiments, Bgh was inoculated onto seedlings to give a density of 90–120 conidia mm−2. For pyrazole experiments a conidial density of 8 conidia mm−2 was used to inoculate Bgh onto detached leaves.
Barley cultivar ‘Golden Promise’ seeds were germinated on filter paper soaked with tap water for 3 d in the dark. Then the germinated seeds were put in a 100 ml glass flask and grown for 3 d on filter paper soaked with tap water and a further 3 d in the presence or absence of 100 mM sucrose (growth chamber conditions as mentioned above). Leaves were inoculated with Bgh using a small brush that was saturated with Bgh spores and moved three times over each individual leaf. Leaf material was harvested after 4–5 d.
Pathogen material
Blumeria graminis (DC) Speer f.sp. hordei Em. Marchal, race A6 (Wiberg, 1974), undergoes a compatible interaction with the barley cultivars ‘Ingrid’ and ‘Golden Promise’, and was maintained on ‘Golden Promise’.
ADH activity assays
Leaf material of barley seedlings was harvested at different time points after Bgh infection, frozen in liquid nitrogen, and ground to a fine powder using a mortar. For extraction of total soluble proteins a 2 ml reaction tube was filled with the powder to about a quarter, and 300 μl of extraction buffer [50 mM HEPES pH 7.5, 15% (v/v) glycerol, 1 mM EDTA, 1 mM dithiothreitol (DTT), 3 mM MgCl2, 1 mM phenylmethylsulphonyl fluoride (PMSF)] was added. After vortexing, debris was removed by centrifugation at 12 000 g for 10 min at 4 °C. The total protein content was determined with the BioRad protein assay (BioRad, München, Germany) using bovine serum albumin (BSA) as standard. ADH assays with 150 μg of total protein were performed immediately after protein extraction in 1 ml volume (50 mM HEPES pH 8.0, 10 mM MgCl2, 1 mM DTT, 300 μM NAD+) in the presence or absence of 150 mM ethanol. The reaction was started by adding ethanol and the absorbance at 340 nm was followed in a spectrophotometer. The linear initial increase in absorbance was used to determine specific enzyme activities with an absorption coefficient of 6.2 mM cm−1.
Semi-quantitative reverse transcription-PCR
Total RNA was extracted from leaf material of barley seedlings and cDNA was synthesized from 1 μg of total RNA using the QuantiTec Reverse Transcription kit (QIAGEN, Hilden, Germany) following the manufacturer's instructions. For semi-quantitative RT-PCR, 1 μl of cDNA served as template in a total volume of 25 μl and specific products were amplified in 26–38 PCR cycles, as indicated, using the following primer pairs (in 5′–3′ orientation): HvADH1 (38 cycles) oligo-1 ATT CAA GGC GAC GCG AAG CAC, oligo-2 TGA AGA GGA TCT TGA CGC GCA C; HvPR1b (26 cycles) oligo-1 GTG TTG GAG CCG TAG TCG TAG T, oligo-2 TGGTATAGAGCAGGCCCATAGAA; and HvUbc2 (26 cycles) oligo-1 TCTCGTCCCTGAGATTGCCCACAT, oligo-2 TTTCTCGGGACAGCAACACAATCTTCT. PCR started with 1 min denaturation at 95 °C followed by 26–38 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 40 s, and a final step at 72 °C for 60 s. A 15 μl aliquot of the PCR products was loaded on a 1.5% agarose gel and stained with ethidium bromide.
Cloning
For transient knock-down, the hairpin construct CaMV35S::antisenseHvADH1-intron-senseHvADH1-nos carrying a partial sequence of HvADH1 was cloned as follows. A partial sequence of HvADH1 was amplified by proofreading PCR with the oligos RNAi_fwd (5′-GACAGAGGTGTGATGATCGGGGATG-3′) and RNAi_rev (5′-TGTATCATAGCATTGACGTTGCCAGTGC-3′). This fragment was cloned into the entry vector pIPKTA38 using the SwaI site, and the HvADH1 fragment was subsequently introduced into the destination vector pIPKTA30N using the standard clonase reaction. Vectors pIPKTA38 and pIPKTA30N were provided by P. Schweizer (IPK Gatersleben, Germany; Douchkov et al., 2005). For transient HvADH1 overexpression, the full-length cDNA was cloned by proofreading PCR with the oligos ADH_fwd (5′-TGGATCCTGTGAAGTGAGAGATC-3′) and ADH_rev (5′- AGTCGACCGATGATCTGGTTCAGAAG-3′), thereby introducing a BamHI and SalI restriction site, respectively, and cloned into the vector pGEM-T (Promega, Madison, WI, USA). After sequencing, the cDNA was released and subcloned into the vector pGY1 with the restriction sites BamHI and SalI to be expressed under the control of the CaMV35S promoter. Cloning of the HvMLO RNAi (RNA interference) construct for transient knock-down of HvMLO is described by Douchkov et al. (2005).
Microscopic analysis
Barley cv. ‘Ingrid’ plants were grown on soil in a growth chamber at 18 °C with 60% relative humidity and a photoperiod of 16 h (150 μmol m−2 s−1 photon flux density). Ballistic transformation of epidermal cells of 1-week-old barley leaf segments was performed using a PDS-1000/He plant transformation gun with a hepta-adaptor (Bio-Rad Laboratories GmbH, Munich, Germany), as described previously (Douchkov et al., 2005; Eichmann et al., 2010). Following bombardment, leaf segments were incubated in a growth chamber at 18 °C with 60% relative humidity and a photoperiod of 16 h (60 μmol m−2 s−1 photon flux density). For each shot, 1 μm gold particles were coated with 7 μg of pGY1–GFP (green flouorescent protein) as a transformation marker, together with 7 μg of empty pIPKTA30N vector, empty pGY1 vector, pPIKTA30N-HvADH1, pIPKTA36 (MLO RNAi), or pGY1-HvADH1. Leaf segments were bombarded with coated gold particles 48 h prior to inoculation with spores of Bgh at a density of 100 conidia mm−2. Interaction outcome was judged 48 h after inoculation by fluorescence microscopy. Transformed GFP-expressing cells were identified under blue light excitation. The susceptibility (haustorial) index was calculated as the number of penetrated GFP cells divided by the total number of GFP cells multiplied by 100. In each individual experiment, between 100 and 350 GFP-expressing cells were evaluated per variant. All together, six independent experiments were conducted.
Pyrazole treatment
The first leaves of 8-day-old barley cultivar ‘Ingrid’ seedlings were infiltrated with 10 mM glucose, 10 mM sucrose, 2 mM pyrazole, 10 mM pyrazole, and combinations of sugars and pyrazole. From the middle part of each leaf a 1 cm fragment was cut out, which resulted in the same leaf area for all samples tested, and floated on the same solution as used for infiltration. Leaf explants were infected with Bgh and incubated for 4 d in the climate chamber. The number of pustules of four-leaf explants in three replications each was counted. Pictures of leaf explants were taken 3 d and 4 d after Bgh inoculation with a digital camera mounted on a stereomicroscope (Stemi 200-C, Zeiss, Germany). Leaf material that was photographed after 3 d was extracted with destaining solution (8 ml of ethanol, 2 ml of chloroform, 0.15 g of trichloroacetic acid) overnight at room temperature, and fungal hyphae were stained for 2 min in staining solution [25% (v/v) acetic acid/blue ink: 9/1 (v/v)]. Stained samples were stored in 50% glycerol prior to photography.
Results
Infection of barley leaves with Bgh results in an increase in ADH activity
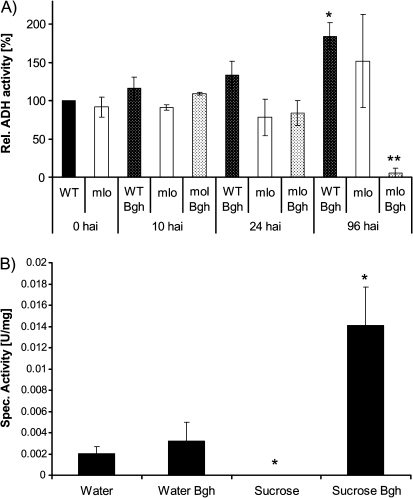
Based on preliminary microarray data, which show an induction of HvADH1 after inoculation with Bgh, a detailed analysis was performed to clarify the role of ADH in the barley–Bgh pathosystem. First, ADH activity in leaves in the course of barley–Bgh interaction was measured at different time points after infection. As ADH activity was developmentally regulated, with decreasing activity in the course of seedling development (data not shown), the specific ADH activity was normalized to the corresponding untreated cultivar Ingrid (susceptible MLO wild type; WT) control at each time point. In Fig. 1A the ADH activity at the start of the experiment, and at 10 h after inoculation (hai), 24 hai, and 96 hai is shown for non-infected and Bgh-infected samples. An induction of ADH activity was observed starting at 24 hai and reaching almost double the activity of non-infected controls at 96 hai. In parallel to the WT, the resistant mlo5 isoline was analysed for ADH activity. Interestingly, other than for the WT, there was no induction, but, in contrast, a pronounced reduction of ADH activity was observed in Bgh-infected samples at 96 hai compared with controls.
Fig. 1.
(A) Barley seedlings (WT cultivar ‘Ingrid’ and the mlo5 isoline) were grown for 8–9 d on soil, inoculated with Bgh, and leaves were harvested at 0, 10, 24, and 96 hai. Inoculated and non-inoculated (control) samples were extracted for total soluble protein, and the specific ADH activity was determined. Data represent the means of three biological replications ±SE. Asterisks indicate significant differences from the control (t-test: *P <0.05, **P <0.01). (B) Barley seedlings (WT cultivar ‘Golden Promise’) were grown for 3 d in flasks on filter paper soaked with tap water and a further 3 d in the presence or absence of 100 mM sucrose. Following this, seedlings were inoculated with Bgh, and ADH activity of leaf samples was assayed 4–5 dai. Data represent the means of three biological replications ±SE. For sucrose-treated samples, no activity was detectable. Asterisks indicate significant differences from the control (t-test: *P <0.05).
Induction of ADH activity by Bgh is enhanced in the presence of sucrose
Further experiments were aimed at increasing the available endogenous sugar content in the plants to analyse the barley–Bgh interaction under a modified metabolic status. To this end barley seeds were germinated on moistened (tap water) filter paper for 3 d and transferred to glass flasks. The seedlings were grown for 3 d on moistened (tap water) filter paper and a further 3 d in the presence or absence of 100 mM sucrose, the major transport form of sugars in higher plants. Subsequently the hydroponically grown seedlings were infected with Bgh and the ADH activity of leaf samples was determined 4–5 d thereafter. In the absence of sucrose the specific ADH activity was observed to be in the same range as acquired for soil-grown plants (0.002 U mg−1 and 0.0014 U mg−1, respectively), with a similar induction following Bgh infection by a factor of 1.6 and 1.8, respectively (Fig. 1A, B). In the presence of sucrose, however, Bgh infection resulted in a much higher ADH activity, reaching ∼7 times higher absolute values (0.014 U mg−1) compared with non-infected water controls. Thus ADH activity after Bgh challenge reaches >4 times higher values in the presence of sucrose compared with the absence of sucrose (Fig. 1B). Hence, the presence of sucrose in hydroponically grown seedlings strongly supports the Bgh-mediated induction of ADH activity.
Bgh infection results in an induction of HvADH1 expression
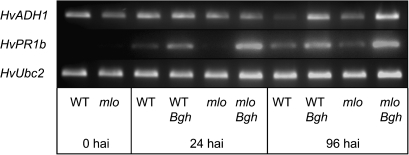
The expression of HvADH1, the ADH isoform which was previously identified in a microarray experiment to be Bgh inducible, was followed in response to Bgh treatment. As shown in Fig. 2, Bgh treatment resulted in a pronounced induction of HvADH1 at 96 hai compared with non-infected controls. At higher inoculation densities, an induction of HvADH1 already starting at 12 hai (data not shown) was observed. The above-mentioned reduction in ADH activity during seedling development is reflected in a reduced HvADH1 expression at later time points in non-infected samples. As a marker for the pathogen response, HvPR1b expression was analysed, with the expected results of a strong Bgh-triggered induction at 24 hai and 96 hai. Similar to the WT, the resistant mlo5 line showed an induction of HvADH1 expression after Bgh treatment at 96 hai compared with non-infected controls. The HvPR1b expression seems to be slightly more strongly induced in mlo5 by Bgh compared with the WT, which is in agreement with previous results (Peterhänsel et al., 1997).
Fig. 2.
Transcript levels of HvADH1, HvPR1b, and HvUbc2 (housekeeping gene) in barley leaves. Leaf material of the WT cultivar ‘Ingrid’ and the mlo5 isoline was harvested at 0, 24, and 96 h after Bgh infection together with material of non-infected controls, extracted for RNA, and analysed by semi-quantitative RT-PCR. Images show ethidium bromide-stained gels. Experiments were repeated in three biological replications, and representative data are shown.
HvADH1 modulates susceptibility to Bgh
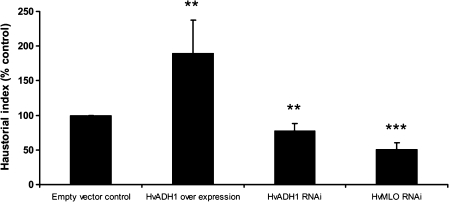
To address specifically the role of HvADH1 in susceptibility to Bgh, a hairpin construct for HvADH1 knock-down and an overexpression construct with the HvADH1 cDNA under the control of the 35S promoter was designed. These constructs were used for transient biolistic transformation in barley epidermal cells and assayed for the penetration success of Bgh. The RNAi-mediated transient knock-down of HvADH1 resulted in a reduction of fungal penetration success, and, vice versa, the overexpression of HvADH1 resulted in an increase of fungal penetration success (Fig. 3). Hence, the expression level of host ADH1 appeared to be positively associated with fungal success in penetrating barley epidermal cells.
Fig. 3.
Frequency of successful cell wall penetration and establishment of fungal haustorium at the interaction sites of transiently transformed barley leaves (WT cultivar ‘Ingrid’) with Bgh. Values are normalized to the empty vector control for HvADH1 overexpression, HvADH1 RNAi, and HvMLO RNAi (positive control for maximal reduction in penetration success). Error bars show the confidence intervals of the means (n=5) at P=0.05. Asterisks indicate significant differences (t-test: **P <0.01, ***P <0.001).
Inhibition of ADH inhibits fungal development
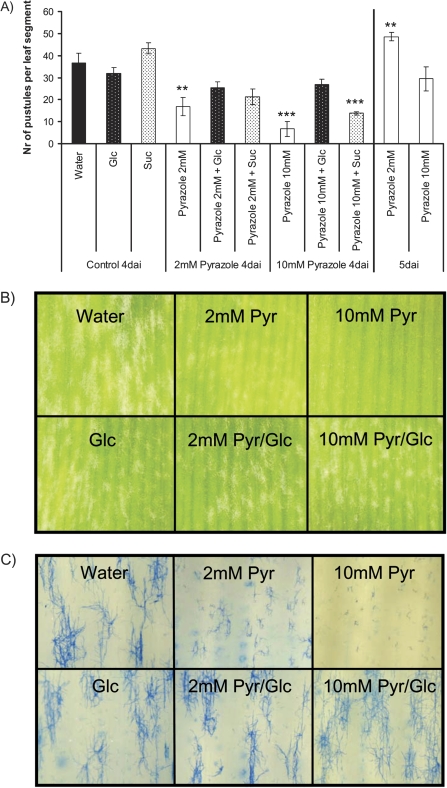
Macroscopic phenotypes in pustule development in the course of Bgh interaction were analysed using the chemical ADH inhibitor pyrazole. To this end, first leaves of 8-day-old barley seedlings were infiltrated with 10 mM glucose, 10 mM sucrose, 2 mM pyrazole, 10 mM pyrazole, and combinations of sugars and pyrazole, and floated on the same solution as used for infiltration. Subsequently, leaf explants were infected with Bgh and incubated for 4 d in the climate chamber. As detailed in Fig. 4A, inhibition of ADH activity by pyrazole resulted in a clear delay in pustule formation at 4 days after inoculation (dai). This effect increased with increasing pyrazole concentrations. At 5 dai the number of pustules in pyrazole-treated samples is the same or even higher than that of non-treated samples at 4 dai, indicating that pyrazole delays pustule formation but has no toxic effect on the plant or fungus. Non-treated samples at 5 dai are completely overgrown with fungal mycelia (data not shown). Delayed pustule formation in pyrazole-treated samples can be circumvented by the simultaneous application of glucose or sucrose. Thus glucose has a more profound effect than sucrose (Fig. 4A). Pictures of leaf explants 4 d (Fig. 4B) and 3 d (Fig. 4C) after Bgh inoculation illustrate the glucose-meditated complementation of the pyrazole effect. Data suggest a requirement for ADH activity for nutrition of Bgh on barley.
Fig. 4.
(A) Leaves of 8-day-old barley seedlings (WT cultivar ‘Ingrid’) were infiltrated with 10 mM glucose (Glc), 10 mM sucrose (Suc), 2 mM pyrazole (Pyr), 10 mM pyrazole, and combinations of sugars and pyrazole as indicated, cut into pieces of 1 cm in length, and floated on the same solution as used for infiltration. Leaf explants were infected with Bgh and incubated for 4 d in the climate chamber. The number of pustules of four leaf explants in three replications each was counted and the means ±SE are shown. Asterisks indicate significant differences (t-test: **P <0.01, ***P <0.001). (B) Images of leaf explants in A 4 d after Bgh inoculation. The height of the panels represents 3 mm. (C) Images of leaf explants treated as described in A 3 d after Bgh inoculation. Independent leaf samples were extracted with ethanol/chloroform to remove chlorophyll, and fungal hyphae were stained with blue ink. The height of the panels represents 1 mm. (This figure is available in colour at JXB online.)
Discussion
Besides the well established function of ADH in flooding stress and seed development, there is mounting evidence for a role for ADH in plant–pathogen interactions (Wildermuth, 2010). In this study the role of ADH in the course of the barley–Bgh interaction was analysed. ADH activity increased after Bgh infection starting at 24 hai and reached almost double the activity at 96 hai when compared with non-infected controls. This ADH induction is reflected in enhanced HvADH1 gene expression at 96 hai in Bgh-infected samples compared with non-infected controls. Non-infected leaves show a decrease in HvADH1 gene expression at later time points, which reflects a reduction of ADH activity in the course of seedling development. Interestingly, the resistant mlo5 line shows a similar pattern of enhanced HvADH1 transcription in Bgh-infected samples at 96 dai; however, in contrast to the WT, the ADH activity is strongly reduced at 96 hai. Bucciarelli et al. (2009) showed that the activity of a yeast ADH is redox regulated. In the active state ADH forms tetramers with Cys278 being in the reduced state. Under oxidising conditions, two ADH subunits are covalently linked via formation of disulfid bridges at Cys278. Those tetramers, consisting of two covalently linked dimers, are metabolically inactive. One explanation for the differential regulation pattern of ADH activity between the WT and mlo5 could be a similar redox regulation of barley ADHs. Barley mlo genotypes are characterized by an increased Bgh-triggered formation of hydrogen peroxide compared with the WT (Hückelhoven et al., 1999; Piffanelli et al., 2002). This increased oxidative status could result in inactivation of ADHs. An interesting further aspect of this hypothesis is contributed by Baxter-Burrell et al. (2002), who showed that a rheostat may regulate fermentative metabolism by redox regulation via NADPH oxidases. This would link ADH function with NADPH oxidases, which have a critical role in plant–pathogen interactions (Torres et al., 2002, 2005; Yoshie et al., 2005; Pogany et al., 2009) and in particular in the barley–Bgh interaction (Proels et al., 2010). Senescence is also accelerated in mlo plants (Piffanelli et al., 2002), which could contribute to reduced ADH activity in the mlo5 line, because ADH activity is usually low in senescent tissue. This would not apply to the susceptible WT line where senescence is retarded by the biotrophic fungus. A role for fermentative metabolism in the barley–Bgh interaction is plausible, as it was shown that Bgh infection results in a progressive reduction in photosynthetic rates at the site of Bgh attack (Scholes et al., 1994; Swarbrick et al., 2006), which in all probability results in a reduced oxygen pressure. Moreover, pathogen-infected tissues are characterized by altered fluxes of carbon within the infected leaf, in particular by an increased hexose level (Roitsch et al., 2003; Biemelt and Sonnewald, 2006; Berger et al., 2007). This was explicitly shown for the interaction of powdery mildew in barley and Arabidopsis (Fotopolous et al., 2003; Swarbrick et al., 2006). Such an increase in hexose levels could also help in maintaining energy metabolism under conditions of fermentation, as under limited O2 availability ATP production is mediated via glycolysis, which yields significantly less ATP per hexose compared with oxidative phosphorylation. For future in-depth analysis it would be interesting to take a closer look at the spatial distribution of ADH induction at the site of pathogen attack versus adjacent areas at different distances from the site of attack. It is assumed that a gradient of oxygen pressure would be seen in the tissue, being lowest directly at the site of attack (photosynthesis is down-regulated) and increasing with distance from the site of attack.
With the use of hydroponically grown seedlings it was substantiated that the metabolic status has an effect on the barley–Bgh interaction. Bgh infection resulted in a >4 times higher ADH activity in the presence compared with the absence of sucrose. It is speculated that the additional carbohydrates in sucrose-treated samples favour pathogen nutrition as they allow the plant to mount and maintain an induced sink metabolism, and hence a higher hexose level at the site of pathogen attack. This is in line with the observation of increased sink strength mediated by elevated invertase and hexose transporter expression at the site of pathogen attack (Fotopolous et al., 2003; Roitsch et al., 2003; Swarbrick et al., 2006). An alternative explanation could be that sucrose is directly exploited by the fungus for a better nutrient supply, as certain fungi up-regulate their own invertase in the course of the infection process (Heisterüber et al., 1994; Chou et al., 2000).
To address specifically the role of HvADH1 in susceptibility to Bgh, an RNAi-mediated transient knock-down of HvADH1 and an overexpression of HvADH1 in single barley epidermal cells was performed. The results show a reduction of fungal penetration success in the case of the HvADH1 knock-down and, inversely, an increase of fungal penetration success in the case of HvADH1 overexpression. From these data and in agreement with the notion of an increased glycolytic/fermentative metabolic activity at the site of pathogen infection (Wildermuth, 2010), it can be concluded that HvADH1 may function as a susceptibility factor and is an important component controlling the host primary metabolism in the course of Bgh infection. In particular, ADH seems to be important to maintain the primary energy metabolism of the host cell under conditions of an increased metabolic flux and an assumed low oxygen pressure at the site of infection. As biotrophic pathogens depend on viable host cells for nutrient acquisition, ADH overexpression, and hence priming of the host cell for glycolytic and fermentative metabolism, could result in even higher penetration rates. The reported clearcut effects after biolistic transformation are already manifest at early time points (48 hai or earlier), in addition to the increase in ADH activity, which yields significant differences at 96 hai. This discrepancy might be explained by the fact that biolistic transformation is a method that allows for analysing the sites of interaction with single transformed cells. Regarding enzyme activities, the whole leaf, including non-infected epidermal cells and mesophyll, was sampled, which results in a ‘dilution effect’, as probably only infected epidermal and/or a few surrounding cells contribute to the observed changes in ADH activity at early time points.
Modulation of ADH activity by the chemical inhibitor pyrazole at concentrations of 2 mM and 10 mM results in a clear delay in fungal development (pustule formation). In the macroscopic analysis, a clear reduction in the number of pustules formed was observed at 4 dai in the presence of pyrazole, indicating that the modulation of ADH activity has an additional effect on symptoms in the barley–Bgh interaction. Inhibition of ADH activity obviously interferes with metabolic reprogramming after Bgh attack. This effect can be circumvented by application of glucose or sucrose together with pyrazole. The authors speculate that the fungus metabolizes the applied sugars to maintain metabolism and hence mycelial growth, and is therefore less dependent on nutrients obtained from the host cell. An inhibition of ADH by pyrazole and a concomitant interference with host metabolism therefore has less effect on fungal development in the presence of externally applied sugar. Thus glucose has a more profound effect than sucrose, which corresponds to the finding that powdery mildew fungi preferably take up and metabolize hexose sugars (Sutton et al., 1999). Taken together, the reversibility of the observed phenotype by application of sugars clearly points towards a specific nutritional effect of pyrazole. A direct effect on the fungus thus appears very unlikely because Bgh itself appears to lack a fermentative pathway (Spanu et al., 2010).
In summary, the presented data show a function of ADH in the compatible interaction of barley and Bgh. In particular, misexpression of HvADH1, a pathogen-responsive barley ADH gene, modulates susceptibility to Bgh, with overexpression of HvADH1 resulting in higher susceptibility and knock-down of HvADH1 resulting in lower susceptibility to Bgh. Hence, HvADH1 can be regarded as a potential susceptibility factor in the barley–Bgh pathosystem. Consequently, modulating alcoholic fermentative metabolism or conditions that regulate its activity may offer the possibility to improve plant performance under biotic stress conditions. ADH may fulfil a function in maintaining the glycolytic/fermentative pathway in pathogen-stressed plants, which could be crucial for hexose supply and biotrophy of the powdery mildew fungus.
Acknowledgments
The authors are grateful to Angelika Muhr for excellent technical assistance.
Glossary
Abbreviations
- ADH
alcohol dehydrogenase
- Bgh
Blumeria graminis f.sp. hordei
- dai
days after inoculation
- hai
hours after inoculation
References
- Bailey-Serres J, Voesenek LACJ. Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology. 2008;59:313–339. doi: 10.1146/annurev.arplant.59.032607.092752. [DOI] [PubMed] [Google Scholar]
- Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J. ROPGAP4-dependent Rop GTPase rheostat controls of Arabidopsis oxygen deprivation tolerance. Science. 2002;296:2026–2028. doi: 10.1126/science.1071505. [DOI] [PubMed] [Google Scholar]
- Berger S, Sinha AK, Roitsch T. Plant physiology meets phytopathology: plant primary metabolism and plant–pathogen interactions. Journal of Experimental Botany. 2007;58:4019–4026. doi: 10.1093/jxb/erm298. [DOI] [PubMed] [Google Scholar]
- Biemelt S, Sonnewald U. Plant–microbe interactions to probe regulation of plant carbon metabolism. Journal of Plant Physiology. 2006;163:307–318. doi: 10.1016/j.jplph.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Bucciarelli T, Saliola M, Brisdelli F, Bozzi A, Falcone C, Di Ilio C, Martini F. Oxidation of Cys278 of ADH I isozyme from Kluyveromyces lactis by naturally occuring disulfides causes its reversible inactivation. Biochimicia et Biophysica Acta. 2009;1794:563–568. doi: 10.1016/j.bbapap.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Bucher M, Brander KA, Sbicego S, Mandel T, Kuhlemeier C. Aerobic fermentation in tobacco pollen. Plant Molecular Biology. 1995;28:739–750. doi: 10.1007/BF00021197. [DOI] [PubMed] [Google Scholar]
- Chandran D, Inada N, Hather G, Kleindt CK, Wildermuth MC. Laser microdissection of Arabidopsis cells at the powdery mildew infection site reveals site-specific processes and regulators. Proceedings of the National Academy of Sciences, USA. 2010;107:460–465. doi: 10.1073/pnas.0912492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou H-M, Bundock N, Rolfe SA, Scholes JD. Infection of Arabidopsis thaliana leaves with Albugo candida white blister rust causes a reprogramming of host metabolism. Molecular Plant Pathology. 2000;1:99–113. doi: 10.1046/j.1364-3703.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- Douchkov D, Nowara D, Zierold U, Schweizer P. A high throughput gene-silencing system for the functional assessment of defense-related genes in barley epidermal cells. Molecular Plant-Microbe Interactions. 2005;18:755–761. doi: 10.1094/MPMI-18-0755. [DOI] [PubMed] [Google Scholar]
- Drew MC. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- Eichmann R, Bischof M, Weis C, Shaw J, Lacomme C, Schweizer P, Duchkov D, Hensel G, Kumlehn J, Hückelhoven R. BAX INHIBITOR-1 is required for full susceptibility of barley to powdery mildew. Molecular Plant-Microbe Interactions. 2010;23:1217–1227. doi: 10.1094/MPMI-23-9-1217. [DOI] [PubMed] [Google Scholar]
- Fotopoulos V, Gilbert MJ, Pittman JK, Marvier AC, Buchanan AJ, Sauer N, Hall JL, Williams LE. The monosaccharide transporter gene, AtSTP4, and the cell-wall invertase, Atβfruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum. Plant Physiology. 2003;132:821–829. doi: 10.1104/pp.103.021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J. Plant responses to hypoxia—is survival a balancing act? Trends in Plant Science. 2004;9:449–456. doi: 10.1016/j.tplants.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Geigenberger P. Response of plant metabolism to too little oxygen. Current Opinion in Plant Biology. 2003;6:247–256. doi: 10.1016/s1369-5266(03)00038-4. [DOI] [PubMed] [Google Scholar]
- Good AG, Crosby WL. Induction of alcohol dehydrogenase and lactate dehydrogenase in hypoxically induced barley. Plant Physiology. 1989;90:860–866. doi: 10.1104/pp.90.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson AD, Brown AHD. Three alcohol dehydrogenase genes in wild and cultivated barley: characterization of the products of variant alleles. Biochemical Genetics. 1984;22:495–515. doi: 10.1007/BF00484519. [DOI] [PubMed] [Google Scholar]
- Hanson AD, Jacobsen JV, Zwar JA. Regulated expression of three alcohol dehydrogenase genes in barley aleurone layers. Plant Physiology. 1984;75:573–581. doi: 10.1104/pp.75.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harberd NP, Edwards KJR. Further studies on the alcohol dehydrogenases of barley: evidence for a third alcohol dehydrogenase locus and data on the effect of an alcohol dehydrogenase-1 null mutation in homozygous and in heterozygous condition. Genetical Research. 1983;41:109–116. [Google Scholar]
- Heisterüber D, Schulte P, Moerschbacher BM. Soluble carbohydrates and invertase activity in stem rust infected, resistant and susceptible near-isogenic wheat leaves. Physiological and Molecular Plant Pathology. 1994;45:111–123. [Google Scholar]
- Hückelhoven R, Fodor J, Preis C, Kogel K- H. Hypersensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with H2O2 but not with salicylic acid accumulation. Plant Physiology. 1999;119:1251–1260. doi: 10.1104/pp.119.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RA, Rumpho ME, Fox TC. Anaerobic metabolism in plants. Plant Physiology. 1992;100:1–6. doi: 10.1104/pp.100.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol PK, Jacobsen JV. Endosperm acidification and related metabolic processes in the developing barley grain. Plant Physiology. 1992;98:1098–1104. doi: 10.1104/pp.98.3.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol PK, Jacobsen JV. Regulation of alcohol dehydrogenase gene expression in barley aleurone by gibberellin and abscisic acid. Physiologia Plantarum. 2001;111:533–539. doi: 10.1034/j.1399-3054.2001.1110414.x. [DOI] [PubMed] [Google Scholar]
- Peterhänsel C, Freialdenhoven A, Kurth J, Kolsch R, Schulze-Lefert P. Interaction analyses of genes required for resistance responses to powdery mildew in barley reveal distinct pathways leading to leaf cell death. The Plant Cell. 1997;9:1397–1409. doi: 10.1105/tpc.9.8.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piffanelli P, Zhou F, Casais C, Orme J, Jarosch B, Schaffrath U, Collins NC, Panstruga R, Schulze-Lefert P. The barley MLO modulator of defense and cell death is responsive to biotic and abiotic stress stimuli. Plant Physiology. 2002;129:1076–1085. doi: 10.1104/pp.010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogány M, von Rad U, Grün S, Dongó A, Pintye A, Simoneau P, Bahnweg G, Kiss L, Barna B, Durner J. Dual roles of reactive oxygen species and NADPH oxidase RBOHD in an Arabidopsis–Alternaria pathosystem. Plant Physiology. 2009;151:1459–1475. doi: 10.1104/pp.109.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proels RK, Roitsch T. Extracellular invertase LIN6 of tomato: a pivotal enzyme for the integration of metabolic, hormonal, and stress signals is regulated by a diurnal rhythm. Journal of Experimental Botany. 2009;60:1555–1567. doi: 10.1093/jxb/erp027. [DOI] [PubMed] [Google Scholar]
- Proels RK, Oberhollenzer K, Pathuri IP, Hensel G, Kumlehn J, Hückelhoven R. RBOHF2 of barley is required for normal development of basal penetration resistance to the parasitic fungus Blumeria graminis f.sp. hordei. Molecular Plant-Microbe Interactions. 2010;23:1143–1150. doi: 10.1094/MPMI-23-9-1143. [DOI] [PubMed] [Google Scholar]
- Roitsch T, Balibrea ME, Hofmann M, Proels R, Sinha AK. Extracellular invertase: key metabolic enzyme and PR protein. Journal of Experimental Botany. 2003;54:513–524. doi: 10.1093/jxb/erg050. [DOI] [PubMed] [Google Scholar]
- Scholes JD, Lee PJ, Horton P, Lewis DH. Invertase: understanding changes in the photosynthetic and carbohydrate metabolism of barley leaves infected with powdery mildew. New Phytologist. 1994;126:213–222. [Google Scholar]
- Spanu PD, Abbott JC, Amselem J, et al. Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science. 2010;330:1543–1546. doi: 10.1126/science.1194573. [DOI] [PubMed] [Google Scholar]
- Sutton PN, Henry MJ, Hall JL. Glucose, and not sucrose, is transported from wheat to wheat powdery mildew. Planta. 1999;208:426–430. [Google Scholar]
- Swarbrick P, Schulze-Lefert P, Scholes JD. Metabolic consequences of susceptibility and resistance (race-specific and broad-spectrum) in barley leaves challenged with powdery mildew. Plant, Cell and Environment. 2006;29:1061–1076. doi: 10.1111/j.1365-3040.2005.01472.x. [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proceedings of the National Academy of Sciences, USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nature Genetics. 2005;37:1130–1134. doi: 10.1038/ng1639. [DOI] [PubMed] [Google Scholar]
- Trick M, Dennis ES, Edwards KJR, Peacock WJ. Molecular analysis of the alcohol dehydrogenase gene family of barley. Plant Molecular Biology. 1988;11:147–160. doi: 10.1007/BF00015667. [DOI] [PubMed] [Google Scholar]
- Wiberg A. Genetical studies of spontaneous sources of resistance to powdery mildew in barley. Hereditas. 1974;77:89–148. doi: 10.1111/j.1601-5223.1974.tb01357.x. [DOI] [PubMed] [Google Scholar]
- Wildermuth MC. Modulation of host nuclear ploidy: a common plant biotroph mechanism. Current Opinion in Plant Biology. 2010;13:1–10. doi: 10.1016/j.pbi.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Yoshie Y, Goto K, Takai R, Iwano M, Takayama S, Isogai A, Che FS. Function of the rice gp91phox homologs OsrbohA and OsrbohE genes in ROS-dependent plant immune responses. Plant Biotechnology. 2005;22:127–135. [Google Scholar]