Abstract
Phototropic curvature results from differential growth on two sides of the elongating shoot, which is explained by asymmetrical indole-3-acetic acid (IAA) distribution. Using 2 cm maize coleoptile segments, 1st positive phototropic curvature was confirmed here after 8 s irradiation with unilateral blue light (0.33 μmol m−2 s−1). IAA was redistributed asymmetrically by approximately 20 min after photo-stimulation. This asymmetric distribution was initiated in the top 0–3 mm region and was then transmitted to lower regions. Application of the IAA transport inhibitor, 1-N-naphthylphthalamic acid (NPA), to the top 2 mm region completely inhibited phototropic curvature, even when auxin was simultaneously applied below the NPA-treated zone. Thus, lateral IAA movement occurred only within the top 0–3 mm region after photo-stimulation. Localized irradiation experiments indicated that the photo-stimulus was perceived in the apical 2 mm region. The results suggest that this region harbours key components responsible for photo-sensing and lateral IAA transport. In the present study, it was found that the NPH3- and PGP-like genes were exclusively expressed in the 0–2 mm region of the tip, whereas PHOT1 and ZmPIN1a, b, and c were expressed relatively evenly along the coleoptile, and ZmAUX1, ZMK1, and ZmSAURE2 were strongly expressed in the elongation zone. These results suggest that the NPH3-like and PGP-like gene products have a key role in photo-signal transduction and regulation of the direction of auxin transport after blue light perception by phot1 at the very tip region of maize coleoptiles.
Keywords: Coleoptiles. indole-3-acetic acid, maize, NPH3, PGP, phototropism
Introduction
Plant growth responses such as phototropism have fascinated plant biologists since the pioneering work of Charles and Francis Darwin (Darwin and Darwin, 1880) more than 125 years ago. Later, studies on growth and tropism using grass coleoptiles resulted in the characterization of indole-3-acetic acid (IAA), the major native auxin. Since then, IAA has been shown to be involved in many physiological events (Woodward and Bartel, 2005). The IAA produced at the tip region of grass coleoptiles is transported downwards, and participates in the plant bending response. As explained by the Cholodny–Went Hypothesis, this tropic response is the result of differential growth on the two sides of the elongating shoot, which can be explained by asymmetrical IAA distribution (Went and Thimann, 1937; see also a recent review by Holland et al., 2009).
In the last decade, studies on the model plant Arabidopsis thaliana have advanced our understanding of the mechanisms of phototropism. Research on several mutants impaired in hypocotyl phototropism has yielded some important new findings; phot1 was identified as the major photoreceptor, and NPH3 and its homologue RPT2 were shown to be involved in tropic responses (Holland et al., 2009). The NPH3 protein, which directly interacts with phot1, was identified as a signalling component essential for the phototropic response (Inada et al., 2004). In addition, it was shown that AtENP/MAB4/NPY1, a NPH3-like protein, functions as a signal transducer during organogenesis and regulates auxin distribution by modifying cellular PIN localization (Cheng et al., 2007; Furutani et al., 2007). In spite of these advances, the precise sites of photo-sensing and lateral auxin redistribution in dicots are still unknown.
Monocot coleoptiles, particularly maize, are an ideal model for researching the Cholodny–Went Hypothesis (Went and Thimann, 1937). One advantage of the maize coleoptile system is that the tissue is relatively large, much larger than Arabidopsis roots, and thus it is possible to quantify endogenous IAA and to conduct detailed analyses of IAA movements. Moreover, coleoptile tips are known to be the site of IAA production (Iino and Carr, 1982; Koshiba et al., 1995; Bennett et al., 2000; Mori et al., 2005; Nishimura et al., 2009). The generally accepted model of phototropism is that the tip region detects the direction of the photo-stimulus, and lateral IAA transport at the tip establishes the asymmetric IAA distribution that moves basipetally, causing the lower region to bend (Briggs et al., 1957; Iino, 1991, 1995; Fuchs et al., 2003). These physiological studies suggest that the very tip region of monocot coleoptiles harbours a specific photo-sensing system and a lateral IAA transport system that orchestrate the phototropic response. CPT1, a rice orthologue of Arabidopsis NPH3, was identified from the cpt1 mutant, which exhibited a non-phototropic coleoptile phenotype. CPT1 may act upstream of auxin redistribution induced by unilateral illumination (Haga et al., 2005). Fuchs and coleagues reported that expression of the K+-channel protein ZMK1 is regulated by asymmetric auxin distribution during blue-light-induced tropic curvature of maize coleoptiles (Philippar et al., 1999; Fuchs et al., 2003). It was previously reported that ZmSAUR has an important role in differential growth at the elongation zone of maize coleoptiles induced by IAA asymmetric distribution after gravi-stimulus (Nishimura et al., 2009). Although these factors have been studied in monocot coleoptiles, there is little information about the key factor(s) that determine tip-region specific photo-sensing and lateral IAA redistribution.
Regarding the regulation of IAA transport, there is a large body of evidence that PIN proteins (auxin efflux carriers) function in the transport of IAA. In Arabidopsis, it is postulated that AUX1, PIN2, and PIN3 are involved in relocating IAA in response to tropic stimuli (Friml and Palme, 2002; Moore, 2002). Among these proteins, AtPIN3 is known to have a role in IAA redistribution in the tropic response of Arabidopsis hypocotyls (Friml and Palme, 2002). In the monocot maize, ZmPIN1a, b, and c are involved in auxin transport during embryogenesis and vegetative development (Carraro et al., 2006; Gallavotti et al., 2008; Forestan et al., 2010). It was shown previously that IAA is synthesized from Trp at the very tip region of maize coleoptiles, and the synthesized IAA moves to basal parts via polar transport regulated by ZmPIN1 proteins (Nishimura et al., 2009). After gravi-stimulus, there was no significant change in the cellular localization of ZmPIN1, but IAA was immediately redistributed in an asymmetrical manner. These results indicate that other PIN protein(s) or PGP-like transporter(s) are involved in lateral IAA movements, as proposed for Arabidopsis (Noh et al., 2003; Li et al., 2007; Nagashima et al., 2008). Since the gravity stimulus is evenly received by every cell within the plant tissue, it is difficult to establish an experimental system to apply a localized gravi-stimulus treatment. By contrast, a photo-stimulus can be applied to localized regions by irradiating specific parts. Using detached 2 cm maize coleoptile segments, we report here on (i) the formation of asymmetrical IAA distribution after photo-stimulation, (ii) the precise region of photo-sensing in coleoptiles, and (iii) the tip-specific expression of NPH3- and PGP-like genes. The role of the coleoptile tip region in light-perception and signal transduction, which lead to the formation of asymmetric IAA distribution during phototropic curvature of maize coleoptiles, is discussed here. The close relationship between tip-specific IAA biosynthesis and blue light perception in maize coleoptiles is also discussed.
Materials and methods
Plant materials and growth conditions
Seeds of maize (Zea mays L. cv. Golden Cross Bantam 70) were rinsed in running tap water for 10 h, sown on moist paper towels, and allowed to germinate at 25 °C under red light (0.45 μmol m−2 s−1) for 2.5 d. All experiments were performed under red light.
Phototropic and gravitropic stimulation
To investigate the phototropic response, 2 cm coleoptile segments were detached from the seedlings, and the base of each segment was clamped in 1% agar as described previously (Nishimura et al., 2009) (see Supplementary Fig. S1 at JXB online). The coleoptile segment was oriented along the plane passing through the two vascular bundles vertical to the direction of blue light (see Supplementary Fig. S2 at JXB online). The segment was irradiated unilaterally with blue light (LED, ISL-150X150-BB, CCS Inc., Kyoto, Japan) for 8 s at 0.33 μmol m−2 s−1 [first pulse-induced positive phototropism (1st positive curvature)], for 200 s at 0.25 μmol m−2 s−1 (no curvature), or with continuous blue light at 10 μmol m−2 s−1 [time-dependent phototropism (2nd positive curvature)]. The fluence rate was measured with a quantum photometer (Li-Cor, Lincoln, NE). To avoid the effects of natural gravity, the photo-stimulated segments were rotated at 2 rpm on a clinostat. During treatments, coleoptiles were kept under red light and the degree of curvature was observed by taking photos at appropriate times. To investigate gravitropic curvature, the segments were tilted horizontally as described previously (Nishimura et al., 2009).
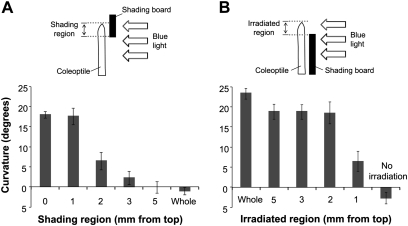
In some experiments, the coleoptile segment was shaded during blue light irradiation with a shading board (two pieces of black paper either side of a sheet of aluminium foil) (see Fig. 4). We confirmed that the shading board did not transmit any light between 200 nm and 590 nm (0% transmittance). To determine the phototropic curvature of individual coleoptiles, photographs were taken at appropriate time intervals after blue light stimulation, and curvature was quantified by drawing a line tangent to the tip to the long axis of the coleoptile and measuring the angular deviation of the tangent from the one obtained before phototropic induction.
Fig. 4.
Phototropic curvature of maize coleoptiles induced by localized irradiation with unilateral blue light. Effects of tip shading (A) and of localized irradiation (B) on 1st positive phototropism. Diagram illustrating the method of blue light irradiation is shown at the top of each figure. The degree of curvature was determined at 90 min after blue light stimulation. Data were obtained from three or more independent experiments and values are means ±SE.
Chemical treatments were applied to the inside surface of the coleoptile segments with a capillary tube: 25 mM potassium phosphate buffer (KPB) pH 6.7 (mock) or 100 μM NPA in KPB was applied to the 0–2 mm region. Simultaneously, 100 μM IAA, NAA or 2,4-D in 25 mM KPB was applied to the 3–4 mm region. The coleoptile segments were placed on an agar block and the photo- and gravi-curvature was observed (see Supplementary Fig. S4 at JXB online).
Measurement of IAA distribution after phototropic stimulation
Free IAA was extracted from tissue samples and quantified by GC-SIM-MS as described previously (Nishimura et al., 2009). There were some differences in growth conditions between this study and our previous work, for example, continuous red light irradiation for 2.5 d in this study, but 2 d red light and 1 d in darkness in our previous study. Thus, it was assessed whether these changes affected the steady levels of IAA in maize coleoptile tissue (see Supplementary Fig. S3 at JXB online). There were some differences in the amounts of free IAA diffusing into the agar block and in the tissue, but these differences were not substantial. IAA was quantified at various time points and compared with that determined at the zero time point. After 0, 10, 20, 30, and 60 min light stimulation, the coleoptile segments were separated into 0–3, 3–6, 6–9, and 9–12 mm sections (measured from the top), and these sections were further separated into irradiated and shaded sides. The free IAA content was measured in three replicates of each type of tissue segment.
Quantitative real-time RT-PCR
Total RNA was extracted using the Qiagen RNeasy Plant Mini Kit, and contaminating DNA was removed using Promega RQ1 RNase-Free DNase. Reverse transcription of RNA was carried out using the Promega M-MLV Reverse Transcriptase, RNase H Minus, Point Mutant following the manufacturer's instructions. Real-time PCR was performed with a LightCycler 480 (Roche Diagnostics) using SYBR Green I Master mix and the primers listed below. The reference gene used was ubiquitin. All real-time RT-PCR experiments were performed in biological duplicate and technical duplicate. The melting and standard curves were determined for each real-time PCR.
Real-time PCR primers were as follows: PHOT1 fw (5'-CCACGGTCATTGTCAG-3'), PHOT1 rev (5'-CTTCCCTTGGTTTTAGAATG-3'); NPH3-like gene fw (5'-CAGGAGGATCTTCGACAAGC-3'), NPH3-like gene rev (5'-GGGCAGCAGTGATTAGCTTC-3'); PGP-like gene fw (5'-GACCTGAACGGCAAAGAAAA-3'), PGP-like gene rev (5'-AATGAGACAACAGCCGTTCC-3'); ZmPIN1a fw (5'-ATAATCGCGTGCGGGAACAA-3'), ZmPIN1a rev (5'-TCCTGCTCCACATCCCCATC-3'); ZmPIN1b fw (5'-ATCATCGCGTGCGGGAACAA-3'), ZmPIN1b rev (5'-ACCCACGGGTCGGTCACAGG-3'); ZmPIN1c fw (5'-TCATCCCCATGGAGTCGAGGATGCCACC-3'), ZmPIN1c rev (5'-GGATCCACCCAGACCCAATCCCCATACCTACTTCT-3'); ZmAUX1 fw (5'-AGTCGAGGGAGAACGCCGTG-3'), ZmAUX1 rev (5'-GGTGGTGGCGGAGGAAGAAG-3'); ZmSAUR2 fw (5'-CAAGAAGTGGCAGAGGATGG-3'), ZmSAUR2 rev (5'-GCTAATATCTTTGCTCCACT-3'); and ZMK1 fw (5'-ATAACAATGGGCATACAG-3'), ZMK1 rev (5'-TTCCGTCTTTCATTGAG-3'). All quantifications were normalized against the signal of ubiquitin cDNA fragments generated using the following primers: ubiquitin fw (5'-TAAGCTGCCGATGTGCCTGCGTCG-3') and ubiquitin rev (5'-CTGAAAGACAGAACATAATGAGCACAG-3').
Results
Phototropic curvature in the maize coleoptile 2 cm segment system
In many previous studies on monocot coleoptiles, phototropic curvature in response to various light conditions, including unilateral blue light irradiation, has been studied in ‘whole seedlings’ grown in darkness and/or under red light (Briggs, 1960; Zimmerman and Briggs, 1963; Liu and Iino, 1996). An experimental method has been developed to study gravitropic curvature using detached 2-cm maize coleoptile sections (Nishimura et al., 2009). In the present study, it was assessed first whether this experimental system was also suitable for research on phototropic curvature. Segments (2 cm) of maize coleoptiles were excised from the seedlings and the bases were fixed in 1% agar (see Supplementary Fig. S1 at JXB online). The coleoptile segments bent most effectively when the thinner side was oriented towards the shaded side, and the plane of the two vascular bundles within the coleoptile was vertical to the direction of blue light (see Supplementary Fig. S2 at JXB online). All of the following experiments were carried out in these conditions.
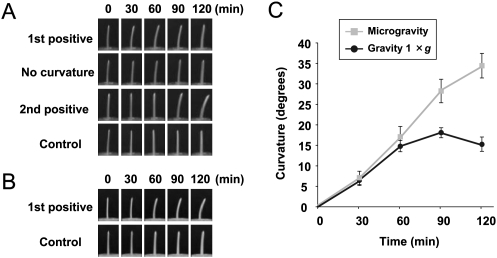
The segments were irradiated with unilateral blue light for 8 s at 0.33 μmol m−2 s−1 [first pulse-induced positive phototropism (1st positive curvature)], for 200 s at 0.25 μmol m−2 s−1 (no curvature), or with continuous blue light at 10.0 μmol m−2 s−1 [time-dependent phototropism (2nd positive curvature)]. As shown in Fig. 1A, the responses were characteristic of the phototropic responses reported previously for maize seedlings (Iino, 1987). Thus, in the following experiments, segments were irradiated unilaterally with blue light for 1st positive curvature.
Fig. 1.
Phototropic curvature of coleoptile 2 cm segments. Phototropic fluence-response of 2 cm coleoptile segments under normal gravity conditions (1 g) (A) and microgravity conditions on a clinostat (B). Time-courses of phototropic curvature (C). Coleoptile segments were irradiated with unilateral (from the right side) blue light for 8 s at 0.33 μmol m−2 s−1 (1st positive), for 200 s at 0.25 μmol m−2 s−1 (no curvature), with continuous blue light at 10 μmol m−2 s−1 (2nd positive), or no irradiation (control). Degree of curvature was determined at the indicated times after blue light stimulation. Data were obtained from four or more independent experiments and values are means ±SE.
In the case of 1st positive curvature, phototropic curvature was visible within 30 min, and increased until 60 min before gradually decreasing (Fig. 1A, C). This pattern of movement appeared to show effects of gravity, because the phototropic stimulus makes the segments bend away from the vertical axis (Nick and Schäfer, 1988). To confirm that gravity was affecting the response, 1st positive phototropic curvature was compared between normal gravity conditions (1 g) and microgravity conditions on a clinostat (Fig. 1). In microgravity conditions the curvature continued after 60 min and persisted until at least 120 min (Fig. 1C).
Changes in free IAA levels after photo-stimulus
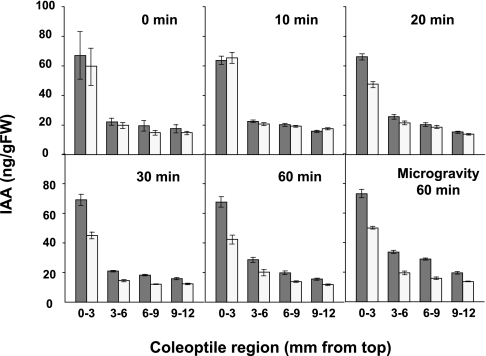
To investigate the distribution and movement of IAA in detail, the free IAA levels in each irradiated and shaded half of various sections of the coleoptile (from the tip: 0–3, 3–6, 6–9, and 9–12 mm sections) were determined before and after blue light stimulation (1st positive) (Fig. 2). Before stimulation (0 min), IAA was evenly distributed between the irradiated and shaded half, and the top 0–3 mm section contained more than 2.5-fold greater levels of IAA than the lower sections. A similar pattern of distribution was observed after 10 min of photo-stimulation, but after 20 min the distribution of IAA was asymmetrical, especially in the 0–3 mm tip region; IAA levels were greater on the shaded side than on the stimulated side. Thus, lateral movement of IAA in the top region appeared to occur between 10 min and 20 min after photo-stimulus. After 30 min, the asymmetrical distribution of IAA was more pronounced, and was also detected in lower parts (3–12 mm). The asymmetrical IAA distribution persisted until 60 min under normal gravity conditions (Fig. 2). Under microgravity conditions, the distribution of IAA became more asymmetrical from 30–60 min after the photo-stimulus (Fig. 2).
Fig. 2.
Redistribution of IAA in coleoptiles after photo-stimulation. IAA redistribution during phototropic curvature under normal gravity conditions (0–60 min), and under microgravity conditions on a clinostat after photo-stimulation (60 min). Endogenous free IAA was extracted from each 3 mm section from the top to the basal parts of the shaded and irradiated sides of the coleoptiles after photo-stimulation and IAA levels were determined by GC-SIM-MS. White bar, irradiated side of the coleoptiles; black bar, shaded side of the coleoptiles. Data and error bars represent means ±SE (15≤n≤27).
Effect of local applications of NPA and auxin on phototropic curvature
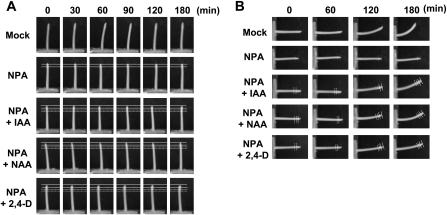
The effects of simultaneous NPA and auxin applications to the coleoptile tip region (see Supplementary Fig. S4 at JXB online) were investigated next. The application of NPA to the top 0–2 mm region completely inhibited phototropic and gravitropic curvatures (Fig. 3). However, an additional treatment with auxin at the 4–5 mm region restored gravitropic curvature, but not phototropic curvature (Fig. 3B and 3A, respectively). These results suggested that the formation of asymmetric IAA distribution occurs at the very tip after photo-stimulation, but along a greater length of the coleoptile after gravi-stimulation as proposed previously (Iino, 1991).
Fig. 3.
Effect of localized NPA and auxin treatment on phototropic and gravitropic curvature of 2 cm coleoptile segments. NPA (100 μM) and auxins [IAA, NAA, 2,4-D (100 μM)] were applied locally to the inside of the top 2 mm and 3–4 mm regions, respectively (see Supplementary Fig. S4 at JXB online), then the phototropic response under normal gravity conditions (A) and the gravitropic response (B) of the segments were observed. NPA and auxins were pretreated for 30 min before the initiation of stimuli. Solid and broken lines represent the regions of NPA and auxin treatment, respectively.
Identification of the photo-sensing region for the phototropic response
To identify the photo-sensing region in the coleoptile tip, the effects of localized irradiation on curvature were determined (Fig. 4). When the apical 5, 3, or 2 mm region was shaded, phototropic curvature was strongly inhibited. While, shading of the top 1 mm region did not decrease the degree of phototropic curvature. Phototropic curvature occurred almost normally when only the 2–5 mm tip region was irradiated with blue light, but it decreased slightly when the 1 mm tip region was stimulated (Fig. 4B). These results suggested that the apical 2 mm region is the critical area for photo-sensing for pulse-induced phototropism.
Expression of genes related to phototropism in maize coleoptiles
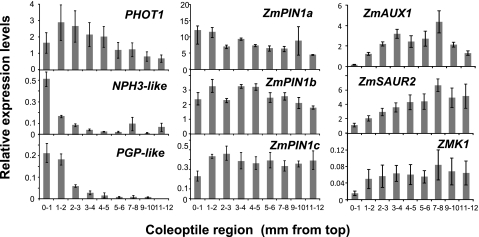
The involvement of certain genes in tip-specific photo-sensing, signal transduction, and/or the formation of asymmetrical IAA distribution were investigated next. A DNA microarray with 44k maize array plates was constructed. By comparing expressions between the tip (top 0–2 mm) and subapical regions (4–6 mm from the top), two genes related to blue light sensing/signalling and IAA transport were found that were highly expressed in the coleoptile tip region; an NPH3 (non-phototropic hypocotyl 3)-like gene and a PGP-like gene. Therefore, the expression of these two genes, as well as the blue light receptor PHOT1, IAA influx and efflux transporters, ZmAUX1 and ZmPIN1s, respectively, and auxin-responsive ZmSAUR and ZMK1 genes in the coleoptile, were investigated. The pattern of expression of each gene was determined along the length of the coleoptile section. As shown in Fig. 5, ZmPIN1a, b, and c were expressed relatively evenly throughout the coleoptile, while expression of PHOT1 was 1.5–2 times higher in the 0–5 mm tip region than in the lower 5–12 mm region. In contrast, NPH3- and PGP-like genes were specifically expressed in the 0–1 or 0–2 mm tip region, while ZmAUX1, ZmSAUR, and ZMK1 were expressed weakly in the tip region, but strongly in the elongation zone.
Fig. 5.
Expression of PHOT1, NPH3-like, PGP-like, ZmAUX1, ZmPIN1s, ZmSAUR, and ZMK1 genes in maize coleoptiles. Total mRNA was obtained from 20 sections of each 1 mm region indicated. The relative ratio of gene expression is normalized against expression of the ubiquitin gene. All real-time RT-PCR experiments were performed in biological duplicate and technical duplicate, and values are means ±SE.
Discussion
Maize coleoptile segments provide a simpler system for analysing mechanisms of photo-sensing and auxin redistribution
In general, whole seedlings have been used in previous studies on tropic curvature of monocot coleoptiles (Briggs et al., 1957; Liu and Iino, 1996; Nick et al., 1992; Fuchs et al., 2003). A simpler system was developed with 2 cm coleoptile segments detached from maize seedlings to investigate the gravitropic response (Nishimura et al., 2009). Because the IAA content can be quantified in 0.5–1.0 mm sections in this system, the pattern of IAA distribution and its fluctuations over time within the tissue can be accurately determined (Fig. 2). Furthermore, it is easy to localize chemical treatments at both the outside and the inside regions of coleoptile tips (Fig. 3). The relationship between coleoptile position and the degree of tropic curvature was also defined; coleoptile segments bend most effectively when the orientation of the light stimulus is at right angles to the plane of the two vascular bundles and the thinner side is oriented towards the shaded side (see Supplementary Fig. S2 at JXB online). This orientation is the same as that for optimal gravitropic curvature as reported previously (Nishimura et al., 2009). In addition, because 2 cm segments are used, it is easy to manipulate light stimuli and microgravity conditions. The degrees of curvature observed during a light-fluence test (1st positive, no curvature, and 2nd positive) and under normal and microgravity conditions (Fig. 1) were similar to those reported previously using whole seedlings (Iino, 1987; Nick and Schäfer, 1988).
Tip-specific IAA biosynthesis and lateral transport in maize coleoptiles
The Darwins stated in their book, ‘We must therefore conclude that when seedlings are freely exposed to a lateral light some influence is transmitted from the upper to the lower part, causing the latter to bend’ (Darwin and Darwin, 1880). There is strong evidence that this ‘influence’ is auxin. In monocot coleoptiles, particularly, IAA is known to be synthesized from Trp at the tip region, and then transported to lower parts (Iino, 1982; Weiler and Wischnewski, 1984; Koshiba et al., 1995; Nishimura et al., 2009). In our research on maize coleoptiles, detailed analyses of quantitative IAA distribution and movement within the coleoptile have been conducted (Mori et al., 2005; Nishimura et al., 2009). The results of those studies showed that de novo synthesis of IAA from Trp occurs in the 0–2 mm tip region, and that the synthesized IAA is transported mainly by ZmPIN1(s). Recently, IAA immuno-histochemical techniques were used to visualize IAA localization in maize coleoptile tips (T Nishimura et al., unpublished data), and it was found that IAA synthetic cells are localized in the top 0.5–1.5 mm region. These findings clearly confirm that the very tip region of the coleoptile is a specific site of IAA biosynthesis, and that synthesized IAA is transported to lower elongating regions to regulate coleoptile growth and/or tropic curvature.
However, the results of some studies contradict the auxin theory. There is evidence that some growth inhibitor(s) increased in the light-irradiated half of oat coleoptiles (Hasegawa and Sakoda, 1988; Togo and Hasegawa, 1991). One of the reasons for this controversy may be that it is very difficult to quantify IAA in small tissue segments. In fact, there is only one report of precise quantification of IAA during photo-curvature. In that report, GC-MS was used to determine IAA in 0–5 mm and 0–20 mm maize coleoptile sections separated into shaded and irradiated sides under 2nd positive light conditions (Fuchs et al., 2003). In the present study, IAA localization was accurately determined in 0–3, 3–6, 6–9, and 9–12 mm sections of maize coleoptiles, comparing irradiated and shaded sides under 1st positive light under normal gravity and microgravity conditions. The results showed that IAA is first redistributed asymmetrically in the 0–3 mm tip region between 10 and 20 min after light stimulation, and then the asymmetric IAA distribution moves into lower parts of the coleoptile (Fig. 2). This result indicates that light-dependent IAA lateral movement occurs mainly within the top 3 mm region. This was also evident from simultaneous NPA and auxin treatments (Fig. 3). It appears that lateral auxin movement takes place in a very restricted region during phototropic curvature. These results are consistent with previous studies, in which the movement of exogenously applied radiolabelled IAA was determined (Pickard and Thimann, 1964; Iino, 1991). Thus, the results presented here illustrate in detail the formation of the asymmetrical IAA gradient in maize coleoptiles after unilateral blue light stimulation.
The 0–2 mm tip region, the IAA synthetic site, is a key region for phototropic curvature in maize coleoptile
It is generally accepted that the very tip region is an important region for the phototropic response of monocot coleoptiles. The photo-sensing region for 1st pulse-induced positive phototropism was investigated by decapitating maize coleoptiles at various positions (up to 2 mm from the top) immediately after blue light stimulation and applying IAA to cut surfaces (Iino, 1995). In that study, the most active area of photo-sensing was the apical 1 mm region, but the region below the 2 mm position also showed weak photo-sensing capacity. Similar results were obtained in the present work. Using an LED blue light source and 2 cm maize coleoptile segments, it was confirmed that the 0–2 mm region is the key region for sensing unilateral blue light (Fig. 4).
Our results and those of other studies show that the coleoptile tip region is not only the site of IAA biosynthesis, but also the specific sensing site of environmental stimuli. This finding suggests that stimulus perception is co-ordinated with auxin biosynthesis and transport within the very tip region. If this is the case, the very tip region should contain some specific key factor(s). Several candidates have been identified in monocots, including the photo-sensing phot1 and the signal transducer CPT1/NPH3 (Knieb et al., 2004; Haga et al., 2005). Previous studies have reported that phot1 is a tip-specific blue-light-sensitive kinase, and its localization has been studied in maize and oat coleoptiles. However, these investigations were carried out using 5 mm coleoptile sections (Palmer et al., 1993; Hager, 1996; Knieb et al., 2004). In the present study, it was found that expression of PHOT1 was higher in the 0–5 mm tip region than in the 5–12 mm region (Fig. 5). However, expression of PHOT1 was lower in the top 0–1 mm region than in the 1–3 mm region. The microarray followed by quantitative RT-PCR analyses showed strong expressions of NPH3-like and PGP-like genes in the tip 0–1 mm and 0–2 mm region, respectively (Fig. 5). Database searches indicated that the NPH3-like gene encodes a protein similar to AtENP/MAB4 and that the PGP-like gene encodes an ABC transporter with similarities to AtPGP7, 9, and 19. The AtENP/MAB4/NPY1 protein is a member of the Arabidopsis NPH3 family, and it is proposed to function as a regulator of IAA distribution in organogenesis (Cheng et al., 2007; Furutani et al., 2007). In addition, direct binding of AtNPH3 and its homologue AtRPT2 to the phot1 protein was also reported (Motchoulski and Liscum, 1999; Sakamoto and Briggs, 2002; Inada et al., 2004). Therefore, it is possible that the NPH3-like gene has some role(s) in phototropic signal transduction between photo-perception and regulation of IAA dynamics in maize coleoptiles. On the other hand, there were reports that phototropic curvature is enhanced in mdr1/pgp19 (Noh et al., 2003) and that AtABCB19 is involved in the phototropic response of Arabidopsis hypocotyls (Nagashima et al., 2008). Therefore, it is likely that AtNPH3s act downstream of phot1 and regulates the direction of IAA transport by modifying PIN or related protein(s), including MDR1/PGP19 (Blakeslee et al., 2004). However, it has not been shown that these genes act as primary signalling components in the photo-perception pathway even in Arabidopsis. There is a possibility that other components participate in the signalling pathway. In our microarray analysis with maize coleoptiles, it was found that several other AtABCB19 orthologues did not show tip-specific expression. At present, there is little information about maize NPH3 and MDR/PGP genes, although it is known that the MDR/PGP gene encoding ABCB1/BR2 has a role in shoot growth, as revealed from analyses of the maize dwarf mutant br2 (Multani et al., 2003; Knöller et al., 2010). Further analyses are required to determine the functions of phot1, NPH3-like, PINs, and PGP-like proteins, and the protein–protein interactions that occur before and after blue light stimulation in maize coleoptiles. Such studies will clarify the mechanisms by which blue light perception is linked to the formation of the asymmetric IAA distribution in the 0–2 mm tip of maize coleoptiles (the site of IAA biosynthesis) during the phototropic response.
Supplementary Material
Acknowledgments
We thank Drs T Okamoto and T Komano from our laboratory for helpful discussions and technical assistance. This work was supported in part by the ‘Initiatives for Attractive Education in Graduate School (IAGE)’ grant awarded to the Department of Biological Sciences, Tokyo Metropolitan University, from the Ministry of Education, Culture, Sports, Science and Technology of Japan. This work was also supported by Grants-in-Aid for Scientific Research in Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), No. 21027030 (TK), and by a Grant-in Aid from the Japanese Society for the Promotion of Science (JSPS), No. 19 7171 (TN).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/2.0/uk/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- Bennett MJ, Roberts J, Palme K. Moving on up: auxin-induced K+ channel expression regulates gravitropism. Trends in Plant Science. 2000;5:85–86. doi: 10.1016/s1360-1385(00)01557-0. [DOI] [PubMed] [Google Scholar]
- Blakeslee JJ, Bandyopadhyay A, Peer WA, Makam SN, Murphy AS. Relocalization of the PIN1 auxin efflux facilitator plays a role in phototropic responses. Plant Physiology. 2004;134:28–31. doi: 10.1104/pp.103.031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR. Light dosage and phototropic responses of corn and oat coleoptiles. Plant Physiology. 1960;35:951–962. doi: 10.1104/pp.35.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR, Tocher RD, Wilson JF. Phototropic auxin redistribution in corn coleoptiles. Science. 1957;126:210–212. doi: 10.1126/science.126.3266.210. [DOI] [PubMed] [Google Scholar]
- Carraro N, Forestan C, Canova S, Traas J, Varotto S. ZmPIN1a and ZmPIN1b encode two novel putative candidates for polar auxin transport and plant architecture determination of maize. Plant Physiology. 2006;142:254–264. doi: 10.1104/pp.106.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Qin G, Dai X, Zhao Y. NPY1, a BTB-NPH3-like protein, plays a critical role in auxin-regulated organogenesis in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2007;104:18825–18829. doi: 10.1073/pnas.0708506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C, Darwin F. The power of movement in plants. London: John Murray Publisher; 1880. [Google Scholar]
- Forestan C, Meda S, Varotto S. ZmPIN1-mediated auxin transport is related to cellular differentiation during maize embryogenesis and endosperm development. Plant Physiology. 2010;152:1373–1390. doi: 10.1104/pp.109.150193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Palme K. Polar auxin transport-old questions and new concepts? Plant Molecular Biology. 2002;49:273–284. [PubMed] [Google Scholar]
- Fuchs I, Philippar K, Ljung K, Sandberg G, Hedrich R. Blue light regulates an auxin-induced K+-channel gene in the maize coleoptile. Proceedings of the National Academy of Sciences, USA. 2003;100:11795–11800. doi: 10.1073/pnas.2032704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani M, Kajiwara T, Kato T, Treml BS, Stockum C, Torres-Ruiz RA, Tasaka M. The gene MACCHI-BOU 4/ENHANCER OF PINOID encodes a NPH3-like protein and reveals similarities between organogenesis and phototropism at the molecular level. Development. 2007;134:3849–3859. doi: 10.1242/dev.009654. [DOI] [PubMed] [Google Scholar]
- Gallavotti A, Yang Y, Schmidt RJ, Jackson D. The relationship between auxin transport and maize branching. Plant Physiology. 2008;147:1913–1923. doi: 10.1104/pp.108.121541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga K, Takano M, Neumann R, Iino M. The Rice COLEOPTILE PHOTOTROPISM1 gene encoding an ortholog of Arabidopsis NPH3 is required for phototropism of coleoptiles and lateral translocation of auxin. The Plant Cell. 2005;17:103–115. doi: 10.1105/tpc.104.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A. Properties of a blue-light-absorbing photoreceptor kinase localized in the plasma membrane of the coleoptile tip region. Planta. 1996;198:294–299. doi: 10.1007/BF00206256. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Sakoda M. Distribution of endogenous indole-3-acetic acid and growth inhibitor(s) in phototropically responding oat coleoptiles. Plant and Cell Physiology. 1988;29:1159–1164. [Google Scholar]
- Holland JJ, Roberts D, Liscum E. Understanding phototropism: from Darwin to today. Journal of Experimental Botany. 2009;60:1969–1978. doi: 10.1093/jxb/erp113. [DOI] [PubMed] [Google Scholar]
- Iino M. Action of red light on indole-3-acetic-acid status and growth in coleoptiles of etiolated maize seedlings. Planta. 1982;156:21–32. doi: 10.1007/BF00393439. [DOI] [PubMed] [Google Scholar]
- Iino M. Kinetic modelling of phototropism in maize coleoptiles. Planta. 1987;171:110–126. doi: 10.1007/BF00395074. [DOI] [PubMed] [Google Scholar]
- Iino M. Mediation of tropisms by lateral translocation of endogenous indole-3-acetic acid in maize coleoptiles. Plant, Cell and Environment. 1991;14:279–286. [Google Scholar]
- Iino M. Gravitropism and phototropism of maize coleoptiles: evaluation of the Cholodny–Went theory through effects of auxin application and decapitation. Plant and Cell Physiology. 1995;36:361–367. [Google Scholar]
- Iino M, Carr DJ. Estimation of free, conjugated, and diffusible indole-3-acetic acid in etiolated maize shoots by the indolo-α-pyrone fluorescence method. Plant Physiology. 1982;69:950–956. doi: 10.1104/pp.69.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada S, Ohgishi M, Mayama T, Okada K, Sakai T. RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. The Plant Cell. 2004;16:887–896. doi: 10.1105/tpc.019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knieb E, Salomon M, Rudiger W. Tissue-specific and subcellular localization of phototropin determined by immuno-blotting. Planta. 2004;218:843–851. doi: 10.1007/s00425-003-1164-7. [DOI] [PubMed] [Google Scholar]
- Knöller AS, Blakeslee JJ, Richards EL, Peer WA, Murphy AS. Brachytic2/ZmABCB1 functions in IAA export from intercalary meristems. Journal of Experimental Botany. 2010;61:3689–3696. doi: 10.1093/jxb/erq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Kamiya Y, Iino M. Biosynthesis of indole-3-acetic acid from L-tryptophan in coleoptile tips of maize (Zea mays L) Plant and Cell Physiology. 1995;36:1503–1510. [Google Scholar]
- Li P, Wang Y, Qian Q, Fu Z, Wang M, Zeng D, Li B, Wang X, Li J. LAZY1 controls rice shoot gravitropism through regulating polar auxin transport. Cell Research. 2007;17:402–410. doi: 10.1038/cr.2007.38. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Iino M. Phytochrome is required for the occurrence of time-dependent phototropism in maize coleoptiles. Plant, Cell and Environment. 1996;19:1379–1388. doi: 10.1111/j.1365-3040.1996.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Moore I. Gravitropism lateral thinking in auxin transport. Current Biology. 2002;12:R452–R454. doi: 10.1016/s0960-9822(02)00943-0. [DOI] [PubMed] [Google Scholar]
- Mori Y, Nishimura T, Koshiba T. Vigorous synthesis of indole-3-acetic acid in the apical very tip leads to a constant besipetal flow of the hormone in maize coleoptiles. Plant Science. 2005;168:467–473. [Google Scholar]
- Motchoulski A, Liscum E. Arabidopsis NPH3: a NPH1 photoreceptor-interacting protein essential for phototropism. Science. 1999;286:961–964. doi: 10.1126/science.286.5441.961. [DOI] [PubMed] [Google Scholar]
- Multani DS, Briggs SP, Chamberlin MA, Blakeslee JJ, Murphy AS, Johal GS. Loss of an MDR transporter in compact stalks of maize br2 and sorghum dw3 mutants. Science. 2003;302:81–84. doi: 10.1126/science.1086072. [DOI] [PubMed] [Google Scholar]
- Nagashima A, Uehara Y, Sakai T. The ABC subfamily B auxin transporter AtABCB19 is involved in the inhibitory effects of N-1-naphthylphthalamic acid on the phototropic and gravitropic responses of Arabidopsis hypocotyls. Plant and Cell Physiology. 2008;49:1250–1255. doi: 10.1093/pcp/pcn092. [DOI] [PubMed] [Google Scholar]
- Nick P, Schäfer E. Interaction of gravi- and phototropic stimulation in the response of maize (Zea mays L.) coleoptiles. Planta. 1988;173:213–220. doi: 10.1007/BF00403013. [DOI] [PubMed] [Google Scholar]
- Nick P, Schäfer E, Furuya M. Auxin redistribution during first positive phototropism in corn coleoptiles. Microtubule reorientation and the Cholodny–Went theory. Plant Physiology. 1992;99:1302–1308. doi: 10.1104/pp.99.4.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Nakano H, Hayashi K, Niwa C, Koshiba T. Differential downward stream of auxin synthesized at the tip has a key role in gravitropic curvature via TIR1/AFBs-mediated auxin signaling pathways. Plant and Cell Physiology. 2009;50:1874–1885. doi: 10.1093/pcp/pcp129. [DOI] [PubMed] [Google Scholar]
- Noh B, Bandyopadhyay A, Peer WA, Spalding EP, Murphy AS. Enhanced gravi- and phototropism in plant mdr mutants mislocalizing the auxin efflux protein PIN1. Nature. 2003;423:999–1002. doi: 10.1038/nature01716. [DOI] [PubMed] [Google Scholar]
- Palmer JM, Short TW, Briggs WR. Correlation of blue light-induced phosphorylation to phototropism in Zea mays L. Plant Physiology. 1993;102:1219–1225. doi: 10.1104/pp.102.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippar K, Fuchs I, Lüthen H, et al. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proceedings of the National Academy of Sciences, USA. 1999;96:12186–12191. doi: 10.1073/pnas.96.21.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard BG, Thimann KV. Transport and distribution of auxin during tropistic response. II. The lateral migration of auxin in phototropism of coleoptiles. Plant Physiology. 1964;39:341–350. doi: 10.1104/pp.39.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Briggs WR. Cellular and subcellular localization of phototropin 1. The Plant Cell. 2002;14:1723–1735. doi: 10.1105/tpc.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo S, Hasegawa K. Phototropic stimulation does not induce unequal distribution of indole-3-acetic acid in maize coleoptiles. Physiologia Plantarum. 1991;81:555–557. [Google Scholar]
- Weiler EW, Wischnewski S. The relationship between diffusible, extractable and conjugated (base-labile) forms of indole-3-acetic acid in isolated coleoptile tips of Zea mays L. Planta. 1984;162:30–32. doi: 10.1007/BF00397417. [DOI] [PubMed] [Google Scholar]
- Went FW, Thimann KV. Phytohormones. New York: Macmillan; 1937. [Google Scholar]
- Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Annals of Botany. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman BK, Briggs WR. Phototropic dosage-response curves for oat coleoptiles. Plant Physiology. 1963;38:248–253. doi: 10.1104/pp.38.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.