Abstract
Spring geophytes produce larger storage organs and present delayed leaf senescence under lower growth temperature. Bulb and leaf carbon metabolism were investigated in Erythronium americanum to identify some of the mechanisms that permit this improved growth at low temperature. Plants were grown under three day/night temperature regimes: 18/14 °C, 12/8 °C, and 8/6 °C. Starch accumulated more slowly in the bulb at lower temperatures probably due to the combination of lower net photosynthetic rate and activation of a ‘futile cycle’ of sucrose synthesis and degradation. Furthermore, bulb cell maturation was delayed at lower temperatures, potentially due to the delayed activation of sucrose synthase leading to a greater sink capacity. Faster starch accumulation and the smaller sink capacity that developed at higher temperatures led to early starch saturation of the bulb. Thereafter, soluble sugars started to accumulate in both leaf and bulb, most probably inducing decreases in fructose-1,6-bisphosphatase activity, triose-phosphate utilization in the leaf, and the induction of leaf senescence. Longer leaf life span and larger bulbs at lower temperature appear to be due to an improved equilibrium between carbon fixation capacity and sink strength, thereby allowing the plant to sustain growth for a longer period of time before feedback inhibition induces leaf senescence.
Keywords: Bulb, carbohydrate, carbon allocation, carbon metabolism, Erythronium americanum, sink strength, starch, sucrose cleaving enzymes
Introduction
Low-growth temperatures are usually known to reduce growth rate and final biomass, even for plants well-adapted to low-temperature regimes. Yet, there are exceptions, such as some geophytes where growth is increased at low temperatures and a much larger storage organ results (Daymond et al., 1997; Wheeler et al., 2004; Badri et al., 2007). This constitutes a notable case among temperate species. This positive effect on growth of the storage organ is correlated with greater leaf longevity, and therefore, a longer period of carbon assimilation, which could partly explain higher storage organ biomass. However, corm growth and leaf longevity in Crocus vernus, a spring ephemeral, are more greatly affected by soil temperatures than by air temperatures (Badri et al., 2007), supporting the idea that leaf life duration in spring ephemerals is mainly influenced by sink activity. Thus leaf senescence is induced by a reduction in carbohydrate sink demand once the storage organ is filled with carbohydrates (Lapointe, 2001). Therefore, low temperatures most likely increase the overall sink strength leading to larger storage organs in these species.
Sink activity and capacity define the ability of the sink organ to import carbohydrates, i.e. its sink strength (Marcelis, 1996). Starch synthesis and respiratory rate determine sink activity whereas cell growth determines sink capacity. Sucrose metabolism plays a key role in controlling the C allocation patterns to these different metabolic pathways. As enzymes involved in sucrose metabolism exhibit differential responses to temperature, C allocation pattern could thus be impacted by temperature. Sucrose phosphate synthase (SPS) activity generally increases at low temperatures (Guy et al., 1992). In potato tuber sweetening, low temperature activates amylase, releasing more hexoses that induce an increase in SPS activity (Malone et al., 2006). Geigenberger et al. (1998) suggest that the inhibition of ADP-glucose pyrophosphorylase (AGPase) activity and starch synthesis under high temperature, is due to decreased availability of their substrates in response to increased respiratory rates. Sucrose synthase (Susy) activity is reduced when potato tubers are subjected to higher growth temperatures whereas invertase activity is not affected (Lafta and Lorenzen, 1995). Temperature can also affect sink capacity. Indeed, numerous studies have reported an increase in cell division and cell expansion rates with increasing temperature in fruit (Bertin, 2005), leaves (Tardieu et al., 2000), and roots (Pritchard, 1994). Changes in both sink activity and capacity may thus explain the positive response of spring ephemeral plant growth to low temperature.
Once sink strength decreases and sink limitation starts to build up, feedback inhibition of photosynthesis is expected as starch starts to accumulate in the chloroplasts (Paul and Foyer, 2001) and eventually induces leaf senescence. Feedback inhibition of photosynthesis is sensed not only as a decrease in net photosynthetic rate (Pn) but also as a decrease in photochemical quenching and a concurrent increased loss of photochemical energy (i.e., non-photochemical quenching). Sugar-dependent signals have been described as one major pathway that links source activity to sink limitation (Paul and Driscoll, 1997).
The main objective of the present study is to investigate regulatory steps in carbon metabolism that could be linked to the modulation of sink strength caused by growth temperature in spring geophytes. Erythronium americanum Ker Gawl. (trout lily) plants were grown under three temperature regimes: 18/14 °C day/night, 12/8 °C, and 8/6 °C. In addition to responding positively to low temperatures, E. americanum provides an interesting biological model for the study of whole-plant carbon allocation because of its simple morphology; the plant is composed of a single leaf and single bulb (i.e. one source versus one sink; Fig. 1). Harvests were performed throughout the period of epigeous growth to measure plant growth in relation to phenological status. Gas exchange, chlorophyll fluorescence, plant growth, bulb cell size, and carbohydrate concentrations were assessed as measures of source and sink activities. The activities of several sucrose-related enzymes were also assayed to provide insight into the steps regulating carbon metabolism of the source and sink. This work identifies some of the potential mechanisms that lead to increased storage organ production of spring ephemerals under low-temperature regimes.
Fig. 1.
Representative illustration of Erythronium americanum plants during the different phenological stages of epigeous growth. (This figure is available in colour at JXB online.)
Materials and methods
Plant material and growing conditions
Bulbs of E. americanum were collected in autumn in a sugar maple forest near Saint-Augustin-de-Desmaures (QC, Canada; 46°48' N, 71°23' W). Bulbs of similar biomass (0.40–0.45 g fresh weight) were selected and planted in plastic pots containing Turface (Applied Industrial Materials Corp., Buffalo Grove, IL, USA) as substrate and stored in a cold chamber for 4–5 months of cold stratification. Plants were then randomly allocated to growth chambers (PGW36, Conviron Inc., Winnipeg, MB, Canada) under the following light conditions: photoperiod of 14 h and a photon flux density (PPFD) of 400 μmol m−2 s−1. E. americanum was exposed to three growth temperatures: 18/14 °C day/night, 12/8 °C, and 8/6 °C, with relative humidities (RH) of 75%, 65%, and 50%, respectively. The two higher temperature regimes corresponded to the daily (day and night) mean temperatures encountered at the beginning (12/8 °C) and end (18/14 °C) of the E. americanum growing season under natural conditions in this area. RH was modulated as a function of growth temperature to maintain a constant vapour pressure deficit (VPD) across chambers. Plants were watered daily and fertilized weekly with 10% Hoagland's solution for optimal growth (Lapointe and Lerat, 2006). The experiment was repeated over two years and treatments were switched among growth chambers between years.
Plant growth measurements
Six plants per chamber were harvested at the following stages: (i) the beginning of the experiment (day 0), (ii) initiation of leaf unfolding (days 3, 4, and 5 at 18/14 °C, 12/8 °C, and 8/6 °C, respectively), (iii) complete leaf unfolding (days 5, 7 or 9), (iv) the first visual signs of leaf senescence (days 22, 29 or 33), and (v) complete leaf senescence (days 29, 39 or 45, Fig. 1). Between completed leaf unfolding and leaf senescence, harvesting was staggered among the three temperature regimes, i.e. every 2 d for 18/14 °C, 3 d for 12/8 °C, and 4 d for 8/6 °C. Leaf area was measured using a Li-Cor 3100 area meter (Li-Cor Inc, Lincoln, NE, USA). Then, leaves, bulbs, and roots were lyophilized for 24 h and weighed separately.
Gas exchange and fluorescence measurements
Gas exchange measurements were carried out on the single leaf of five plants per chamber using a Li-Cor 6400 Portable Photosynthesis System (Li-Cor Inc, Lincoln, NE, USA). Measurements were performed on the same plants from leaf unfolding to complete leaf senescence. Measurements were conducted under three light conditions: 0, 400, and 1000 μmol m−2 s−1. A background CO2 concentration of 400 μmol mol−1 and airflow of 200 μmol s−1 were maintained. Temperature and RH conditions were similar to those in the growth chamber. Pn, stomatal conductance (gs), intercellular CO2 concentration (Ci), and dark respiration (Rd) were recorded. Measurements of chlorophyll fluorescence were carried out simultaneously with gas exchange using a Li-Cor 6400-40 pulse-modulated fluorometer. The intensity of the pulse was set at 14 000 μmol m−2 s−1 for 1 s. Photochemical quenching (qP), non-photochemical quenching (qN), PSII maximum efficiency (Fv/Fm), and electron flux across PSII (PhiPSII) were subsequently estimated. Total electron flux (JT) was also calculated and used to estimate photorespiratory rates (PR) as: PR=1/12[JT–4(Pn+Rd)] (Genty et al., 1989). Furthermore, dark respiration measurements were recorded for the bulbs of five plants in each chamber. The entire bulb fit into the gas analyser chamber. Conditions of measurement were similar to those for leaf measurements. All measurements were randomized among treatments between 11.00 h and 13.00 h (i.e. middle of the light period).
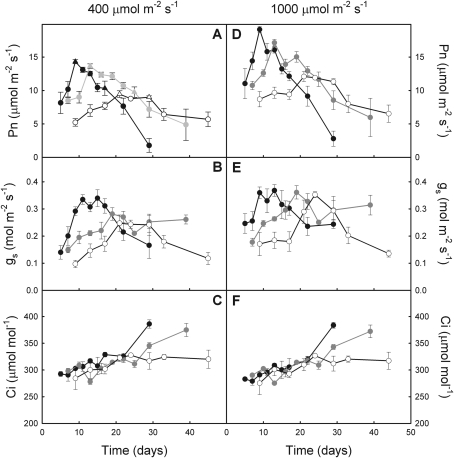
Photosynthetic CO2 response curves (A–Ci curves) were recorded on five plants per treatment at maximum Pn (days 9, 13, and 21 at 18/14 °C, 12/8 °C, and 8/6 °C, respectively; Fig. 3A) and subsequently at 17, 25, and 29 d for the three respective temperature regimes (Fig. 3A). Maximum carboxylation rate (Vcmax), maximum electron transport rate (Jmax), and triose phosphate utilization (TPU) were calculated using Photosynthesis software (Li-Cor Inc, Lincoln, NE, USA; Sharkey et al., 2007).
Fig. 3.
Evolution of net photosynthetic rate (A, D), stomatal conductance (B, E), and intercellular CO2 concentration (C, F) in E. americanum plants grown at 18/14 °C (black), 12/8 °C (grey), and 8/6 °C (white). Gas exchange data (mean ±SE) were recorded at both 400 μmol m−2 s−1 (A, B, C) and at 1000 μmol m−2 s−1 (D, E, F) (n=2; five plants per growing season). Triangles indicate, for each temperature, the date to which A–Ci curves were carried out.
Carbohydrates
Starch, sucrose, and reducing sugar (glucose and fructose) concentrations were determined from the bulbs and leaves of three plants per chamber, per harvest date. Leaves and bulbs were harvested and flash-frozen in liquid nitrogen. Plant material was stored at –80 °C until extraction. Frozen tissues were lyophilized for 24 h and weighed before maceration in a solution of methanol, chloroform, and water (12:5:3 by vol.) for 20 min at 65 °C (Blakeney and Mutton, 1980). The mixture was dispersed and homogenized with a Polytron (Kinematica, Lucerne, Switzerland) and centrifuged at 3500 rpm for 10 min at 4 °C. Starch contained in the pellet was gelatinized in boiling water for 90 min then hydrolysed at 55 °C for 60 min in the presence of amyloglucosidase. The supernatant was analysed pre- and post-invertase digestion to estimate reducing sugars and sucrose concentrations, respectively. Finally, all reducing sugars were quantified colorimetrically at 415 nm after reaction with p-hydroxybenzoic acid hydrazide (Sigma Chemical Co., St Louis, MO, USA).
Enzyme extraction and assays
Soluble proteins were extracted from leaves and bulbs of three plants per chamber, per harvest date. As in the previous assay, the leaves and bulb of each plant were separated, flash-frozen in liquid N2, and stored at –80 °C until extraction. Frozen leaf and bulb tissues (300 mg FW) were ground in liquid nitrogen with a mortar and pestle. Crude enzyme extractions were performed at 4 °C in 3 ml 0.1 M HEPES-KOH buffer (pH 7.5) containing 7% w/w polyethylene glycol 20000, 2 mM dithiothreitol, 5 mM MgCl2, 5 mM ethylene glycol-bis-(β-aminoethyl ether)-tetraacetic acid, 10% v/v glycerol, 1 mM phenylmethylsulphonyl fluoride, 9% w/v polyvinylpyrrolidone 25 000 (PVP25), 1 μM pepstatin, and 1 μM leupeptin. The homogenate was centrifuged at 18 000 rpm for 20 min at 4 °C. The supernatant was collected and filtered through a Sephadex-G25 column (Pharmacia Company, Uppsala, Sweden). Enzymes were eluted with HEPES-KOH buffer and a volume of 3.5 ml containing the enzymes was collected and used as enzymatic extract for the determination of fructose-1,6-bisphosphatase (F1,6BPase) in the leaf, and for neutral invertase (NInv), Susy, vacuolar invertase (VInv), SPS, and AGPase activities in the bulb. The pellet was washed twice with 3 ml of 100 mM HEPES containing 0.5% (m/v) PVP25, 5 mM MgCl2, 5 mM EGTA, and 2 mM DTT and centrifuged at 13 000 rpm for 20 min at 4 °C. The pellet was then treated with the same medium plus 1 M NaCl overnight and centrifuged at 13 000 rpm for 20 min at 4 °C. The supernatant was collected and used for the determination of cell wall invertase (CWInv) activity in the bulb. Protein concentrations in the different supernatants were measured according to Bradford (1976).
Enzymatic activities were determined spectrophotometrically in a microplate reader (Spectra Max 190, Molecular Devices, Sunnyvale, CA, USA). F1,6BPase was assayed according to Vassey and Sharkey (1989), with modifications. The assay medium contained 200 μl of 50 mM HEPES-NaOH (pH 7), 100 mM KCl, 4 mM MgCl2, 1.2 mM NADP, 3 U ml−1 glucose-6-phosphate dehydrogenase (EC 1.1.1.49), 5 U ml−1 phosphoglucoisomerase (EC 5.3.1.9), and 10 μl of enzymatic extract. The addition of 100 μM of fructose-1,6-bisphosphate initiated the reaction. AGPase assay was prepared according to Smith et al. (1989). The assay medium contained 200 μl of 50 mM HEPES-NaOH (pH 7.8), 5 mM MgCl2, 1.2 mM NADP, 500 μM ADP-glucose, 5 U. ml−1 glucose-6-phosphate dehydrogenase (EC 1.1.1.49), 2.5 U ml−1 phosphoglucomutase (EC 5.4.2.2), and 10 μl of enzymatic extract. The addition of 3.5 mM of NaPPi initiated the reaction. NInv and Susy were assayed according to Gandin et al. (2009). NInv was assayed with 200 μl of 50 mM HEPES-NaOH (pH 7) containing 2.8 mM ATP, 1.2 mM NADP, 12 U ml−1 hexokinase (EC 2.7.1.1), 3.5 U ml−1 phosphoglucoisomerase (EC 5.3.1.9), 1.75 U ml−1 glucose-6-phosphate dehydrogenase (EC 1.1.1.49), and 10 μl of enzymatic extract. The addition of 100 mM sucrose initiated the reaction. A similar medium with 2 mM UDP was used for the Susy assay. Susy activity was estimated via the difference between the reactions described above, both with and without UDP. VInv was assayed according to Tang et al. (1999), with modifications. The assay medium (200 μl) comprised 50 mM sodium citrate (pH 5.6), 10 μl of enzymatic extract and 100 mM sucrose to initiate the reaction. CWInv was assayed with 200 μl of 50 mM sodium citrate (pH 5), 10 μl of enzymatic extract, and 150 mM sucrose. Glucose produced in both VInv and CWInv assays was then measured with glucose oxidase-peroxidase reagent (Sigma-Aldrich, St Louis, MO, USA). SPS was assayed with 200 μl of 50 mM HEPES (pH 7.5) containing 10 mM UDP-glucose, 8 mM fructose-6-phosphate, and 32 mM glucose-6-phosphate. Phenyl-β-glucoside (20 mM) was used to inhibit Susy activity. UDP released by SPS activity was quantified according to Bergmeyer and Bernt (1974) following NADH consumption by pyruvate kinase/lactate dehydrogenase activity at 340 nm. Controls without substrates were run for all assays.
Determination of cell size
Cell size was determined on the bulbs of three plants per chamber, per harvest. Bulbs were immediately fixed in FAA (formaldehyde-acetic acid-alcohol) solution (Sass, 1958) and then embedded in paraffin. Three transversal thin sections from each bulb were mounted on microscope slides and stained with 0.01% (w/v) toluidine blue. Slides were then observed under light microscope (Olympus, Lake Success, NY, USA). Diameter was measured for each cell along four transects (horizontal, vertical, and two diagonals) per thin section for a total of two thin sections per bulb. Cell size in μm2 was then calculated from the diameter of each cell, assuming that the cells were round.
Statistical analysis
The change in all variables through time were analysed by comparing the slope of linear regressions during both increasing and decreasing phases to test the effect of temperature on the rate of change of these variables through time (Statistix 8.2, Analytical Software, Tallahassee, FL, USA). Furthermore, one-way ANOVAs were carried out on either maximal or minimal values. When either maximal or minimal values formed a single peak, ANOVAs were applied on data from the single harvest composing the peak. When maximal or minimal values formed a plateau, the ANOVA analysis included all data points composing this plateau. Effects were considered significant when P <0.05. A posteriori multiple comparisons tests were performed using Fisher's LSD. In order to correct for ‘chamber effects’, treatments were switched from one growth chamber to another between years. The two years are thus true replicates.
Results
Plant growth
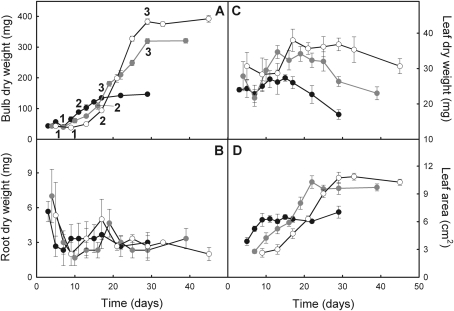
Erythronium americanum growth was affected by temperature, especially growth kinetics and final biomass of the bulb. Bulb final dry mass was 2.2-fold higher for 12/8 °C and 2.7-fold higher for 8/6 °C than for the highest growth temperature regime (Fig. 2A; see Supplementary Table S1 at JXB online). Biomass gain of the bulb ranged from 166% (18/14 °C) to 795% (8/6 °C) from leaf unfolding (i.e. days 3, 4, and 5 at 18/14 °C, 12/8 °C, and 8/6 °C, respectively) to the first visible signs of leaf senescence. During the first days of growth, the kinetics of biomass accumulation were faster at the highest temperature than under cooler temperatures (Table 1). Thereafter, mean growth rate decreased by 35% at 18/14 °C, whereas it increased by 106% and 244% at 12/8 °C and 8/6 °C, respectively. Growth rate was thus inversely related to growth temperature between state 2 and state 3 (as defined in Fig. 2). Leaf life span also decreased as growth temperature increased. The initiation of leaf senescence occurred after 22, 29, and 33 d at 18/14 °C, 12/8 °C, and 8/6 °C, respectively; complete leaf senescence occurred after 29, 39, and 45 d at 18/14 °C, 12/8 °C, and 8/6 °C, respectively (Table 1). Leaf senescence became visible a few days after bulb growth rate decreased. Root biomass kinetics were not affected by the growth temperature regime (Fig. 2B). Leaf growth thus lasted longer at lower temperatures, leading to higher maximum leaf biomass and area at the onset of leaf yellowing (Fig. 2C, D).
Fig. 2.
Evolution of bulb mass (A), root mass (B), leaf mass (C), and leaf area (D) throughout the epigeous growth period in E. americanum plants grown at 18/14 °C (black), 12/8 °C (grey), and 8/6 °C (white). The first data points correspond to the time when leaves were completely unfolded. The penultimate data points correspond to the first visual signs of leaf senescence and the last data points correspond to complete leaf senescence. The data points identified 1, 2, and 3 on each curve are the lower and upper thresholds used to estimate two different growth rates (see Table 1) during bulb growth. The means ±SE (n=2; six plants per growing season) are presented.
Table 1.
Growth rate (mg d−1) during two different Growth phases (see Fig. 2) and leaf longevity of E. americanum grown at three growth temperatures: 18/14 °C, 12/8 °C, and 8/6 °C
| Growth phase | Temperature |
|||
| 18/14 °C | 12/8 °C | 8/6 °C | ||
| Growth rate (mg d−1) | 1–2 | 11.9 | 7.4 | 7.0 |
| 2–3 | 7.7 | 13.9 | 24.1 | |
| Leaf longevity (d) | 29 | 39 | 45 | |
Gas exchange measurements
Pn measured at 400 μmol m−2 s−1 (PPFD)—similar to irradiance conditions for growth—exhibited a hyperbolic form through time; the parameters of these hyperbolic forms varied with temperature (Fig. 3A). The initial increase of Pn was faster as growth temperature increased, leading to a maximum at 9, 13, and 21 d at 18/14 °C, 12/8 °C, and 8/6 °C, respectively (see Supplementary Table S1 at JXB online). Maximum Pn was 5% lower at 12/8 °C and 34% lower at 8/6 °C when compared with 18/14 °C. Afterwards, Pn decreased more rapidly as temperature increased until complete leaf senescence was achieved. Despite a lower maximum Pn, the amount of C fixed during the entire life of the leaf was 59% higher at 12/8 °C and 85% higher at 8/6 °C, compared to 18/14 °C, because of the longer photosynthetically active phase at cooler temperatures (Table 2). Stomatal conductance exhibited a higher initial increase and a higher maximum at 18/14 °C than at the two cooler temperatures (Fig. 3B). Maximum gs was reached later than the maximum Pn at the two higher temperatures, whereas gs was synchronized with Pn at 8/6 °C. However, Ci was not affected by growth temperature (Fig. 3C; see Supplementary Table S1 at JXB online). Pn measured under saturating irradiance (i.e. 1000 μmol m−2 s−1) exhibited a faster initial increase at warmer temperatures and a faster decrease after the maximum had been reached (Fig. 3D; see Supplementary Table S1 at JXB online). Maximum Pn was 10% lower at 12/8 °C and 37% lower at 8/6 °C than at 18/14 °C. Pn was 34% higher under saturating irradiance than under 400 μmol m−2 s−1 at 18/14 °C, whereas Pn was light-stimulated by 25% at 12/8 °C and by 27% at 8/6 °C. Moreover, the decrease of Pn was 1.3-fold faster under 1000 μmol m−2 s−1 than under 400 μmol m−2 s−1 for plants growing at 18/14 °C (P <0.041), whereas it was not different between the two PPFDs at the two cooler temperatures (12 °C: P=0.185; 8 °C: P=0.219). gs and Ci that were measured under saturating irradiance exhibited a similar evolution over time as under growth irradiance conditions. Growth temperature affected gs only when measured under growth irradiance conditions; gs measured under saturating light conditions and Ci under both light conditions were unaffected (Fig. 3E, F). Thus, stomatal limitation did not occur in E. americanum plants at either growth temperatures.
Table 2.
Whole-plant C budget of E. americanum grown at three temperatures: 18/14 °C, 12/8 °C, and 8/6 °C
| Temperature |
|||
| 18/14 °C | 12/8 °C | 8/6 °C | |
| C fixed by the leaf (mg) | 105 | 167 | 194 |
| C respired by the leaf (mg) | 40.5 | 48.4 | 50.2 |
| C respired by the bulb (mg) | 12.4 | 20.7 | 29.2 |
| Net C incorporated (mg) | 52.1 | 97.9 | 114.6 |
| Starch accumulated (mg C) | 50.1 | 107.5 | 116.3 |
| C accumulation efficiency (%) | 96 | 110 | 101 |
Total amount of C fixed in leaf and C respired in leaf and bulb during the entire epigeous growth period are indicated. Total net amount of C incorporated was estimated as [leaf C fixed–(leaf C respirated+Bulb C respirated)] and then compared with the amount of C accumulated in the bulb as starch at the end of leaf senescence. C accumulation efficiency was estimated as the ratio of C accumulated as starch/Net C incorporated.
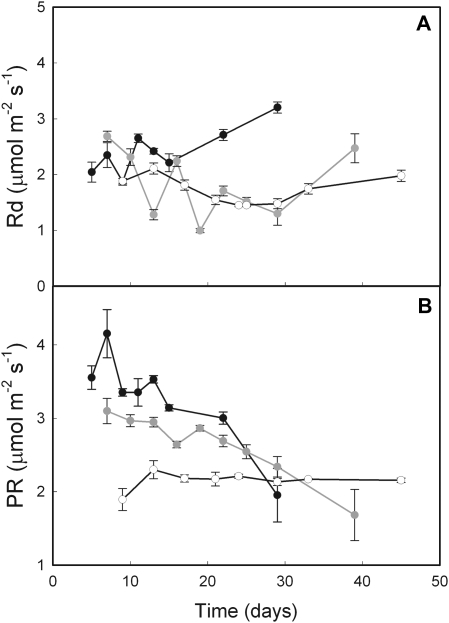
Leaf respiratory rate tended to increase over time at 18/14 °C, whereas it decreased continuously at cooler temperatures (Fig. 4A). However, respiratory rates did not significantly differ with growth temperature and increased during leaf senescence. The amount of C lost through respiration throughout the epigeous growth period increased both in the leaf and bulb as growth temperature decreased (Table 2). The amount of C accumulated in the bulb as starch represented 96%, 110%, and 101% of net C incorporated by plants growing at 18/14 °C, 12/8 °C, and 8/6 °C, respectively. Thus, almost all net C assimilated by the plant was stored in the bulb as starch. The accumulated amounts of C were very similar to the net amounts that were incorporated, suggesting that the punctual gas exchange measurements reported here truly reflect the evolution of plant gas exchange throughout the season.
Fig. 4.
Evolution of leaf respiratory (A) and photorespiratory rate (B) over time in E. americanum plants grown at 18/14 °C (black), 12/8 °C (grey), and 8/6 °C (white). Photorespiratory rates were estimated from measures taken at 400 μmol m−2 s−1. The means ±SE (n=2; five plants per growing season) are presented.
Photorespiration reached maximum rates soon after leaf unfolding, which was 13% lower at 12/8 °C and 47% lower at 8/6 °C, compared with 18/14 °C (Fig. 4B; see Supplementary Table S1 at JXB online). Photorespiratory rate then decreased until complete leaf senescence at warmer temperatures, whereas it remained fairly constant at 8/6 °C. Photorespiratory rates exhibited changes through time similar to Pn, except at 8/6 °C where Pn slowly decreased while photorespiratory rates remained fairly constant.
A–Ci curves indicated that Vcmax, Jmax, and TPU increased with growth temperature when measured at the time Pn was maximal (Table 3). However, a few days prior to the beginning of leaf senescence, Vcmax and Jmax had decreased at the two warmer temperatures to the point that both rates were similar among temperature regimes. TPU decreased even more at warm temperatures than at cold temperatures, leading to lower TPU as growth temperature increased.
Table 3.
Parameters estimated from A–Ci curves for E. americanum grown at three temperatures: 18/14 °C, 12/8 °C, and 8/6 °C
| Temperature |
ANOVA | |||
| 18/14 °C | 12/8 °C | 8/6 °C | P-value | |
| A–Ci curves at maximum Pna | ||||
| Vcmax (μmol m−2 s−1) | 37.3±0.7 | 27.1±3.8 | 20.3±2.1 | 0.004 |
| Jmax (μmol m−2 s−1) | 123±1 | 111±1 | 105±2 | 0.024 |
| TPU (μmol m−2 s−1) | 9.33±0.11 | 7.09±1.04 | 6.22±0.68 | <0.001 |
| A–Ci curves before leaf senescencea | ||||
| Vcmax (μmol m−2 s−1) | 21.0±0.9 | 22.1±1.1 | 20.6±1.4 | 0.523 |
| Jmax (μmol m−2 s−1) | 95.4±1.3 | 89.9±0.9 | 92.5±1.8 | 0.391 |
| TPU (μmol m−2 s−1) | 4.48±0.52 | 5.42±0.99 | 5.99±0.92 | 0.037 |
Maximum rate of carboxylase activity of Rubisco (Vcmax), maximum electron flux (Jmax), and triose phosphate utilization (TPU) are indicated. Values represent the means of three measures ±SE.
See triangles on Fig. 2A.
Chlorophyll fluorescence
At 400 μmol m−2 s−1, qP was fairly constant over the first days of growth (Fig. 5A) while qP was 8% lower at 12/8 °C and 22% lower at 8/6 °C than at 18/14 °C (see Supplementary Table S1 at JXB online). Photochemical quenching then decreased after 9, 13, and 29 d at 18/14 °C, 12/8 °C, and 8/6 °C, respectively. Indeed, qP remained fairly constant throughout the growing season until the initiation of leaf senescence in plants growing at 8/6 °C, whereas it decreased in the two warmer temperatures and the negative slope was more pronounced at the warmest temperature. By contrast, qN exhibited an earlier and greater increase at 18/14 °C than at 12/8 °C, whereas at 8/6 °C, it remained fairly constant throughout the season and higher than at the two other temperature regimes (Fig. 5B). Maximum quantum efficiency of PSII (Fv/Fm) increased during the first few days at the three temperatures (Fig. 5C). Maximum Fv/Fm was attained by day 13 at 18/14 °C and 12/8 °C and at day 24 at 8/6 °C. Thereafter, Fv/Fm decreased faster at higher temperature until complete leaf senescence. Fv/Fm at 8/6° C appeared relatively constant from day 13 onwards. Nevertheless, except in completely senescent leaves, Fv/Fm was always higher than 0.73, suggesting a fully functional photosynthetic apparatus under all three growth temperature conditions. Electron flux density through PSII (PhiPS2) exhibited a more rapid increase at 18/14 °C than at 12/8 °C during the first days, whereas it was stable at 8/6 °C until day 29 (Fig. 5D). Maximum PhiPS2 was 9% lower at 12/8 °C and 35% lower at 8/6 °C than 18/14 °C. Thereafter, PhiPS2 decreased faster as temperature increased. The reduction in qP and PhiPS2 through time matched the reductions observed with Pn (Fig. 2A).
Fig. 5.
Evolution of photochemical quenching (A), non-photochemical quenching (B), PSII maximum efficiency (C), and electron flux across PSII (D) over time in E. americanum plants grown at 18/14 °C (black), 12/8 °C (grey), and 8/6 °C (white). Fluorescence data (mean ±SE) were recorded at 400 μmol m−2 s−1 (n=2; five plants per growing season).
Carbohydrates
Starch concentration in the bulb decreased at all temperatures during leaf unfolding (Fig. 6A). Thereafter, bulb starch concentration increased with time, but the duration of starch accumulation differed among temperature treatments. Starch accumulated during 12, 18, and 20 d at 18/14 °C, 12/8 °C, and 8/6 °C, respectively. Starch accumulation rate increased with temperature, but final starch concentration, around 88% of bulb dry mass, was similar among temperatures (see Supplementary Table S1 at JXB online). Starch reached maximum concentration a few days before the initiation of leaf senescence regardless of growth temperature. By contrast, changes in soluble sugar concentration in the bulb—sucrose and reducing sugars—indicated a continuous decline over time in all treatments (Fig. 6B, C). The proportion of starch present as amylose increased continuously until leaf senescence with no significant difference among temperatures in terms of its rate of change (Fig. 6D). The increase lasted longer at cooler temperatures, leading to a maximum 7% higher at 12/8 °C and 15% higher at 8/6 °C before leaf senescence than at 18/14 °C. During leaf senescence, amylose decreased at the three growth temperatures and reached a similar final content. Given the dilution effect caused by increasing starch concentrations, soluble sugar concentrations were also presented as a function of bulb biomass from which starch accumulation has been subtracted (mg g−1 DW-Starch, Fig. 6E, F). These values are likely more representative of the concentration that can be sensed by the plant. Starchless soluble sugar indicated a transient increase in concentration at the time starch stopped accumulating. An initial peak was more obvious for reducing sugar than for sucrose and the height of this peak increased with growth temperature. Moreover, high soluble sugar concentrations were maintained until complete leaf senescence at 18/14 °C, while they decreased at the two lower temperatures.
Fig. 6.
Evolution of starch (A), sucrose (B, E, G), reducing sugar concentrations (C, F, H), and fraction of amylose (D) over time in the bulb (A–F) and leaf (G, H) of E. americanum plants grown at 18/14 °C (black), 12/8 °C (grey), and 8/6 °C (white). Concentration of sucrose and reducing sugars are presented as a function of total bulb biomass (mg g−1; B, C) and as a function of bulb biomass other than starch (mg g−1 DW-Starch; E, F). The means ±SE of three plants are presented.
In the leaf, sucrose concentrations remained fairly constant during the initial growth phase then strongly increased at days 15, 25, and 33 at 18/14 °C, 12/8 °C, and 8/6 °C, respectively, i.e. a few days before the first visual signs of leaf senescence (Fig. 6G). Sucrose concentration reached similar maximum concentration regardless of growth temperature. Growth temperature did not affect reducing sugar concentrations, which remained fairly stable through most of the growth period (Fig. 6H). Starch did not accumulate to a detectable amount in E. americanum leaves.
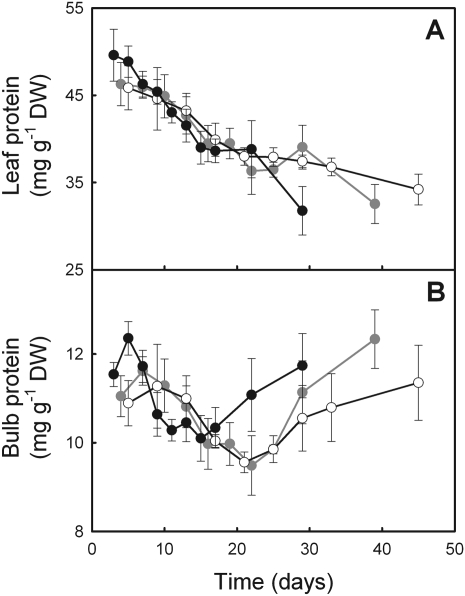
Soluble protein concentration
The initial concentration of total soluble proteins in the leaf appeared fairly similar among growth temperatures and decreased at a constant rate over time (Fig. 7A). Therefore, reduction of leaf protein did not coincide with the reduction of Pn over time. In the bulb, protein concentration was maximal soon after leaf unfolding and then decreased until a few days before leaf senescence (Fig. 7B). No difference among treatments was observed when bulb protein concentration was at a minimum at 9 days for 18/14 °C and after 22 d and 21 d for 12/8 °C and 8/6 °C, respectively. Neither the final increase nor the final concentration of protein was subsequently affected by temperature.
Fig. 7.
Evolution of leaf (A) and bulb (B) protein concentration over time in E. americanum plants grown at 18/14 °C (black), 12/8 °C (grey), and 8/6 °C (white). The means ±SE of three plants are presented.
Enzymes of carbon metabolism
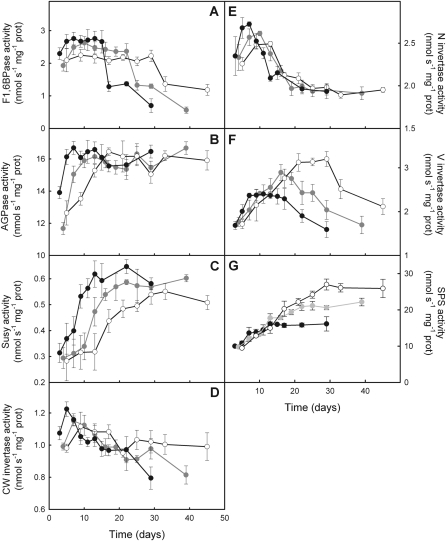
In the leaf, F1,6BPase activity exhibited a short increase after leaf unfolding, leading to maximum activity that was maintained during most of the epigeous growth phase (Fig. 8A). Maximum activity of F1,6BPase was 9% lower at 12/8 °C and 20% lower at 8/6 °C than at 18/14 °C (see Supplementary Table S1 at JXB online). F1,6BPase decreased drastically after 17, 25, and 33 d at 18/14 °C, 12/8 °C, and 8/6 °C, respectively, i.e. a few days prior to the first visual sign of leaf senescence. In the bulb, AGPase activity increased during the first few days to reach maximal activity early in the growing season (Fig. 8B). The initial increase tended to be faster at warmer temperatures, although there was no significant difference among the treatments (P=0.086). Thereafter, activity remained constant over time until complete leaf senescence with no difference among growth temperatures. Susy activity increased strongly during the first few days of the growth season and reached a maximum at days 13, 22, and 33 at 18/14 °C, 12/8 °C, and 8/6 °C, respectively (Fig. 8C). The increase was significantly faster as growth temperature increased. Moreover, maximum Susy activity increased with growth temperature. By contrast, CWInv activity was high at leaf unfolding with no difference among growth temperatures (Fig. 8D). Thereafter, the activity of the CWInv decreased continuously until complete leaf senescence. The decrease occurred faster at warmer temperatures. NInv increased quickly after leaf unfolding, exhibiting a maximum rate at days 7, 10, and 13 at 18/14 °C, 12/8 °C, and 8/6 °C, respectively (Fig. 8E). The increase was significantly faster and the maximum higher as growth temperature increased. Yet the decrease of NInv activity, which occurred shortly after it had reached maximum values, was not affected by growth temperature. VInv activity increased during the first few days to reach a maximum at days 7, 16, and 29 at 18/14 °C, 12/8 °C, and 8/6 °C, respectively (Fig. 8F). Maximum activity of VInv was maintained for a longer period of time at 18/14 °C than at the two cooler temperatures. Contrary to most other enzymes, the maximum rate attained by VInv decreased with an increase in growth temperature. Neither the initial increase nor the decrease before leaf senescence was affected by growth temperature. Finally, SPS started to increase at a similar rate at all three growth temperatures as soon as plants were moved to the growth cabinets (Fig. 8G). SPS activity reached a maximum value earlier and was thus lower as growth temperature increased. The maximum activity of SPS was maintained until complete leaf senescence.
Fig. 8.
Activities of fructose-1,6-bisphosphatase (A) in the leaf and of ADP-glucose pyrophosphorylase (B), sucrose synthase (C), cell wall invertase (D), neutral invertase (E), vacuolar invertase (F), and sucrose phosphate synthase (G) throughout the growth period in the bulb of E. americanum plants grown at 18/14 °C (black), 12/8 °C (grey), and 8/6 °C (white). The means ±SE of three plants are presented.
Each sucrose hydrolytic enzyme presented a different pattern of activity overtime. CWInv was the first one to be fully activated, replaced a few days later by maximum NInv activity. Subsequently, while NInv activity was declining, VInv reached a maximum that was maintained until a few days before leaf senescence. Once the activity of the VInv started to decline, Susy reached maximum activity, which remained high until the end of the epigeous growth period. Susy activity exhibited the lowest rates among the four sucrose hydrolytic enzymes. Growth temperature stimulated the maximum activity of Susy and NInv but reduced the activity of the VInv and SPS, suggesting that the latter two enzymes might play a role in the overall growth stimulation of the bulb at lower temperature.
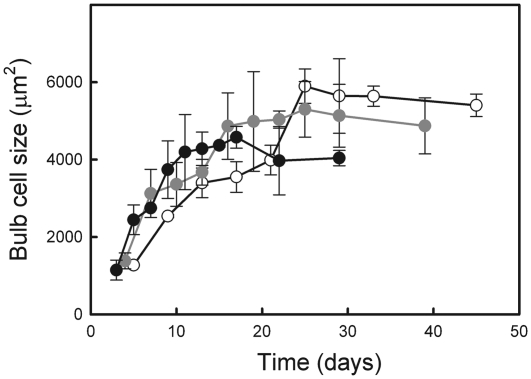
Bulb cell growth
Cell growth rate tended to increase with temperature (P=0.067), but cell expansion ceased much earlier as temperature increased (Fig. 9). The final size was thus reached much more rapidly at high temperature, leading to a smaller cell size at the end of the epigeous growth period at the warmer temperatures than at the cooler ones (see Supplementary Table S1 at JXB online).
Fig. 9.
Evolution of bulb cell size in E. americanum plants grown at 18/14 °C (black), 12/8 °C (grey), and 8/6 °C (white). The means ±SE of three plants are presented.
Discussion
Positive effect of low growth temperature
Low growth temperatures strongly increased the final bulb biomass as well as leaf longevity. Reports of positive effects of low temperature on growth are scarce in temperate zone higher plants and even in arctic herbs. Nevertheless, some high-arctic species, as well as some onion cultivars, exhibit optimal growth at temperatures around 12 °C (Heide, 1992; Daymond et al., 1997; Heide and Gauslaa, 1999). Moreover, positive responses of plant growth to low growth temperatures have previously been reported for E. americanum (Lapointe and Lerat, 2006), Crocus vernus (Badri et al., 2007) and a few other ornamental spring geophytes (De Hertogh and Le Nard, 1993). Our results thus confirmed the larger bulb size at 12/8 °C than at 18/14 °C (Lapointe and Lerat, 2006) and, in addition, presented evidence that the bulb can be even larger when the plant is grown at 8/6 °C. The initial growth rate increased with growth temperature, but bulb growth rate was reduced earlier in the warmest growth temperature, several days before the first visible signs of leaf senescence. Furthermore, at the time the leaf started to senesce at 18/14 °C (22 d), the bulb was already larger at either 12/8 °C or 8/6 °C. These results support the idea that the positive effect of low growth temperature on bulb growth is partially independent of its effect on leaf life duration (Badri et al., 2007).
Pn increased more rapidly and reached a higher maximum at warmer temperatures than at cooler ones. Yet, given the difference in leaf lifespan, there was more C incorporated over the whole epigeous growth period at lower temperatures, despite the greater cumulative losses of C by photorespiration and respiration. Carbohydrates are apparently more rapidly photosynthesized and available for translocation towards the bulb at 18/14 °C than at cooler growth temperatures, but the shorter leaf lifespan reduced the total amount of C that was translocated to the bulb.
Sucrose metabolism modulates sink activity and capacity
Our results indicated a sequential induction through time of sucrose-cleaving enzymes, which constitutes the first step in C metabolism at the sink level. Invertase activities peaked at the beginning of the growth period in the following order: CWInv, NInv, and VInv—while bulb development was initiated and starch accumulated. On the other hand, Susy reached its maximum activity a few days before leaf senescence, at a time when starch concentrations had reached maximum values and cell growth had ceased. Invertase contributions are well known to occur during initiation and cell expansion of the sink whereas Susy contribution occurs during cell maturation (Koch, 2004). The activity of Susy increased far earlier at high growth temperatures as compared to low growth temperatures, suggesting early maturation of bulb cells. Indeed, cell size measurements indicated a shorter duration of cell expansion as growth temperature increased, whereas the expansion rate was only slightly affected by temperature. Timing of induction of the different sucrose-cleaving enzymes could explain the faster shift from elongation to maturation in E. americanum bulb cells growing at warmer temperatures. A faster shift from cell elongation to cell maturation was also suggested for Crocus vernus (Lundmark et al., 2009) as they reported an earlier increase in C allocation to cell walls at a warmer growth temperature. An increased allocation to cell walls would fit with an earlier induction of the activity of Susy at warmer temperatures, given the close relation between Susy and cellulose synthase activities (Amor et al., 1995). The early contribution of Susy could thus explain the overall smaller sink capacity that develops at higher growth temperatures.
In the bulb, starch was initially consumed to provide C required for new bulb formation and leaf expansion. Thereafter, it accumulated more quickly as growth temperature increased. Starch synthesis is generally stimulated at warmer temperatures, as reported for potato tuber (Geigenberger et al., 1998). VInv and SPS activities increased as growth temperature decreased. Nguyen-Quoc and Foyer (2001) suggested that Vinv and SPS activities may be involved in a futile cycle, inducing sucrose degradation and resynthesis. Induction of such cycle could restrict the availability of the hexose pool for starch synthesis, via carbon accumulation in the vacuole at low temperature. The stimulation of this futile cycle and the resulting carbohydrate accumulation in the vacuole could sustain carbon sink demand, and thus sink strength, while sink capacity is being built up. Furthermore, the restricted hexose availability could account for the slower starch accumulation that was measured at low growth temperatures, despite similar AGPase capacity across temperature treatments. Faster starch accumulation at warmer growth temperatures encountered limited sink capacity induced by early cell maturation. This would explain the earlier cessation of starch accumulation as temperature increased. Moreover, the proportion of amylose decreased at the time cells stopped growing and starch accumulation slowed down. Amylose is interspersed with amylopectin in amorphous regions of starch and depends on the space available within the matrix of amyloplast (Denyer et al., 2001). Changes in the proportion of amylose without changes in starch concentration suggest that starch synthesis continued but in presence of a similar rate of starch degradation. This could explain why AGPase activity remained high up to the end of the epigeous growth despite no additional increase in starch concentration.
Response of leaf metabolism to sink activity
Under saturating light conditions, Pn decreased more rapidly at the warmest temperatures than under growth light conditions, suggesting an inhibition of the photosynthetic carboxylation phase over time. In the spring ephemeral Podophyllum peltatum, the maximum rate of rubisco carboxylation decreases with leaf ageing and as starch concentration in rhizomes increases (Constable et al., 2007). Even though leaf protein concentration decreased steadily during the growth cycle, it cannot explain the reduction in Pn since leaf protein concentrations decreased at a similar rate at the three temperatures, whereas the decrease in Pn occurred earlier and much faster at warmer temperatures. Furthermore, total leaf protein content decreased later in the season (data not shown), suggesting that the initial decrease in protein concentration was due to leaf expansion rather than to net protein degradation. Photochemical quenching decreased earlier and faster as temperature increased. A higher proportion of absorbed photons was lost through non-radiative energy dissipation mechanisms instead of being used to drive photosynthesis (Maxwell and Johnson, 2000). Although the response of qN was not as clear as the response of qP, it also suggested that Pn was down-regulated earlier at warmer than at cooler temperatures. Moreover, effective quantum yield of PSII (PhiPS2) decreased faster as growth temperature increased. This fast decrease could be due to lower triose phosphate utilisation that induces a large pH gradient known to inhibit electron transport rates (Pammenter et al., 1993). Thus, it appeared that the rate of C assimilation at warmer temperatures rapidly became too high for the capacity to incorporate C in the sink, leading to feedback inhibition of Pn. In single-rooted sweet potato (Ipomoea batatas cv. Koganesengan), Sawada et al. (2003) suggested that photosynthetic activity depends on the development of sink activity, highlighting the feedback effects of sink activity on source activity. Early decreases in Pn at warmer temperatures could thus well be a response to an imbalance between source and sink activity. Further studies are necessary to identify the cascade of events leading to the modulation of Pn early in the season and the metabolic links between source–sink balance and rate of C assimilation.
Vcmax and TPU decreased to a greater extent over time at the warmer temperatures, such that TPU became lower at warm than at cooler temperatures a few days before leaf senescence. This suggests a slower transport rate of photosynthetic product at warm temperatures toward the end of the growing season (Sharkey et al., 2007). Moreover, F1,6BPase activity rapidly decreased a few days before the initiation of leaf senescence, regardless of growth temperature. F1,6BPase is usually inhibited in response to fructose-2,6-bisphosphate synthesis, which is stimulated when sucrose translocation decreases (Paul and Foyer, 2001). The decrease of F1,6BPase activity could thus be induced by the accumulation of sucrose in the leaf that took place a few days before leaf senescence. Sucrose accumulation in the leaf can also be linked to the concomitant accumulation of soluble sugars (starchless concentrations) in the bulb. Thus, the decrease in sink strength, once bulb cells were filled with starch, would cause an accumulation of sucrose in the bulb and leaf. This would then lead to a decrease in F1,6BPase activity and of TPU thereby inducing leaf senescence.
In conclusion, the production of a larger storage organ at low temperatures was confirmed in E. americanum by sampling plants grown under the 8/6 °C regime. The enhanced growth at lower temperatures appeared to be due to a slower starch accumulation that was apparently more in rhythm with the evolution of sink capacity at low temperature, leading to less source–sink imbalance and thus delayed leaf senescence.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Table S1. Effects of growth temperature on the rates of change through time and on either maximum or minimum values reached for different parameters related to growth, gas exchange, chlorophyll fluorescence, bulb carbohydrates, and enzyme activites in Erythronium americanum.
Acknowledgments
This work was financially supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) grant to LL. The authors acknowledge Kevin Thomas, Cécile Durand, and Caroline Mercier for technical assistance and William Parsons and Dave Onorato for English language revision.
References
- Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proceedings of the National Academy of Sciences, USA. 1995;92:9353–9357. doi: 10.1073/pnas.92.20.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri MA, Minchin PE, Lapointe L. Effects of temperature on the growth of spring ephemerals: Crocus vernus. Physiologia Plantarum. 2007;130:67–76. [Google Scholar]
- Bergmeyer HU, Bernt E. Methods of enzymatic analysis. New York: Academic Press Inc; 1974. [Google Scholar]
- Bertin N. Analysis of the tomato fruit growth response to temperature and plant fruit load in relation to cell division, cell expansion and DNA endoreduplication. Annals of Botany. 2005;95:439–447. doi: 10.1093/aob/mci042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeney AB, Mutton LL. A simple colorimetric method for the determination of sugars in fruit and vegetables. Journal of the Science of Food and Agriculture. 1980;31:889–897. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Constable JVH, Peffer BJ, DeNicola DM. Temporal and light-based changes in carbon uptake and storage in the spring ephemeral Podophyllum peltatum (Berberidaceae) Environmental and Experimental Botany. 2007;60:112–120. [Google Scholar]
- Daymond AJ, Wheeler TR, Hadley P, Ellis RH, Morison JIL. The growth, development and yield of onion (Allium cepa L.) in response to temperature and CO2. Journal of Horticultural Science. 1997;72:135–145. [Google Scholar]
- De Hertogh A, Le Nard M. The physiology of flower bulbs. A comprehensive treatise on the physiology and utilization of ornamental flowering bulbous and tuberous plants. Amsterdam: Elsevier Science Publishers; 1993. [Google Scholar]
- Denyer K, Johnson P, Zeeman S, Smith AM. The control of amylose synthesis. Journal of Plant Physiology. 2001;158:479–487. [Google Scholar]
- Gandin A, Lapointe L, Dizengremel P. The alternative respiratory pathway allows sink to cope with changes in carbon availability in the sink-limited plant Erythronium americanum. Journal of Experimental Botany. 2009;60:4235–4248. doi: 10.1093/jxb/erp255. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Geiger M, Stitt M. High-temperature perturbation of starch synthesis is attributable to inhibition of ADP-glucose pyrophosphorylase by decreased levels of glycerate-3-phosphate in growing potato tubers. Plant Physiology. 1998;117:1307–1316. doi: 10.1104/pp.117.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Guy CL, Huber JLA, Huber SC. Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant Physiology. 1992;100:502–508. doi: 10.1104/pp.100.1.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide OM. Flowering strategies of the high-arctic and high-alpine snow bed grass species Phippsia algida. Physiologia Plantarum. 1992;85:606–610. [Google Scholar]
- Heide OM, Gauslaa Y. Developmental strategies of Koenigia islandica, a high-arctic annual plant. Ecography. 1999;22:637–642. [Google Scholar]
- Koch KE. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Current Opinion in Plant Biology. 2004;7:235–246. doi: 10.1016/j.pbi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Lafta AM, Lorenzen JH. Effect of high temperature on plant growth and carbohydrate metabolism in potato. Plant Physiology. 1995;109:637–643. doi: 10.1104/pp.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe L. How phenology influences physiology in deciduous forest spring ephemerals. Physiologia Plantarum. 2001;113:151–157. doi: 10.1034/j.1399-3054.2001.1130201.x. [DOI] [PubMed] [Google Scholar]
- Lapointe L, Lerat S. Annual growth of the spring ephemeral Erythronium americanum as a function of temperature and mycorrhizal status. Canadian Journal of Botany. 2006;84:39–48. [Google Scholar]
- Lundmark M, Hurry V, Lapointe L. Low temperature maximizes growth of Crocus vernus (L.) Hill via changes in carbon partitioning and corm development. Journal of Experimental Botany. 2009;60:2203–2213. doi: 10.1093/jxb/erp103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone JG, Mittova V, Ratcliffe RG, Kruger NJ. The response of carbohydrate metabolism in potato tubers to low temperature. Plant and Cell Physiology. 2006;47:1309–1322. doi: 10.1093/pcp/pcj101. [DOI] [PubMed] [Google Scholar]
- Marcelis LFM. Sink strength as a determinant of dry matter partitioning in the whole plant. Journal of Experimental Botany. 1996;47:1281–1291. doi: 10.1093/jxb/47.Special_Issue.1281. [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence: a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Nguyen-Quoc B, Foyer CH. A role for ‘futile cycles’ involving invertase and sucrose synthase in sucrose metabolism of tomato fruit. Journal of Experimental Botany. 2001;52:881–889. doi: 10.1093/jexbot/52.358.881. [DOI] [PubMed] [Google Scholar]
- Pammenter NW, Loreto F, Sharkey TD. End product feedback effects on photosynthetic electron transport. Photosynthesis Research. 1993;35:5–14. doi: 10.1007/BF02185407. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Driscoll SP. Sugar repression of photosynthesis: the role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant, Cell and Environment. 1997;20:110–116. [Google Scholar]
- Paul MJ, Foyer CH. Sink regulation of photosynthesis. Journal of Experimental Botany. 2001;52:1383–1400. doi: 10.1093/jexbot/52.360.1383. [DOI] [PubMed] [Google Scholar]
- Pritchard J. The control of cell expansion in roots. New Phytologist. 1994;127:3–26. doi: 10.1111/j.1469-8137.1994.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Sass JE. Botanical microtechnique. Ames: Iowa State University Press; 1958. [Google Scholar]
- Sawada S, Sato M, Kasai A, Yaochi D, Kameya Y, Matsumoto I, Kasai M. Analysis of the feed-forward effects of sink activity on the photosynthetic source–sink balance in single-rooted sweet potato leaves. I. Activation of RuBPcase through the development of sinks. Plant and Cell Physiology. 2003;44:190–197. doi: 10.1093/pcp/pcg024. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell and Environment. 2007;30:1035–1040. doi: 10.1111/j.1365-3040.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- Smith AM, Bettey M, Bedford ID. Evidence that the rb locus alters the starch content of developing pea embryos through an effect on ADP-glucose pyrophosphorylase. Plant Physiology. 1989;89:1279–1284. doi: 10.1104/pp.89.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang GQ, Luscher M, Sturm A. Antisense repression of vacuolar and cell wall invertase in transgenic carrot alters early plant development and sucrose partitioning. The Plant Cell. 1999;11:177–190. doi: 10.1105/tpc.11.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu F, Reymond M, Hamard P, Granier C, Muller B. Spatial distributions of expansion rate, cell division rate and cell size in maize leaves: a synthesis of the effects of soil water status, evaporative demand and temperature. Journal of Experimental Botany. 2000;51:1505–1514. doi: 10.1093/jexbot/51.350.1505. [DOI] [PubMed] [Google Scholar]
- Vassey TL, Sharkey TD. Mild water stress of Phaseolus vulgaris plants leads to reduced starch synthesis and extractable sucrose phosphate synthase activity. Plant Physiology. 1989;89:1066–1070. doi: 10.1104/pp.89.4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler TR, Daymond AJ, Morison JIL, Ellis RH, Hadley P. Acclimation of photosynthesis to elevated CO2 in onion (Allium cepa) grown at a range of temperatures. Annals of Applied Biology. 2004;144:103–111. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.