Abstract
Despite the economic importance of grasses as food, feed, and energy crops, little is known about the genes that control their cell wall synthesis, assembly, and remodelling. Here a detailed transcriptome analysis that allowed the identification of genes involved in grass cell wall biogenesis is provided. Differential gene expression profiling, using maize oligonucleotide arrays, was used to identify genes differentially expressed between an elongating internode, containing cells exhibiting primary cell wall synthesis, and an internode that had just ceased elongation and in which many cells were depositing secondary cell wall material. This is one of only a few studies specifically aimed at the identification of cell wall-related genes in grasses. Analysis identified new candidate genes for a role in primary and secondary cell wall biogenesis in grasses. The results suggest that many proteins involved in cell wall processes during normal development are also recruited during defence-related cell wall remodelling events. This work provides a platform for studies in which candidate genes will be functionally tested for involvement in cell wall-related processes, increasing our knowledge of cell wall biogenesis and its regulation in grasses. Since several grasses are currently being developed as lignocellulosic feedstocks for biofuel production, this improved understanding of grass cell wall biogenesis is timely, as it will facilitate the manipulation of traits favourable for sustainable food and biofuel production.
Keywords: Biofuel, cell wall, defence, grasses, lignocellulose, microarray, Zea mays
Introduction
The plant cell wall is a highly dynamic structure that, besides providing mechanical support, needs to respond to various environmental and developmental cues and fulfils important functions in signalling events, the defence against biotic and abiotic stresses, and growth. Importantly, the cell wall represents a major energy storage compartment as much of the solar energy captured by plants is photosynthetically converted into chemical energy locked into the cell wall polymers: cellulose, hemicellulose, and lignin. The energy content and portability of plant-derived biofuels, and their compatibility with the existing petroleum-based transportation infrastructure, explains the attractiveness of lignocellulosic biomass as a renewable and sustainable source of mixed sugars for fermentation to biofuels. Indeed maize stover residues and several perennial grasses, including Miscanthus and switchgrass (Panicum virgatum), are currently being developed as lignocellulosic feedstocks for biofuel production. Perennial C4 grasses are considered superior potential feedstocks because of their efficient photosynthesis and long growing season, their ability to sequester nutrients in rhizomes at the end of the growing season, and their high water-use efficiency (Lewandowski et al., 2003).
However, current conversion of lignocellulosic biomass into fermentable sugars is inefficient and costly as plant cell walls have evolved to resist microbial and enzymatic deconstruction, collectively known as ‘biomass recalcitrance’ (Himmel et al., 2007). Optimizing cell wall composition and cross-linking to improve digestibility is critical for enhancing conversion efficiency and is a major breeding goal for biofuel crops. To achieve this optimization will require a detailed understanding of the dynamic architectural structure and function of the grass cell wall.
The grasses represent one of the most important families of flowering plants, the Poaceae, which includes the cereals, many important feed and forages, and more recently feedstocks for biofuel and biopower. However, relatively little is known about the genes that control cell wall synthesis, assembly, and remodelling in these monocots. Grass cell walls are distinct in composition from all other flowering plants, making a so-called type II cell wall, as opposed to the type I cell wall present in all dicots and non-grass monocots (see Carpita, 1996 for a detailed description). It has been estimated that 10% of plant genomes are devoted to cell wall-related processes and that at least a third of cell wall-related genes in grasses could have no, or few, orthologues in Arabidopsis (Carpita and McCann, 2008). This makes the study of a grass model system essential. Differences in cell wall-related gene family structure and expression, between Arabidopsis and the grasses (Penning et al., 2009), underscores the need for a grass model for the functional analysis of type II cell wall biogenesis.
Genetic resources for dedicated bioenergy crops such as switchgrass and Miscanthus are currently limited. Maize (Zea mays), like switchgrass and Miscanthus, belongs to the panicoid subfamily of the grasses (Lawrence and Walbot, 2007). This close phylogenetic relationship, combined with the long-standing genetic tools and completed genome sequence available (Schnable et al., 2009), make maize an attractive model for the identification and functional analysis of genes involved in cell wall biogenesis. Subsequently this information can be translated to the more genetically recalcitrant, and undomesticated, energy grasses.
The internodes of a maize stalk represent a developmental profile in which the successive internodes from the base to the apex become progressively younger. These internodes, therefore, provide a useful model for the identification of genes involved in cell wall synthesis, assembly, and remodelling during internode elongation and thickening. Here the identification of maize genes differentially expressed between an internode undergoing active elongation, which predominantly contained cells exhibiting primary cell wall synthesis, and an internode that had just ceased elongation and in which many cells were depositing secondary cell wall material is described. Transcriptome analysis, using maize oligonucleotide arrays, focused on genes and gene families potentially involved in cell wall biogenesis. This study confirmed the involvement of known cell wall genes and identified new candidates for a role in primary and secondary cell wall-related processes in grasses. To our knowledge this study describes the first total expression profiling in maize which specifically focuses on genes involved in cell wall biogenesis, and is one of only a few studies aimed at the identification of cell wall-related genes in grasses. The data presented provide a platform for the selection and functional analysis of candidate genes involved in grass cell wall biogenesis.
Materials and methods
Plant material and growth conditions
Maize inbred line B73 plants were grown in a greenhouse. Daylight was supplemented with overhead lighting using 400W high-pressure sodium lamps for 16 h daily. Minimum temperatures were maintained at 25 °C (day) and 18 °C (night). Internode development was assessed using the vegetative identification system (Ritchie et al., 1993). At stage V13, the plants were harvested, the leaves and leaf sheath were removed, internodes 9–13 (IN9–IN13) were excised, avoiding 1cm either side of the node, frozen in liquid nitrogen, and stored at –80°C. The average length of experimental IN9s at collection was 93.5 mm (±7.1), similar to the maximum length reached by these internodes in control plants at the silking stage. Collected IN13s showed an average length of 39.2 mm (±7.8), ∼40% of their maximum size at the silking stage.
RNA isolation
IN9 and IN13 from six maize plants were ground in liquid nitrogen. Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and purified using RNeasy MinElute Cleanup Kit columns (Qiagen, Valencia, CA, USA) according to a protocol recommended at www.maizearray.org. Following extraction, the quality and quantity of the RNA samples was determined by absorbance measurements at 230, 260, and 280 nm. RNA integrity was evaluated on an agarose gel.
RNA labelling and microarray procedures
Maize 70-mer oligonucleotide arrays, printed at the University of Arizona, were used to determine the expression profile of RNA extracted from IN9 and IN13. Each array contained 46,128 oligos printed on a single slide. The experiment consisted of six biological replicates: three slides in which IN9 RNA was labelled with Cy3 and IN13 RNA from the same plant was labelled with Cy5; and three slides in which IN13 RNA was labelled with Cy3 and IN9 RNA with Cy5, to ensure dye balance. RNA labelling, hybridization, and scanning were conducted at the University of Arizona by the Maize Oligonucleotide Array Project using their optimized protocols.
Data normalization and analysis
The microarray data were analysed within the statistical programming language R (version 2.7.0) using the Bioconductor library limma (Smyth, 2005). Loess normalization was applied to the raw data to remove intensity-dependent dye effects. A linear model with internode and dye as main effects was fitted and the limma-specific moderated F-test (Smyth, 2004) was applied to obtain P-values for testing the internode effect. As limma models the log ratio of the two channels on the same array this analysis automatically takes the pairing of samples into account. The Benjamini–Hochberg method (Benjamini and Hochberg, 1995) was used to adjust P-values for multiple testing. Genes were selected as significant if they had an adjusted P-value <0.01 [i.e. the false discovery rate (FDR) was controlled at 1%]. As an additional criterion only genes were selected with at least a 2-fold change in the primary analysis and a minimum of a 4-fold change for further detailed analysis. The microarray data have been deposited in NCBI's Gene Expression Omnibus (Edgar et al., 2002), accession number GSE24014.
Gene annotation and functional categories
Although gene annotation for the 46K maize oligonucleotide array was provided in the GenePix Array List (GAL-file, January 2007), the 70-mer oligos which showed >4-fold differential expression were subjected to a more thorough annotation strategy by submitting the oligo probes to Blast searches using the Maize Genome Browser (Release 4a.53) (http://www.maizesequence.org) and GenBank (http://blast.ncbi.nlm.nih.gov). The closest maize accession and Arabidopsis thaliana homologue were identified, and the InterPro and Pfam domains contained in the predicted protein sequences were determined. Genes were assigned to functional categories using the MIPS Functional Catalogue Database (Ruepp et al., 2004).
Quantitative RT-PCR
Gene-specific primers were designed using Primer Express software (Applied Biosystems). Whenever possible, primer pairs spanning one or more exon–intron junction were selected. Alternatively, at least one of the primers of a pair was located in the 3'-untranslated region. Primer sequences are listed in Supplementary Table S6 available at JXB online. RNA was isolated from IN9 and IN13 as described above from three randomly selected maize plants. First-strand cDNA synthesis was performed using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer's instructions, using 1 μg of total RNA and oligo(dT) primers. Based on the array data, the following reference genes were selected: cyclophilin (MZ00016819), peptidase C14 (MZ00027363), and ribosomal L11 (MZ00016094). The primer pairs for these reference genes exhibited primer efficiencies with a correlation coefficient >0.99 over a 10-fold dilution series and showed no differential expression between IN9 and IN13. Validation experiments showed that the slope of log input amount versus ΔCT was <0.1, demonstrating that the efficiencies of target and reference were approximately equal, confirming that the comparative CT method (ΔΔCT) could be used for quantitation. The fold change was calculated from 2–ΔΔCT where ΔΔCT represents ΔCT (IN9)–ΔCT (IN13). Quantitative RT-PCR was performed on an ABI 7500 Real Time PCR system using a SYBR Green I master mix (Applied Biosystems) with cDNA of three biological replicates. All reactions were performed in triplicate.
Histochemical staining of lignin
Maize internode sections of 10 μm thickness embedded in Agar Scientific JB-4 resin were stained for syringyl lignin using the Maule colour reaction. Sections were immersed in 1% neutral KMnO4 for 3 min and rinsed in distilled water. The sections were decolorized with 3% HCl and washed thoroughly in water. Sections were mounted in concentrated NH4OH and examined immediately by bright-field microscopy using a Leica CTR6500 fluorescence microscope.
Results and Discussion
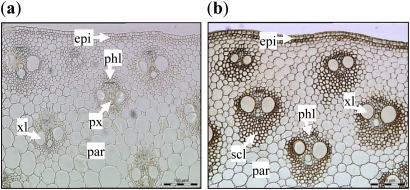
To profile differentially the expression of genes in an actively elongating internode versus an internode that had just ceased elongation, the development of maize internodes was assessed using the vegetative identification system described by Ritchie et al. (1993). Based on RT-PCR data for cell wall-related genes expressed during the elongation phase, IN9 and IN13 were selected for the expression profiling experiment (data not shown). These internodes were harvested from six maize plants at 50 d after sowing, representing six biological replicates. IN9 represented a non-elongating internode, with an average length of 93.5 mm (±7.1), and IN13 represented an elongating internode with an average length of 39.2 mm (±7.8). Stem cross-sections showed that the cells within non-elongating IN9 contained significant amounts of lignin when compared with those of elongating IN13 (Fig. 1). RNA was extracted from these internodes and hybridized in a pairwise pattern to the Maize 46K Oligonucleotide microarrays, with a dye swap.
Fig. 1.
Cross-section of the elongating internode IN13 (a) and the non-elongating internode IN9 (b) stained with Maule reagent. Dark coloration indicates the presence of syringyl lignin units. epi, epidermis; xl, xylem; par, parenchyma; phl, phloem; px, protoxylem; scl, sclerenchyma. Scale bar=200 μm. (This figure is available in colour at JXB online.)
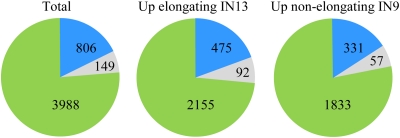
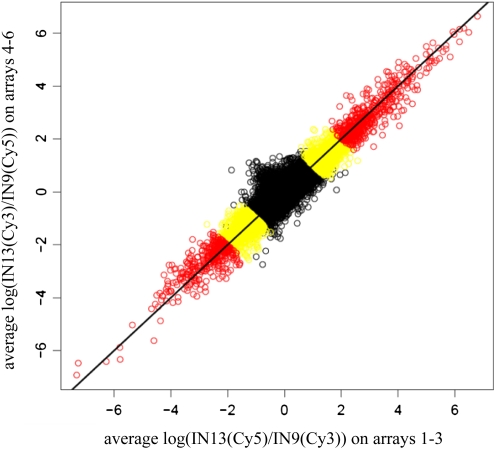
A total of 3988 oligonucleotide probes (8.6%) out of the 46,128 70-mer oligos printed on the slide exhibited >2-fold differential expression (Benjamini–Hochberg adjusted P-value <0.01) between IN13 and IN9 (Fig. 2; see Supplementary Table S1 at JXB online for a full list). The high number of differentially expressed genes is in agreement with other studies; for instance, >2000 genes were differentially expressed during stem development of Arabidopsis (Minic et al., 2009) and >3000 in poplar (Populus trichocarpa) (Dharmawardhana et al., 2010). A quality check of the array data is presented in Fig. 3 and Supplementary Fig. S1. The fact that points scatter close around the diagonal line in the Dye-Swap plot (Fig. 3) indicates that (i) the normalization has removed any systematic dye biases and (ii) the results are very consistent between the two dye settings. An MA-plot, which indicates at which overall level of expression significant changes occur, is shown in Supplementary Fig. S1. In order to validate the differential expression profiles obtained from the microarray analysis, transcripts of 13 selected genes were analysed using quantitative real-time PCR. For all the genes tested, the expression profile was the same as obtained with the microarrays (Table 1). This confirmed the robustness of the differential expression profiles obtained between IN9 and IN13 using the maize oligonucleotide arrays.
Fig. 2.
The number of differentially expressed genes between the elongating internode IN13 and the non-elongating internode IN9. The green and blue areas represent the number of >2-fold (P <0.01) and >4-fold (P <0.005) differentially expressed genes, respectively. The light grey area shows the number of genes represented more than once in the >4-fold differentially expressed gene list.
Fig. 3.
Dye-swap plot depicting the log-fold change for IN13 versus IN9 from the first three arrays (IN13 on Cy5) on the x-axis against the corresponding log-fold change from the three dye-swapped arrays (IN13 on Cy3) on the y-axis. Significant genes with at least a 2-fold change are labelled in yellow and significant genes with >4-fold changes are labelled in red. A few non-significant (black coloured) spots can be seen in the >2-fold change region. These are the ones furthest away from the diagonal, which shows that the statistical test successfully eliminates genes with non-consistent changes.
Table 1.
Quantitative RT-PCR results for selected transcripts
| Annotation | Maize gene ID | Oligo ID | Array | Q-RT-PCRa | ||
| IN9 | IN13 | IN9 | IN13 | |||
| NAC TF | GRMZM2G162739 | MZ00028246 | 5.9 | 1 | 17.2 (11.2–26.4) | 1 (0.8–1.2) |
| NAC TF | GRMZM2G068973 | MZ00026127 | 6.2 | 1 | 21.0 (17.3–25.5) | 1 (0.7–1.4) |
| NAC TF | GRMZM2G167018 | MZ00022590 | 2.9 | 1 | 7.0 (5.6–8.7) | 1 (0.8–1.2) |
| MYB TF | AC197146.3_FG002 | MZ00030474 | 5.9 | 1 | 23.0 (15.7–33.5) | 1 (0.4–2.5) |
| MYB TF | GRMZM2G037650 | MZ00040639 | 5.3 | 1 | 10.6 (7.4–15.2) | 1 (0.8–1.3) |
| MYB TF | GRMZM2G088524 | MZ00055303 | 5.2 | 1 | 11.0 (9.5–12.9) | 1 (0.9–1.2) |
| GH16/XTH | GRMZM2G004699 | MZ00024711 | 1 | 42.9 | 1 (0.4–2.6) | 274.0 (245–306) |
| GID1L2 | GRMZM2G049675 | MZ00020346 | 1 | 6.4 | 1 (0.8–1.2) | 16.9 (14.8–19.2) |
| RF2C | GRMZM2G071021 | MZ00044059 | 1 | 5.3 | 1 (0.9–1.1) | 12.8 (10.8–15.1) |
| GT47 | GRMZM2G100143 | MZ00033488 | 4.9 | 1 | 6.7 (4.7–9.5) | 1 (0.7–1.4) |
| GT47 | GRMZM2G059825 | MZ00036858 | 4.1 | 1 | 4.1 (3.4–5.0) | 1 (0.7–1.5) |
| GT43 | gb|BT036881.1|b | MZ00056172 | 1 | 4.6 | 1 (0.8–1.2) | 13.0 (10.4–16.4) |
| GT43 | GRMZM2G150302 | MZ00015783 | 2.9 | 1 | 3.3 (2.4–4.5) | 1 (0.8–1.2) |
Values in parentheses indicate the range of fold differential expression by incorporating the standard deviation of the ΔΔCT into the fold difference calculation.
No gene associated with the oligo using the maize genome browser.
Annotation and functional classification
Given that the existing annotation of the maize oligonucleotide array dated to January 2007, and a draft genome sequence for maize B73 was recently published (Schnable et al., 2009), the gene targets for the oligo probes that showed >4-fold differential expression in the array experiment (955 oligo probes) were re-annotated. Each 70-mer oligo sequence that showed a >4-fold differential hybridization signal was analysed using BLAST in the Maize Genome Browser (Release 4a.53) to query the B73 reference genome version 1, and to identify the corresponding maize gene ID and the InterPro and Pfam domains contained in the predicted protein sequence. The closest maize accession and A. thaliana homologue in the National Center for Biotechnology Information (NCBI) database was also determined (Supplementary Table S2 at JXB online). The analysis revealed some degree of redundancy within the oligo probes, as 149 maize gene IDs (15.6%) were represented more than once in the >4-fold differentially expressed target group of genes (Fig. 2). Unless stated otherwise, further analyses and descriptions will be confined to the genes that were >4-fold differentially expressed between IN13 and IN9.
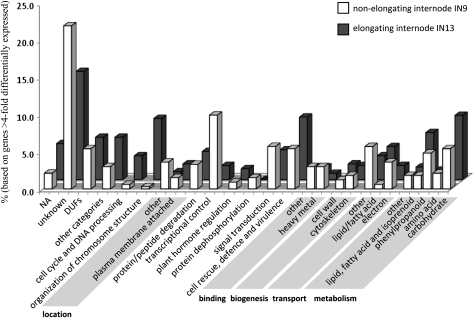
Using the MIPS Functional Catalogue Database (Ruepp et al., 2004), the transcripts were functionally categorized (Fig. 4). More genes involved in carbohydrate metabolism were preferentially expressed in the elongating IN13 (n=41) compared with the non-elongating IN9 (n=18). Many genes predicted to be involved in lipid, fatty acid, and isoprenoid metabolism were also preferentially expressed in IN13 (n=30) compared with IN9 (n=6), indicating that lipid metabolic processes are important during elongation. This was confirmed by the high number of genes up-regulated in IN13 (n=21) with functions related to lipid/fatty acid transport, and which all contained a protease inhibitor/seed storage/LTP family domain. Although lipid transfer proteins (LTPs) were long thought to function as lipid carriers between intracellular organelles, LTPs are in fact small secreted proteins with a range of biological functions including defence signalling in plant systemic acquired resistance (Jung et al., 2009) and the export of lipids to the cuticle (DeBono et al., 2009). It has been shown that LTPs can promote cell expansion (Nieuwland et al., 2005), although the mechanism of action is not clear.
Fig. 4.
Functional categories of genes showing >4-fold differential expression between the elongating internode IN13 and the non-elongating internode IN9.
The functional category ‘cell rescue, defence, and virulence’ contained many differentially expressed genes, with approximately twice as many genes up-regulated in IN13 (n=40) compared with IN9 (n=18). This is consistent with the need for more defence-related genes to be expressed in fragile elongating tissues. Many of the proteins involved in cell wall-related processes might also function in cell wall remodelling during a defence response. Examples of this included the secretory plant peroxidases with 11 genes up-regulated in IN13 and six in IN9. Such class III peroxidases are involved in multiple processes including cell wall loosening, cross-linking, and responses to wounding (Passardi et al., 2004) and some are believed to catalyse lignin polymerization (Fagerstedt et al., 2010). Thaumatins are specifically up-regulated in IN13 (n=7). The expression of plant thaumatins is induced by environmental stress and some thaumatin-like proteins exhibit β-1,3-glucanase activity and xylanase inhibitor activity (reviewed in Liu et al., 2010). Several thaumatin-like proteins were also differentially expressed in developing stems of Arabidopsis (Minic et al., 2009), suggesting that these defence-related secretory proteins could also function in cell wall remodelling during development. The high number (n=39) of ‘nucleosome assembly’-related genes preferentially expressed in IN13 is potentially surprising. Almost all of these genes encode histones (n=36). Maize internodes do not contain a vascular meristem, so involvement in secondary growth is unlikely. One explanation could be that some cells undergoing cell division were still present in IN13 samples, even though the 1 cm basal part, containing the active intercalary meristem, was not included during sample collection.
The largest numbers of differentially expressed genes were classified as of unknown function, with n=69 and n=74 in IN13 and IN9, respectively. A third of the genes of unknown function up-regulated in IN13 contained at least one identifiable conserved domain, and the equivalent figure was 21% for IN9. For only 39% of the unknown genes >4-fold up-regulated in IN13 could an Arabidopsis homologue be identified based on e <1−10; this figure was even lower for IN9 unknowns (26%), while these values were 94% and 88%, respectively, for the genes in the other functional categories.
Cell wall biogenesis is a complex process involving the action of many protein families directly involved in the synthesis of cell wall polysaccharides and the rearrangement of cell wall polymers, but also proteins with indirect involvement such as regulatory genes including transcription factors (TFs). In the remaining part of this section a more detailed analysis of selected genes and gene families with known or potential involvement in cell wall biogenesis is reported.
Carbohydrate metabolism: glycosyl transferases and hydrolases
Glycosyl transferases (GTs) constitute a large family of enzymes involved in the biosynthesis of oligosaccharides, polysaccharides, and glycoconjugates. Several genes encoding GTs showed >4-fold differential expression, including GT1, GT2, GT8, GT31, GT43, and GT47 family members (Table 2). Mutants for several Arabidopsis homologues to the GTs up-regulated in IN9 have been extensively studied and show an irregular xylem (IRX) phenotype. The Arabidopsis homologues for the two GT2 genes preferentially expressed in IN9, IRX1 and IRX5, both encode cellulose synthases (AtCesA8 and AtCesA4) involved in secondary cell wall cellulose synthesis (Taylor et al., 2003). The third maize cellulose synthase (ZmCesA12) suggested to be involved in secondary wall formation (Appenzeller et al., 2004) was not >4-fold preferentially expressed in IN9, assuming that an oligo probe was available for this gene. The GT2 up-regulated in IN13 suggests involvement in primary cell wall biosynthesis. The Arabidopsis homologue to this GT2, AtCSLC12, is a potential β-1,4-glucan synthase involved in the synthesis of the xyloglucan backbone rather than cellulose (www.uniprot.org), supporting this hypothesis. None of the presumed primary cell wall-associated CesAs (ZmCesA1–ZmCesA9; Appenzeller et al., 2004) showed >4-fold preferential expression in IN13. Upon querying the maize oligonucleotide annotation file, expression data for all but one of these CesAs could be retrieved (see Supplementary Table S3 at JXB online). Only ZmCesA1 was >2-fold preferentially expressed in IN13, while the remaining CesAs showed only a minor preferential expression pattern for the elongating internode. Based on phylogenetic analysis it has been suggested that some of these CesAs (ZmCesA6– ZmCesA8) are involved in primary cell wall synthesis later in development before the onset of secondary wall formation (Appenzeller et al., 2004), which could explain the observed expression pattern for some of the CesAs. Experimental evidence is still needed to confirm that ZmCesA1– ZmCesA9 are indeed involved in primary cell wall cellulose synthesis. A bioinformatics approach identified the GT43, GT47, and GT61 families, and proteins containing the PF02458 domain, as the most likely candidates to encode enzymes involved in the synthesis of arabinoxylan and its side chains in the grasses (Mitchell et al., 2007). It was found that genes belonging to these families were differentially expressed (Table 2 and Supplementary Table S2). For example, the orthologous GT61 gene (GRMZM2G354610) for the locus identified as the most promising candidate for a feruloyl-arabinoxylan β-1,2-xylosyl transferase in rice (Os06g27560), was 5.3-fold up-regulated in IN9 compared with IN13 (Table 2). The differentially expressed GT43 showed the highest homology to Arabidopsis IRX9, believed to encode a xylan synthase responsible for adding β-xylosyl residues to the nascent glucuronoxylan (Pena et al., 2007). However, the maize GT43 was up-regulated in elongating tissue and therefore not involved in secondary cell wall synthesis as was the case for IRX9 in Arabidopsis. The Arabidopsis homologues of the two GT47 genes up-regulated in IN9 correspond to IRX10 and IRX10-L and are believed to be required for xylan chain elongation (Brown et al., 2009) as an irx10/irx10-L double mutant exhibited a large reduction of xylan in the secondary cell walls and a severe reduction in β-(1,4) xylosyltransferase activity. The two maize GT47 genes, GRMZM2G100143 and GRMZM2G059825, preferentially expressed in the non-elongating internode, therefore represent excellent candidates for involvement in the biosynthetic process of secondary cell wall xylans in maize.
Table 2.
Glycosyl transferases (GTs) and glycosyl hydrolases (GHs) >4-fold differentially expressed between IN9 and IN13
| Family | Oligo ID | Signal intensity | Fold changea | Maize gene ID | Arabidopsis homologue gene | E-value | Putative annotation |
| GT1 | MZ00015539 | 9.9 | 4.8 | GRMZM2G008263 | AT1G32900 | 0E+00 | Granule bound starch synthase IIa precursor |
| GT1 | MZ00012710 | 10.8 | –4.6 | GRMZM2G051683 | AT3G16520 | 3E-105 | Glycosyltransferase |
| GT2 | MZ00037072 | 13.4 | –8.0 | GRMZM2G055795 | AT4G18780 (IRX1) | 0E+00 | Cellulose synthase 11 |
| GT2 | MZ00020258 | 13.5 | –5.5 | GRMZM2G445905 | AT5G44030 (IRX5) | 0E+00 | Cellulose synthase 10 |
| GT2 | MZ00044509 | 11.1 | 4.0 | GRMZM2G074792 | AT4G07960 | 0E+00 | Putative glucosyltransferase/cellulose synthase |
| GT8 | MZ00026735 | 11.4 | –9.7 | GRMZM2G165919 | AT2G47180 | 3E-149 | Galactinol synthase |
| GT8 | MZ00044441 | 12.6 | –8.3 | GRMZM2G131697 | AT2G47180 | 5E-148 | Galactinol synthase |
| GT8 | MZ00028554 | 9.9 | 4.5 | GRMZM2G036918 | AT2G38650 | 3E-137 | GT8-like transferase, transferring glycosyl groups |
| GT31 | MZ00044747 | 10.3 | –5.3 | GRMZM2G057779 | AT5G57500 | 7E-58 | Galactosyltransferase/glycosyltransferase family 31 |
| GT31 | MZ00032181 | 10.5 | –4.5 | GRMZM2G072406 | AT5G57500 | 5E-54 | Transferase, transferring glycosyl groups |
| GT43 | MZ00056172 | 10.8 | 4.6 | gb|BT036881.1|b | AT2G37090 (IRX9) | 5E-43 | GT43 [Os03g0287800 (2e-74)] |
| GT47 | MZ00033488 | 11.5 | –4.9 | GRMZM2G100143 | AT1G27440 (IRX10) | 0E+00 | Secondary cell wall-related GT47 [Os01g0926700 (0E+00)] |
| GT47 | MZ00036858 | 13.5 | –4.1 | GRMZM2G059825 | AT5G61840 (IRX10-L) | 0E+00 | Secondary cell wall-related GT47 [Os01g0926700 (0E+00)] |
| pGH17 | MZ00018424 | 10.0 | –5.3 | GRMZM2G354610 | AT3G18180 | 9E-79 | Glycosyltransferase |
| GH1 | MZ00057235 | 12.7 | 63.6 | GRMZM2G016890 | AT5G44640 | 2E-128 | Beta-D-glucosidase [Os4bglu12 (5E-134)] |
| GH1 | MZ00023504 | 11.5 | 60.4 | GRMZM2G014844 | AT5G44640 | 1E-135 | Beta-D-glucosidase [Os6bglu24 and Os4bglu12 (3E-137)] |
| GH1 | MZ00026498 | 12.6 | 34.1 | GRMZM2G120962 | AT5G44640 | 2E-137 | Beta-D-glucosidase [Os4bglu12 (6E-145)] |
| GH1 | MZ00039183 | 10.8 | 21.1 | GRMZM2G008247 | AT5G44640 | 3E-135 | Beta-D-glucosidase [Os4bglu12 (6E-136)] |
| GH1 | MZ00035426 | 10.9 | 17.5 | ref|NM_001111984.1|c | AT5G44640 | 2E-128 | Beta-D-glucosidase [Os4bglu12 (6E-136)] |
| GH1 | MZ00023721 | 12.5 | 16.8 | GRMZM2G118003 | AT3G18080 | 4E-131 | Beta-D-glucosidase [Os3bglu7 (0E+00)] |
| GH1 | MZ00032041 | 11.1 | –7.7 | GRMZM2G457040 | AT4G21760 | 0E+00 | Beta-D-glucosidase [Os4bglu16 (0E+00)] |
| GH1 | MZ00016333 | 11.1 | 5.9 | AC217401.3_FG002 | AT3G18080 | 1E-176 | Beta-D-glucosidase [Os3bglu8 (0E+00)] |
| GH5 | MZ00016790 | 10.1 | 5.0 | GRMZM2G140201 | AT2G20680 | 4E-152 | Cellulase family protein |
| GH16 | MZ00024711 | 12.2 | 42.9 | GRMZM2G004699 | AT5G13870 | 1E-131 | XTH |
| GH16 | MZ00013946 | 11.5 | 34.4 | GRMZM2G180870 | AT4G03210 | 8E-93 | XTH |
| GH16 | MZ00036398 | 10.9 | –15.2 | GRMZM2G026980 | AT4G25810 | 2E-102 | XTH |
| GH16 | MZ00052416 | 9.8 | 6.3 | GRMZM2G039919 | AT1G14720 | 4E-75 | XTH |
| GH16 | MZ00055592 | 10.0 | 5.3 | GRMZM2G030173 | AT2G06850 | 6E-21 | XTH |
| GH16 | MZ00013272 | 9.9 | 5.1 | GRMZM2G175598 | AT5G57530 | 5E-38 | XTH |
| GH16 | MZ00004529 | 12.1 | 4.8 | GRMZM2G413044 | AT2G36870 | 5E-103 | XTH |
| GH16 | MZ00018500 | 11.4 | 4.9 | GRMZM2G413006 | AT5G57560 | 1E-108 | XTH |
| pGH17 | MZ00029303 | 12.7 | 18.7 | GRMZM2G074811 | AT3G13560 | 7E-05 | Glucan endo-1,3-beta-glucosidase |
| GH17 | MZ00028373 | 11.1 | 14.1 | GRMZM2G046101 | AT4G34480 | 4E-153 | Glucan endo-1,3-beta-glucosidase |
| GH17 | MZ00042022 | 11.2 | 12.1 | GRMZM2G046459 | AT2G05790 | 3E-161 | Glycosyl hydrolase family 17 protein |
| GH17 | MZ00037281 | 11.1 | –7.7 | GRMZM2G137535 | AT4G16260 | 1E-85 | Lichenase |
| GH17 | MZ00028849 | 11.0 | 6.7 | GRMZM2G335111 | AT4G26830 | 6E-69 | Glucan endo-1,3-beta-glucosidase |
| pGH17 | MZ00026915 | 11.4 | 4.4 | GRMZM2G447691 | AT2G43670 | 2E-24 | Glucan endo-1,3-beta-glucosidase |
| GH18 | MZ00032065 | 9.7 | 5.1 | GRMZM2G141456 | AT4G19810 | 6E-52 | Class V plant chitinase |
| pGH17 | MZ00004170 | 10.1 | –5.2 | GRMZM2G130276 | AT5G24090 | 3E-14 | Hevamine-A/class III endochitinase |
| GH19 | MZ00043887 | 10.3 | –4.7 | GRMZM2G145461 | AT3G12500 | 2E-30 | Chitinase |
| GH20 | MZ00027331 | 10.9 | 7.0 | GRMZM2G121514 | AT1G65600 | 0E+00 | Beta-N-acetylhexosaminidase/hexosaminidase |
| GH28 | MZ00018754 | 11.1 | 15.7 | AC231180.2_FG006 | AT4G23820 | 2E-157 | Polygalacturonase |
| GH28 | MZ00041598 | 11.1 | 4.1 | GRMZM2G052844 | AT4G23820 | 1E-163 | Polygalacturonase |
| GH35 | MZ00015016 | 10.0 | 7.7 | GRMZM2G038281 | AT5G63810 | 0E+00 | Beta-galactosidase |
| GH35 | MZ00027220 | 10.3 | 7.0 | GRMZM2G127123 | AT2G28470 | 0E+00 | Beta-galactosidase |
| GH35 | MZ00019862 | 11.8 | 4.6 | GRMZM2G417455 | AT4G36360 | 0E+00 | Beta-galactosidase |
| GH35 | MZ00039458 | 11.5 | 4.3 | GRMZM2G178106 | AT4G36360 | 0E+00 | Putative galactosidase |
| GH36 | MZ00019029 | 11.9 | –4.0 | GRMZM2G127147 | AT5G20250 | 0E+00 | Alkaline alpha galactosidase/raffinose synthase |
| pGH17 | MZ00022215 | 10.6 | 9.6 | GRMZM2G077299 | AT3G10740 | 0E+00 | Alpha-N-arabinofuranosidase |
Positive values indicate fold higher expression in IN13 compared with IN9. Negative values indicate higher expression in IN9 compared with IN13.
No gene associated with the oligo using the maize genome browser.
The oligo has too low homology using the maize genome browser.
p, putative; [ ] indicates the closest rice homologue.
Glycosyl hydrolases (GHs) represent some of the most extensive gene families in plants, many involved in cell wall remodelling (Minic, 2008). Several GH1 β-glucosidases were highly up-regulated in elongating IN13 (Table 2). β-Glucosidases have been implicated in several functions including responses to biotic and abiotic stresses, lignification, and cell wall remodelling and metabolism (Cairns and Esen, 2010). Analysis of the five most differentially expressed β-glucosidases identified At5g44640 as the closest homologue in Arabidopsis and Os4bglu12 as the closest homologue in rice (see Table 2). Os4bglu12 has the highest sequence similarity to a cell wall-bound β-glucosidase containing high exoglucanase activity, consistent with a role in cell wall biogenesis (Opassiri et al., 2006).
Xyloglucan endotransglucosylase/hydrolases (XTHs) are GH16 family hydrolases acting on the xyloglucan chains that cross-link the cellulose microfibrils in the cell wall, catalysing endotransglucosylase (XET) and/or xyloglucan endohydrolase activities. XTHs acting in XET mode perform molecular grafting reactions by cleaving donor xyloglucan chains and rejoining the newly formed ends (Rose et al., 2002). This creates the potential ability to alter and loosen the cell wall matrix, and studies have shown strong correlations between XTH expression and cell elongation activity (Uozu et al., 2000; Vissenberg et al., 2000). In agreement with this, seven genes encoding XTHs showed >4-fold higher expression in IN13 compared with IN9 (Table 2). However, XTH expression does not always correlate with growth rate. XTH activity has been detected in tissues in which expansion has ceased, and involvement in the formation of secondary cell walls of vascular tissues has previously been suggested (Bourquin et al., 2002). Thus, the XTH highly up-regulated in IN9 might fulfil such a secondary cell wall-related function (Table 2). The exact function of XTH in the grasses remains to be elucidated as type II cell walls contain a relatively low amount of xyloglucan. There is evidence that XTH can use a range of donor and acceptor substrates in addition to xyloglucan for its XET activity (Hrmova et al., 2007), suggesting that certain XTH/XET isoforms might catalyse the formation of covalent linkages between different types of wall polysaccharides in grasses (Fincher, 2009).
Other GHs that were mostly up-regulated in IN13 and implicated in cell wall loosening during growth include members of the GH17 family, encoding putative β-1,3-glucanases, several GH35 family members, encoding β-galactosidases, and GH28 polygalacturonases (Table 2). Two potential chitinases were up-regulated in IN9 while one was up-regulated in IN13. Two Arabidopsis chitinases, AtCTL1 and AtCTL2, are believed to be involved in secondary plant cell wall biosynthesis since mutations in these genes cause ectopic deposition of lignin (Zhong et al., 2002) and increase lignin accumulation in dark-grown seedlings (Hossain et al., 2010), respectively. A putative GH51 family, α-N-arabinofuranosidase (GRMZM2G077299), was up-regulated in IN13. GH51 enzymes catalyse the hydrolysis of terminal non-reducing α-L-arabinofuranosyl residues, although they are also capable of hydrolysing β-D-xylosyl residues and thus might be considered as bi-functional arabinofuranosidase/β-D-xylosidase enzymes. GRMZM2G077299 showed the highest homology with Arabidopsis At3g10740, one of the few α-N-arabinofuranosidases that have been studied in plants (Montes et al., 2008). In grasses, the removal of arabinofuranosyl residues from arabinoxylans by α-N-arabinofuranosidases leads to significant changes in the physicochemical properties of the cell wall (Lee et al., 2003). Arabinoxylans consist of a (1,4)-β-D-xylan backbone substituted with α-L-arabinofuranosyl units, which can be esterified with hydroxycinnamic acids, in particular ferulic acid, which may form cross-bridges between adjacent arabinoxylan chains, or with lignin, by oxidative dimerization (Hatfield et al., 1999). Such esterification can significantly contribute to the recalcitrance of biomass and the conversion of cell wall polysaccharides into fermentable sugars and thus the production of biofuels. The putative maize arabinofuranosidase identified therefore represents a priority candidate for further functional analysis. Modification of endogenous arabinofuranosidase activity might provide a tool to decrease the number of α-L-arabinofuranosyl residues available for substitution with ferulic acids and thereby reduce cross-linking in the cell wall matrix.
Transcriptional control of cell wall biogenesis
Because TFs act as master regulators of cellular processes, they are predicted to be excellent candidates for modifying complex traits in crop plants. TF-based technologies are therefore likely to be a prominent part of the next generation of biotechnological crops.
It has recently been shown that a number of NAC and MYB TFs regulate the formation of secondary cell wall synthesis in Arabidopsis and a number of woody species including poplar, pine, and eucalyptus (Rogers and Campbell, 2004; Goicoechea et al., 2005; Mitsuda et al., 2007; Bomal et al., 2008; Zhong et al., 2008, 2010). However, to date, only a few TFs regulating cell wall-associated processes in the grasses have been identified.
A wide range of TFs, including several MYB and NAC TFs, were differentially expressed between IN9 and IN13 (Table 3). The majority of these were up-regulated in IN9, thus representing excellent candidates for further functional analysis to confirm if these genes are involved in regulating secondary cell wall biogenesis in grasses. One of the few, if not only, TF so far reported to be involved in regulating secondary cell wall synthesis in grasses is the maize MYB TF ZmMYB42 (Fornale et al., 2006; Sonbol et al., 2009). Overexpression of ZmMYB42 in Arabidopsis repressed lignin biosynthesis, reducing the lignin content of lignified tissues, and increased cell wall degradability. A close homologue of ZmMYB42, GRMZM2G419239, with 95% identity at the amino acid level, was 6-fold more highly expressed in IN9 than in IN13 (Table 3). This is somewhat surprising as it might be expected that there would be no need for repression of lignin biosynthesis in tissue undergoing secondary cell wall thickening. However, ZmMYB42 has only been studied by heterologous expression in Arabidopsis using the strong, and constitutively active, 35S promoter. For an accurate functional analysis, regulators of lignin biosynthetic genes should be expressed in cells actively undergoing lignification. Thus, it is uncertain whether ZmMYB42, as well as some of the other MYBs that have been studied using expression in heterologous systems driven by strong constitutive promoters, are indeed regulators of lignin biosynthesis (Zhong and Ye, 2009). The alternative interpretation is that their effects on lignin biosynthesis observed following overexpression are indirect.
Table 3.
Transcription factors >4-fold differentially expressed between IN9 and IN13
| Transcription factor class | Oligo ID | Signal intensity | Fold changea | Maize gene ID | Arabidopsis homologue gene | E-value |
| NAC | MZ00023972 | 10.9 | –7.1 | GRMZM2G079632 | AT5G08790/ATAF2 | 3E-65 |
| NAC | MZ00026127 | 10.7 | –6.2 | GRMZM2G068973 | AT5G08790/ATAF2 | 6E-69 |
| NAC | MZ00028246 | 10.8 | –5.9 | GRMZM2G162739 | AT5G08790/ATAF2 | 1E-65 |
| NAC | MZ00035947 | 10.8 | –4.8 | GRMZM2G347043 | AT5G08790/ATAF2 | 8E-71 |
| NAC | MZ00027155 | 11.4 | –4.7 | GRMZM2G054252 | AT2G33480 | 3E-29 |
| NAC | MZ00039846 | 11.3 | –4.3 | GRMZM2G018553 | AT5G08790/ATAF2 | 1E-68 |
| NACb | MZ00018291 | 9.5 | –3.6 | GRMZM2G123667 | AT5G08790/ATAF2 | 4E-74 |
| NACb | MZ00022590 | 10.3 | –2.9 | GRMZM2G167018 | AT1G56010/NAC021 | 1E-62 |
| NACb | MZ00036503 | 12.0 | –2.9 | GRMZM2G014653 | AT1G01720/ATAF1 | 2E-92 |
| NACb | MZ00043228 | 11.5 | –2.0 | AC202396.4_FG010 | AT1G01720/ATAF1 | 1E-07 |
| MYB | MZ00032119 | 11.1 | –6.0 | GRMZM2G419239 | AT4G38620/MYB4 | 2E-75 |
| MYB | MZ00030474 | 11.0 | –5.9 | AC197146.3_FG002 | AT3G28910/MYB30 | 3E-69 |
| MYB | MZ00040603 | 10.0 | –5.4 | GRMZM2G037650 | AT4G22680/MYB85 | 4E-66 |
| MYB | MZ00055303 | 10.9 | –5.2 | GRMZM2G088524 | AT5G61620 | 6E-30 |
| MYB | MZ00040616 | 10.2 | –4.2 | GRMZM2G162434 | AT1G08810 | 8E-70 |
| AP2-EREBP | MZ00012977 | 11.2 | –6.0 | GRMZM2G069146 | AT4G25470/DREB1C | 7E-33 |
| AP2-EREBP | MZ00016032 | 12.7 | –5.6 | GRMZM2G174347 | AT5G44210/ERF9 | 2E-29 |
| AP2-EREBP | MZ00016033 | 13.3 | –4.9 | GRMZM2G020150 | AT3G15210/ERF4 | 9E-27 |
| AP2-EREBP | MZ00025004 | 14.1 | –4.5 | GRMZM2G052667 | AT1G72360/ERF073 | 2E-28 |
| AP2-EREBP | MZ00005099 | 11.0 | –4.5 | GRMZM2G124011 | AT4G25480/DREB1A | 5E-30 |
| AP2-EREBP | MZ00018542 | 12.3 | –4.5 | gb|EU955981.1|c | AT1G72360/ERF073 | 3E-29 |
| AP2-EREBP | MZ00004814 | 10.2 | –4.1 | GRMZM2G129674 | AT3G16770/RAP2-3 | 5E-20 |
| AUX/IAA | MZ00029389 | 11.3 | 6.3 | GRMZM2G366373 | AT1G04240/IAA3 | 1E-40 |
| AUX/IAA | MZ00004631 | 10.8 | –5.8 | GRMZM2G079200 | AT4G14550/IAA14 | 4E-35 |
| AUX/IAA | MZ00037052 | 12.1 | –4.5 | GRMZM2G115357 | AT5G43700/IAA4 | 4E-43 |
| AUX/IAA | MZ00024726 | 13.3 | –4.2 | GRMZM2G004696 | AT1G04250/IAA17 | 2E-46 |
| C2H2 | MZ00029551 | 10.5 | –5.9 | GRMZM2G400714 | AT5G67450/AZF1 | 1E-30 |
| C2H2 | MZ00021052 | 13.7 | –4.8 | GRMZM2G086835 | AT1G24625/ZFP7 | 4E-17 |
| C2H2 | MZ00020958 | 10.2 | –4.5 | GRMZM2G035103 | AT3G19580/AZF2 | 1E-29 |
| C3H | MZ00037166 | 12.6 | –9.3 | GRMZM2G173124 | AT2G19810 | 9E-70 |
| C3H | MZ00044553 | 13.7 | –5.7 | gb|EU958052.1|d | AT5G58620 | 1E-21 |
| bZIP | MZ00042491 | 9.8 | 5.2 | GRMZM2G052102 | AT3G58120/BZIP61 | 5E-57 |
| bZIP | MZ00028419 | 11.6 | –4.8 | GRMZM2G073427 | AT5G28770 | 4E-29 |
| WRKY | MZ00042508 | 10.8 | –10.2 | ref|NM_001154079.1|c | AT2G38470/WRKY33 | 1E-89 |
| WRKY | MZ00035864 | 13.0 | 5.1 | GRMZM2G069668 | AT1G29860/WRKY71 | 3E-19 |
| PLATZ | MZ00029580 | 11.0 | –4.4 | GRMZM2G131280 | AT4G17900 | 2E-75 |
| MADS | MZ00022435 | 10.4 | 7.5 | GRMZM2G137510 | AT5G60910/AGL8 | 6E-43 |
| HB | MZ00019195 | 10.1 | 7.5 | GRMZM2G097349 | AT1G69780/ATHB-13 | 2E-46 |
| HB | MZ00030241 | 9.5 | 4.3 | GRMZM2G106276 | AT5G06710/HAT14 | 2E-49 |
| HB | MZ00042036 | 13.2 | –4.2 | GRMZM2G028041 | AT4G08150/KNAT1 | 1E-98 |
| bHLH | MZ00033503 | 10.2 | 4.6 | GRMZM2G035156 | AT3G47710 | 2E-25 |
| bHLH | MZ00048554 | 10.0 | 4.5 | GRMZM2G180452 | AT1G10120/BHLH74 | 2E-37 |
Positive values indicate fold higher expression in IN13 compared with IN9. Negative values indicate higher expression in IN9 compared with IN13.
Identified using the annotation file for the maize oligonucleotide array.
The oligo has too low homology using the maize genome browser.
No gene associated with the oligo using the maize genome browser.
Although most attention has been focused on the MYB and NAC TFs, TFs belonging to other families might also be involved in regulating cell wall biogenesis. For instance, it has been shown that WRKY TFs increase the levels of soluble and wall-bound phenolic compounds and lignin. Such increases were observed when Medicago WRKY genes were overexpressed in tobacco (Naoumkina et al., 2008), and a grapevine WRKY TF has been shown to be involved in regulating lignification (Guillaumie et al., 2010). AUX/IAA TFs have been shown to regulate the developmental processes involved in secondary growth (Oh et al., 2003; Scarpella and Meijer, 2004). Moreover, it has recently been shown that transcriptional regulators including C2H2 zinc finger and AP2-EREBP TFs were more highly expressed during cell wall thickening during cotton fibre development, compared with the earlier elongation stage (Al-Ghazi et al., 2009). A regulatory role for AP2-EREBPs in poplar and Arabidopsis secondary cell wall metabolism has also been suggested (van Raemdonck et al., 2005; Lasserre et al., 2008). Thus, it is possible that some of the other differentially expressed TFs listed in Table 3 are also involved in regulating secondary cell wall biogenesis in grasses. TFs specifically involved in regulating the biosynthetic pathways of cellulose and xylan synthesis remain elusive. Some of these regulatory functions might well be fulfilled by some of the other TF family members.
Many TFs are involved in regulating resistance to biotic and abiotic stress in model plants as well as in crop plants (for a review, see Century et al., 2008). As the cell wall plays an intrinsic role in conferring resistance to many biotic and abiotic stresses (Bray, 2004; Fan et al., 2006; Konno et al., 2008; Moore et al., 2008), it is possible that some of the TFs identified, in this and other studies, might be involved in regulating interacting pathways of stress-related responses and normal developmental regulation of cell wall biogenesis.
Lignin synthesis
Lignin plays an important role in plant growth and development. This heterogeneous hydrophobic phenolic polymer impregnates the cellulose and hemicellulose networks, thereby strengthening the cell wall and providing a stable, waterproof coating that protects the secondary wall from physical and biological attacks. The presence of lignin is one of the main obstacles in the conversion of lignocellulosic biomass into fermentable sugars as it impedes the enzymatic digestion of the cell wall biomass (Himmel et al., 2007), hence lignin modification is one of the priorities for the improvement of bioenergy feedstocks. Recently, genetic genomics approaches have been used in maize recombinant inbred lines to identify candidate genes associated with lignin content and cell wall digestibility (Shi et al., 2007; Thomas et al., 2010), but further investigations are needed to identify genes underlying quantitative trait loci (QTLs) involved in these complex traits. Although lignification is spatially and temporally regulated within maize internodes, most of the lignification takes place during post-elongation secondary cell wall deposition (Jung and Casler, 2006). Lignin precursors are produced by the phenylpropanoid pathway, and many genes belonging to this pathway exhibited higher expression in IN9 (Table 4), in agreement with active secondary cell wall synthesis and lignification occurring in this internode. Because the biochemical pathways of monolignol biosynthesis are highly conserved throughout vascular plants (Xu et al., 2009), the >4-fold differentially expressed gene list was extended to include potential phenylpropanoid pathway genes queried from the maize oligonucleotide annotation file showing >2-fold differential expression (Table 4).
Table 4.
Genes involved in phenylpropanoid metabolism >2-fold differentially expressed between IN9 and IN13
| Family | Oligo ID | Signal intensity | Fold changea | Gene ID | Arabidopsis homologuegene | E-value | Putative annotation |
| ALDH | MZ00039837 | 10.5 | 5.5 | GRMZM2G071021 | AT3G24503 | 0E+00 | Cytosolic aldehyde dehydrogenase RF2C |
| CCR/DFR | MZ00000662 | 11.1 | –21.1 | gb|BT064982.1|b | AT1G15950 | 2E-125 | Cinnamoyl-CoA reductase/dihydroflavonol-4-reductase |
| CCR/DFR | MZ00000962 | 10.9 | –6.5 | GRMZM2G001991 | AT5G14700 | 7E-81 | Dihydroflavonol-4-reductase/cinnamoyl-CoA reductase-related |
| CCR/DFR | MZ00027625 | 11.5 | –5.8 | GRMZM2G050076 | AT2G33590 | 1E-58 | Dihydroflavonol-4-reductase/putative cinnamoyl-CoA reductase |
| CCR/DFR | MZ00034340 | 10.1 | 4.0 | GRMZM2G033555 | AT2G33590 | 2E-91 | Dihydroflavonol-4-reductase/putative cinnamoyl-CoA reductase |
| CCR/DFR | MZ00036789 | 11.3 | 4.0 | GRMZM2G034069 | AT2G33590 | 1E-100 | Dihydroflavonol-4-reductase/putative cinnamoyl-CoA reductase |
| CCR1 | MZ00015899 | 13.5 | –3.1 | GRMZM2G131205 | AT1G15950 | 2E-134 | Cinnamoyl CoA reductase1 |
| CCR/DFR | MZ00035958 | 9.4 | 2.8 | GRMZM2G034360 | AT2G33590 | 1E-100 | Dihydroflavonol-4-reductase |
| CCR/DFR | MZ00023228 | 9.9 | 2.4 | GRMZM2G057328 | AT1G15950 | 1E-103 | Dihydroflavonol-4-reductase |
| CCR/DFR | MZ00012815 | 9.2 | –2.3 | gb|BT036278.1|c | AT2G33590 | 2E-85 | Dihydroflavonol-4-reductase |
| CCR/DFR | MZ00030523 | 10.7 | –2.2 | GRMZM2G009681 | AT2G33590 | 4E-94 | Dihydroflavonol-4-reductase |
| OMT | MZ00044446 | 12.5 | 37.0 | GRMZM2G041866 | AT4G35160 | 4E-50 | O-Methyltransferase ZRP4-like |
| OMT | MZ00016324 | 11.7 | 14.7 | GRMZM2G140996 | AT4G35160 | 7E-53 | O-Methyltransferase ZRP4-like |
| OMT | MZ00042149 | 12.4 | –14.3 | GRMZM2G097297 | AT4G35160 | 2E-49 | O-Methyltransferase ZRP4 |
| OMT | MZ00026069 | 11.3 | 9.7 | ref|NM_001157182.1|c | AT4G35160 | 3E-25 | O-Methyltransferase ZRP4-like |
| OMT | MZ00042974 | 13.4 | 6.9 | GRMZM2G085924 | AT4G35160 | 1E-26 | O-Methyltransferase-like protein |
| FOMT | MZ00043408 | 15.1 | –4.2 | AC196475.3_FG004 | AT5G54160 | 3E-128 | Flavonoid O-methyltransferase |
| OMT | MZ00051351 | 9.7 | –2.2 | GRMZM2G141026 | AT4G35160 | 4E-52 | O-Methyltransferase ZRP4-like |
| CHI | MZ00036732 | 13.0 | –18.5 | GRMZM2G175076 | AT5G05270 | 1E-57 | Chalcone flavonone isomerase |
| CHI | MZ00026366 | 11.9 | –2.7 | GRMZM2G155329 | AT3G55120 | 6E-73 | Chalcone flavanone isomerase 1 |
| IFR | MZ00029320 | 14.0 | –6.4 | GRMZM2G326116 | AT1G32100 | 3E-122 | Isoflavone reductase-like protein |
| F3'H | MZ00019364 | 14.6 | –5.9 | gb|EU971853.1|b | AT5G07990 | 1E-65 | Cytochrome P450/flavonoid 3'-hydroxylase-like protein |
| F3'H | MZ00021196 | 10.7 | –5.5 | GRMZM2G146234 | AT5G24530 | 2E-61 | Flavanone 3-hydroxylase-like protein |
| F3'H | MZ00005460 | 11.2 | –5.4 | GRMZM2G160763 | AT5G07990 | 4E-161 | Cytochrome P450/ flavonoid 3-monooxygenase |
| CCoAOMT | MZ00000781 | 10.0 | –4.0 | GRMZM2G033952 | AT4G34050 | 5E-81 | Caffeoyl-CoA O-methyltransferase 1 |
| CCoAOMT | MZ00017952 | 11.8 | –2.4 | GRMZM2G004138 | AT4G34050 | 1E-80 | Caffeoyl-CoA O-methyltransferase 1 like |
| CCoAOMT | MZ00057269 | 14.0 | –2.0 | GRMZM2G099363 | AT4G34050 | 1E-112 | Caffeoyl-CoA O-methyltransferase 2 |
| PAL | MZ00014292 | 12.6 | –4.9 | GRMZM2G160541 | AT3G10340 | 0E+00 | Phenylalanine ammonia-lyase |
| PAL | MZ00039256 | 14.5 | –4.1 | GRMZM2G074604 | AT2G37040 | 0E+00 | Phenylalanine ammonia-lyase |
| PAL | MZ00025090 | 11.3 | –2.3 | GRMZM2G029048 | AT2G37040 | 0E+00 | Phenylalanine ammonia-lyase |
| PAL | MZ00034925 | 12.4 | –2.2 | GRMZM2G081582 | AT2G37040 | 0E+00 | Phenylalanine ammonia-lyase |
| 4CL | MZ00016350 | 13.1 | –3.8 | gb|BT067847.1|c | AT3G21240 | 0E+00 | 4-Coumarate coenzyme A ligase |
| 4CL | MZ00001892 | 10.4 | –3.0 | GRMZM2G019746 | AT5G63380 | 1E-135 | 4-Coumarate coenzyme A ligase family protein |
| 4CL | MZ00020111 | 10.1 | 2.9 | GRMZM2G054013 | AT1G65060 | 0E+00 | 4-Coumarate coenzyme A ligase, 4CL3 |
| 4CL | MZ00018351 | 10.4 | 2.1 | GRMZM2G080663 | AT5G63380 | 2E-70 | 4-Coumarate coenzyme A ligase family protein |
| Laccase | MZ00004658 | 9.8 | 4.8 | GRMZM2G132169 | AT5G05390 | 0E+00 | Laccase/L-ascorbate oxidase |
| Laccase | MZ00018473 | 10.2 | –4.1 | GRMZM2G031117 | AT5G05390 | 4E-147 | Laccase 1 |
| Laccase | MZ00004270 | 12.0 | –4.0 | GRMZM2G072780 | AT5G60020 | 0E+00 | Laccase |
| Laccase | MZ00055128 | 9.6 | –2.4 | GRMZM2G329311 | AT3G09220 | 1E-68 | Laccase |
| CAD | MZ00014812 | 13.9 | 2.4 | AC234163.1_FG004 | AT4G37980 | 6E-105 | Putative cinnamyl alcohol dehydrogenase |
| FLS | MZ00026581 | 11.7 | 3.3 | GRMZM2G152801 | AT5G08640 | 1E-101 | Flavonol synthase/flavanone 3-hydroxylase |
Positive values indicate fold higher expression in IN13 compared with IN9. Negative values indicate higher expression in IN9 compared with IN13.
THe oligo has too low homology using the maize genome browser.
No gene is associated with the oligo using the maize genome browser.
ALDH, aldehyde dehydrogenase; CAD, cinnamyl alcohol dehydrogenase, CCoAOMT, caffeoyl-CoA O-methyltransferase; CCR, cinnamoyl-CoA reductase; CHI, chalcone flavonone isomerase; 4CL, 4-coumarate:CoA ligase; DFR, dihydroflavonol-4-reductase; FLS, flavonol synthase; FOMT, flavonoid O-methyltransferase; F3'H, flavonoid 3'-hydroxylase; IFR, isoflavone reductase; OMT, O-methyltransferase; PAL, phenylalanine ammonia-lyase.
Four putative phenylalanine ammonia-lyases (PALs), predicted to catalyse the first step in the phenylpropanoid pathway, showed a >2-fold increase in expression in IN9. Two 4-coumarate-CoA ligase (4CL) family members were >2-fold preferentially expressed in IN13 and two >2-fold preferentially expressed in IN9. The 4CL maize homologue to 4CL2 in Arabidopsis (At3g21240) showed the highest level of 4CL expression in the non-elongating internode, in agreement with transcriptome analysis results obtained from macroarray gene expression profiling during maize internode development (Guillaumie et al., 2007). In addition, their study showed that different 4CLs exhibit different expression patterns, some being preferentially expressed in young stem tissues. Several O-methyltransferases (OMTs) showed >2-fold differential expression, most of these being classified as ZRP4-like OMT genes. ZRP4 is involved in suberin synthesis (Held et al., 1993) and, although ZRP4-like OMTs have been associated with lignin synthesis, their involvement in the lignin pathway has not yet been firmly established. Three caffeoyl-CoA O-methyltransferases (CCoAOMTs), including maize CCoAOMT1 and CCoAOMT2, were >2-fold up-regulated in IN9. ZmCAD2, a cinnamyl alcohol dehydrogenase (CAD) catalysing the last step in monolignol synthesis and the activity of which is affected by the maize brown-midrib1 mutation (bm1) (Halpin et al., 1998), was not identified as being >2-fold differentially expressed. Likewise, the gene encoding caffeic acid O-methyl transferase (COMT), which is mutated in bm3 plants (Vignols et al., 1995), could not be detected as being >2-fold differentially expressed. Several putative cinnamoyl-CoA reductases (CCRs) were differentially expressed, although it is difficult to distinguish CCRs from dihydroflavonol-4-reductases (DFRs). CCR1 was the only confirmed CCR identified as being differentially expressed and exhibited the highest absolute expression level (Table 4). In agreement with expression data for ZmCCR1 showing highest expression during the period of active lignification (Pichon et al., 1998), CCR1 showed 3.1-fold higher expression in the lignifying IN9 compared with the elongating IN13. Based on expression patterns that correlate with regions undergoing lignification, laccases have been proposed to be involved in the oxidative polymerization of monolignols, produced by the phenylpropanoid pathway, into lignins (Caparros-Ruiz et al., 2006; Guillaumie et al., 2007). The up-regulation of three laccases in the lignifying IN9 suggests an involvement in the lignification process.
Aldehyde dehydrogenase (ALDH), RF2C, was up-regulated in IN13. The closest Arabidopsis homologue, At3g24503/REF1, was shown to be involved in the formation of both soluble and cell wall-linked ferulate esters (Nair et al., 2004). REF1 is more closely related to RF2C in maize than other Arabidopsis ALDHs, suggesting that RF2C, like REF1, is involved in the biosynthesis of ferulic acid, a major cell wall-esterified hydroxycinnamic acid in the grasses which impedes the hydrolysis of the cell wall biomass.
Cell wall and plasma membrane proteins
Many leucine-rich proteins (LRPs), in particular those containing extensin-like motifs, called leucine-rich extensins (LRXs), and arabinogalactan proteins (AGPs) are involved in cell wall-related processes. Several LRXs and other LRPs showed >4-fold differential expression (Table 5), most being up-regulated in the elongating IN13. A function related to cell wall biogenesis has been either shown or implicated for most of the Arabidopsis homologues (Table 5). For instance, AtLRX1, the closest homologue to the IN13 preferentially expressed GRMZM2G082823, is involved in regulating cell wall formation and assembly in elongating root hairs (Baumberger et al., 2001). The plasma membrane/membrane-attached multicopper oxidase, GRMZM2G049693, whose Arabidopsis homologue, SKU5, is involved in cell wall expansion in roots (Sedbrook et al., 2002), was also preferentially expressed in IN13. Fasciclin-like arabinogalactan proteins (FLAs) are a subclass of AGPs that contain putative cell adhesion domains known as fasciclin domains. Expression of some FLAs has been correlated with the onset of secondary wall cellulose synthesis in Arabidopsis stems (Ito et al., 2005), and also with wood formation in trees (Lafarguette et al., 2004; Andersson-Gunneras et al., 2006), suggesting a role in secondary cell wall formation. The FLA up-regulated in IN9, EU962845, showed highest homology to Arabidopis AtFLA11 (Table 5). A recent study has shown that a T-DNA knockout mutant for AtFLA11 affects the tensile strength and stiffness of the stem as well as secondary cell wall composition (MacMillan et al., 2010). Four FLAs were >4-fold up-regulated in IN13, suggesting that these might be involved in primary cell wall biogenesis. This hypothesis is supported by the high level of expression of the Populus trichocarpa homologue for AC234156.1_FG005, gw1.XIV.3668.1, in elongating internodes (Dharmawardhana et al., 2010). Furthermore, based on the expression profile of FLAs during cotton fibre development and Arabidopsis stem development, a function in either primary cell wall development or secondary cell wall deposition has been suggested (Liu et al., 2008; Minic et al., 2009).
Table 5.
Selected genes encoding cell wall and plasma membrane proteins >4-fold differentially expressed between IN9 and IN13
| Annotation | Oligo ID | Signal intensity | Fold changea | Maize gene ID | Arabidopsis homologue gene | E-value | Suggested function |
| LRR protein | MZ00019529 | 10.6 | 10.3 | GRMZM2G149201 | AT4G13340/LRX3 | 6E-127 | Expression associated with elongating cells1 |
| LRR protein | MZ00028889 | 9.8 | 5.6 | GRMZM2G042181 | AT4G06744 | 3E-74 | Indirectly associated with cell wall biosynthesis2 |
| LRR protein | MZ00046729 | 10.2 | –5.6 | GRMZM2G341410 | AT3G22800/LRX6 | 9E-101 | Potentially involved in cell wall development3 |
| LRR protein | MZ00041948 | 12.5 | –5.5 | GRMZM2G012031 | AT2G34680/AIR9 | 0E+00 | Microtubule-Associated Protein4 |
| LRR protein | MZ00005954 | 10.5 | 5.3 | GRMZM2G342509 | AT4G06744 | 2E-97 | Indirectly associated with cell wall biosynthesis2 |
| LRR protein | MZ00044323 | 11.2 | 5.3 | GRMZM2G082823 | AT1G12040/LRX1 | 8E-113 | Cell wall formation and assembly in elongating root hairs5 |
| LRR protein | MZ00018762 | 10.6 | 4.9 | GRMZM2G022897 | AT1G25570 | 0E+00 | Unknown |
| LRR protein | MZ00016499 | 12.4 | –4.0 | GRMZM2G366150 | AT1G15740 | 0E+00 | Unknown |
| Fasciclin-like AGP | MZ00016317 | 11.0 | 15.6 | AC234156.1_FG005 | AT2G04780/FLA7 | 8E-53 | Poplar homologue expressed in elongating internode6 |
| Fasciclin-like AGP | MZ00018923 | 11.4 | 9.2 | GRMZM2G301908 | AT5G55730/FLA1 | 4E-37 | Unknown |
| Fasciclin-like AGP | MZ00032716 | 12.7 | -6.6 | gb|EU962845.1|b | AT5G03170/FLA11 | 2E-20 | Involved in secondary cell wall formation7 |
| Fasciclin-like AGP | MZ00016177 | 10.6 | 6.0 | GRMZM2G003752 | AT2G45470/FLA8 | 3E-105 | Unknown |
| Fasciclin-like AGP | MZ00039834 | 12.1 | 5.4 | GRMZM2G084812 | AT4G12730/FLA2 | 5E-52 | Unknown |
| Cobra-like protein | MZ00012936 | 10.0 | 6.4 | GRMZM2G167497 | AT5G15630/IRX6 | 0E+00 | Zm brittle stalk-2-like protein 7 / Unknown |
| Cobra-like protein | MZ00005183 | 12.7 | –7.7 | GRMZM2G109326 | AT5G15630/IRX6 | 0E+00 | Zm brittle stalk2: involved in cell wall biogenesis8 |
| Multicopper oxidase | MZ00015277 | 10.1 | 4.5 | GRMZM2G049693 | AT4G12420/SKU5 | 0E+00 | Cell wall expansion in roots9 |
Positive values indicate fold higher expression in IN13 compared with IN9. Negative values indicate higher expression in IN9 compared with IN13.
No gene associated with the oligo using the maize genome browser.
The maize britle stalk2 (ZmBK2) gene is a member of the COBRA gene family that encodes glycosylphosphatidylinositol (GPI)-anchored proteins. Several members of this family are involved in cell wall biogenesis in both dicots and monocots. Mutations in ZmBK2 affect stalk strength in maize by interfering with the deposition of cellulose in the secondary cell wall in fibre cells (Ching et al., 2006). The exact function of COBRA proteins is still unknown, although a function for BK2 in the patterning of lignin–cellulosic interactions that maintain organ flexibility has been suggested (Sindhu et al., 2007). The location of COBRA proteins at the interface of the plasma membrane and the cell wall, and their association with lipid microdomains (rafts) suggest a potential function in the regulation or integration of secretion and wall assembly (Martin et al., 2005). In accordance with a secondary cell wall-related function, ZmBK2 (GRMZM2G109326) was 7.7 fold up-regulated in IN9 (Table 5). Surprisingly, the ZmBK2-like gene, ZmBK2L7 (GRMZM2G167497), was 6.4-fold up-regulated in IN13, suggesting a role in primary rather than secondary cell wall biogenesis. Although phylogenetic analysis has suggested that ZmBK2 and ZmBK2L7 are co-orthologues of Arabidopsis AtCOBL4/ IRX6, expression analysis already suggested that ZmBK2L7 does not carry out the same function as ZmBK2 (Brady et al., 2007).
Members of the receptor-like kinase (RLK) family, membrane-bound signalling molecules with an extracellular receptor domain, are good candidates for sensing and transducing the ‘status’ of the cell wall (Shiu and Bleecker, 2001), and it is perhaps not surprising that recently several RLKs have been implicated in cell wall integrity sensing (Kohorn et al., 2006; Hématy et al., 2007; Xu et al., 2008; Guo et al., 2009). Supplementary Table S4 at JXB online contains a list and analysis of the protein kinases that were >4-fold differentially expressed.
Genes specifically involved in grass cell wall biogenesis
The potential functionality of newly identified genes in grasses can currently often only be inferred from information available from studies in Arabidopsis due to its unique molecular genetic resources. Hence, many of the genes and gene families discussed so far contain Arabidopsis homologues with small E-values (Tables 2–5). However, functionality cannot always be implied from the closest homologues in Arabidopsis. Indeed, comparative genomics and expression analysis of cell wall-related genes and gene families between the grasses and Arabidopsis revealed that orthology often cannot be inferred from homology (Cao et al., 2008; Penning et al., 2009).
Comparative analysis of the maize genome with that of rice, sorghum, and Arabidopsis indicated that 71.5% of the 11 892 gene families identified in maize were shared between these grasses and Arabidopsis, while 17.5% (2077 gene families) appeared to be specific to the grasses (Schnable et al., 2009). The differences in cell wall structure and composition between the dicots and the grasses suggest the relative contribution of specific genes and gene families involved in grass cell wall biogenesis to be even higher. Indeed it has been estimated that at least a third of cell wall-related genes of grasses could have no, or few, orthologues in Arabidopsis, making genetic functional analyses in a grass model system essential (Carpita and McCann, 2008).
In an attempt to identify some of the potential genes specifically involved in grass cell wall-related processes, the list of genes that showed >4-fold differential expression was filtered for maize genes with no significant Arabidopsis homologue based on an E-value >1e−20 (Supplementary Table S5 at JXB online). The filtered list contains two jacalin-like lectin domain-containing proteins highly up-regulated in IN13. One of them, GRMZM2G402417, was one of the most highly differentially expressed genes in the experiment (105-fold). Although lectins are generally considered to play a role in defence signalling, a mannose-binding jacalin-related lectin was involved in the regulation of rice growth and development (Jiang et al., 2007). Two glycine-rich cell wall proteins (GRPs) up-regulated in IN13 and one in IN9 might be involved in cell wall sensing. Other genes up-regulated in IN13 included a number of proline-rich cell wall proteins, a putative GH17 family member, a WRKY TF, and two rapid alkalinization factor (RALF) domain-containing proteins, which are polypeptide hormones involved in regulating plant stress, growth, and development (Pearce et al., 2001). A RALF domain-containing protein was also highly up-regulated in non-elongating internodes.
A number of genes encoding ‘grass-specific’ ABA/WDS-induced proteins were highly up-regulated in IN9. These plant defence-related proteins are induced by water deficit stress (WDS) or abscisic acid (ABA) stress and ripening. The role of these proteins at the molecular level is unclear. The plant cell wall plays an important role in conferring drought tolerance as this involves restructuring of the cell wall to allow growth at lower water content. Previous studies have shown that water deficit stress changes the expression of cell wall-related genes (Bray, 2004; Harb et al., 2010), the activity of cell wall matrix enzymes (Konno et al., 2008), and the accumulation of cell wall phenolics such as lignin and ferulic acid (Fan et al., 2006; Hura et al., 2009). It is possible that these ABA/WDS-induced proteins play a role in regulating cell wall-related processes, possibly by acting as an architectural transcriptional regulator. For example, an ABA/WDS-containing protein from Solanum lycopersicum (formerly Lycopersicon esculentum) has been shown to bind double-stranded DNA (Maskin et al., 2007), and a Vitis vinifera ABA/WDS protein was able to interact with another TF (Saumonneau et al., 2008), suggesting a recruitment function for other regulatory proteins.
Most of the genes in the filtered list without obvious Arabidopsis homologues were categorized as unknown (57% and 62% of the genes that were >4-fold up-regulated in IN13 and IN9, respectively). Judged by their pattern of expression, many of these genes might be involved in cell wall-related processes specific to the grasses and are therefore interesting candidates for further functional analysis.
Concluding remarks
The analysis of genes differentially expressed between an elongating internode (IN13) and a non-elongating internode (IN9) demonstrated that this profiling experiment succeeded in identifying genes associated with primary and secondary cell wall processes. A significant overlap between genes associated with cell wall biogenesis and genes implicated in responses to biotic and/or abiotic stresses was also identified. This suggests that many of the proteins involved in cell wall-related processes during normal development are also recruited during defence-related cell wall remodelling events. This is not necessarily surprising given that the plant cell wall goes through extensive remodelling and reconstruction processes to ensure an adequate defence against biotic attacks and severe environmental conditions (Sarkar et al., 2009; Moura et al., 2010).
Several thousand gene products are estimated to participate in the synthesis, deposition, and function of cell walls, but very few cell wall-associated genes have been identified in grasses. The data reported in this paper provide a platform for the functional testing of candidate genes for involvement in cell wall-related processes. This testing could include forward and reverse genetic-based approaches in model grasses such as again maize or Brachypodium. Such studies will enable a better understanding of type II cell wall biogenesis and its regulation in the grasses which is necessary to enable the manipulation of traits that contribute to biomass yield and quality. The optimization of energy crop cell walls will enable the matching of lignocellulosic feedstock chemistry to a range of biopower and biofuel end uses.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. MA-plot depicting the average log-intensity on the x-axis against the average log-fold change on the y-axis.
Table S1. List of genes showing >2-fold differential expression between internode 9 and internode 13.
Table S2. List of genes showing >4-fold differential expression between internode 9 and internode 13.
Table S3. Cellulose synthase (CesA) and cellulose synthase-like (CSL) genes preferentially expressed in elongating internode 13.
Table S4. List of protein kinases >4-fold differentially expressed between internode 9 and internode 13.
Table S5. List of >4-fold differentially expressed genes without apparent Arabidopsis homologues.
Table S6. Primers used for quantitative RT-PCR.
Note S1. References used in the supplementary data but not listed in the main manuscript.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programme Grant on Bioenergy and Biorenewables [BBSEG00003134].
References
- Al-Ghazi Y, Bourot S, Arioli T, Dennis ES, Llewellyn DJ. Transcript profiling during fiber development identifies pathways in secondary metabolism and cell wall structure that may contribute to cotton fiber quality. Plant and Cell Physiology. 2009;50:1364–1381. doi: 10.1093/pcp/pcp084. [DOI] [PubMed] [Google Scholar]
- Andersson-Gunneras S, Mellerowicz EJ, Love J, Segerman B, Ohmiya Y, Coutinho PM, Nilsson P, Henrissat B, Moritz T, Sundberg B. Biosynthesis of cellulose-enriched tension wood in populus: global analysis of transcripts and metabolites identifies biochemical and developmental regulators in secondary wall biosynthesis. The Plant Journal. 2006;45:144–165. doi: 10.1111/j.1365-313X.2005.02584.x. [DOI] [PubMed] [Google Scholar]
- Appenzeller L, Doblin M, Barreiro R, Wang HY, Niu XM, Kollipara K, Carrigan L, Tomes D, Chapman M, Dhugga KS. Cellulose synthesis in maize: isolation and expression analysis of the cellulose synthase (CesA) gene family. Cellulose. 2004;11:287–299. [Google Scholar]
- Baumberger N, Ringli C, Keller B. The chimeric leucine-rich repeat/extensin cell wall protein lRX1 is required for root hair morphogenesis in Arabidopsis thaliana. Genes and Development. 2001;15:1128–1139. doi: 10.1101/gad.200201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B: Methodological. 1995;57:289–300. [Google Scholar]
- Bomal C, Bedon F, Caron S, et al. Involvement of Pinus taeda MYB1 and MYB8 in phenylpropanoid metabolism and secondary cell wall biogenesis: a comparative in planta analysis. Journal of Experimental Botany. 2008;59:3925–3939. doi: 10.1093/jxb/ern234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourquin V, Nishikubo N, Abe H, Brumer H, Denman S, Eklund M, Christiernin M, Teeri TT, Sundberg B, Mellerowicz EJ. Xyloglucan endotransglycosylases have a function during the formation of secondary cell walls of vascular tissues. The Plant Cell. 2002;14:3073–3088. doi: 10.1105/tpc.007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SM, Song S, Dhugga KS, Rafalski JA, Benfey PN. Combining expression and comparative evolutionary analysis. The COBRA gene family. Plant Physiology. 2007;143:172–187. doi: 10.1104/pp.106.087262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray EA. Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. Journal of Experimental Botany. 2004;55:2331–2341. doi: 10.1093/jxb/erh270. [DOI] [PubMed] [Google Scholar]
- Brown DM, Zhang ZN, Stephens E, Dupree P, Turner SR. Characterization of IRX10 and IRX10-like reveals an essential role in glucuronoxylan biosynthesis in Arabidopsis. The Plant Journal. 2009;57:732–746. doi: 10.1111/j.1365-313X.2008.03729.x. [DOI] [PubMed] [Google Scholar]
- Buschmann H, Chan J, Sanchez-Pulido L, Andrade-Navarro MA, Doonan JH, Lloyd CW. Microtubule-associated AIR9 recognizes the cortical division site at preprophase and cell-plate insertion. Current Biology. 2006;16:1938–1943. doi: 10.1016/j.cub.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Cairns JRK, Esen A. 2010. Beta-glucosidases. Cellular and Molecular Life Sciences. 67:3389–3405. doi: 10.1007/s00018-010-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao PJ, Bartley LE, Jung KH, Ronald PC. Construction of a rice glycosyltransferase phylogenomic database and identification of rice-diverged glycosyltransferases. Molecular Plant. 2008;1:858–877. doi: 10.1093/mp/ssn052. [DOI] [PubMed] [Google Scholar]
- Caparros-Ruiz D, Fornale S, Civardi L, Puigdomenech P, Rigau J. Isolation and characterisation of a family of laccases in maize. Plant Science. 2006;171:217–225. [Google Scholar]
- Carpita NC. Structure and biogenesis of the cell walls of grasses. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:445–476. doi: 10.1146/annurev.arplant.47.1.445. [DOI] [PubMed] [Google Scholar]
- Carpita NC, McCann MC. Maize and sorghum: genetic resources for bioenergy grasses. Trends in Plant Science. 2008;13:415–420. doi: 10.1016/j.tplants.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Century K, Reuber TL, Ratcliffe OJ. Regulating the regulators: the future prospects for transcription-factor-based agricultural biotechnology products. Plant Physiology. 2008;147:20–29. doi: 10.1104/pp.108.117887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching A, Dhugga KS, Appenzeller L, Meeley R, Bourett TM, Howard RJ, Rafalski A. Brittle stalk 2 encodes a putative glycosylphosphatidylinositol-anchored protein that affects mechanical strength of maize tissues by altering the composition and structure of secondary cell walls. Planta. 2006;224:1174–1184. doi: 10.1007/s00425-006-0299-8. [DOI] [PubMed] [Google Scholar]
- Dauwe R, Morreel K, Goeminne G, et al. Molecular phenotyping of lignin-modified tobacco reveals associated changes in cell-wall metabolism, primary metabolism, stress metabolism and photorespiration. The Plant Journal. 2007;52:263–285. doi: 10.1111/j.1365-313X.2007.03233.x. [DOI] [PubMed] [Google Scholar]
- DeBono A, Yeats TH, Rose JKC, Bird D, Jetter R, Kunst L, Samuelsa L. Arabidopsis LTPG is a glycosylphosphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface. The Plant Cell. 2009;21:1230–1238. doi: 10.1105/tpc.108.064451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmawardhana P, Brunner AM, Strauss SH. Genome-wide transcriptome analysis of the transition from primary to secondary stem development in Populus trichocarpa. BMC Genomics. 2010;11:150. doi: 10.1186/1471-2164-11-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diet A, Link B, Seifert GJ, Schellenberg B, Wagner U, Pauly M, Reiter WD, Ringli C. The Arabidopsis root hair cell wall formation mutant LRX1 is suppressed by mutations in the RHM1 gene encoding a UDP-l-rhamnose synthase. The Plant Cell. 2006;18:1630–1641. doi: 10.1105/tpc.105.038653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Research. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstedt KV, Kukkola EM, Koistinen VVT, Takahashi J, Marjamaa K. Cell wall lignin is polymerised by class III secretable plant peroxidases in Norway spruce. Journal of Integrative Plant Biology. 2010;52:186–194. doi: 10.1111/j.1744-7909.2010.00928.x. [DOI] [PubMed] [Google Scholar]
- Fan L, Linker R, Gepstein S, Tanimoto E, Yamamoto R, Neumann PM. Progressive inhibition by water deficit of cell wall extensibility and growth along the elongation zone of maize roots is related to increased lignin metabolism and progressive stelar accumulation of wall phenolics. Plant Physiology. 2006;140:603–612. doi: 10.1104/pp.105.073130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher GB. Revolutionary times in our understanding of cell wall biosynthesis and remodeling in the grasses. Plant Physiology. 2009;149:27–37. doi: 10.1104/pp.108.130096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornale S, Sonbol FM, Maes T, Capellades M, Puigdomenech P, Rigau J, Caparros-Ruiz D. Down-regulation of the maize and Arabidopsis thaliana caffeic acid o-methyl-transferase genes by two new maize R2R3-MYB transcription factors. Plant Molecular Biology. 2006;62:809–823. doi: 10.1007/s11103-006-9058-2. [DOI] [PubMed] [Google Scholar]
- Goicoechea M, Lacombe E, Legay S, et al. EgMYB2, a new transcriptional activator from Eucalyptus xylem, regulates secondary cell wall formation and lignin biosynthesis. The Plant Journal. 2005;43:553–567. doi: 10.1111/j.1365-313X.2005.02480.x. [DOI] [PubMed] [Google Scholar]
- Guillaumie S, Mzid R, Méchin V, Léon C, Hichri I, Destrac-Irvine A, Trossat-Magnin C, Delrot S, Lauvergeat V. The grapevine transcription factor WRKY2 influences the lignin pathway and xylem development in tobacco. Plant Molecular Biology. 2010;72:215–234. doi: 10.1007/s11103-009-9563-1. [DOI] [PubMed] [Google Scholar]
- Guillaumie S, San-Clemente H, Deswarte C, Martinez Y, Lapierre C, Murigneux A, Barriere Y, Pichon M, Goffner D. Maizewall. Database and developmental gene expression profiling of cell wall biosynthesis and assembly in maize. Plant Physiology. 2007;143:339–363. doi: 10.1104/pp.106.086405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HQ, Li L, Ye HX, Yu XF, Algreen A, Yin YH. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proceedings of the National Academy of Sciences. USA. 2009;106:7648–7653. doi: 10.1073/pnas.0812346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin C, Holt K, Chojecki J, Oliver D, Chabbert B, Monties B, Edwards K, Barakate A, Foxon GA. Brown-midrib maize (bm1)—a mutation affecting the cinnamyl alcohol dehydrogenase gene. The Plant Journal. 1998;14:545–553. doi: 10.1046/j.1365-313x.1998.00153.x. [DOI] [PubMed] [Google Scholar]
- Harb A, Krishnan A, Ambavaram MMR, Pereira A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiology. 2010;154:1254–1271. doi: 10.1104/pp.110.161752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield RD, Ralph J, Grabber JH. Cell wall cross-linking by ferulates and diferulates in grasses. Journal of the Science of Food and Agriculture. 1999;79:403–407. [Google Scholar]
- Held BM, Wang HQ, John I, Wurtele ES, Colbert JT. An mRNA putatively coding for an O-methyltransferase accumulates preferentially in maize roots and is located predominantly in the region of the endodermis. Plant Physiology. 1993;102:1001–1008. doi: 10.1104/pp.102.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hématy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, Pelletier S, Renou JP, Höfte H. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Current Biology. 2007;17:922–931. doi: 10.1016/j.cub.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Himmel ME, Ding SY, Johnson DK, Adney WS, Nimlos MR, Brady JW, Foust TD. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science. 2007;315:804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Noh HN, Kim KI, Koh EJ, Wi SG, Bae HJ, Lee H, Hong SW. Mutation of the chitinase-like protein-encoding AtCTL2 gene enhances lignin accumulation in dark-grown Arabidopsis seedlings. Journal of Plant Physiology. 2010;167:650–658. doi: 10.1016/j.jplph.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Hrmova M, Farkas V, Lahnstein J, Fincher GB. A barley xyloglucan xyloglucosyl transferase covalently links xyloglucan, cellulosic substrates, and (1,3;1,4)-beta-d-glucans. Journal of Biological Chemistry. 2007;282:12951–12962. doi: 10.1074/jbc.M611487200. [DOI] [PubMed] [Google Scholar]
- Hura T, Hura K, Grzesiak S. Possible contribution of cell-wall-bound ferulic acid in drought resistance and recovery in triticale seedlings. Journal of Plant Physiology. 2009;166:1720–1733. doi: 10.1016/j.jplph.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Irshad M, Canut H, Borderies G, Pont-Lezica R, Jamet E. A new picture of cell wall protein dynamics in elongating cells of Arabidopsis thaliana: confirmed actors and newcomers. BMC Plant Biology. 2008;8:94. doi: 10.1186/1471-2229-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, Suzuki Y, Miyamoto K, Ueda J, Yamaguchi I. AtFLA11, a fasciclin-like arabinogalactan-protein, specifically localized in screlenchyma cells. Bioscience Biotechnology and Biochemistry. 2005;69:1963–1969. doi: 10.1271/bbb.69.1963. [DOI] [PubMed] [Google Scholar]
- Jiang JF, Xu YY, Chong K. Overexpression of OsJAC1, a lectin gene, suppresses the coleoptile and stem elongation in rice. Journal of Integrative Plant Biology. 2007;49:230–237. [Google Scholar]
- Jung HG, Casler MD. Maize stem tissues: cell wall concentration and composition during development. Crop Science. 2006;46:1793–1800. [Google Scholar]
- Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT. Priming in systemic plant immunity. Science. 2009;324:89–91. doi: 10.1126/science.1170025. [DOI] [PubMed] [Google Scholar]
- Kohorn BD, Kobayashi M, Johansen S, Friedman HP, Fischer A, Byers N. Wall-associated kinase 1 (WAK1) is crosslinked in endomembranes, and transport to the cell surface requires correct cell-wall synthesis. Journal of Cell Science. 2006;119:2282–2290. doi: 10.1242/jcs.02968. [DOI] [PubMed] [Google Scholar]
- Konno H, Yamasaki Y, Sugimoto M, Takeda K. Differential changes in cell wall matrix polysaccharides and glycoside-hydrolyzing enzymes in developing wheat seedlings differing in drought tolerance. Journal of Plant Physiology. 2008;165:745–754. doi: 10.1016/j.jplph.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Lafarguette F, Leple JC, Dejardin A, Laurans F, Costa G, Lesage-Descauses MC, Pilate G. Poplar genes encoding fasciclin-like arabinogalactan proteins are highly expressed in tension wood. New Phytologist. 2004;164:107–121. doi: 10.1111/j.1469-8137.2004.01175.x. [DOI] [PubMed] [Google Scholar]
- Lasserre E, Jobet E, Llauro C, Delseny M. AtERF38 (At2g35700), an AP2/ERF family transcription factor gene from Arabidopsis thaliana, is expressed in specific cell types of roots, stems and seeds that undergo suberization. Plant Physiology and Biochemistry. 2008;46:1051–1061. doi: 10.1016/j.plaphy.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Lawrence CJ, Walbot V. Translational genomics for bioenergy production from fuelstock grasses: maize as the model species. The Plant Cell. 2007;19:2091–2094. doi: 10.1105/tpc.107.053660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Hrmova M, Burton RA, Lahnstein J, Fincher GB. Bifunctional family 3 glycoside hydrolases from barley with α-l-arabinofuranosidase and β-d-xylosidase activity—characterization, primary structures, and COOH-terminal processing. Journal of Biological Chemistry. 2003;278:5377–5387. doi: 10.1074/jbc.M210627200. [DOI] [PubMed] [Google Scholar]
- Lewandowski I, Scurlock JMO, Lindvall E, Christou M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass and Bioenergy. 2003;25:335–361. [Google Scholar]
- Liu DQ, Tu LL, Li YJ, Wang L, Zhu LF, Zhang XL. Genes encoding fasciclin-like arabinogalactan proteins are specifically expressed during cotton fiber development. Plant Molecular Biology Reporter. 2008;26:98–113. [Google Scholar]
- Liu JJ, Sturrock R, Ekramoddoullah AKM. The superfamily of thaumatin-like proteins: its origin, evolution, and expression towards biological function. Plant Cell Reports. 2010;29:419–436. doi: 10.1007/s00299-010-0826-8. [DOI] [PubMed] [Google Scholar]
- MacMillan CP, Mansfield SD, Stachurski ZH, Evans R, Southerton SG. Fasciclin-like arabinogalactan proteins: specialization for stem biomechanics and cell wall architecture in Arabidopsis and Eucalyptus. The Plant Journal. 2010;62:689–703. doi: 10.1111/j.1365-313X.2010.04181.x. [DOI] [PubMed] [Google Scholar]
- Martin SW, Glover BJ, Davies JM. Lipid microdomains—plant membranes get organized. Trends in Plant Science. 2005;10:263–265. doi: 10.1016/j.tplants.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Maskin L, Frankel N, Gudesblat G, Demergasso MJ, Pietrasanta LI, Iusem ND. Dimerization and DNA-binding of ASR1, a small hydrophilic protein abundant in plant tissues suffering from water loss. Biochemical and Biophysical Research Communications. 2007;352:831–835. doi: 10.1016/j.bbrc.2006.11.115. [DOI] [PubMed] [Google Scholar]
- Minic Z. Physiological roles of plant glycoside hydrolases. Planta. 2008;227:723–740. doi: 10.1007/s00425-007-0668-y. [DOI] [PubMed] [Google Scholar]
- Minic Z, Jamet E, San-Clemente H, Pelletier S, Renou JP, Rihouey C, Okinyo DPO, Proux C, Lerouge P, Jouanin L. Transcriptomic analysis of Arabidopsis developing stems: a close-up on cell wall genes. BMC Plant Biology. 2009;9:6. doi: 10.1186/1471-2229-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RAC, Dupree P, Shewry PR. A novel bioinformatics approach identifies candidate genes for the synthesis and feruloylation of arabinoxylan. Plant Physiology. 2007;144:43–53. doi: 10.1104/pp.106.094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuda N, Iwase A, Yamamoto H, Yoshida M, Seki M, Shinozaki K, Ohme-Takagi M. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. The Plant Cell. 2007;19:270–280. doi: 10.1105/tpc.106.047043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes RAC, Ranocha P, Martinez Y, et al. Cell wall modifications in Arabidopsis plants with altered α-l-arabinofuranosidase activity. Plant Physiology. 2008;147:63–77. doi: 10.1104/pp.107.110023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JP, Vicre-Gibouin M, Farrant JM, Driouich A. Adaptations of higher plant cell walls to water loss: drought vs desiccation. Physiologia Plantarum. 2008;134:237–245. doi: 10.1111/j.1399-3054.2008.01134.x. [DOI] [PubMed] [Google Scholar]
- Moura J, Bonine CAV, Viana JDF, Dornelas MC, Mazzafera P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. Journal of Integrative Plant Biology. 2010;52:360–376. doi: 10.1111/j.1744-7909.2010.00892.x. [DOI] [PubMed] [Google Scholar]
- Nair RB, Bastress KL, Ruegger MO, Denault JW, Chapple C. The Arabidopsis thaliana reduced epidermal fluorescence1 gene encodes an aldehyde dehydrogenase involved in ferulic acid and sinapic acid biosynthesis. The Plant Cell. 2004;16:544–554. doi: 10.1105/tpc.017509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoumkina MA, He XZ, Dixon RA. Elicitor-induced transcription factors for metabolic reprogramming of secondary metabolism in Medicago truncatula. BMC Plant Biology. 2008;8:132. doi: 10.1186/1471-2229-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwland J, Feron R, Huisman BAH, Fasolino A, Hilbers CW, Derksen J, Mariani C. Lipid transfer proteins enhance cell wall extension in tobacco. The Plant Cell. 2005;17:2009–2019. doi: 10.1105/tpc.105.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Park S, Han KH. Transcriptional regulation of secondary growth in Arabidopsis thaliana. Journal of Experimental Botany. 2003;54:2709–2722. doi: 10.1093/jxb/erg304. [DOI] [PubMed] [Google Scholar]
- Opassiri R, Pomthong B, Onkoksoong T, Akiyama T, Esen A, Cairns JRK. Analysis of rice glycosyl hydrolase family I and expression of Os4bglu12 β-glucosidase. BMC Plant Biology. 2006;6:33. doi: 10.1186/1471-2229-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passardi F, Penel C, Dunand C. Performing the paradoxical: how plant peroxidases modify the cell wall. Trends in Plant Science. 2004;9:534–540. doi: 10.1016/j.tplants.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Pearce G, Moura DS, Stratmann J, Ryan CA. RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proceedings of the National Academy of Sciences. USA. 2001;98:12843–12847. doi: 10.1073/pnas.201416998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena MJ, Zhong RQ, Zhou GK, Richardson EA, O'Neill MA, Darvill AG, York WS, Ye ZH. Arabidopsis irregular xylem8 and irregular xylem9: implications for the complexity of glucuronoxylan biosynthesis. The Plant Cell. 2007;19:549–563. doi: 10.1105/tpc.106.049320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning BW, Hunter CT, Tayengwa R, et al. Genetic resources for maize cell wall biology. Plant Physiology. 2009;151:1703–1728. doi: 10.1104/pp.109.136804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichon M, Courbou I, Beckert M, Boudet AM, Grima-Pettenati J. Cloning and characterization of two maize cDNAs encoding cinnamoyl-CoA reductase (CCR) and differential expression of the corresponding genes. Plant Molecular Biology. 1998;38:671–676. doi: 10.1023/a:1006060101866. [DOI] [PubMed] [Google Scholar]
- Ritchie SW, Hanway JJ, Benson GO. How a corn plant develops, special report no. 48. Ames, IA: Iowa State University of Science and Technology Cooperative Extension Service. 1993 [Google Scholar]
- Rogers LA, Campbell MM. The genetic control of lignin deposition during plant growth and development. New Phytologist. 2004;164:17–30. doi: 10.1111/j.1469-8137.2004.01143.x. [DOI] [PubMed] [Google Scholar]
- Rose JKC, Braam J, Fry SC, Nishitani K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant and Cell Physiology. 2002;43:1421–1435. doi: 10.1093/pcp/pcf171. [DOI] [PubMed] [Google Scholar]
- Ruepp A, Zollner A, Maier D, et al. The FunCat, a functional annotation scheme for systematic classification of proteins from whole genomes. Nucleic Acids Research. 2004;32:5539–5545. doi: 10.1093/nar/gkh894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar P, Bosneaga E, Auer M. Plant cell walls throughout evolution: towards a molecular understanding of their design principles. Journal of Experimental Botany. 2009;60:3615–3635. doi: 10.1093/jxb/erp245. [DOI] [PubMed] [Google Scholar]
- Saumonneau A, Agasse A, Bidoyen MT, Lallemand M, Cantereau A, Medici A, Laloi M, Atanassova R. Interaction of grape ASR proteins with a DREB transcription factor in the nucleus. FEBS Letters. 2008;582:3281–3287. doi: 10.1016/j.febslet.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Scarpella E, Meijer AH. Pattern formation in the vascular system of monocot and dicot plant species. New Phytologist. 2004;164:209–242. doi: 10.1111/j.1469-8137.2004.01191.x. [DOI] [PubMed] [Google Scholar]
- Schnable PS, Ware D, Fulton RS, et al. The B73 maize genome: complexity, diversity, and dynamics. Science. 2009;326:1112–1115. doi: 10.1126/science.1178534. [DOI] [PubMed] [Google Scholar]
- Sedbrook JC, Carroll KL, Hung KF, Masson PH, Somerville CR. The Arabidopsis SKU5 gene encodes an extracellular glycosyl phosphatidylinositol-anchored glycoprotein involved in directional root growth. The Plant Cell. 2002;14:1635–1648. doi: 10.1105/tpc.002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Uzarowska A, Ouzunova M, Landbeck M, Wenzel G, Lübberstedt T. Identification of candidate genes associated with cell wall digestibility and eQTL (expression quantitative trait loci) analysis in a Flint×Flint maize recombinant inbred line population. BMC Genomics. 2007;8:22. doi: 10.1186/1471-2164-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu S-H, Bleecker AB. Plant receptor-like kinase gene family: diversity, function, and signaling. Sciences's STKE. 2001;2001:re22. doi: 10.1126/stke.2001.113.re22. [DOI] [PubMed] [Google Scholar]
- Sindhu A, Langewisch T, Olek A, Multani DS, McCann MC, Vermerris W, Carpita NC, Johal G. Maize brittle stalk2 encodes a COBRA-like protein expressed in early organ development but required for tissue flexibility at maturity. Plant Physiology. 2007;145:1444–1459. doi: 10.1104/pp.107.102582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Statistical Applications in Genetics and Molecular Biology. 2004 doi: 10.2202/1544-6115.1027. 3, Article 3. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, editors. Bioinformatics and computational biology solutions using r and bioconductor. New York: Springer; 2005. pp. 397–420. [Google Scholar]
- Sonbol FM, Fornale S, Capellades M, et al. The maize ZmMYB42 represses the phenylpropanoid pathway and affects the cell wall structure, composition and degradability in Arabidopsis thaliana. Plant Molecular Biology. 2009;70:283–296. doi: 10.1007/s11103-009-9473-2. [DOI] [PubMed] [Google Scholar]
- Taylor NG, Howells RM, Huttly AK, Vickers K, Turner SR. Interactions among three distinct CesA proteins essential for cellulose synthesis. Proceedings of the National Academy of Sciences. USA. 2003;100:1450–1455. doi: 10.1073/pnas.0337628100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Guillaumie S, Verdu C, Denoue D, Pichon M, Barriere Y. Cell wall phenylpropanoid-related gene expression in early maize recombinant inbred lines differing in parental alleles at a major lignin QTL position. Molecular Breeding. 2010;25:105–124. [Google Scholar]
- Uozu S, Tanaka-Ueguchi M, Kitano H, Hattori K, Matsuoka M. Characterization of XET-related genes of rice. Plant Physiology. 2000;122:853–859. doi: 10.1104/pp.122.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Raemdonck D, Pesquet E, Cloquet S, Beeckman H, Boerjan W, Goffner D, El Jaziri M, Baucher M. Molecular changes associated with the setting up of secondary growth in aspen. Journal of Experimental Botany. 2005;56:2211–2227. doi: 10.1093/jxb/eri221. [DOI] [PubMed] [Google Scholar]
- Vignols F, Rigau J, Torres MA, Capellades M, Puigdomenech P. The brown midrib3 (bm3) mutation in maize occurs in the gene encoding caffeic acid O-methyltransferase. The Plant Cell. 1995;7:407–416. doi: 10.1105/tpc.7.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissenberg K, Martinez-Vilchez IM, Verbelen JP, Miller JG, Fry SC. In vivo colocalization of xyloglucan endotransglycosylase activity and its donor substrate in the elongation zone of Arabidopsis roots. The Plant Cell. 2000;12:1229–1237. doi: 10.1105/tpc.12.7.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SL, Rahman A, Baskin TI, Kieber JJ. Two leucine-rich repeat receptor kinases mediate signaling, linking cell wall biosynthesis and ACC synthase in Arabidopsis. The Plant Cell. 2008;20:3065–3079. doi: 10.1105/tpc.108.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZY, Zhang DD, Hu J, et al. Comparative genome analysis of lignin biosynthesis gene families across the plant kingdom. BMC Bioinformatics. 2009;10((Suppl 11):S3. doi: 10.1186/1471-2105-10-S11-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong RQ, Kays SJ, Schroeder BP, Ye ZH. Mutation of a chitinase-like gene causes ectopic deposition of lignin, aberrant cell shapes, and overproduction of ethylene. The Plant Cell. 2002;14:165–179. doi: 10.1105/tpc.010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong RQ, Lee CH, Ye ZH. Functional characterization of poplar wood-associated NAC domain transcription factors. Plant Physiology. 2010;152:1044–1055. doi: 10.1104/pp.109.148270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong RQ, Lee CH, Zhou JL, McCarthy RL, Ye ZH. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis. The Plant Cell. 2008;20:2763–2782. doi: 10.1105/tpc.108.061325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Ye Z- H. Transcriptional regulation of lignin biosynthesis. Plant Signalling and Behavior. 2009;4:1028–1034. doi: 10.4161/psb.4.11.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.