Abstract
Wood formation in trees is a dynamic process that is strongly affected by environmental factors. However, the impact of ozone on wood is poorly documented. The objective of this study was to assess the effects of ozone on wood formation by focusing on the two major wood components, cellulose and lignin, and analysing any anatomical modifications. Young hybrid poplars (Populus tremula×alba) were cultivated under different ozone concentrations (50, 100, 200, and 300 nl l−1). As upright poplars usually develop tension wood in a non-set pattern, the trees were bent in order to induce tension wood formation on the upper side of the stem and normal or opposite wood on the lower side. Biosynthesis of cellulose and lignin (enzymes and RNA levels), together with cambial growth, decreased in response to ozone exposure. The cellulose to lignin ratio was reduced, suggesting that cellulose biosynthesis was more affected than that of lignin. Tension wood was generally more altered than opposite wood, especially at the anatomical level. Tension wood may be more susceptible to reduced carbon allocation to the stems under ozone exposure. These results suggested a coordinated regulation of cellulose and lignin deposition to sustain mechanical strength under ozone. The modifications of the cellulose to lignin ratio and wood anatomy could allow the tree to maintain radial growth while minimizing carbon cost.
Keywords: Cellulose, lignin, ozone, poplar, tension wood
Introduction
Wood is of primary importance for various industrial purposes such as paper manufacturing and construction (Plomion et al., 2001), but also as a renewable source of energy for biofuels (Carroll and Somerville, 2009). The two main components of wood are cellulose and lignin. Their deposition in the cell wall occurs in a regulated manner during wood formation, which includes cambial cell division, cell expansion, secondary wall formation, and cell death (Hertzberg et al., 2001; Mellerowicz et al., 2001).
Cellulose comprises 40–50% of wood dry matter and, being the main component of the cell wall, constitutes a strong carbon sink within the plant (Delmer and Haigler, 2002). Cellulose is a linear polymer composed of 500–14 000 (1→4)-linked β-D-glucose residues (Somerville, 2006). In plant cell walls, the glucan chains are linked by hydrogen bonds to form insoluble cellulose microfibrils. Cellulose is synthesized at the plasma membrane by 36 cellulose synthase (CesA) subunits assembled in a rosette complex (Doblin et al., 2002; Somerville, 2006; Joshi and Mansfield, 2007). The precursor of the β-1,4-glucan chain is uridine diphosphoglucose (UDPG) which results from the cleavage of sucrose by sucrose synthase (SuSy) or is derived from glucose-1-P via UDPG pyrophosphorylase (UGPase) (Delmer and Haigler, 2002). CesA proteins are encoded by a gene superfamily. Characterization of cellulose-deficient mutants and genome sequencing revealed 10 CesA genes in Arabidopsis thaliana (Richmond and Somerville, 2000). Some of these are essential for primary cell wall synthesis and others are involved in secondary cell wall deposition (Taylor, 2008). Eighteen CesA genes have been identified in Populus trichocarpa. Seven of these genes are specific to or are highly expressed in xylem tissue (Suzuki et al., 2006).
Lignin is the second most abundant component of wood after cellulose and accounts for 15–35% of wood dry matter. Lignin provides hydrophobicity and structural support that allows water transport in the vascular system. After the start of secondary wall formation, lignification begins in the middle lamella and primary wall and then continues in the secondary wall (Donaldson, 2001). Lignin is a phenylpropanoid derivative and heteropolymer of three monolignols: p-coumaryl, coniferyl, and sinapyl alcohols. Over the past two decades, the monolignol biosynthetic pathway has been redrawn several times (Humphreys and Chapple, 2002; Boudet et al., 2004; Davin et al., 2008). Significant information has been obtained by altering the expression of individual genes in the phenylpropanoid and monolignol biosynthetic pathway and studying the consequences on lignin content and composition (Vanholme et al., 2008). The shikimate pathway supplies phenylalanine that is converted to monolignols through a metabolic grid of 10 enzyme families (Humphreys and Chapple, 2002). In A. thaliana, 12 candidate genes for vascular lignification were identified (Raes et al., 2003). In poplar, 15–23 genes potentially involved in wood monolignol synthesis have been identified, based on transcript abundance in the xylem (Hamberger et al., 2007; Shi et al., 2010).
Although wood formation is commonly said to be highly influenced by the environment (Mellerowicz and Sundberg, 2008), few reports have dealt with the impact of abiotic stress on wood components and their biosynthesis. Most studies of abiotic stress have addressed variations in wood anatomy. Water-limited trees showed a decrease in vessel or tracheid diameter (February et al., 1995; Abe and Nakai, 1999; Abe et al., 2003; Corcuera et al., 2004; Sheriff and Whitehead, 2006) compensated by an increase in vessel number in poplar (Arend and Fromm, 2007), eucalyptus (Searson et al., 2004), and a shrub, Calligonum comosum (Al-Khalifah et al., 2006), which resulted in enhanced wood density (Searson et al., 2004). A similar response was observed in poplars (Junghans et al., 2006; Escalante-Perez et al., 2009) subjected to salt stress. High temperatures increased wood density and fibre length but had no effect on wood polymer composition in scots pine (Kilpelainen et al., 2005). Analyses of wood composition in response to abiotic stress are limited. Drought led to a reduction of lignification of the middle lamella and an altered distribution of lignin in the secondary wall of Pinus radiata (Donaldson, 2002).
Trees now have to cope with new stresses such as ozone, which has been apparent since the pre-industrial era and is predicted to increase even more (IPCC, 2007). Tropospheric ozone is now considered to be the most important air pollutant affecting vegetation (Karnosky et al., 2007). Ozone causes cellular damage in leaves, reduces photosynthesis, decreases carbon allocation to sink tissues, and affects plant biomass and radial growth (Wittig et al., 2009). In particular, leaf metabolism is reoriented to repair processes and defence mechanisms such as stress lignins (Cabané et al., 2004; Betz et al., 2009). Nevertheless, knowledge of the effects of ozone on wood formation is limited. All studies have been performed in free-air ozone fumigation experiments and the results are contradictory (Kaakinen et al., 2004; Kostiainen et al., 2006, 2008). Elevated ozone increased lignin concentration in trembling aspen and birch in a 3 year experiment (Kaakinen et al., 2004), but this increase was not observed after 5 years at the same site (Kostiainen et al., 2008). The differences observed in this experiment were in part attributed to non-controlled climatic variations. Although free-air experiments have a certain ecological relevance (Matyssek et al., 2010), they provide limited information about the underlying mechanisms because of interactions with other stresses (biotic or abiotic) or specific site conditions. Indeed, experiments in controlled chambers are needed to study the mechanisms of wood response to ozone.
The purpose of this work was to determine whether high ozone concentrations could alter cellulose and lignin synthesis during wood formation in poplars (Populus tremula×alba clone INRA 717-1-B4) cultivated under completely controlled conditions.
When growing upright, some trees such as poplar and eucalyptus produce heterogeneous wood which contains, in addition to normal wood, a peculiar tissue called tension wood (TW) (Wilson and Archer, 1977; Jourez et al., 2001; Washusen et al., 2002; Badia et al., 2006). The distribution of TW follows no set pattern in such trees (Isebrands and Bensend, 1972). The production of TW could be related to internal axial stresses in fast-growing species (Isebrands and Bensend, 1972; Badia et al., 2006) and high sensitivity to the gravity stimulus (Jourez et al., 2001; Jourez and Avella-Shaw, 2003). In fact, TW typically occurs in response to the gravity stimulus in bent trees and develops on the upper side of stems in order to restore the verticality of their axis (Pilate et al., 2004; Mellerowicz and Sundberg, 2008). At an anatomical level, TW differs from normal wood or the opposite wood (OW) formed on the lower side of bent stems. In TW of many species, including poplar, the fibres develop a specialized cell wall layer known as the gelatinous layer (G-layer). Most of the G-layer (95%) consists of crystalline cellulose which therefore results in a higher cellulose content and lower proportion of lignin in TW compared with OW or normal wood.
The effect of ozone was therefore investigated in both kinds of wood (TW and OW) encountered in poplar. Moreover, the trees were bent so as to induce the formation of TW in the upper part of the stem and OW in the lower part, and thereby to analyse biochemical, chemical, and anatomical properties in well-defined wood. Young hybrid poplars were bent and exposed to four different ozone concentrations (50, 100, 200, and 300 nl l−1) in controlled chambers. Cellulose and lignin composition, enzyme activities, and the genes involved in the cellulose and lignin biosynthesis pathways were analysed in TW and OW and any variations in wood anatomy were investigated.
Materials and methods
Plant material and growth conditions
Micropropagated hybrid poplar plants (Populus tremula×alba, clone INRA 717-1-B4) were transplanted into 5 l pots containing compost fertilized with 20 g of slow-release 13:13:13 N:P:K (Nutricot T 100, Fertil, Boulogne-Billancourt, France). The plants were cultivated in controlled chambers at 75/85% relative humidity (day/night) with a 14 h light period (Sun T Agro, Philips, Eindhoven, The Netherlands; intensity: 250–300 μmol m−2 s−1) and 22/18 °C day/night temperatures. The young trees were artificially tilted at 42 ° from the vertical using a rigid stick in order to stimulate TW formation on the upper side of the stems. The development of TW was checked by cutting fresh transverse sections at different levels of the stem by hand and staining them with safranin O/Astra blue according to Vazquez-Cooz and Meyer (2002).
Ozone treatment
Plants developing six fully expanded leaves were subjected to ozone treatment in phytotrons used for plant acclimation. The young trees were exposed either to charcoal-filtered air (control) or to different ozone concentrations (50±5, 100±10, 150±10, 200±10, and 300±10 nl l−1) for 46 d. Ozone was generated from pure O2 with a CMG3-3 ozone generator (Innovatec II, Rheinbach, Germany) and supplied to the fumigation chambers during the 14 h light period. Ambient air in the different chambers was continuously analysed by an ozone analyser (O341M, Environment SA, Paris).
Biomass and growth measurements
Leaves and stems were collected at the end of the experiment (46 d) and were dried at 60 °C for 3 weeks before biomass determination. Tree height and diameter were measured throughout the fumigation. Radial growth was measured 10 cm above the collar with a Vernier caliper. Analyses were done on six plants per treatment.
Preparation of enzyme extracts
Stems were harvested in the middle of the day. After removal of the bark, TW was sampled from the upper quarter of the section of the trees and OW from the lower quarter. Samples were then frozen in liquid nitrogen, and stored at –80 °C until analysis. Frozen tissues (∼300 mg) were ground in a mortar chilled with liquid nitrogen and extracts were obtained from the powders as described by Cabané et al. (2004). The resulting desalted extracts were used for enzyme assays.
Enzyme activities
The activities of enzymes involved in cellulose biosynthesis, namely SuSy (EC 2.4.1.13) and UGPase (EC 2.7.7.9), were determined with a Beckman DU 640 spectrophotometer (Beckman Coulter, Roissy, France). SuSy activity was based on the reduction of NADP+ at 340 nm with an enzyme coupling reaction (Hauch and Magel, 1998). UGPase activity was assayed at 340 nm by following the reduction of NAD+ with an enzyme coupling reaction (Ciereszko et al., 2001).
The activities of enzymes connected to lignin biosynthesis were measured as follows. Shikimate dehydrogenase (SHDH; EC 1.1.1.25) and cinnamyl alcohol dehydrogenase (CAD; EC 1.1.1.195) activities were determined at 30 °C with a microplate reader (Elx 808 iu BIO-TEX INSTRUMENTS). SHDH activity was determined by following the reduction of NADP+ at 340 nm (Fiedler and Schultz, 1985). CAD activity was monitored at 400 nm as described by O'Malley et al. (1992). Phenylalanine ammonia-lyase (PAL; EC 4.3.1.5) activity was assayed by measuring the release of cinnamate at 290 nm (Havir, 1987) with a Beckman DU 640 spectrophotometer (Beckman Coulter).
The protein content of enzyme extracts was determined with Bio-Rad Bradford protein reagent dye using bovine serum albumin as standard. The enzyme activities are reported as the mean value from three trees per treatment.
RNA extraction and cDNA synthesis
Tissues (∼100 mg) were placed in teflon jars chilled with liquid nitrogen and ground to a fine powder for 2 min using a mixer mill MM301 (Retsch, France). Total RNA was isolated with TRIzol (Invitrogen), according to the manufacturer's instructions. Any contaminating genomic DNA was removed by treating the RNA samples with DNase I, Amp Grade, (Invitrogen), then cleaning with RNeasy MinElute CleanUp columns (Qiagen). The absence of genomic DNA was checked by testing RNA samples (standard PCR) for amplification of sequences encoding ubiquitin-conjugated enzyme E2-17 kDa 10/12 (UBC10) (Sterky et al., 2004) with the following primers: UBC146A (5′-CCCGGCTCTAACCATATCCA-3′) and UBC146B (5′-GGGTCCAGCTTCTTGCAGTC-3′). A 1 μg aliquot of total RNA was reverse transcribed using the iScript cDNA Synthesis Kit (Biorad) to generate cDNA.
Quantitative real-time PCR
Quantitative real-time PCRs were carried out using iQ SYBR Green Supermix (Biorad) in a MyiQ Single-Color Real-Time PCR Detection System ICycler (Bio-Rad). The real-time PCR conditions were as follows: denaturation by hot start at 95 °C for 3 min, followed by 40 cycles of a two-step program consisting of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 45 s. The transcript abundance of genes involved in cellulose and lignin synthesis were quantified, namely CesA, PtCesA4, PtCesA5, PtCesA7, PtCesA8, PtCesA13, PtCesA17, and PtCesA18 [primers according to Suzuki et al. (2006)]; SuSy, PtSUS1 (JGI Protein ID:835735), PtSUS1F (5′-TGTTGAGGAGTTGCGTGTTG-3′), PtSUS1R (5′-TGGGCGAGGGAAAGATGC-3′), and PtSUS2 (JGI Protein ID:826368), PtSUS2F (5′-TGTATCCCCTGGTGCTGACG-3′), PtSUS2R (5′-GGCTTGTTGCGGTCTTTTAGC-3′); UGPase, UGP1 and UGP2 [primers according to Meng et al., (2007)]; PAL, PAL1; cinnamoyl CoA reductase, CCR2; and cinnamyl alcohol dehydrogenase, CAD1 [primers according to Shi et al. (2010)]. The genes selected for transcript analysis were based on previously reported high levels or specific expression in poplar xylem (Li et al., 2005; Suzuki et al., 2006; Barakat et al., 2009).
Four genes were used as endogenous controls to normalize transcript quantity: 18S rRNA [primers according to Meng et al. (2007)], polyubiquitin (UBQ11), actin (ACT2) [primers according to Brunner et al. (2004)], and UBC10 (primers described above in the cDNA synthesis section). Critical thresholds (Ct) for all genes were quantified in triplicate and normalized with GeNorm software (Vandesompele et al., 2002). Transcript relative abundance was calculated as the mean of three biological replicates (three trees per condition) and three analytical replicates.
Wood compounds
Cellulose content was determined on lyophilized wood samples. After grinding, the powders (10 mg) were subjected to acetic acid/nitric acid digestion as described by Updegraff (1969). The acid-insoluble material (cellulose) was recovered by centrifuging at 4000 g for 15 min. The dried pellets were resuspended in 67% sulphuric acid. Cellulose content was determined on a glucose basis by colorimetry using the phenol–sulphuric acid method (Dubois et al., 1956).
Lignin analyses were carried out on dry extract-free samples. These extract-free samples were prepared by subjecting the dried ground wood to exhaustive solvent extraction in a Soxhlet apparatus (toluene:ethanol, 1:1, then ethanol, and finally water). The lignin content was determined by the Klason method from 300 mg of sample according to the standard procedure (Dence, 1992) and was calculated as the weight percentage of the cell wall residue. All the Klason analyses were run in duplicate and with three trees per sample type. In addition, the acid-soluble lignin was determined according to the standard procedure and showed no differences between treatments. Thioacidolysis was performed as previously reported (Lapierre et al., 1999). The lignin-derived monomers were determined by gas chromatography–mass spectrometry (GC-MS) of their silylated derivatives (Lapierre et al., 1999).
Lignin and cellulose contents are reported as the mean values obtained for each series of three individually analysed trees.
Wood density
Wood samples (2 cm long) were saturated in distilled water. TW and OW were separated. The maximum volume of the wood fragment was determined from the apparent increase in mass using an electronic balance. Samples were then placed at 103 °C for 24 h to obtain the minimum dry mass. The density corresponded to the ratio of the minimum dry mass versus the maximum water-saturated volume. Analyses were performed on three different trees per condition. Two measurements were done on each tree.
Wood anatomy
Wood samples were dried in ambient air for 1 week. Transverse sections were prepared with a sliding microtome. Air-dried samples were analysed by environmental scanning electron microscopy (ESEM; FEI Quanta 200). Images were taken under a pressure of 1 Torr. Three poplars from each treatment were collected for wood anatomy analyses. For each tree, six random scanning zones were defined in the wood formed during the treatment: three in TW and three in OW. Vessel number, fibre number, and vessel lumen diameter were analysed on each scanning image using different types of software (Image J and Scion Image).
Cambium activity was analysed by saturating the stems with water under a vacuum, soaking progressively in polyethyleneglycol 1500, then obtaining transverse sections with a sliding microtome. The sections were stained with safranin O/Astra blue (Vazquez-Cooz and Meyer, 2002) and observed under a light microscope. The number of cambium cell layers was recorded on three trees per treatment.
Statistical analysis
The significance of ozone effects compared with controls was assessed in a two-way analysis of variance (ANOVA) followed by Tukey's test.
Results
Young hybrid poplars (Populus tremula×alba, clone INRA 717-1-B4) growing upright (staked or not) in controlled chambers developed TW without any defined pattern (Supplementary Fig. S1 at JXB online). The young trees were therefore bent to limit the development of TW to the upper side of the stem (Supplementary Fig. S1) and were grown in charcoal-filtered air (control) or were fumigated with ozone (50, 100, 200, or 300 nl l−1) in phytotronic chambers.
Biomass and plant development
Foliar and stem biomass were severely affected during the 46 d of ozone fumigation (Table 1). Biomass loss was significant at 100, 200, and 300 nl l−1, with a reduction of up to 37% for leaves and 48% for stems. Leaf fall was enhanced at the same time as the reduction of foliar biomass. Leaf fall in hybrid poplars subjected to 300 nl l−1 ozone was 2-fold higher than in control plants.
Table 1.
Dry matter and percentage of fallen leaves of hybrid poplars cultivated for 46 d under different ozone treatments
| Treatment | Dry weight (g) |
Fallen leaves (% total leaves) | |
| Leaves | Stem | ||
| Control | 23.52±2.5 (100) | 22.61±2.8 (100) | 12.70±1.9 |
| Ozone, 50 nl l−1 | 19.50±4.1 (83) | 15.60±6.1 (69) | 13.73±2.4 |
| Ozone, 100 nl l−1 | 15.13±2.5* (64) | 12.23±3.2* (54) | 15.42±2.4 |
| Ozone, 200 nl l−1 | 15.10±0.9* (64) | 11.67±1.6* (52) | 18.53±1.5 |
| Ozone, 300 nl l−1 | 14.86±1.5* (63) | 13.03±1.4* (58) | 31.44±1.4* |
Data are mean values ±SD (n=6). The normalized values relative to the control are shown in parentheses.
Significant differences (P <0.05) between control and ozone treatments.
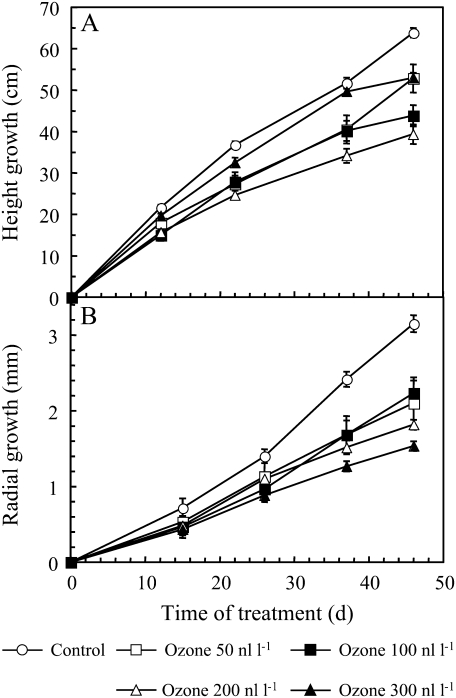
Tree height and radial growth were also reduced by ozone fumigation (Fig. 1). Height growth was decreased by 38% in plants subjected to 200 nl l−1 ozone compared with the controls. The reduction of height growth was delayed at 300 nl l−1 and corresponded to a growth break (visual observation of apical bud). A significant decrease in radial growth was also observed in plants exposed to ozone. This decline in radial growth attained 50% at 300 nl l−1.
Fig. 1.
Height (A) and radial (B) growth of hybrid poplars since the beginning of the treatment, i.e. height and diameter differences compared with the height and diameter at time zero. Trees were cultivated under control conditions or different ozone concentrations. Data represent means ±SD (n=6).
Cellulose biosynthesis
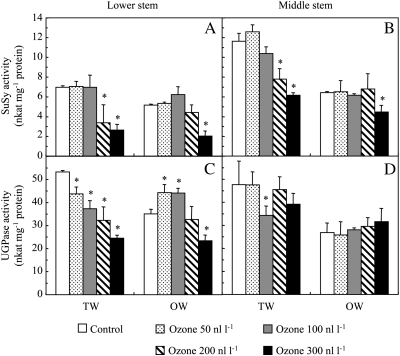
Two stem levels corresponding to two different developmental stages were sampled. The lower stem (LS) was located 10 cm above the collar and corresponded to wood that developed before and during ozone fumigation. The middle stem (MS) developed just after the beginning of ozone fumigation. TW from the upper side and OW from the lower side of the stem were collected from each level, and separated. Under control conditions, SuSy and UGPase activities were higher in TW than in OW at both LS and MS levels (Fig. 2). The transcript levels of SuSy genes (SUS1 and SUS2) were higher in TW than in OW (Table 2). The RNA levels for one UGPase gene, UGP1, were similar between TW and OW, whereas the transcript abundance of UGP2 was twice as high in TW than in OW. Among the 18 CesA genes identified in P. trichocarpa (Suzuki et al., 2006), seven genes specific to or highly expressed in xylem were selected for this study (PtCesA4, PtCesA5, PtCesA7, PtCesA8, PtCesA13, PtCesA17, and PtCesA18). The CesA5 and CesA13 genes are reported to be involved in cellulose synthesis in the primary cell wall, whereas the CesA4, CesA7, CesA8, CesA17, and CesA18 genes are believed to be involved in cellulose production in the secondary cell wall. The transcript abundance of CesA7 and CesA18 genes was higher in TW than in OW, but the RNA levels of the other CesA genes were unchanged between TW and OW.
Fig. 2.
SuSy (A, B) and UGPase (C, D) activity in hybrid poplar wood after 46 d of culture under control conditions or different ozone concentrations. Analyses were performed in tension wood (TW) and opposite wood (OW) at different stem levels. Data represent means ±SD (n=3). *Significant differences (P <0.05) between control and ozone treatments.
Table 2.
Transcript abundance of cellulose and lignin biosynthetic genes in hybrid poplar wood (LS) cultivated for 46 d under control or ozone (300 nl l−1) conditions
| Gene | Tension wood |
Opposite wood |
||
| Control | Ozone, 300 nl l−1 | Control | Ozone, 300 nl l−1 | |
| Cellulose biosynthesis | ||||
| SUS1 | 1.50±0.35 | 0.41±0.14 | 1±0.16 | 0.32±0.05 |
| SUS2 | 1.64±0.22 | 0.47±0.16 | 1±0.23 | 0.34±0.05 |
| UGP1 | 1.22±0.16 | 0.48±0.27 | 1±0.37 | 0.82±0.03 |
| UGP2 | 2.28±0.71 | 1.59±0.42 | 1±0.34 | 1.54±0.18 |
| CesA4 | 0.89±0.18 | 0.56±0.19 | 1±0.13 | 0.64±0.18 |
| CesA5 | 0.81±0.03 | 0.60±0.16 | 1±0.22 | 0.77±0.07 |
| CesA7 | 1.59±0.17 | 0.61±0.27 | 1±0.15 | 0.46±0.18 |
| CesA8 | 0.95±0.26 | 0.31±0.13 | 1±0.06 | 0.38±0.09 |
| CesA13 | 0.76±0.13 | 0.82±0.09 | 1±0.19 | 0.85±0.1 |
| CesA17 | 0.85±0.15 | 0.46±0.16 | 1±0.11 | 0.55±0.1 |
| CesA18 | 2.06±0.47 | 0.82±0.37 | 1±0.13 | 0.43±0.11 |
| Lignin biosynthesis | ||||
| PAL1 | 0.47±0.19 | 0.37±0.06 | 1±0.27 | 0.55±0.03 |
| CCR2 | 0.84±0.13 | 0.56±0.1 | 1±0.07 | 0.56±0.05 |
| CAD1 | 1.46±0.31 | 0.64±0.12 | 1±0.31 | 0.60±0.07 |
Transcript levels were normalized and expressed as fold changes compared with values of opposite wood in control conditions. Data are means ±SD of three biological replicates.
Under ozone conditions, the SuSy and UGPase activities in TW were decreased in both LS and MS (Fig. 2). The transcript levels of the SUS1 and SUS2 genes were reduced in both TW and OW tissues (Table 2). The transcript levels of UGP1 and UGP2 were reduced in TW. However, the UGP1 transcript seemed to remain unchanged in OW, whereas the expression of UGP2 was stimulated. All seven CesA genes, with the exception of CesA13, were affected by ozone in both TW and OW. A substantial decrease in transcript level was observed for CesA7, CesA8, CesA17, and CesA18.
Lignin biosynthesis
The activity of CAD, which catalyses the reduction of p-hydroxycinnamaldehydes to p-hydroxycinnamyl alcohols, was measured. At the same time the activity of two enzymes involved in earlier steps of phenolic metabolism were monitored. These were SHDH, an enzyme of the shikimate pathway associated with phenylalanine synthesis, and PAL, the first enzyme of the phenylpropanoid pathway. Transcript levels were analysed by real-time quantitative PCR for PAL, CAD, and for cinnamoyl CoA reductase (CCR), the enzyme that provides substrates to CAD.
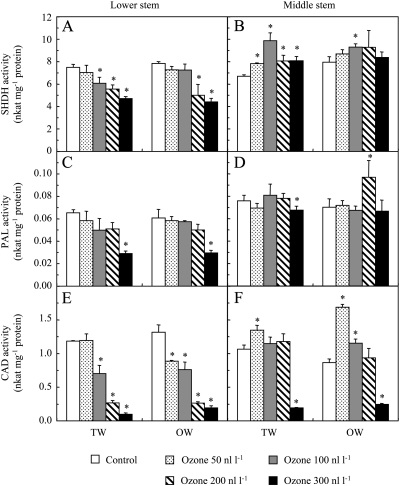
The enzymes involved in lignin metabolism showed similar activities in TW and OW under control conditions (Fig. 3) and similar CAD1 and CCR2 expression levels (Table 2). The PAL1 transcript level was higher in OW than in TW.
Fig. 3.
SHDH (A, B), PAL (C, D), and CAD (E, F) activity in hybrid poplar wood after 46 d of culture under control conditions or different ozone concentrations. Analyses were performed in tension wood (TW) and opposite wood (OW) at different stem levels. Data represent means ±SD (n=3). *Significant differences (P <0.05) between control and ozone treatments.
Under ozone, the LS was more affected than the MS. In the TW and OW of LS, the SHDH, PAL, and CAD activities were lower than in the controls. The PAL1, CAD1, and CCR2 transcript levels were also reduced by ozone, except PAL1 in TW. In MS, the PAL and SHDH activities were slightly enhanced at some ozone concentrations while CAD activity was only reduced at the highest ozone concentration.
Cellulose and lignin content
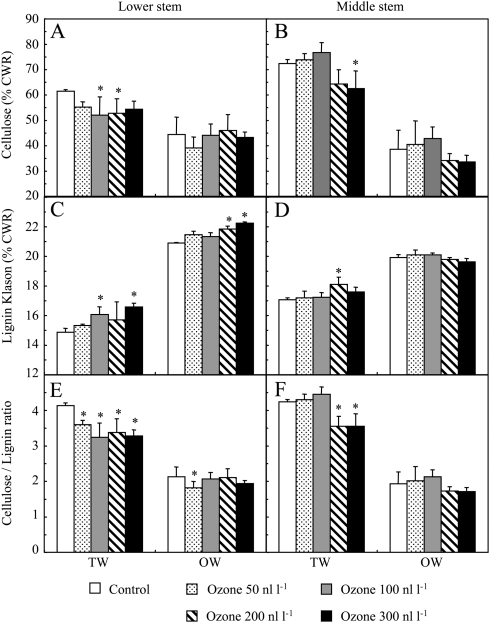
The cellulose content under control conditions and at both stem levels was higher in TW than in OW, whereas the TW Klason lignin content was lower (Fig. 4). Accordingly, the cellulose to lignin ratio was 2-fold higher in TW than in OW.
Fig. 4.
Cellulose content (A, B), lignin content (C, D), and cellulose to lignin ratio (E, F) in hybrid poplar wood after 46 d of culture under control conditions or different ozone concentrations. Analyses were performed in tension wood (TW) and opposite wood (OW) at different stem levels. Cellulose and lignin content are expressed as a percentage of cell wall residue (CWR). Data represent means ±SD (n=3). *Significant differences (P <0.05) between control and ozone treatments.
OW composition remained unchanged under ozone, but TW was affected. Cellulose content was reduced by ozone, and Klason lignin was enhanced in LS TW (Fig. 4). Similar results were obtained when the acid-soluble lignin fraction was considered together with Klason lignin (data not shown). Ozone significantly reduced the cellulose to lignin ratio at every ozone concentration in LS and at the highest concentrations (200 nl l−1 and 300 nl l−1) in MS. In contrast, lignin composition was not affected by ozone, as revealed by the S/G thioacidolysis ratios (Supplementary Table S1 at JXB online).
Wood density and anatomy
Wood density was higher in TW than in OW in both MS and LS under each condition (Table 3). Ozone reduced the ratio between TW and OW densities at both levels of the stem, compared with control plants.
Table 3.
Tension wood to opposite wood density ratio of hybrid poplar stem after 46 d of fumigation with different ozone concentrations
| Level | Treatment | Wood density (TW/OW) |
| Lower stem | Control | 1.209±0.007 |
| Ozone, 50 nl l−1 | 1.145±0.013 | |
| Ozone, 100 nl l−1 | 1.111±0.032* | |
| Ozone, 200 nl l−1 | 1.075±0.010* | |
| Ozone, 300 nl l−1 | 1.089±0.018* | |
| Middle stem | Control | 1.088±0.032 |
| Ozone, 50 nl l−1 | 1.050±0.005 | |
| Ozone, 100 nl l−1 | 1.033±0.037* | |
| Ozone, 200 nl l−1 | 1.039±0.017* | |
| Ozone, 300 nl l−1 | 1.037±0.007* |
Data are mean values ±SD (n=3, individual replicates).
*Significant differences (P <0.05) between control and ozone treatments.
TW/OW, tension wood to opposite wood ratio.
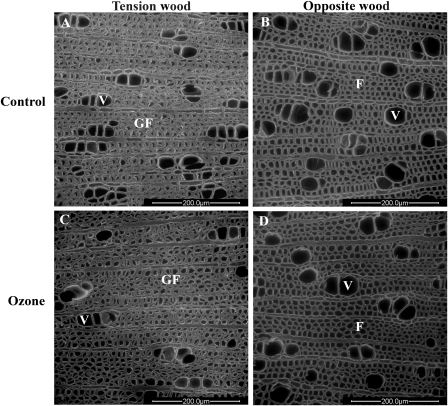
Images of wood anatomy were recorded by ESEM. Images were taken in the most external part of LS that was formed during fumigation. The TW images (Fig. 5) showed characteristic fibres with an additional G-layer. Under control conditions, TW contained fewer vessels with smaller lumens than OW. The cambial cell layers (Supplementary Fig. S2 at JXB online) and fibre frequencies were similar in TW and OW (Table 4).
Fig. 5.
Environmental scanning electron microphotographs of the transverse surface of tension wood (A, C) and opposite wood (B, D) in stems (LS) of hybrid poplars cultivated for 46 d under control conditions (A, B) or 200 nl l−1 ozone (C, D). V, vessel; F, fibre; GF, G-layer fibre. Scale bars=200 μm.
Table 4.
Wood anatomy in hybrid poplar stem (LS) after 46 days of treatment with different ozone concentrations
| Treatment | Tension wood |
Opposite wood |
||||||
| Cambium cell layers (number) | Fibre frequency (number mm−2) | Vessel frequency (number mm−2) | Vessel lumen diameter (μm) | Cambium cell layers (number) | Fibre frequency (number mm−2) | Vessel frequency (number mm−2) | Vessel lumen diameter (μm) | |
| Control | 6.4±0.5 | 2890±200 | 173±33 | 40.7±2.8 | 6.2±0.3 | 2970±200 | 200±13 | 49.8±3.1 |
| Ozone, 50 nl l−1 | ND | 3760±510* | 142±25* | 47.4±1.2* | ND | 3120±480 | 179±12 | 49.7±3.6 |
| Ozone, 100 nl l−1 | ND | 4560±730* | 126±35* | 45.1±4.5* | ND | 3340±410 | 220±27 | 49.1±1.3 |
| Ozone, 200 nl l−1 | 3.3±0.4* | 3790±170* | 124±15* | 47.2±2.6* | 3.0±0.5* | 3500±180 | 188±22 | 49.4±0.8 |
| Ozone, 300 nl l−1 | ND | 4050±240* | 105±62* | 47.9±2.1* | ND | 3450±500 | 188±17 | 49.6±2.6 |
Data are mean values ±SD (n=3, individual replicates).
*Significant differences (P <0.05) between control and ozone treatments.
ND, not determined.
Ozone reduced cambial growth in both TW and OW at 200 nl l−1 (Table 4). Other parameters were not affected by ozone in OW. Wood anatomy was altered by ozone in TW. Vessel lumen diameter increased while vessel frequency decreased. Fibre frequency was enhanced in TW in hybrid poplars fumigated with ozone.
Discussion
Cellulose and lignin biosynthesis in tension wood formation
High proportions of TW develop in upright hybrid poplars cultivated in growth chambers, so the trees in the present experiment were bent so that TW formation would be limited to the upper side of the stem (Supplementary Fig. S1 at JXB online). In fact, many features of TW such as high wood density, low vessel frequency, fibres with a G-layer, and high cellulose content (Tables 2, 4, and Figs 4, 5) have been found in this part as compared with the OW (or normal wood) formed on the lower side of the hybrid poplar stem (Plomion et al., 2001; Pilate et al., 2004; Mellerowicz and Sundberg, 2008).
As described (Pilate et al., 2004), it was found that TW contained less lignin than OW and that their lignin S/G (syringyl/guaiacyl) ratios were slightly higher (Fig. 4, and Supplementary Table S1 at JXB online). However, the activities of different enzymes involved in lignin synthesis (CAD), and in supply pathways, the phenylpropanoid pathway (PAL), and the shikimate pathway (SHDH), were similar in TW and OW (Fig. 3). Additionally, CAD1 and CCR2 expression levels remained unchanged (Table 2). These findings are in accordance with different authors who concluded that TW cell walls contained the same quantity of lignin as OW and that the apparent decrease in lignin content resulted only from the increase of cellulose on a dry matter basis (Bentum et al., 1969; Joseleau et al., 2004; Pilate et al., 2004; Xu et al., 2006).
Reduction of cellulose and lignin biosynthesis activities under ozone
The present growth data revealed a decrease in biomass production and growth rate of young hybrid poplars exposed to ozone (Table 1 and Fig. 1). A marginal response, which would correspond to high sensitivity or high resistance, was avoided by using four different ozone levels (50, 100, 200, and 300 nl l−1). Most of the responses showed the same trend, whatever the ozone level, and were more pronounced at the highest levels. The reduction of tree growth under ozone is a well-known effect (Wittig et al., 2009) and is generally attributed to a decrease in photosynthesis (Wittig et al., 2007). It was shown here that stems were more affected than leaves and that the reduction in stem diameter was stronger for the highest ozone concentration. Biomass allocation to stem was therefore reduced under ozone treatment since the leaf to stem biomass ratio was significantly enhanced. Indeed poplars exposed for 35 d at 200 nl l−1 ozone and labelled for 4 h with 13CO2 showed a decrease in carbon allocation to stems. Newly incorporated carbon was reduced by nearly half in lower stems (data not shown). Carbon retention was probably increased in the leaves, resulting in a stronger reduction of carbon allocation to the stems. This greater retention in leaves may be explained by a higher carbon demand for repair and defence in damaged foliage (Sandermann, 2000).
Wood formation is initiated in the vascular cambium. In the present experiment, the decrease in radial growth under ozone probably resulted from reduced cambial activity (Table 4, and Supplementary Fig. S2 at JXB online), as previously suggested by Matyssek et al. (2002) and as observed in reaction to many other stresses (Savidge, 2001). Cambial derivatives develop into xylem cells through division, expansion, secondary wall formation, lignification, and finally cell death. This study is, to our knowledge, the first which combines analyses of the composition and metabolism (enzyme activities and RNA levels) of the two main cell wall components, cellulose and lignin, in relation to TW and OW formation under ozone fumigation. The results show that enzyme activities involved in cellulose and lignin metabolism decreased in poplar stems exposed to ozone (Figs 2, 3). Enzyme activities and transcript levels were in good agreement (Table 2) and support the hypothesis of a transcriptional control of cellulose and lignin metabolism in such stems. Moreover, these results suggest a coordinated regulation of metabolism for the biosynthesis of secondary walls which could be a direct or indirect consequence of carbon availability (Rogers et al., 2005). Hertzberg et al. (2001) showed that genes involved in lignin and cellulose synthesis were expressed in the secondary wall formation zone of poplar wood. In the present experiment, the decreased xylem differentiation zone associated with a reduction in cambial activity could explain the reduced cellulose and lignin biosynthesis activities.
Modification of wood anatomy under ozone
Ozone-induced variations in wood anatomy occurred mainly in TW (Table 4) which is a major consumer of carbon and may therefore be more susceptible to the reduced allocation of carbon to the stem under ozone. Vessel lumen diameter was enhanced by ozone, the vessel frequency was decreased, and the vessel lumen fraction was maintained. Other environmental conditions may also influence wood anatomy. The effects of drought and salt stress, unlike those of ozone, have been well characterized. Both stresses result in decreased vessel area and increased vessel frequency (Junghans et al., 2006; Arend and Fromm, 2007; Escalante-Perez et al., 2009). The formation of smaller vessels in response to drought appears to be an adaptive response to drought-induced xylem embolism. Conversely, the modifications in vessel anatomy found in this experiment might improve water transport efficiency and induce a greater risk of embolism (Tyree et al., 1994; Zanne et al., 2010).
Decrease of the cellulose to lignin ratio in wood under ozone
It was shown that cellulose and lignin biosynthesis activities (enzymes and transcripts) were reduced by ozone in hybrid poplar wood as a consequence of the decrease in cambial activity and cell wall production. Because the synthesis of both components was altered in the same manner, the content of cellulose or lignin could not be predicted from these results. Indeed, cellulose and lignin contents express the relative abundance of each polymer in the cell wall. Since cellulose and lignin represent the main components of the cell wall, the variation in abundance of one component impacts the content of the other. In the present case, the decrease of cellulose and lignin biosynthesis activities suggested that the amounts of both lignin and cellulose decreased, but do not allow their relative abundance to be deduced. Interestingly, the present data indicate that ozone exposure led to a modification of cell wall composition (Fig. 4). The cellulose content was reduced in accordance with the decrease of biosynthesis activities (enzymes and transcripts). Conversely, lignin content increased, in opposition to enzyme activities and transcript levels. An apparent increase in lignin content can be explained by a strong decrease in cellulose content. All these results suggest that cellulose and lignin biosynthesis was reduced under ozone and that cellulose biosynthesis was more affected than lignin biosynthesis. Accordingly, the cellulose to lignin ratio of the cell wall was reduced by ozone.
Like the anatomical changes, the modifications of the cellulose to lignin ratio were mainly observed in TW, thereby reinforcing the hypothesis that this tissue shows higher sensitivity to ozone than OW. The LS exhibited greater alteration than the MS, although MS only developed during ozone fumigation. However, MS was closer to the carbon source organs (leaves) and might be less affected than LS by carbon deficiency under ozone fumigation.
The response of lignin in stems was completely different from that observed in poplar or beech leaves under ozone (Cabané et al., 2004; Betz et al., 2009). Lignin biosynthesis (enzymes and transcripts) was highly stimulated in leaves, resulting in an increase in lignin content. The newly synthesized lignin was structurally different from constitutive lignin and was thought to be involved in defence mechanisms.
The reduced cellulose to lignin ratio of ozone-treated hybrid poplar stem suggests that the tree would promote lignification rather than cellulose biosynthesis under ozone stress. Lignin and cellulose deposition might be regulated in a compensatory manner in order to sustain the mechanical strength of the cell wall, as already suggested for transgenic poplars (Hu et al., 1999; Li et al., 2003). Although the metabolic cost of lignin synthesis is high (Amthor, 2003), in the context of an absence of carbon skeleton, the carbon cost of lignin strengthening the cell wall would be moderate compared with that of cellulose reinforcement. Moreover, the present results showed that ozone reduced wood density in TW (Table 3), suggesting a decrease in cell wall production per unit volume. By reducing the cellulose to lignin ratio, the tree could continue some radial growth and save carbon. The maintenance of radial growth would allow an increase in height and thence a renewal of photosynthesis which would have been impaired in ozone-damaged leaves. Thus, reduction of the cellulose to lignin ratio in wood might serve as a specific adaptation to maintain photosynthesis in ozone-injured trees.
In conclusion, the first results concerning the effect of ozone on cellulose and lignin deposition during wood formation are reported here. Ozone altered cellulose and lignin biosynthesis in hybrid poplar wood, resulting in a lower cellulose to lignin ratio, together with modifications of anatomy and density. This response appeared to be coordinated and to maintain radial growth despite the lack of carbon, as an indirect consequence of ozone impact on leaves. The results suggest that the mechanical properties of the wood are affected by high concentrations of ozone.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Photomicrographs of a stem cross-section stained with safranin O/Astra blue showing tension wood crescents in hybrid poplar cultivated vertically or bent in phytotronic chambers.
Figure S2. Photomicrographs of a cross-section showing the cambium zone in tension wood and opposite wood in stems (LS) of hybrid poplars cultivated for 46 d under control conditions or 200 nl l−1 ozone.
Table S1. Lignin-derived monomers (H, G, and S) and S/G ratio after thioacidolysis of tension wood or opposite wood at different stem levels from hybrid poplars cultivated for 46 d under control conditions or different ozone concentrations.
Acknowledgments
This work was supported by the French Environment and Energy Management Agency (ADEME) and Région Lorraine. We sincerely thank Frédéric Legée (UMR 1318 AgroParisTech-INRA) and Laurent Cézard (UMR 1318 AgroParisTech-INRA) for the lignin analyses.
Glossary
Abbreviations
- LS
lower stem
- MS
middle stem
- OW
opposite wood
- TW
tension wood
References
- Abe H, Nakai T. Effect of the water status within a tree on tracheid morphogenesis in Cryptomeria japonica D. Don. Trees: Structure and Function. 1999;14:124–129. [Google Scholar]
- Abe H, Nakai T, Utsumi Y, Kagawa A. Temporal water deficit and wood formation in Cryptomeria japonica. Tree Physiology. 2003;23:859–863. doi: 10.1093/treephys/23.12.859. [DOI] [PubMed] [Google Scholar]
- Al-Khalifah NS, Khan PR, Al-Abdulkader AM, Nasroun T. Impact of water stress on the sapwood anatomy and functional morphology of Calligonum comosum. IAWA Journal. 2006;27:299–312. [Google Scholar]
- Amthor JS. Efficiency of lignin biosynthesis: a quantitative analysis. Annals of Botany. 2003;91:673–695. doi: 10.1093/aob/mcg073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend M, Fromm J. Seasonal change in the drought response of wood cell development in poplar. Tree Physiology. 2007;27:985–992. doi: 10.1093/treephys/27.7.985. [DOI] [PubMed] [Google Scholar]
- Badia MA, Constant T, Mothe F, Nepveu G. Tension wood occurrence in three cultivars of Populus×euramericana. Part I: inter-clonal and intra-tree variability of tension wood. Annals of Forest Science. 2006;63:23–30. [Google Scholar]
- Barakat A, Bagniewska-Zadworna A, Choi A, Plakkat U, DiLoreto DS, Yellanki P, Carlson JE. The cinnamyl alcohol dehydrogenase gene family in Populus: phylogeny, organization, and expression. BMC Plant Biology. 2009;9:26. doi: 10.1186/1471-2229-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentum A, Côté W, Day A, Timell T. Distribution of lignin in normal and tension wood. Wood Science and Technology. 1969;3:218–231. [Google Scholar]
- Betz GA, Knappe C, Lapierre C, Olbrich M, Welzl G, Langebartels C, Heller W, Sandermann H, Ernst D. Ozone affects shikimate pathway transcripts and monomeric lignin composition in European beech (Fagus sylvatica L.) European Journal of Forest Research. 2009;128:109–116. [Google Scholar]
- Boudet AM, Hawkins S, Rochange S. The polymorphism of the genes/enzymes involved in the last two reductive steps of monolignol synthesis: what is the functional significance? Comptes Rendus Biologies. 2004;327:837–845. doi: 10.1016/j.crvi.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Brunner AM, Yakovlev IA, Strauss SH. Validating internal controls for quantitative plant gene expression studies. BMC Plant Biology. 2004;4:14. doi: 10.1186/1471-2229-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabané M, Pireaux JC, Leger E, Weber E, Dizengremel P, Pollet B, Lapierre C. Condensed lignins are synthesized in poplar leaves exposed to ozone. Plant Physiology. 2004;134:586–594. doi: 10.1104/pp.103.031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A, Somerville C. Cellulosic biofuels. Annual Review of Plant Biology. 2009;60:165–182. doi: 10.1146/annurev.arplant.043008.092125. [DOI] [PubMed] [Google Scholar]
- Ciereszko I, Johansson H, Hurry V, Kleczkowski LA. Phosphate status affects the gene expression, protein content and enzymatic activity of UDP-glucose pyrophosphorylase in wild-type and pho mutants of Arabidopsis. Planta. 2001;212:598–605. doi: 10.1007/s004250000424. [DOI] [PubMed] [Google Scholar]
- Corcuera L, Camarero JJ, Gil-Pelegrin E. Effects of a severe drought on Quercus ilex radial growth and xylem anatomy. Trees: Structure and Function. 2004;18:83–92. [Google Scholar]
- Davin LB, Jourdes M, Patten AM, Kim KW, Vassao DG, Lewis NG. Dissection of lignin macromolecular configuration and assembly: comparison to related biochemical processes in allyl/propenyl phenol and lignan biosynthesis. Natural Product Reports. 2008;25:1015–1090. doi: 10.1039/b510386j. [DOI] [PubMed] [Google Scholar]
- Delmer DP, Haigler CH. The regulation of metabolic flux to cellulose, a major sink for carbon in plants. Metabolic Engineering. 2002;4:22–28. doi: 10.1006/mben.2001.0206. [DOI] [PubMed] [Google Scholar]
- Dence C. The determination of lignin. In: Lin S, Dence C, editors. Methods in lignin chemistry. Berlin: Springer-Verlag; 1992. pp. 33–62. [Google Scholar]
- Doblin MS, Kurek I, Jacob-Wilk D, Delmer DP. Cellulose biosynthesis in plants: from genes to rosettes. Plant and Cell Physiology. 2002;43:1407–1420. doi: 10.1093/pcp/pcf164. [DOI] [PubMed] [Google Scholar]
- Donaldson LA. Lignification and lignin topochemistry—an ultrastructural view. Phytochemistry. 2001;57:859–873. doi: 10.1016/s0031-9422(01)00049-8. [DOI] [PubMed] [Google Scholar]
- Donaldson LA. Abnormal lignin distribution in wood from severely drought stressed Pinus radiata trees. IAWA Journal. 2002;23:161–178. [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28:350–356. [Google Scholar]
- Escalante-Perez M, Lautner S, Nehls U, et al. Salt stress affects xylem differentiation of grey poplar (Populus×canescens) Planta. 2009;229:299–309. doi: 10.1007/s00425-008-0829-7. [DOI] [PubMed] [Google Scholar]
- February E, Stock W, Bond W, Le Roux D. Relationships between water availability and selected vessel characteristics in Eucalyptus grandis and two hybrids. IAWA Journal. 1995;16:269–276. [Google Scholar]
- Fiedler E, Schultz G. Localization, purification, and characterization of shikimate oxidoreductase-dehydroquinate hydrolase from stroma of spinach chloroplasts. Plant Physiology. 1985;79:212–218. doi: 10.1104/pp.79.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger B, Ellis M, Friedmann M, Souza CDA, Barbazuk B, Douglas CJ. Genome-wide analyses of phenylpropanoid-related genes in Populus trichocarpa, Arabidopsis thaliana, and Oryza sativa: the Populus lignin toolbox and conservation and diversification of angiosperm gene families. Canadian Journal of Botany. 2007;85:1182–1201. [Google Scholar]
- Hauch S, Magel E. Extractable activities and protein content of sucrose-phosphate synthase, sucrose synthase and neutral invertase in trunk tissues of Robinia pseudoacacia L. are related to cambial wood production and heartwood formation. Planta. 1998;207:266–274. [Google Scholar]
- Havir EA. L-Phenylalanine ammonia-lyase from soybean cell suspension cultures. Methods in Enzymology. 1987;142:248–253. [Google Scholar]
- Hertzberg M, Aspeborg H, Schrader J, et al. A transcriptional roadmap to wood formation. Proceedings of the National Academy of Sciences, USA. 2001;98:14732–14737. doi: 10.1073/pnas.261293398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WJ, Harding SA, Lung J, Popko JL, Ralph J, Stokke DD, Tsai CJ, Chiang VL. Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nature Biotechnology. 1999;17:808–812. doi: 10.1038/11758. [DOI] [PubMed] [Google Scholar]
- Humphreys JM, Chapple C. Rewriting the lignin roadmap. Current Opinion in Plant Biology. 2002;5:224–229. doi: 10.1016/s1369-5266(02)00257-1. [DOI] [PubMed] [Google Scholar]
- IPCC. Climate change 2007: synthesis report. Geneva, Switzerland: IPCC; 2007. [Google Scholar]
- Isebrands J, Bensend D. Incidence and structure of gelatinous fibers within rapid-growing eastern cottonwood. Wood Science and Technology. 1972;4:61–71. [Google Scholar]
- Joseleau JP, Imai T, Kuroda K, Ruel K. Detection in situ and characterization of lignin in the G-layer of tension wood fibres of Populus deltoides. Planta. 2004;219:338–345. doi: 10.1007/s00425-004-1226-5. [DOI] [PubMed] [Google Scholar]
- Joshi CP, Mansfield SD. The cellulose paradox—simple molecule, complex biosynthesis. Current Opinion in Plant Biology. 2007;10:220–226. doi: 10.1016/j.pbi.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Jourez B, Avella-Shaw T. Effect of gravitational stimulus duration on tension wood formation in young stems of poplar (Populus euramericana cv ‘Ghoy’) Annals of Forest Science. 2003;60:31–41. [Google Scholar]
- Jourez B, Riboux A, Leclercq A. Anatomical characteristics of tension wood and opposite wood in young inclined stems of poplar (Populus euramericana cv ‘Ghoy’) IAWA Journal. 2001;22:133–157. [Google Scholar]
- Junghans U, Polle A, Duchting P, Weiler E, Kuhlman B, Gruber F, Teichmann T. Adaptation to high salinity in poplar involves changes in xylem anatomy and auxin physiology. Plant, Cell and Environment. 2006;29:1519–1531. doi: 10.1111/j.1365-3040.2006.01529.x. [DOI] [PubMed] [Google Scholar]
- Kaakinen S, Kostiainen K, Ek F, Saranpaa P, Kubiske ME, Sober J, Karnosky DF, Vapaavuori E. Stem wood properties of Populus tremuloides, Betula papyrifera and Acer saccharum saplings after 3 years of treatments to elevated carbon dioxide and ozone. Global Change Biology. 2004;10:1513–1525. [Google Scholar]
- Karnosky DF, Skelly JM, Percy KE, Chappelka AH. Perspectives regarding 50 years of research on effects of tropospheric ozone air pollution on US forests. Environmental Pollution. 2007;147:489–506. doi: 10.1016/j.envpol.2006.08.043. [DOI] [PubMed] [Google Scholar]
- Kilpelainen A, Peltola H, Ryyppo A, Kellomaki S. Scots pine responses to elevated temperature and carbon dioxide concentration: growth and wood properties. Tree Physiology. 2005;25:75–83. doi: 10.1093/treephys/25.1.75. [DOI] [PubMed] [Google Scholar]
- Kostiainen K, Jalkanen H, Kaakinen S, Saranpaa P, Vapaavuori E. Wood properties of two silver birch clones exposed to elevated CO2 and O3. Global Change Biology. 2006;12:1230–1240. [Google Scholar]
- Kostiainen K, Kaakinen S, Warsta E, Kubiske ME, Nelson ND, Sober J, Karnosky DF, Saranpaa P, Vapaavuori E. Wood properties of trembling aspen and paper birch after 5 years of exposure to elevated concentrations of CO2 and O3. Tree Physiology. 2008;28:805–813. doi: 10.1093/treephys/28.5.805. [DOI] [PubMed] [Google Scholar]
- Lapierre C, Pollet B, Petit Conil M, et al. Structural alterations of lignins in transgenic poplars with depressed cinnamyl alcohol dehydrogenase or caffeic acid O-methyltransferase activity have an opposite impact on the efficiency of industrial kraft pulping. Plant Physiology. 1999;119:153–163. doi: 10.1104/pp.119.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LG, Cheng XF, Lu SF, Nakatsubo T, Umezawa T, Chiang VL. Clarification of cinnamoyl co-enzyme a reductase catalysis in monolignol biosynthesis of aspen. Plant and Cell Physiology. 2005;46:1073–1082. doi: 10.1093/pcp/pci120. [DOI] [PubMed] [Google Scholar]
- Li L, Zhou Y, Cheng X, Sun J, Marita JM, Ralph J, Chiang VL. Combinatorial modification of multiple lignin traits in trees through multigene cotransformation. Proceeding of National Academy of Science USA. 2003;100:4939–4944. doi: 10.1073/pnas.0831166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyssek R, Gunthardt-Goerg MS, Maurer S, Christ R. Tissue structure and respiration of stems of Betula pendula under contrasting ozone exposure and nutrition. Trees: Structure and Function. 2002;16:375–385. [Google Scholar]
- Matyssek R, Karnosky DF, Wieser G, Percy K, Oksanen E, Grams TEE, Kubiske M, Hanke D, Pretzsch H. Advances in understanding ozone impact on forest trees: messages from novel phytotron and free-air fumigation studies. Environmental Pollution. 2010;158:1990–2006. doi: 10.1016/j.envpol.2009.11.033. [DOI] [PubMed] [Google Scholar]
- Mellerowicz E, Baucher M, Sundberg B, Boerjan W. Unravelling cell wall formation in the woody dicot stem. Plant Molecular Biology. 2001;47:239–274. [PubMed] [Google Scholar]
- Mellerowicz EJ, Sundberg B. Wood cell walls: biosynthesis, developmental dynamics and their implications for wood properties. Current Opinion in Plant Biology. 2008;11:293–300. doi: 10.1016/j.pbi.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Meng M, Geisler M, Johansson H, Mellerowicz EJ, Karpinski S, Kleczkowski LA. Differential tissue/organ-dependent expression of two sucrose- and cold-responsive genes for UDP-glucose pyrophosphorylase in Populus. Gene. 2007;389:186–195. doi: 10.1016/j.gene.2006.11.006. [DOI] [PubMed] [Google Scholar]
- O'Malley DM, Porter S, Sederoff RR. Purification, characterization, and cloning of cinnamyl alcohol dehydrogenase in loblolly pine (Pinus taeda L.) Plant Physiology. 1992;98:1364–1371. doi: 10.1104/pp.98.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilate G, Chabbert B, Cathala B, Yoshinaga A, Leple JC, Laurans F, Lapierre C, Ruel K. Lignification and tension wood. Comptes Rendus Biologies. 2004;327:889–901. doi: 10.1016/j.crvi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Plomion C, Leprovost G, Stokes A. Wood formation in trees. Plant Physiology. 2001;127:1513–1523. [PMC free article] [PubMed] [Google Scholar]
- Raes J, Rohde A, Christensen JH, Van de Peer Y, Boerjan W. Genome-wide characterization of the lignification toolbox in Arabidopsis. Plant Physiology. 2003;133:1051–1071. doi: 10.1104/pp.103.026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond TA, Somerville CR. The cellulose synthase superfamily. Plant Physiology. 2000;124:495–498. doi: 10.1104/pp.124.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LA, Dubos C, Cullis IF, Surman C, Poole M, Willment J, Mansfield SD, Campbell MM. Light, the circadian clock, and sugar perception in the control of lignin biosynthesis. Journal of Experimental Botany. 2005;56:1651–1663. doi: 10.1093/jxb/eri162. [DOI] [PubMed] [Google Scholar]
- Sandermann H. Ozone/biotic disease interactions: molecular biomarkers as a new experimental tool. Environmental Pollution. 2000;108:327–332. doi: 10.1016/s0269-7491(99)00211-0. [DOI] [PubMed] [Google Scholar]
- Savidge RA. Intrinsic regulation of cambial growth. Journal of Plant Growth Regulation. 2001;20:52–77. [Google Scholar]
- Searson MJ, Thomas DS, Montagu KD, Conroy JP. Wood density and anatomy of water-limited eucalypts. Tree Physiology. 2004;24:1295–1302. doi: 10.1093/treephys/24.11.1295. [DOI] [PubMed] [Google Scholar]
- Sheriff D, Whitehead D. Photosynthesis and wood structure in Pinus radiata D. Don during dehydration and immediately after rewatering. Plant, Cell and Environment. 2006;7:53–62. [Google Scholar]
- Shi R, Sun Y-H, Li Q, Heber S, Sederoff R, Chiang VL. Towards a systems approach for lignin biosynthesis in Populus trichocarpa: transcript abundance and specificity of the monolignol biosynthetic genes. Plant and Cell Physiology. 2010;51:144–163. doi: 10.1093/pcp/pcp175. [DOI] [PubMed] [Google Scholar]
- Somerville C. Cellulose synthesis in higher plants. Annual Review of Cell and Developmental Biology. 2006;22:53–78. doi: 10.1146/annurev.cellbio.22.022206.160206. [DOI] [PubMed] [Google Scholar]
- Sterky F, Bhalerao RR, Unneberg P, et al. A Populus EST resource for plant functional genomics. Proceedings of the National Academy of Sciences, USA. 2004;101:13951–13956. doi: 10.1073/pnas.0401641101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Li LG, Sun YH, Chiang VL. The cellulose synthase gene superfamily and biochemical functions of xylem-specific cellulose synthase-like genes in Populus trichocarpa. Plant Physiology. 2006;142:1233–1245. doi: 10.1104/pp.106.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NG. Cellulose biosynthesis and deposition in higher plants. New Phytologist. 2008;178:239–252. doi: 10.1111/j.1469-8137.2008.02385.x. [DOI] [PubMed] [Google Scholar]
- Tyree M, Davis S, Cochard H. Biophysical perspectives of xylem evolution: is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction. IAWA Journal. 1994;15:335–360. [Google Scholar]
- Updegraff DM. Semi micro determination of cellulose in biological materials. Analytical Biochemistry. 1969;32:420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3:34. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanholme R, Morreel K, Ralph J, Boerjan W. Lignin engineering. Current Opinion in Plant Biology. 2008;11:278–285. doi: 10.1016/j.pbi.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Vazquez-Cooz I, Meyer RW. A differential staining method to identify lignified and unlignified tissues. Biotechnic & Histochemistry. 2002;77:277–282. doi: 10.1080/bih.77.5-6.277.282. [DOI] [PubMed] [Google Scholar]
- Washusen R, Ades P, Vinden P. Tension wood occurrence in Eucalyptus globulus Labill. I. The spatial distribution of tension wood in one 11-year-old tree. Australian Forestry. 2002;65:120–126. [Google Scholar]
- Wilson BF, Archer RR. Reaction wood: induction and mechanical action. Annual Review of Plant Physiology and Plant Molecular Biology. 1977;28:23–43. [Google Scholar]
- Wittig VE, Ainsworth EA, Long SP. To what extent do current and projected increases in surface ozone affect photosynthesis and stomatal conductance of trees? A meta-analytic review of the last 3 decades of experiments. Plant, Cell and Environment. 2007;30:1150–1162. doi: 10.1111/j.1365-3040.2007.01717.x. [DOI] [PubMed] [Google Scholar]
- Wittig VE, Ainsworth EA, Naidu SL, Karnosky DF, Long SP. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: a quantitative meta-analysis. Global Change Biology. 2009;15:396–424. [Google Scholar]
- Xu F, Sun RC, Lu Q, Jones GL. Comparative study of anatomy and lignin distribution in normal and tension wood of Salix gordejecii. Wood Science and Technology. 2006;40:358–370. [Google Scholar]
- Zanne AE, Westoby M, Falster DS, Ackerly DD, Loarie SR, Arnold SEJ, Coomes DA. Angiosperm wood structure: global patterns in vessel anatomy and their relation to wood density and potential conductivity. American Journal of Botany. 2010;97:207–215. doi: 10.3732/ajb.0900178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.