Abstract
Nepenthes pitchers are specialized leaves that function as insect traps. Several pitcher components may contribute to trapping, including the pitcher fluid, slippery wax crystals and downward-pointing epidermal cells on the inner pitcher wall, and the wetness-dependent pitcher rim (peristome), but the relative importance of these traits is unclear. Mechanisms of prey capture and retention in the field were investigated by quantifying the effect of ‘knock-out’ manipulations of individual pitcher structures, and by testing the ability of pitcher fluids and water to retain insects. Two forms of Nepenthes rafflesiana Jack (‘elongate’ and ‘typical’) with contrasting combinations of pitcher traits were compared. Wax crystals on the inner pitcher wall were found to be the most important trapping structure in the elongate form, whereas the typical form relied primarily on the peristome. The pitcher fluids of both forms, differing markedly in the degree of viscoelasticity, retained significantly more ants than water. The present results show that pitcher plants utilize several mechanisms for prey capture and retention, varying in efficiency and relative importance between forms. It is proposed that these differences represent alternative prey capture strategies that may provide a mechanism to reduce competition and facilitate species co-existence in nutrient-limited habitats.
Keywords: Capture mechanism, carnivorous plants, functional morphology, insect aquaplaning, Nepenthes, plant–insect interactions, trait divergence, wax crystals
Introduction
Nepenthes pitcher plants are a highly diverse genus of carnivorous vines distributed across Southeast Asia with a few outlying species in Madagascar, the Seychelles, and New Caledonia (Juniper et al., 1989). Similar to other carnivorous plants, they typically grow in extremely nutrient-poor habitats such as heath forests (kerangas), peat swamp forests, and montane forests. All Nepenthes species bear conspicuous jug-shaped pitchers on the tips of their leaves. These are complex organs which are specialized to attract, capture, retain, and digest mainly arthropod prey. Most species produce two distinct types of pitchers during their ontogeny. The leaves of young plants form compact rosettes and bear ovoid pitchers with straight tendrils. These ‘lower pitchers’ usually rest on the ground. In contrast, mature plants have climbing stems with long internodes and produce funnel-shaped ‘upper pitchers’ with curled tendrils that provide attachment to the surrounding vegetation.
Arthropods are attracted to the traps by means of optical and olfactory cues and secretion of sugary nectar (Joel et al., 1985; Juniper et al., 1989; Moran, 1996; Di Giusto et al., 2008, 2010). Recent findings on Sarracenia purpurea L. (Bennett and Ellison, 2009) suggest that nectar constitutes the most important attractant, at least for ants, the most common prey of Nepenthes pitchers (Jebb, 1991; Moran, 1996; Adam, 1997). Nectar is secreted from extrafloral nectaries (EFNs) on the tendril, the outside of the pitcher, the pitcher lid, and the inner margin of the pitcher rim (peristome) where the EFNs are largest and most densely packed (Merbach et al., 2001).
Several trapping mechanisms have been described for Nepenthes pitchers, including slippery wax crystals on the inner pitcher wall, ‘aquaplaning’ on the fully wettable peristome, and a direction-dependent surface topography of the inner wall. Until recently, the slippery wax crystals were considered the most important component for trapping (Knoll, 1914; Lloyd, 1942; Juniper and Burras, 1962; Gaume et al., 2002). These minute crystals not only minimize the available contact area for insect feet (Scholz et al., 2010), but they also break off easily and thereby contaminate the adhesive pads (Juniper and Burras, 1962; Gaume et al., 2004; Gorb et al., 2005).
The second main trapping mechanism, ‘aquaplaning’ on the peristome, was overlooked for many years, probably because it depends on activation by wetness (Bohn and Federle, 2004; Bauer et al., 2008). Unlike most plant surfaces, the peristome is highly wettable, leading to the formation of thin water films under humid conditions. These water films prevent insects’ adhesive pads from making close contact with the surface, thereby causing them to slip (Bauer and Federle, 2009).
Other pitcher components that have been reported to play a role in prey capture and retention include downward-pointing epidermal cells on the inner pitcher wall and the digestive fluid which fills the bottom part of the pitcher. The downward-pointing cells create a direction-dependent surface topography that provides no grip for claws of insects trying to climb out of the pitcher (Lloyd, 1942; Gorb et al., 2004). They might also cause sliding legs to vibrate, thereby leading to detachment (Knoll, 1914).
The digestive pitcher fluid has viscoelastic properties in some species, including the typical form of Nepenthes rafflesiana Jack (Gaume and Forterre, 2007). Fluid viscoelasticity was found to aid prey retention by causing moving insects to sink, because the elastic relaxation time of the fluid exceeds the typical time scale of insect movements (Gaume and Forterre, 2007). The viscoelastic fluid of N. inermis Danser has been reported to promote a flypaper trapping mechanism by covering the inner pitcher wall completely with a sticky fluid film (Clarke, 1997a, 2001).
While most species possess peristomes and downward-pointing cells, wax crystals and viscoelastic fluids are absent in a number of species (McPherson et al., 2009; Moran et al., 2010). Nevertheless, most of these species successfully trap arthropods (Adam, 1997; Cresswell, 1998; Bohn and Federle, 2004; Bauer et al., 2009). The details of how different capture mechanisms and functional pitcher zones contribute to prey capture under natural conditions are still unknown. Nepenthes rafflesiana represents an ideal study system to address this question as it occurs in several morphologically distinct forms with contrasting pitcher traits (wax crystals present versus absent, fluid viscoelasticity low versus high; see the detailed description in the Materials and methods). Thus, the function of alternative trap designs can be investigated within the same species, eliminating many uncertainties that would arise from interspecific comparisons.
So far, the function of pitcher components has mainly been inferred from their surface microstructure or deduced from laboratory observations of insects placed on pitchers (Knoll, 1914; Juniper and Burras, 1962; Gaume et al., 2002). The fact that the peristome ‘aquaplaning’ mechanism remained undiscovered in laboratory experiments (on dry pitchers), however, demonstrates that pitcher function can be radically different in the field. Moreover, the biological relevance of individual trapping mechanisms cannot be fully clarified by tests with selected insects but requires the study of prey capture under natural conditions. Therefore, this study aims to compare the contribution of individual trapping mechanisms towards prey capture in the field. The experiments test (i) which pitcher components are most important for natural prey capture; (ii) how the presence or absence of wax crystals affects Nepenthes pitchers; and (iii) whether viscoelastic pitcher fluid is more effective for prey retention than watery pitcher fluid or water.
Materials and methods
Study species and field sites
All experiments were conducted during two consecutive field trips in March–May 2008 and February–March 2009. Two morphologically and ecologically distinct forms of N. rafflesiana in Brunei, Northern Borneo, were investigated. Nepenthes rafflesiana is found in lowland habitats throughout Peninsular Malaysia, Sumatra, and Borneo (Clarke, 1997b). The funnel-shaped upper pitchers of the typical form (Fig. 1A) usually lack wax crystals on the inner wall but have a highly viscoelastic fluid, especially when young (Bauer et al., 2009; Gaume and Di Giusto, 2009). They attract potential prey by means of copious nectar secretion from glands lining the inner margin of the peristome, as well as flower-like sweet fragrance (Di Guisto et al., 2010) and UV reflectance patterns (Moran, 1996; Moran et al., 1999). In contrast, the long and slim pitchers of the elongate form (Fig. 1B) are characterized by a well-developed wax crystal layer, mostly watery pitcher fluid, little nectar secretion and odour, and a lack of UV patterns (Moran et al., 1999; UB, unpublished observation). The third form that is found in Brunei, the giant form, superficially resembles the typical form, but the pitchers are ∼2–3 times as large and usually odourless. This form is relatively rare and therefore is not included in the present study.
Fig. 1.
The two forms of N. rafflesiana used in this study. (A) Typical form, upper pitcher. The pitchers are ∼10–15 cm long, lack wax crystals on the inner pitcher wall, and contain viscoelastic fluid. (B) Elongate form, upper pitcher. The pitchers are approximately twice as long as pitchers of the typical form, have a well-developed wax crystal layer on the inner pitcher wall, and mostly contain watery fluid. (This figure is available in colour at JXB online.)
The taxonomic status of the different N. rafflesiana forms is unresolved, and the only currently accepted variety is N. rafflesiana var. elongata Hort. (i.e. the elongate form). However, all three forms form stable populations of distinct morphology and ecology in Brunei, indicating that they are largely reproductively isolated from each other. In the absence of a consistent nomenclature, the forms are referred to as ‘typical form’ and ‘elongate form’ throughout this paper.
The experiments on the typical form of N. rafflesiana took place at a site of degraded tropical heath forest (kerangas) in the Tutong district of Brunei (04°44.643' N, 114°35.888' E). The site is characterized by open vegetation dominated by ferns and shrubs, interspersed with small trees. Ant-plants and carnivorous plants are common throughout the site, comprising members of the myrmecophytic genera Lecanopteris, Myrmecodia, and Dischidia and the carnivorous genera Drosera, Utricularia, and Nepenthes. Three species of pitcher plants are found: N. rafflesiana (typical form), N. gracilis Korth., and N. albomarginata T. Lobb ex Lindl.
The second field site was located near the town of Labi in the Belait district (04°29.786' N, 114°27.591' E). Unfortunately this site has been cleared for development purposes in January 2010 and the vegetation was completely destroyed. The site consisted of a degraded peat swamp forest edge habitat where all mature trees had been logged. Patches of forest re-growth created a mosaic of dense thickets and open fern- and sedge-dominated vegetation. Four Nepenthes species occurred in this site: N. rafflesiana (elongate and giant form), N. bicalcarata Hook., N. ampullaria Jack, and N. gracilis.
Experiments on the contribution of individual trap components towards natural capture success
Two series of experiments in each field season were conducted simultaneously on both forms of N. rafflesiana. Individual trap components of 219 upper pitchers (typical form, n=126, elongate form, n=93) were experimentally disabled and natural prey capture success was monitored over 12–16 d following the manipulation. Each experimental pitcher was on a different plant and between 5 d and 8 d old (counted from the day of opening). Experimental treatments were randomly assigned to pitchers. On each pitcher, one of three manipulations was performed. A fourth group of pitchers remained untreated as a control.
Pitcher surfaces potentially involved in prey capture (peristome, inner pitcher wall, and underside of the pitcher lid) were rendered non-slippery by coating them with a non-toxic, transparent, and odourless poly(dimethyl siloxane) (PDMS) elastomer (Sylgard™ 184, Dow Corning, Midland, MI, USA). The two components of the elastomer were mixed immediately before applying them with a paint brush. The liquid elastomer formed a smooth layer of ∼0.5 mm thickness and polymerized completely within a few minutes. The resulting surface was hydrophobic and not slippery for insects under both dry and wet conditions. The manipulation had no negative effect on the pitchers even when large surface areas were coated with Sylgard 184. Both manipulated and control pitchers were still in good condition by the end of the experiment.
The innermost 3 mm of the peristome were left uncoated to ensure that nectar secretion and thus prey attraction was not affected. In addition to sugary nectar as a direct reward, sweet scent (Di Guisto et al., 2010) and UV reflection patterns (Moran, 1996) are held responsible for the major role of the peristome for prey attraction. As PDMS is transparent for wavelengths >300 nm (Xia and Whitesides, 1998), making the coatings almost invisible to the human eye, it is unlikely that attractive optical cues were affected. The strong correlation between the rate of nectar secretion and the perceived intensity of pitcher odour (Bauer et al., 2009) together with the observation that the nectar itself, when collected in glass vials, emits a scent similar to that of the pitchers (UB, unpublished observation) suggests that most of the attractive volatiles are secreted with the nectar. This assumption was confirmed by the unaltered sweet scent of the manipulated pitchers. Numerous ants and flying insects were observed visiting SylgardTM-coated peristomes and harvesting nectar, confirming the low impact of the treatment on insect attraction.
Immediately before the manipulations, all prey was removed from the pitchers by sucking out the digestive fluid using a 20 ml syringe with an attached silicon tube. The prey was removed by filtering the fluid through a plastic gauze mesh of ∼1 mm pore size. If the fluid contained large amounts of suspended organic matter, it was additionally filtered through a Nuclepore™ track-etch membrane filter (25 mm diameter, 12 μm pore size, Dow Corning). The pitcher interior was rinsed with filtered rain water until all prey was removed, and a polyurethane ear plug (Pura-Fit Moldex 7700, Moldex-Metric, Walddorfhäslach, Germany) was inserted to prevent prey from getting stuck in the elongated, tapered bottom end of the pitcher.
Following the manipulation, prey from all pitchers was collected every 3 d and counted. The same person (UB) sampled both experimental sites on consecutive days. The short sampling intervals ensured that captured prey was not too heavily decomposed and could still be reliably identified and counted. Prey sampling was repeated four or five times per set-up. Overall prey numbers for each pitcher were normalized for the number of days, and daily prey capture was then used for further analysis. The prey capture of pitchers in each experimental group was tested against the untreated control group for each of the two N. rafflesiana forms.
It was originally intended to test the effect of the pitcher fluid in the same experiment by replacing the digestive fluid with water. Preliminary tests (see Supplementary data and Supplementary fig. S1 available at JXB online) showed, however, that even repeated thorough rinsing of the pitcher interior was not sufficient to remove all remnants of the polysaccharide responsible for the viscoelastic properties of the fluid (Gaume and Forterre, 2007). The retentive capacity of the different pitcher fluids in comparison with water was therefore tested in a separate, semi-artificial experiment in glass vials (see below).
Experiments on insect-attracting properties in both forms:
Nectar secretion (in sucrose equivalents) from the peristome was quantified in eight pitchers of each form over the course of 12 d. Prior to the experiment, the peristome surface of each pitcher was thoroughly rinsed with distilled water to remove all nectar. The pitchers were then enclosed in fine-mesh gauze bags and roofed with transparent plastic sheets to protect the nectar from foraging insects and rain. In addition, a small amount of a sticky insect trap coating (Tangle-trap™, The Tanglefoot Company, Grand Rapids, MI, USA) was applied to the tendril to exclude crawling insects.
Nectar was sampled every other day by gently wiping the peristome with a wet, ∼1 cm2 laboratory paper cleaning tissue (Kimwipe™, Kimberley-Clark, Reigate, UK) and absorbing all moisture with small highly absorbing cotton sponges (Sugi™ swabs, Kettenbach Medical, Eschenburg, Germany). Both Kimwipes™ and Sugi™ swabs were handled with clean forceps and collected in Eppendorff™ tubes. The samples were dried over silica gel, re-diluted in 0.2–0.5 ml of distilled water, and the liquid measured using a temperature-compensated handheld refractometer (ATAGO, L. Kübler, Karlsruhe, Germany). Clean Kimwipes™ and Sugi™ swabs were measured as a control and were found to contain no sugar.
Retention experiments.
Pitcher fluid from fully inflated but not yet opened N. rafflesiana pitchers (typical and elongate form, upper pitchers only) was sampled on two consecutive days in the field. Samples were divided into three groups according to N. rafflesiana form and fluid viscoelasticity: (i) typical form, high viscoelasticity (this fluid usually forms long filaments of >30 cm length when pulled apart between fingertips); (ii) elongate form, medium viscoelasticity (forming shorter filaments of 1–10 cm length); and (iii) elongate form, watery (no visible filaments). Fluid samples from 3–5 pitchers per group were pooled and 10 ml of each combined sample was transferred to clean 25 ml glass vials (height=50 mm, diameter=25 mm).
For the retention experiments, three different ant species [Crematogaster sp., body length ∼3–5 mm; Camponotus (Colobopsis) cf. saundersi Emery, ∼8–12 mm; and Anoplolepis gracilipes Smith, ∼5–6 mm] were used, all of which were commonly found as prey in Nepenthes pitchers. Partial colonies of these ants were collected a few days prior to the experiments and kept in plastic containers with the walls coated with slippery Fluon™ (Whitford, Diez, Germany). Retention experiments were conducted under natural climatic conditions over 7 d, starting on the second day of fluid sample collection. Forty ants of each species were tested, 10 in each of the fluids. The ant species were tested in consecutive order and the fluid samples simultaneously.
Each ant was placed in a 1.5 ml Eppendorff™ vial with Fluon™-coated inner walls and dropped from 3 cm height onto the fluid surface in the open glass vial. Only ants that landed completely on the fluid surface were taken into account. Each ant was observed for a maximum of 10 min until one of four possible outcomes was reached: (i) escaped; (ii) swimming actively at the fluid surface; (iii) floating motionless; or (iv) ‘sunken’. Any ant that had managed to leave the fluid completely was counted as escaped. Ants that were moving at the fluid surface after 10 min of observation were counted as ‘swimming’. Ants in the category ‘floating’ were not necessarily dead but were generally inactive and did not react to stimulation by gently blowing at them. ‘Sunken’ ants were completely submerged and in the process of sinking, or had sunk to the bottom of the vial.
The surface tension of pitcher fluids from unopened pitchers of both forms of N. rafflesiana was measured at ∼20 °C using the capillary rise method. To this end, 10 μl micropipettes (BLAUBRAND™ intraMARK, Brand, Wertheim, Germany) were held vertically in a custom-built holder over a glass vial containing the fluid sample. The tip of the capillary was positioned just below the fluid surface and a minimum time of 2 h (6 h in the case of viscoelastic fluid) was allowed for the fluid to rise in the capillary. The height of the meniscus was then measured with a calliper. Distilled water was measured as a reference.
Experiments on the retentive property of the inner pitcher wall.
To investigate the retention efficiency of the inner wall surface in both forms of N. rafflesiana, five pitchers of each form were tested in the field. Immediately before the experiment, the pitcher fluid was removed using a syringe with an attached silicon tube, and some tissue paper was stuffed into the bottom end of the pitchers (without touching the pitcher wall) to create a safe ‘starting platform’ for ants. Ten Camponotus (Colobopsis) cf. saundersi ants were dropped into each pitcher and observed, either until all ants had escaped, or up to a maximum of 6 h. All experiments were started between 11:00 and 12:30.
As the inner wall of pitchers without wax crystals is relatively hydrophilic (Gorb et al., 2004), the degree of surface wetness on both the inner wall and the peristomes in three N. rafflesiana (typical form) pitchers was measured continuously over a period of 2 weeks in the field. Surface wetness was monitored through the electrical resistance between two small electrodes (distance=10 mm) attached to the pitcher wall using small magnets. A second pair of electrodes was attached to the inner and outer edge of the peristome. Resistance was measured with a custom-built circuit and recorded every 30 s to a μLog VL 100S data logger (a.b.i. data, Brussels, Belgium). For a detailed description of the method, see Bauer et al. (2008, 2009). In addition to surface wetness, relative air humidity and temperature were recorded with a Tinytag TGP-4500 data logger (Gemini Data Loggers, Chichester, UK), and precipitation was measured with a tipping bucket rain gauge (Davis Instruments, Hayward, CA, USA) connected to a Tinytag TGPR-1201 data logger (Gemini Data Loggers).
To investigate the effects of surface topography and wetting of the wax-free inner wall surface in the typical form, an additional running experiment with Crematogaster inflata Smith ants was performed. A cylindrical segment of 3.5 cm height was excised from a freshly harvested pitcher (cf. Gaume et al., 2002). The segment comprised the pitcher wall from just above the fluid layer up to 5 mm below the lowest point of the peristome. The inner wall segment was tested under four different conditions: (i) natural orientation, dry; (ii) inverted orientation, dry; (iii) natural orientation, wetted; and (iv) inverted orientation, wetted. The experiments on the dry cylinder were performed first. Filtered rain water was used for surface wetting. The cylinder was rinsed thoroughly until the inner surface was completely wetted. This treatment was repeated before every running test.
For each condition, 30 ants were tested individually by putting the cylinder over an ant while it was running on a level surface. Each ant was only used once. As all ants managed to escape, the time between the first step onto the inner pitcher wall surface and the arrival at the top of the cylinder was recorded, using a stopwatch. If an ant returned to the bottom before reaching the upper rim, the attempt was discarded and the stopwatch was restarted when the ant started to climb up again.
Data analysis.
Statistical comparisons of results were conducted using the software packages BiAS for Windows 8.6 (Epsilon Verlag GmbH, Frankfurt, Germany) and SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA). P-values below a level of α=0.05 were considered significant. Pitcher manipulations were tested against the respective control group using Kruskal–Wallis H tests with selected post-hoc Dunn comparisons, where P-values are corrected for multiple testing using the Bonferroni–Holm procedure (Holm, 1979). The effects of fluid type and ant species on retentive capacity were tested using hierarchical log-linear analysis. In a second step, the different pitcher fluids used in the retention experiments were tested against water as a control using separate Craddock–Flood χ2 tests with Bonferroni correction for each tested ant species.
Results
The two forms of N. rafflesiana rely on different trap components for prey capture
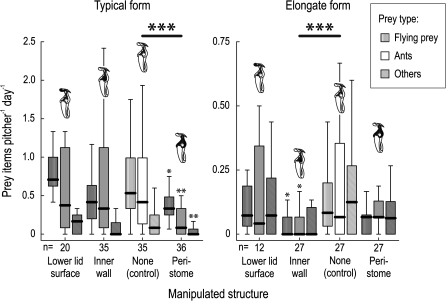
The typical and elongate forms of N. rafflesiana are not only different in outer appearance and trapping-related pitcher traits but they also rely on different pitcher structures to capture their prey (Fig. 2). The typical form captured significantly less prey when the trapping function of the peristome was disabled (Kruskal–Wallis H test with post-hoc Dunn comparisons, for sample sizes see Fig. 2, Z=4.23, P <0.001). Within each individual functional group of prey (flying insects, ants, and other arthropods), the effect of the peristome ‘knock-out’ was still significant (flying insects, Z=2.74, P=0.019; ants, Z=3.07, P=0.006; other arthropods, Z=3.40, P=0.002). None of the other manipulations had a significant effect on prey capture in the typical form.
Fig. 2.
Effect of ‘knock-out’ manipulations of individual pitcher components on the natural prey capture success of N. rafflesiana pitchers (typical and elongate form). The effect of each manipulation (grey boxes) was compared with a control group (white boxes). The plot shows medians (centre lines), interquartile ranges (boxes), and the largest and smallest values (whiskers) that are not outliers, separately for the three major functional groups of prey (flying insects, ants, and other arthropods). Large asterisks denote significant differences in overall prey numbers, while small asterisks mark values for individual prey groups that differ significantly from the respective control value (*P <0.05; **P <0.01; ***P <0.001).
In contrast, ‘knock-out’ of the peristome surface had no significant effect on prey capture in the elongate form (Kruskal–Wallis H test with post-hoc Dunn comparisons, for sample sizes see Fig. 2, Z=1.53, P=0.25), despite the fact that the peristome of this form is a fully functional trapping surface (cf. Supplementary data and Supplementary Fig. S2 at JXB online). Instead, the manipulation of the waxy inner pitcher wall caused a highly significant reduction of overall prey numbers (Z=3.91, P <0.001). This reduction was still significant for flying insects (Z=2.87, P=0.012) and ants (Z=2.79, P=0.016), but not for other arthropods (Z=2.27, P=0.069). In both forms, the manipulation of the underside of the pitcher lid had no effect on prey capture.
Prey numbers and nectar secretion differ between both forms
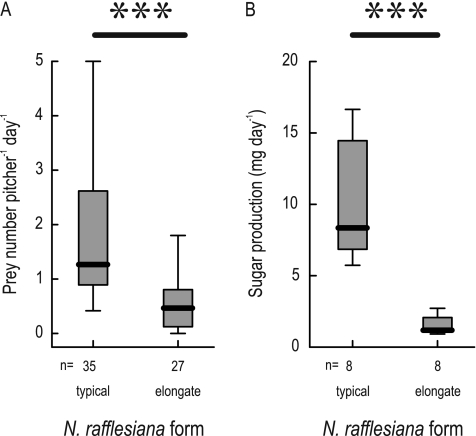
Apart from the dominance of different trapping structures, both forms of N. rafflesiana also differed in overall prey numbers, as visible from the control pitchers (Mann–Whitney U-test, ntypical=35, nelongate=27, U=123.5, P <<0.001; see Fig. 2). The median number of prey items per day was 2.7 times higher in the typical than in the elongate form (Fig. 3A). This is not due to differences in the size of the pitcher opening: the pitcher width at the peristome was not significantly different between the forms (two-sample t-test, mean pitcher width: typical=3.6 cm, elongate=3.2 cm, n=10 pitchers each, df=18, t=1.20, P=0.25). Consistent with previous results, prey capture rates varied drastically between individual pitchers and sample intervals (Bauer et al., 2009). Despite the different trapping frequency, both forms of N. rafflesiana captured similar proportions of flying insects (typical, 22%; elongate, 24%), ants (typical, 36%; elongate, 37%), and other flightless prey (typical, 42%; elongate, 39%; χ2 test of overall prey numbers, n=1644 prey from 35 typical and 27 elongate pitchers; χ2=0.90, df=2, P=0.64). There was a highly significant difference in nectar secretion between both forms (Mann–Whitney U-test, ntypical=8, nelongate=8, U=0, P <0.001; Fig. 3B). Typical pitchers secreted almost seven times as much sugar per day as elongate pitchers.
Fig. 3.
Average daily prey capture (A) and nectar production (B) by unmanipulated pitchers of both forms of N. rafflesiana. The plot shows medians (centre lines), interquartile ranges (boxes), and the largest and smallest values (whiskers) that are not outliers.
Pitcher fluids retain prey more efficiently than water
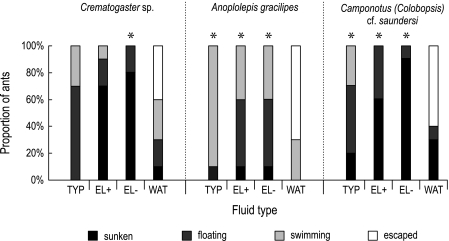
The only fluid from which any of the tested ant species managed to escape was water. All tested pitcher fluids (typical form, highly viscoelastic; elongate form, medium and watery) were 100% efficient in retaining any of the tested ants. All pitcher fluids were significantly different from water, except for Crematogaster sp., where only the watery fluid of the elongate form differed significantly from water (post-hoc χ2 tests, Bonferroni-corrected, n=20, df=3, see Fig. 4).
Fig. 4.
Retention rate for three ant species dropped into glass vials filled with three types of N. rafflesiana pitcher fluid and pure water (TYP=typical form, highly viscoelastic; EL+=elongate form, moderately viscoelastic; EL– =elongate form, watery; WAT=water, see Materials and methods). Each bar represents 10 ants. Asterisks (*P <0.05) indicate significant differences between pitcher fluids and the control (water).
The three different ant species differed in their ability to escape from water and to stay afloat in the different fluids. Hierarchical log-linear analysis showed highly significant partial associations for both fluid×outcome (n=120, df=9, partial χ2=91.22, P <0.001) and ant×outcome (n=120, df=6, partial χ2=42.5, P <0.001). The strong association between fluid and outcome is based on ants sinking earlier and more quickly in the non-viscous fluid of the elongate form.
Ants only sank once they were fully wetted and were no longer held at the fluid surface by surface tension. Wetting was clearly facilitated when the ant was actively swimming. The surface tension for the pitcher fluid of both forms was similar to that of distilled water (typical form, 72.07±0.68 mN m−1; n=9; elongate form, 73.0±0.85 mN m−1; n=8; water, 72.0 mN m−1). The density of the pitcher fluids was similar to that of water (998 mg ml−1) for the elongate form (994.2±5.2 mg ml−1, n=6) and slightly lower for the typical form (982.8±2.6 mg ml−1, n=9).
Wetness-based slipperiness of the inner wall in the typical form of N. rafflesiana
When testing the retention efficiency of the inner pitcher wall surface in both forms of N. rafflesiana, none of the dropped ants was able to climb up the wax crystal layer of the elongate form, indicating that this surface provides a highly efficient escape barrier. For the wax-free typical form, however, the results are less clear. Observations confirmed that at most times during the day, ants and other arthropods could run without difficulty on the inner pitcher wall. However, at night-time when humidity levels were high, insects were repeatedly witnessed to slip on the inner wall surface. In the slippery conditions, no individual droplets were visible on the surface, suggesting that it was covered by a homogeneous water film similar to that on the peristome.
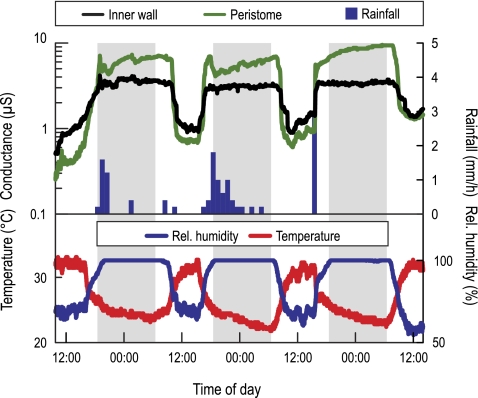
Continuous measurements of surface wetness on the inner wall and the peristome in three N. rafflesiana (typical form) pitchers revealed that the inner pitcher wall shows the same diurnal fluctuations of surface wetness as the peristome, which are strongly influenced by air humidity and rainfall (Fig. 5, cf. Bauer et al., 2008). The changes in wetness appeared to be less pronounced on the inner wall than on the peristome, probably as a result of the less exposed position.
Fig. 5.
Diurnal variation of surface wetness measured on the peristomes and inner walls of three N. rafflesiana (typical form) pitchers (upper graph, curves represent means) in parallel with rainfall (bars), air humidity, and temperature (lower graph). High electrical conductance indicates wetness. (This figure is available in colour at JXB online.)
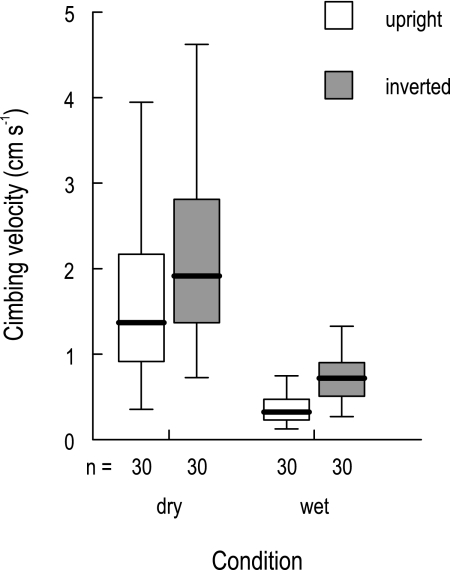
In the running experiments on the inner wall of a cylindrical pitcher segment of N. rafflesiana (typical form), all C. inflata ants managed to escape, but the time needed to reach the top was strongly dependent on both orientation (upright versus inverted) and surface wetness (Fig. 6). Most of the variation was explained by wetness, with ants climbing significantly more slowly on the wet inner wall [two-way analysis of variance (ANOVA) on log-transformed data, Table 1]. However, the effect of orientation was also highly significant, with upright segments being significantly more difficult to climb. These results indicate that both wetness-based slipperiness and the anisotropic surface topography of the inner pitcher wall surface contribute to effective prey retention by N. rafflesiana (typical form) pitchers.
Fig. 6.
Climbing velocity of Crematogaster inflata ants on an inner pitcher wall segment of N. rafflesiana (typical form) under four different conditions. The plot shows medians (centre lines), interquartile ranges (boxes), and the largest and smallest values (whiskers) that are not outliers.
Table 1.
Statistical results showing the influence of wetness and orientation on the retention efficiency of the inner pitcher wall of N. rafflesiana (typical form) for Crematogaster inflata ants (two-way ANOVA on log-transformed data)
| Factor | df | SS | MS | F | P |
| Surface wetness | 1 | 9.07 | 9.07 | 129.29 | <0.001 |
| Segment orientation | 1 | 1.57 | 1.57 | 22.32 | <0.001 |
| Wetness×orientation | 1 | 0.07 | 0.07 | 1.00 | NS |
| Residual | 116 | 8.14 | 0.07 | ||
| Total | 119 | 18.85 |
The daytime field experiments on the retention efficiency of the inner wall confirmed these observations. After being dropped into an empty pitcher, the ants immediately tried to run up the inner wall and escape from the pitcher. Under dry weather conditions, all ants had no problem climbing the inner wall and left the pitcher within the first 15 min of the experiment. In two instances, however, it rained during or immediately before the experiment. Under these circumstances, the ants slipped on the inner wall and were unable to climb up. Only 30–45 min after the rain had stopped and the sun had come out again, the inner wall gradually became less slippery and the ants were able to leave the pitcher.
Discussion
The role of the peristome and the inner pitcher wall
The present results show that both the peristome and the inner pitcher wall play an important role in prey capture by Nepenthes pitchers under natural conditions, and that both the pitcher fluid and inner wall contribute to successful prey retention. In the two forms of N. rafflesiana studied here, different pitcher structures were primarily responsible for prey capture. The peristome was shown to be most important for the typical form while the elongate form depended mainly on the waxy inner wall.
The negative, non-significant results for other pitcher structures, however, do not exclude that they might also contribute to prey capture, for several reasons. First, prey capture by Nepenthes is highly aggregated; that is, prey numbers between individual pitchers (and days) vary greatly (Bauer et al., 2009), rendering all but the largest effects insignificant. Secondly, statistical power was reduced by the low prey numbers in pitchers of the elongate form.
Although the effect of the peristome on prey capture in the elongate form was not significant, the peristomes of this form and other wax-bearing Nepenthes are fully wettable, and they can also trap insects by ‘aquaplaning’ (Supplementary Fig. S2 at JXB online; cf. Bohn and Federle, 2004). It is possible that the presence of wax crystals in the elongate form compensates at least partly for the peristome ‘knock-out’, thus attenuating the effect of this manipulation.
The manipulations of individual pitcher components did not differentiate between initial prey capture and retention. There are, however, indications that the peristome is more important for initial capture while the inner wall (and the fluid) plays a larger role for prey retention. In laboratory experiments on N. alata Blanco, the waxy inner wall was found to be responsible for prey capture only when the peristome was dry, while under wet conditions all captured ants fell from the peristome (Bohn and Federle, 2004). In this context, the elongate form pitcher with peristome ‘knock-out’ resembles the dry condition, where initial trapping occurs via the wax crystals.
In the wet condition, the peristome is a highly efficient trapping device, capturing much more prey than the wax crystals in the dry state (Bohn and Federle, 2004). Field measurements of surface wetness showed that peristomes of N. rafflesiana (typical form) were sufficiently wet to capture prey during at least 60% of the day (Bauer et al., 2008). Interestingly, typical form pitchers showed a non-significant trend towards fewer captures when the inner wall was ‘knocked out’, even though they do not possess a wax crystal layer. This suggests that wetness-based slipperiness may not be confined to the peristome, especially when the present observations of insects slipping on the wet inner wall of the typical form are considered.
The wax crystal layer on the inner pitcher wall of the elongate form of N. rafflesiana is a permanently effective escape barrier (see also Gaume and Di Guisto, 2009). In contrast, the glandular surface of the typical form was only observed to be slippery during rain or periods of high air humidity. This suggests that the inner wall retains insects more effectively in the elongate form. In both forms, retention is further enhanced by the pitcher fluid. High retention efficiency is most important at times of high capture rates. In the typical form, both the peristome and the inner pitcher wall become slippery when wet; hence prey capture and retention efficiency may be conveniently synchronized.
While the secretion of nectar and scent was not affected by the experimental manipulations, these attractive traits may explain the large difference in prey numbers between the two forms of N. rafflesiana, which was already reported by Moran (1996) and Gaume and Di Giusto (2009). Less copious secretion of sugar from the peristome nectaries of elongate form pitchers and the lack of UV patterns and sweet scent (both present in the typical form) should lead to a reduced attractiveness to visitors. Moreover, nectar on the peristome enhances wetting by condensation (Bauer et al., 2008). Therefore, the smaller amount of nectar in the elongate form could also shorten the time where the peristome is wet and thereby reduce trapping success.
The role of the pitcher fluid
All tested pitcher fluids were significantly more effective in retaining different-sized ants than water. This confirms the importance of the viscoelastic properties of the pitcher fluid for prey retention. Gaume and Forterre (2007) showed that insects could not escape even strongly diluted fluids of N. rafflesiana (typical form), even though the shear viscosity of these solutions was almost as low as that of water. However, the diluted fluid still exhibited a high resistance to stretching, suggesting that its extensional viscosity is the key parameter that mediates prey retention. It is likely that the fluid of the elongate form represents such a ‘dilute’ fluid; that is, it may have a shear viscosity similar to that of water but may still exhibit a high extensional viscosity. Observations made by the authors of this study indicate that the unknown polysaccharide causing the viscoelastic properties is also present in the elongate form, but in a much lower and variable concentration (so that fluid filaments were only visible in very few pitchers). Detailed measurements of the extensional viscosity in the watery pitcher fluid of the elongate form are required to test whether this parameter can indeed explain its high retention rate.
The more frequent sinking observed in the fluid of the elongate form cannot be explained by a lower surface tension or density, as both were similar to water (cf. Gaume and Forterre, 2007). Instead, the higher shear viscosity in the typical form may slow down the wetting of prey insects, thus causing them to remain at the surface for longer.
As the fluid of N. rafflesiana (typical form) has been shown to retain its full retention capacity even when diluted by a factor of up to 14 (Gaume and Forterre, 2007), the question arises as to why the plant produces such a high concentration of the relevant polysaccharide. The apparent viscosity in young N. rafflesiana (typical form) pitchers was found to decrease markedly over the first weeks after pitcher opening without affecting the prey capture rate (Bauer et al., 2009). It is possible that the initial polysaccharide concentration is just high enough to compensate the dilution by rain water over the pitcher lifetime. Further research should test whether the initial polysaccharide concentration is related to pitcher longevity.
Ecological relevance and evolutionary diversification of trapping mechanisms
The present results underline the ecological importance of both the peristome and inner pitcher wall (especially in the presence of wax crystals) for prey capture and retention. The dominance of a different trapping mechanism in each form of N. rafflesiana suggests that both forms pursue alternative strategies for prey capture. One possible explanation for the evolution of distinct capture strategies is that certain mechanisms might be more suitable for specific habitats than others. The peristome, for example, is only slippery when wet and might be less efficient in open and dry habitats, while the wax crystal layer is independent of wetness. However, the typical form of N. rafflesiana usually occurs in more open habitats than the elongate form, arguing against this hypothesis. It is nevertheless conceivable that other species have evolved trap adaptations in response to prevailing habitat conditions. For example, N. bicalcarata and N. ampullaria, both without wax crystals, typically grow in the forest understorey where humidity levels are high during most of the day (Phillipps et al., 2008). On the other hand, the peristome mechanism might also be favoured by variable wetness levels: intermittent trap activation could facilitate ant recruitment to the pitchers due to an increased survival rate of scout ants (cf. Bauer et al., 2008).
Alternative trapping mechanisms might also be more or less effective for specific prey types. For example, the ant species Polyrhachis pruinosa Mayr, one of the most frequent pitcher visitors in all field sites covered by this study, is exceptionally successful at escaping from the pitcher fluid. These ants frequently escape from N. bicalcarata pitchers which lack wax crystals (Bohn and Federle, 2004) or from pitchers of the typical form of N. rafflesiana, but are mostly unable to overcome the waxy inner wall of the elongate form (Gaume and Di Guisto, 2009).
Specializations for different prey spectra could help to avoid interspecific competition. As both forms of N. rafflesiana are rarely found together (Gaume and Di Guisto, 2009), their distinct pitcher morphologies might have evolved in response to prey competition with different syntopic Nepenthes species. Some support for interspecific competition for prey is provided by the findings of Moran et al. (1999) who report different frequencies of individual insect orders in the prey of syntopic N. rafflesiana (typical form) and N. gracilis plants, with the former capturing disproportionately more flying prey and the latter more ants. In contrast to the typical form of N. rafflesiana, N. gracilis has a well-developed wax crystal layer and a very narrow peristome (∼1 mm wide).
Significantly different prey spectra have been reported for a variety of Nepenthes species including examples of sympatric taxa (e.g. Adam, 1997; Clarke, 1997b; Moran et al., 2001). Some species growing in habitats with low arthropod densities have even evolved adaptations to obtain nutrients from mammalian faeces (Clarke et al., 2009; Chin et al., 2010; Grafe et al., 2011). The diversity of available prey (in the widest sense) may be mirrored by the extraordinary diversity of pitcher morphology across the genus, suggesting that multiple and strong selection pressures are acting on pitchers.
The pitcher morphology of the common ancestor of modern Nepenthes is unknown as fossil records are missing. However, the most basal modern species (N. pervillei Blume, N. distillatoria L., N. madagascariensis Poir., N. masoalensis Schmid-Holl., and N. khasiana Hook.; see Meimberg et al., 2001) tend to have rather simple, cylindrical pitchers with well-developed wax crystal layers and narrow, inconspicuous peristomes (McPherson et al., 2009). The reduction of the wax crystal layer and the presence of a viscoelastic pitcher fluid are traits that are not unique to N. rafflesiana but occur in a number of species across the genus Nepenthes. The size and shape of the peristome is equally variable: in some species it is massively enlarged (e.g. N. hurreliana Cheek & A.L.Lamb) while it is almost absent in others (e.g. N. inermis). The finding that differences in pitcher design might reflect different trapping strategies calls for a comparative analysis of pitcher morphology and function across the whole genus Nepenthes.
Supplementary data
Supplementary data and figures are available at JXB online.
Supplementary data. Experiment testing the feasibility of replacing N. rafflesiana pitcher fluid with water.
Figure S1. Retention rates for three different ant species in untreated water in comparison with water that had been in pitchers for either 10 min or 3 d, showing a clear effect of the pitchers on the retention efficiency of water.
Figure S2. Experimental comparison of the peristome trapping efficiency of both forms of N. rafflesiana, showing no difference between the forms.
Acknowledgments
The authors are grateful to Laurence Gaume for helpful comments on an earlier version of this manuscript. They also wish to thank Universiti Brunei Darussalam and the Brunei Forestry Department for granting permission to conduct field work. In Brunei, Harith Tinggal and family provided accommodation and extraordinary hospitality. This work was supported by an External Research Studentship from Trinity College Cambridge to UB and by a grant from the Leverhulme Trust (F/09 364/G to WF). The Mark Pryor Fund (Trinity College) and the Balfour Trust (Department of Zoology, University of Cambridge) funded UB's expenses of the 2008 field trip.
References
- Adam JH. Prey spectra of Bornean Nepenthes species (Nepenthaceae) in relation to their habitat. Pertanika Journal of Tropical Agricultural Science. 1997;20:121–134. [Google Scholar]
- Bauer U, Bohn HF, Federle W. Harmless nectar source or deadly trap: Nepenthes pitchers are activated by rain, condensation and nectar. Proceedings of the Royal Society B: Biological Sciences. 2008;275:259–265. doi: 10.1098/rspb.2007.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer U, Federle W. The insect-trapping rim of Nepenthes pitchers: surface structure and function. Plant Signaling and Behavior. 2009;4:1019–1023. doi: 10.4161/psb.4.11.9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer U, Willmes C, Federle W. Effect of pitcher age on trapping efficiency and natural prey capture in carnivorous Nepenthes rafflesiana plants. Annals of Botany. 2009;103:1219–1226. doi: 10.1093/aob/mcp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett KF, Ellison AM. Nectar, not colour, may lure insects to their death. Biology Letters. 2009;5:469–472. doi: 10.1098/rsbl.2009.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn HF, Federle W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proceedings of the National Academy of Sciences, USA. 2004;101:14138–14143. doi: 10.1073/pnas.0405885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Moran JA, Clarke C. Trap geometry in three giant montane pitcher plant species from Borneo is a function of tree shrew body size. New Phytologist. 2010;186:461–470. doi: 10.1111/j.1469-8137.2009.03166.x. [DOI] [PubMed] [Google Scholar]
- Clarke C. Another nice trip to Sumatra. Carnivorous Plant Newsletter. 1997a;26:4–10. [Google Scholar]
- Clarke C. Nepenthes of Borneo. Kota Kinabalu: Natural History Publications; 1997b. [Google Scholar]
- Clarke C. Nepenthes of Sumatra and Peninsular Malaysia. Kota Kinabalu: Natural History Publications; 2001. [Google Scholar]
- Clarke CM, Bauer U, Lee CC, Tuen AA, Rembold K, Moran JA. Tree shrew lavatories: a novel nitrogen sequestration strategy in a tropical pitcher plant. Biology Letters. 2009;5:632–635. doi: 10.1098/rsbl.2009.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell JE. Morphological correlates of necromass accumulation in the traps of an Eastern tropical pitcher plant, Nepenthes ampullaria Jack, and observations on the pitcher infauna and its reconstitution following experimental removal. Oecologia. 1998;113:383–390. doi: 10.1007/s004420050390. [DOI] [PubMed] [Google Scholar]
- Di Giusto B, Bessière J-M, Guéroult M, Lim LBL, Marshall DJ, Hossaert-McKey M, Gaume L. Flower-scent mimicry masks a deadly trap in the carnivorous plant Nepenthes rafflesiana. Journal of Ecology. 2010;98:845–856. [Google Scholar]
- Gaume L, Di Giusto B. Adaptive significance and ontogenetic variability of the waxy zone in Nepenthes rafflesiana. Annals of Botany. 2009;104:1281–1291. doi: 10.1093/aob/mcp238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaume L, Forterre Y. A viscoelastic deadly fluid in carnivorous pitcher plants. PLoS One. 2007;2:e1185. doi: 10.1371/journal.pone.0001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaume L, Gorb S, Rowe N. Function of epidermal surfaces in the trapping efficiency of Nepenthes alata pitchers. New Phytologist. 2002;156:479–489. doi: 10.1046/j.1469-8137.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- Gaume L, Perret P, Gorb E, Gorb S, Labat J-J, Rowe N. How do plant waxes cause flies to slide? Experimental tests of wax-based trapping mechanisms in three pitfall carnivorous plants. Arthropod Structure and Development. 2004;33:103–111. doi: 10.1016/j.asd.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Gorb E, Haas K, Henrich A, Enders S, Barbakadze N, Gorb S. Composite structure of the crystalline epicuticular wax layer of the slippery zone in the pitchers of the carnivorous plant Nepenthes alata and its effect on insect attachment. Journal of Experimental Biology. 2005;208:4651–4662. doi: 10.1242/jeb.01939. [DOI] [PubMed] [Google Scholar]
- Gorb E, Kastner V, Peressadko A, Arzt E, Gaume L, Rowe N, Gorb S. Structure and properties of the glandular surface in the digestive zone of the pitcher in the carnivorous plant Nepenthes ventrata and its role in insect trapping and retention. Journal of Experimental Biology. 2004;207:2947–2963. doi: 10.1242/jeb.01128. [DOI] [PubMed] [Google Scholar]
- Grafe TU, Schöner CR, Kerth G, Junaidi A, Schöner MG. A novel resource–service mutualism between bats and pitcher plants. Biology Letters. 2011 doi: 10.1098/rsbl.2010.1141. doi:10.1098/rsbl.2010.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Jebb M. An account of Nepenthes in New Guinea. Science in New Guinea. 1991;17:7–54. [Google Scholar]
- Joel DM, Juniper BE, Dafni A. Ultraviolet patterns in the traps of carnivorous plants. New Phytologist. 1985;101:585–593. [Google Scholar]
- Juniper BE, Burras JK. How pitcher plants trap insects. New Scientist. 1962;13:75–77. [Google Scholar]
- Juniper BE, Robins RJ, Joel DM. The carnivorous plants. London: Academic Press; 1989. [Google Scholar]
- Knoll F. Über die Ursache des Ausgleitens der Insektenbeine an wachsbedeckten Pflanzenteilen. Jahrbücher für wissenschaftliche Botanik. 1914;54:448–497. [Google Scholar]
- Lloyd FE. The carnivorous plants. Chronica Botanica. Vol. 9. New York: Ronald Press; 1942. [Google Scholar]
- McPherson S, Robinson A, Fleischmann A. Pitcher plants of the Old World. Poole, UK: Redfern Natural History; 2009. [Google Scholar]
- Meimberg H, Wistuba A, Dittrich P, Heubl G. Molecular phylogeny of Nepenthaceae based on cladistic analysis of plastid trnK intron sequence data. Plant Biology. 2001;3:164–175. [Google Scholar]
- Merbach MA, Zizka G, Fiala B, Maschwitz U, Booth WE. Patterns of nectar secretion in five Nepenthes species from Brunei Darussalam, Northwest Borneo, and implications for ant–plant relationships. Flora. 2001;196:153–160. [Google Scholar]
- Moran JA. Pitcher dimorphism, prey composition and the mechanisms of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. Journal of Ecology. 1996;84:515–525. [Google Scholar]
- Moran JA, Booth WE, Charles JK. Aspects of pitcher morphology and spectral characteristics of six Bornean Nepenthes pitcher plant species: implications for prey capture. Annals of Botany. 1999;83:521–528. [Google Scholar]
- Moran JA, Hawkins BJ, Gowen BE, Robbins SL. Ion fluxes across the pitcher walls of three Bornean Nepenthes pitcher plant species: flux rates and gland distribution patterns reflect nitrogen sequestration strategies. Journal of Experimental Botany. 2010;61:1365–1374. doi: 10.1093/jxb/erq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JA, Merbach MA, Livingston NJ, Clarke CM, Booth WE. Termite prey specialization in the pitcher plant Nepenthes albomarginata—evidence from stable isotope analysis. Annals of Botany. 2001;88:307–311. [Google Scholar]
- Phillipps A, Lamb A, Lee CC. Pitcher plants of Borneo. 2nd edn. Kota Kinabalu: Natural History Publications; 2008. [Google Scholar]
- Scholz I, Buckins M, Dolge L, et al. Slippery surfaces of pitcher plants: Nepenthes wax crystals minimize insect attachment via microscopic surface roughness. Journal of Experimental Biology. 2010;213:1115–1125. doi: 10.1242/jeb.035618. [DOI] [PubMed] [Google Scholar]
- Xia Y, Whitesides GM. Soft lithography. Angewandte Chemie International Edition. 1998;37:550–575. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.