Abstract
To examine the efficacy of a cognitive-behavioral intervention (CBT) to prevent depression among methadone maintenance patients undergoing antiviral treatment for hepatitis C (HCV), 29 patients beginning HCV treatment were randomized to CBT or standard care (SC). Study participants did not meet criteria for major depressive disorder at the time of study recruitment. CBT did not result in less depression-related antiviral treatment failure, better adherence to antiviral treatment, or better HCV RNA outcomes. There were no significant treatment group differences on depressive symptoms over time. The CBT group did display a greater and more consistent decline in both BDI-II and HAM-D scores over time (d=.85 on the BDI-II; d=.72 on the HAM-D).
Keywords: Hepatitis C, Antiviral treatment, Depressive symptoms, Cognitive-behavioral treatment, Beck depression inventory, Hamilton rating scale for depression
Introduction
It is estimated that at least 4 million Americans are infected with the hepatitis C virus (HCV) [1,2], and there are an estimated 170 million individuals infected with HCV globally [3]. Furthermore, the number of persons diagnosed with chronic HCV is projected to increase four-fold from 1990 to 2015 [2]. HCV is the most common chronic bloodborne infection in the U.S.[4] and is a leading cause of liver disease in this country [5]. Of those infected with HCV, 60–85% will develop chronic infection [6]. HCV is a common cause of cirrhosis and liver cancer and is the most common reason for liver transplantation [2]. Most studies have found that cirrhosis develops in 10–30% of persons with chronic HCV over a period of 20–30 years [7–9], which places these individuals at risk for liver failure, hepatocellular carcinoma, and liver-related death [10,11]. HCV is the leading cause of death from liver disease [12], and mortality resulting from HCV infection is predicted to rise over the next two decades [13].
The primary route of infection with HCV is through exposure to infected blood. Intravenous drug use currently accounts for most HCV transmission in the U.S. [10] and as much as 70% of all new cases of HCV in Western countries [14,15]. In fact, the prevalence of HCV among intravenous drug users is estimated to be 70–90% [2]. Within one year of beginning needle use, as many as 65% of intravenous drug users are infected with HCV; after 5 years, the rate of HCV infection rises to as much as 90% [16,17].
A combination of the antiviral agents pegylated interferon alpha (IFN) and ribavirin are now the standard treatment for HCV [15]. The rate of sustained virologic response, which refers to an absence of HCV RNA in the serum 6 months after the completion of therapy, varies based on the genotype of HCV with which individuals are infected and the pretreatment viral load [18–20]. Pegylated IFN combined with ribavirin results in a sustained virologic response of nearly 50% for genotype 1 and approximately 80% for genotypes 2 and 3 [20,21].
While treatment holds promise for those with HCV, it carries the risk of a number of side effects. The most common side effects, which occur early in treatment and tend to dissipate as treatment continues, include flu-like symptoms such as fever, tachycardia, headache, malaise, arthralgia, and myalgia [22]. Neuropsychiatric side effects in some cases appear later in treatment and include fatigue, affective changes (depression, anxiety, irritability, manic symptoms, and even suicidal behavior), cognitive changes (decreased concentration, confusion, and delirium), and, rarely, psychosis [23–25].
Neuropsychiatric side effects are the most frequently cited reason for limiting or discontinuing antiviral therapy, occurring in 30–80% of HCV patients undergoing antiviral treatment [26] and leading to a complete discontinuation of treatment in up to 16% of those receiving antiviral treatment for HCV [23,24,27,28]. There is some evidence that the rate of depression and treatment discontinuation is higher among patients with a history of drug use disorders, relative to other patients undergoing IFN treatment [26]. In one study, antiviral therapy was discontinued in two-thirds of the patients who developed psychiatric symptoms during the course of treatment [29]. Of particular concern is the emergence or exacerbation of depressive symptoms [30]. In addition to an increased risk of antiviral treatment discontinuation, depression and other IFN-induced neuropsychiatric symptoms can lead to suicidal ideation and completion, relapse to substance use, impairment in social and occupational functioning, and more general negative effects on quality of life [31–35]. Clinical studies have demonstrated that 10 to 45% of patients receiving antiviral treatment for HCV develop depression during the course of therapy [31,36–40], with the most methodologically rigorous studies yielding depression estimates of approximately 33% [41].
Several studies have examined the efficacy of antidepressant medication during IFN treatment, some of which have taken a prophylactic approach to the use of antidepressants while others have taken a symptomatic approach, prescribing antidepressants only when symptoms have exceeded a predetermined threshold. Most of these studies have had very small sample sizes, and many of them have been open-label studies. However, taken together, there is some support for the efficacy of antidepressants used in a both prophylactic and symptomatic manner during IFN treatment (see Sockalingam and Abbey (2009) [41] for review).
Despite the demonstrated efficacy of antidepressants, particularly selective serotonin reuptake inhibitors, to address depression during IFN treatment, there are some issues that should be considered regarding their use in this population. First, there is some evidence that the IFN-induced depression specific symptoms respond well to serotonergic antidepressants while the neurovegetative symptoms (e.g., sleep changes, appetite changes) do not [41,42]. Second, while data are lacking to address this issue, there are theoretical reasons why SSRIs may negatively impact viral clearance as a result of their anti-inflammatory properties [43]. Third, in addition to the more broadly experienced side effect profile of SSRIs, patients undergoing antiviral treatment for HCV may be at increased risk for certain side effects of SSRIs, such as SSRI-induced mania and gastrointestinal bleeding [41]. As a result of the side effect profile for SSRIs, particularly among patients undergoing treatment for HCV, some patients elect not to receive SSRIs. Furthermore, some patients who begin SSRI treatment experience side effects that necessitate the discontinuation of SSRIs. Therefore, SSRI treatment is simply not an option for some patients undergoing antiviral treatment for HCV.
Psychosocial interventions could be used as an adjunct or alternative to antidepressants to address the sequelae of antiviral treatment for HCV. To date, the use of psychosocial treatments to address the neuropsychiatric symptoms that commonly result from IFN treatment has been largely unexplored. In the current study, we examined the efficacy of a cognitive-behavioral intervention to prevent depression among methadone maintenance treatment (MMT) patients undergoing antiviral treatment for HCV. We chose to focus on MMT patients given the high rate of HCV infection in this population [17] and a greater likelihood of a stabilized lifestyle relative to individuals with opiate dependence who are not engaged in MMT, facilitating the completion of the study intervention sessions and interviews.
Methods
Recruitment
Study participants were recruited from patients seeking antiviral treatment for HCV at an urban hospital-based primary care clinic during the period September 2004 through July 2007. In order to be eligible to receive antiviral treatment, patients had to meet treatment inclusion criteria: (1) at least 18 years of age, (2) HCV antibody positive and detectable HCV RNA in serum, (3) no medical contraindications to IFN and ribavirin, (4) evidence of chronic hepatitis on a liver biopsy, (5) drinking less than “at-risk” levels during the past month (defined as ≤ 14 drinks per week and no more than four drinks on one occasion for men, ≤ 7 drinks per week and no more than three drinks on one occasion for women; NIAAA, 2005). Patients had to meet additional eligibility criteria in order to participate in the study: (1) English speaking, (2) enrolled in MMT for at least six months, (3) no current (past month) major depressive episode, (4) not currently taking an antidepressant medication, (5) not currently suicidal, (6) not currently psychotic, (7) no previous antiviral treatment of HCV, (8) no intention to relocate from the study area in the next 6 months. While HIV infection was not a study exclusion criterion, all study patients were mono-infected with HCV.
During the study recruitment period, 117 patients beginning antiviral treatment for HCV appeared to be eligible for the study and were referred to the study by their treatment provider. All of the 117 patients agreed to be contacted by study staff. A study research assistant contacted each potential participant, typically at the time of his or her medical appointment, to obtain informed consent. This involved providing detailed information about the study, answering questions about the study, obtaining a signed consent form, and providing the participant with a copy of the consent form. Participants were then scheduled for a baseline interview, which included a screening measure to confirm study eligibility. Participants received $25 for the baseline interview regardless of their eligibility.
Of the 117 patients referred to the study, 4 declined study participation, 1 was ineligible because s/he did not speak English, 65 because they were taking antidepressant medication, 10 because they had received previous antiviral treatment for HCV, and 8 because they were both on antidepressant medication and had received previous antiviral treatment for HCV. The remaining 29 patients met all study eligibility criteria, signed a written informed consent statement, and were recruited into the study. The study was approved by the Institutional Review Board of Rhode Island Hospital.
Following the baseline interview, the 29 study participants were randomized to receive either an 8-session cognitive behavior therapy intervention (CBT) or standard care for HCV (SC). Urn randomization was used to balance on sex (female or male), age (under 40 or over 40), and Beck Depression Inventory-II score (BDI-II) [44] (under 20, 20 and over).
Participants in both treatment conditions completed follow-up interviews at 1, 3, and 6 months following the baseline interview. Participants were paid $30 for completing the 1-month follow-up, $35 for the 3-month follow-up, and $50 for the 6-month follow-up. In addition, the BDI-II was administered at each weekly medical visit for antiviral treatment injections.
Hepatitis C treatment
Hepatitis C treatment was administered according to the following protocol. Therapy started with weekly injections of 180 mcg of peginterferon alfa-2a, administered in the clinic by a clinic treatment provider. Genotype 1 and 4 participants received 1000 mg of oral ribavirin daily (for those <75kg) or 1200 mg of oral ribavirin daily (for those >75kg). Genotype 2 and 3 participants received 800 mg of ribavirin daily. Medication dosage adjustments were based on established criteria. Complete blood counts and liver function tests were obtained every two weeks through week 12 and then every four weeks through the end of treatment. Thyroid-stimulating hormone levels were measured every three months during treatment. For patients with genotype 1 or 4, HCV RNA was assessed at baseline and at 12, 24, and 48 weeks. Therapy was given for a total of 48 weeks. Therapy was discontinued early if HCV RNA at 12 weeks decreased less than 100 fold from baseline (< 2 logs) or was still positive at 24 weeks. For patients with genotype 2 or 3, HCV RNA was assessed at baseline and 24 weeks. Therapy was given for a total of 24 weeks.
Intervention condition
The Coping with Depression Course (CWD), co-developed by Drs. Richard Brown and Peter Lewinsohn [45,46] served as the basis of the cognitive-behavioral intervention for depression that was used in this study. CWD is a multicomponent treatment for depression that includes training in several skills that are relevant to depression, such as mood monitoring, pleasant activities, constructive thinking, and social skills and assertiveness. Versions of CWD have been found to be efficacious, including in a sample of alcohol dependent patients [47]. A number of modifications were made to the intervention to meet the unique needs of MMT patients with HCV undergoing IFN treatment. First, we added psychoeducational information concerning the depressive effects of antiviral treatment for HCV. Second, we emphasized the importance of the early identification of depression, using daily mood ratings to track depressive symptoms. The presence of depressive symptoms was used to signal that greater efforts should be made to apply the coping skills taught in the intervention. Third, we added exercises to the cognitive restructuring techniques that allowed for the exploration of patients’ counterproductive thoughts about having HCV, facilitating coping with and adaptation to this medical condition. Fourth, we emphasized depression as a risk factor for relapse to drug use. Therapists helped patients to plan strategies to cope with cravings to use drugs. Fifth, we enhanced the positive activities and behavioral activation component of the intervention.
The CBT intervention in the current study included the treatment components detailed below, divided across eight 50-minute individual treatment sessions. The intervention sessions typically coincided with participants’ weekly IFN injections and began with IFN initiation. No intervention sessions were delivered beyond 12 weeks after the baseline interview. Sessions were delivered by one of two Ph.D.-level clinical psychologists, both of whom had extensive experience in the delivery of cognitive-behavioral interventions for depression.
Treatment rationale and social learning theory model of depression: The interrelationships among behavior, thoughts, and mood were explained. Examples of how changes in behavior and thoughts can impact mood were provided. The importance of learning skills to control depressive symptoms while undergoing IFN treatment and attempting to avoid drug use was discussed, as was the relationship between depressive symptoms and drug use.
Daily mood rating: In order to gain skills in identifying daily mood states and insight into the factors that influence them, participants were asked to monitor their mood on a daily basis, using a 9-point Likert-type scale.
Increasing pleasant activities: Pleasant activities were described as an important, alternative source of non-drug related activities. The Pleasant Events Schedule-Mood Related Form (PES-MR) [48] was used to help participants create an individualized activity schedule. Participants used this schedule to monitor daily pleasant activities and to determine their baseline level of pleasant activities. Participants also plotted the relationship between their daily mood and engagement in pleasant activities. Participants contracted for achievable, systematic increases in pleasant activities to improve their mood or to prevent the onset of depressive symptoms.
Increasing positive/decreasing negative thoughts: This treatment component involved teaching participants to monitor their thoughts daily and to distinguish between positive and negative thoughts and between constructive and destructive thoughts. Cognitive self-management techniques such as thought stopping, increasing positive thoughts by priming and using cues, time projection, and self-talk procedures were also taught.
ABC technique: The Rational-Emotive Therapy principles of Albert Ellis [49] serve as the foundation of this procedure. Instruction in the identification of cognitive distortions, as identified by Beck [50] and popularized by Burns [51], is also incorporated into this treatment component. The procedure involves the recognition that activating events often trigger a series of distorted or irrational beliefs, which result in negative emotional consequences (e.g., depression, anger). Participants were taught techniques for identifying and disputing these distorted, depressive thoughts, which were honed through daily practice.
Social skills/assertiveness: This treatment component involved teaching participants that improving one’s interactions with others and responding more assertively in various situations (including social pressures to use drugs) can have a positive impact on one’s mood. Situations that elicit non-assertive responses were listed, and participants were taught more assertive ways to respond to these situations through modeling, role-playing, and take-home exercises.
Maintaining gains: Participants were encouraged to monitor their mood periodically, to identify skills found to be the most effective, and to use those skills actively to manage their moods without drinking.
Measures
Screening measure
In order to confirm eligibility for the study, the Structured Clinical Interview for DSM-IV-Patient Version (SCID-P) Module A (Affective Disorders) was administered [52] at baseline.
Mood measures
The Beck Depression Inventory-II (BDI-II) [44] assessed baseline and weekly levels of depressive symptoms. It was also administered at the follow-up interviews. The 17-item Hamilton Rating Scale for Depression (HAM-D) was used as a second, complementary measure of depressive symptoms at baseline and follow-up interviews [53].
The Longitudinal Interval Follow-up Evaluation (LIFE) [54] was used to evaluate affective disorder diagnostic changes over the follow-up period. The LIFE was used to determine if patients met criteria for major depressive episode over the course of the 6-month follow-up period, which was one index of treatment failure. The LIFE is a well-established, short interval follow-up interview, which generates a continuous weekly measure of psychopathology, based on research diagnostic criteria [55]. The affective disorders section of the LIFE was administered at 1, 3, and 6 months.
Substance use measures
The Timeline Followback (TLFB) interview [56] was used to assess drug use at baseline, as well as during the follow-up intervals. The TLFB interview is a calendar-assisted structured interview, which provides a way to cue memory so that accurate recall is enhanced. The TLFB assessed the types of drug classes subjects used and the frequency of drug use. At baseline, it was administered for the six months prior to the interview and provided data on the percent illicit drug use days. It was also administered at each follow-up interview, covering the period from the previous interview in order to provide a continuous measure of drug use.
Urine Drug Screens were conducted, using DrugCheck NxStep OnSite test cups, at baseline and each follow-up to detect recent use of illicit drugs. The drug screen tested for the presence of the following classes of drugs: amphetamines, barbiturates, benzodiazepines, cocaine, methadone, methamphetamine, opiates, phencyclidine, and tetrahydrocannabinoids. In instances in which the participant reported no drug use on the TLFB but had a positive urine screen, the participant was considered positive for that class of drugs for the 7 days prior to the interview.
Treatment-related measures
The Number and Dosage of IFN Injections were extracted from participants’ medical records once they completed HCV treatment. This included any reduction or discontinuation of antiviral medication due to depressive symptoms.
HCV RNA Levels were collected from medical records in order to determine the extent to which antiviral treatment lowered HCV viral load.
Patients were also asked to provide information regarding the Use of Medications for psychiatric or substance use indications. At baseline, patients were asked to provide information regarding medications used in the past 6 months. At each follow-up interview, they were asked to provide information concerning use of medications since the previous interview.
Treatment failure
Treatment failure was defined as meeting criteria for a major depressive episode during the course of the study, having antiviral medication reduced or discontinued due to depressive symptoms, or receiving a referral to the study psychiatrist during the course of the study.
Depression symptom monitoring/psychiatry referral
As stated above, the BDI-II was administered at participants’ weekly medical clinic visits. Participants who endorsed any suicidality on the BDI-II (i.e., a score of ≥ 1 on item 9) were assessed for safety by a study clinical psychologist. In addition, participants who met pre-established study cutoffs for elevated total scores on the BDI-II (i.e., BDI-II total score of ≥ 29 or ≥ 20 if it represented a doubling of a participant’s baseline score) were assessed by a study clinical psychologist. Those participants with elevated scores for two consecutive weeks were referred to a study psychiatrist for evaluation and possible antidepressant medication treatment. Study participants receiving antidepressant medications from the psychiatrist were followed in treatment as appropriate. Those participants still being followed for treatment by the study psychiatrist at the end of their study involvement were referred back to their primary care treatment providers for either medication maintenance or referral to a non-study psychiatrist as deemed appropriate.
Data analytic plan
Logistic regression was used to test for treatment differences in the single endpoint primary outcomes of depression-related antiviral treatment failure, antiviral treatment adherence (a dichotomous measure of having received 24 injections vs. receiving fewer than 24 injections), and HCV RNA outcome assessed at 24 weeks and 48 weeks. In the first step of the analyses, we examined unadjusted treatment effects [57–59]. Follow-up analyses examined the same outcome variables, controlling for key baseline characteristics.
Random effects regression analyses with full information maximum likelihood (FIML) estimation were conducted with SAS PROC MIXED to test treatment effects for the primary outcomes assessing depressive symptoms (BDI-II and HAM-D) and illicit drug use during the depression intervention phase (baseline and 1 and 3 months) and post depression intervention (3 and 6 months).BDI-II and HAM-D scores were log transformed to improve normality prior to analysis. Each level-1 model included random effects for initial status and a slope term assessing monthly linear change from baseline to 6 months. The level-2 model tested treatment group differences in these level-1 terms. FIML allows available data from all participants to contribute to the estimation of intercepts and slopes. Follow-up analyses examined adjusted treatment effects for these outcomes by including key baseline and time-varying covariates in the random effects regression models. To characterize the magnitude of change over time for depressive symptoms and illicit drug use, mean difference scores were calculated by subtracting the mean estimated change from baseline to each follow-up for the CBT group from the mean estimated change from baseline to follow-up for the SC group. Predicted values from the random effects regression analyses generated these difference scores. We used a standard method to obtain Cohen’s d treatment effect size by dividing the mean difference scores by the SC group baseline standard deviation (SD; [60]). Cohen’s d values of 0.20, 0.50, and 0.80 represent small, moderate, and large effect sizes, respectively.
Secondary analyses used logistic regression to test the relation between observed change in BDI-II and HAM-D depressive symptom scores from baseline to each of the three follow-up assessments and the likelihood of receiving 24 or more injections (treatment adherence).
Results
The mean age of the participants was 42.4 (+ 9.2) years, and 86% were male (Table 1). In terms of racial identity, 90% identifed as Caucasian, 3% as American Indian or Alaskan Native, and 7% as some “other” race; 17% of the sample were Hispanic. At baseline, 22 of the 29 participants (76%) had BDI scores < 20 (14 out of 15 participants in the standard care condition and 8 out of 14 in the CBT condition). Follow-up interview completion rates were 96.6% at 1 month, 93.1% at 3 months, and 93.1% at 6 months. HCV RNA data were available at 24 weeks for 25 of the 29 participants (86.2%); all four participants who were missing HCV RNA data were in the CBT group. HCV RNA data were available at 48 weeks for 24 of the 29 participants (82.8%); four of the participants who were missing HCV RNA were in the CBT group, and one was in the SC group. Participants assigned to the CBT condition completed a mean of 5.93 (+ 2.73) of the 8 total intervention sessions; 9 of the 14 CBT participants completed 6 or more of the sessions. Of the entire sample, 12 participants had HCV genotype 1 (58.33% were in the CBT group; 41.6% were in the SC group), four participants had genotype 2 (50% were in the CBT group; 50% were in the SC group), 11 participants had genotype 3 (45.45% were in the CBT group; 54.55% were in the control group), and two participants had genotype 4 (100% were in the control group).
Table 1.
Participant Characteristics at Baseline.
| Total (N=29) |
CBT (N=14) |
Standard Care (N=15) |
P-value | |
|---|---|---|---|---|
| Age (SD) | 42.4 (9.2) | 43.2 (7.7) | 41.6 (10.7) | 0.65 |
| % Female | 13.8 | 0.0 | 26.7 | 0.10 |
| % Caucasian | 89.7 | 92.9 | 86.7 | 0.58 |
| % High School Education | 69.0 | 71.4 | 66.7 | 0.78 |
| % Employed Full Time | 44.8 | 28.6 | 60.0 | 0.09 |
| Mean Proportion Illicit Drug Use Days (SD)* |
0.22 (0.33) | 0.14 (0.20) | 0.29 (0.41) | 0.21 |
| Mean BDI-II (SD) | 13.6 (12.4) | 16.9 (15.7) | 10.6 (7.9) | 0.18 |
| Mean HAM-D (SD) | 8.8 (6.4) | 11.1 (7.4) | 6.6 (4.5) | 0.06 |
Calculated based on days in which participant was not in a controlled environment
There were no significant treatment group differences in depression-related treatment failure or HCV RNA outcomes at 24 weeks or 48 weeks, but the CBT group was marginally less likely (p=0.086) to have completed 24 or more antiviral injections (Table 2). Follow-up analyses for depression-related treatment failure indicated that gender and baseline employment status, which were marginally imbalanced across treatment groups, were not related to depression-related treatment failure (p=0.82 and p=0.53 for gender and employment status, respectively), nor was baseline illicit drug use (p=0.25). However, baseline BDI-II depression was marginally related to an increased likelihood of depression-related treatment failure (odds ratio (OR) = 1.83; 95% confidence interval (CI) = 0.89, 44.01; p=0.066). Therefore, we conducted a logistic regression analysis to test for treatment group differences in depression-related treatment failure, controlling for log transformed baseline BDI-II depression. The adjusted odds ratio suggested that the treatment effect was smaller and remained non significant after controlling for baseline BDI-II (Table 2). Baseline BDI-II remained marginally related to depression-related treatment failure (OR=3.30; 95% CI=0.83, 13.22, p=0.092).
Table 2.
Treatment Group Differences on Primary Single Endpoint Outcomes.
| Outcome | CBT (n=14) |
Standard Care (N=15) |
Odds Ratio (95% CI) |
Adjusted Odds Ratio (95% CI) |
|---|---|---|---|---|
| Depression-Related Treatment Failure |
28.6% (n=4) |
13.3% (n=2) | 3.11 (0.47, 20.65) |
1.89 (0.24, 15.11) |
| Complete Treatment Ad- herence (24 injections) |
50.0% (n=7) |
80.0% (n=12) |
0.25 (0.05, 1.29) |
0.19 (0.03, 1.15) |
| Hep C RNA Detectable (24 weeks) |
20.0% (n=2/10) |
20.0% (n=3/15) |
1.24 (0.17, 9.25) |
NA |
| Hep C RNA Detectable (48 weeks) |
20.0% (n=2/10) |
42.9% (n=6/14) |
0.43 (0.07, 2.81) |
NA |
Note: NA=adjusted odds ratios were not calculated for these outcomes
Follow-up analyses for treatment adherence indicated that gender and baseline employment status were not related to treatment adherence (p=0.67 and p=0.25 for gender and employment status, respectively), nor was baseline illicit drug use (p=0.62). However, baseline BDI-II depression was marginally related to an increased likelihood of adherence to 24 or more injections (OR=2.05; 95% CI=0.86, 4.89; p=0.104). Therefore, we conducted a logistic regression analysis to test for treatment group differences in treatment adherence, controlling for log transformed baseline BDI-II depression. The adjusted odds ratio suggested that treatment effect was slightly larger and remained marginally significant (p=0.071) after controlling for baseline BDI-II (Table 2). Baseline BDI-II remained marginally related to treatment adherence (OR=2.29; 95% CI=0.93, 5.66, p=0.072).
Similar follow-up analyses for HCV RNA outcomes indicated that males were marginally less likely to have a positive HCV RNA outcome at 24 weeks (OR=0.17, 95% CI=0.02, 1.68; p=0.109) and 48 weeks (OR=0.11, 95% CI=0.01, 1.33; p=0.058). Baseline employment status (p=0.24 and 0.77 for 24-week and 48-week HCV RNA, respectively), BDI-II score (p=0.77 and 0.74), and illicit drug use (p=0.54 and 0.35) were unrelated to both HCV RNA outcomes. Because there were no women in the CBT group and no other potential covariates were significantly related to the HCV RNA outcomes, we did not test logistic regressions with covariates for these outcomes.
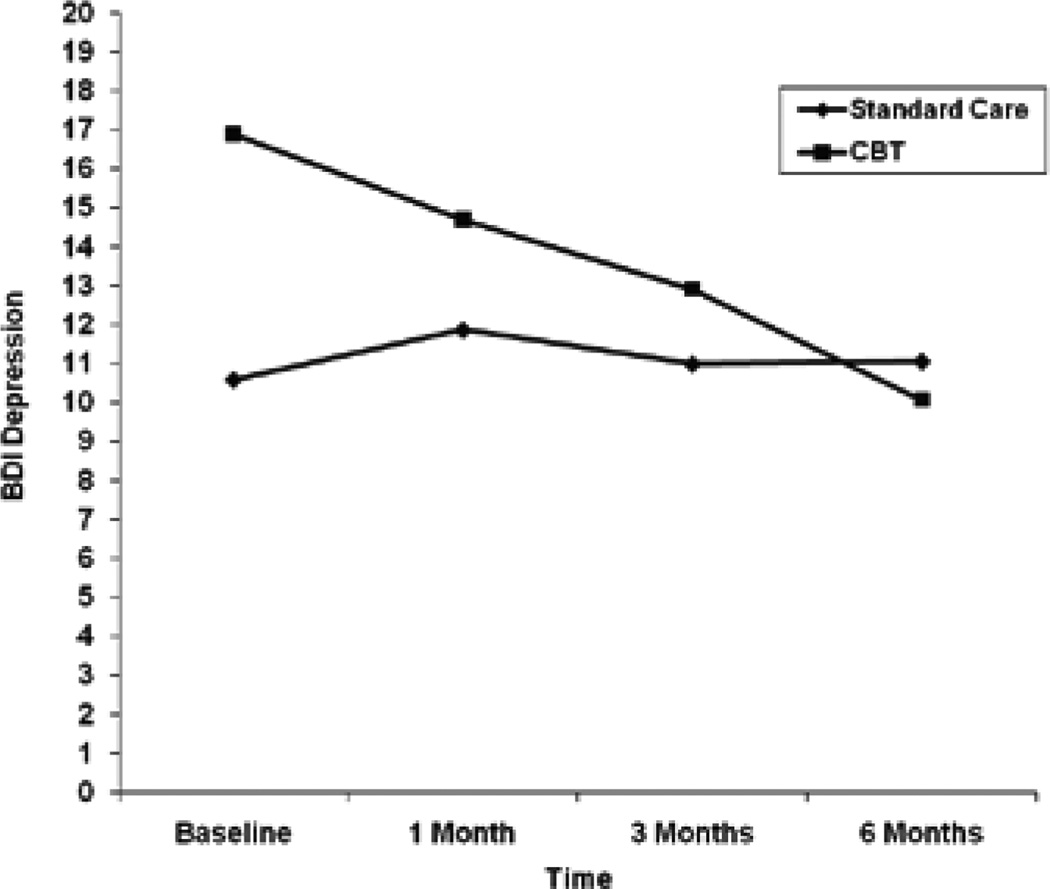
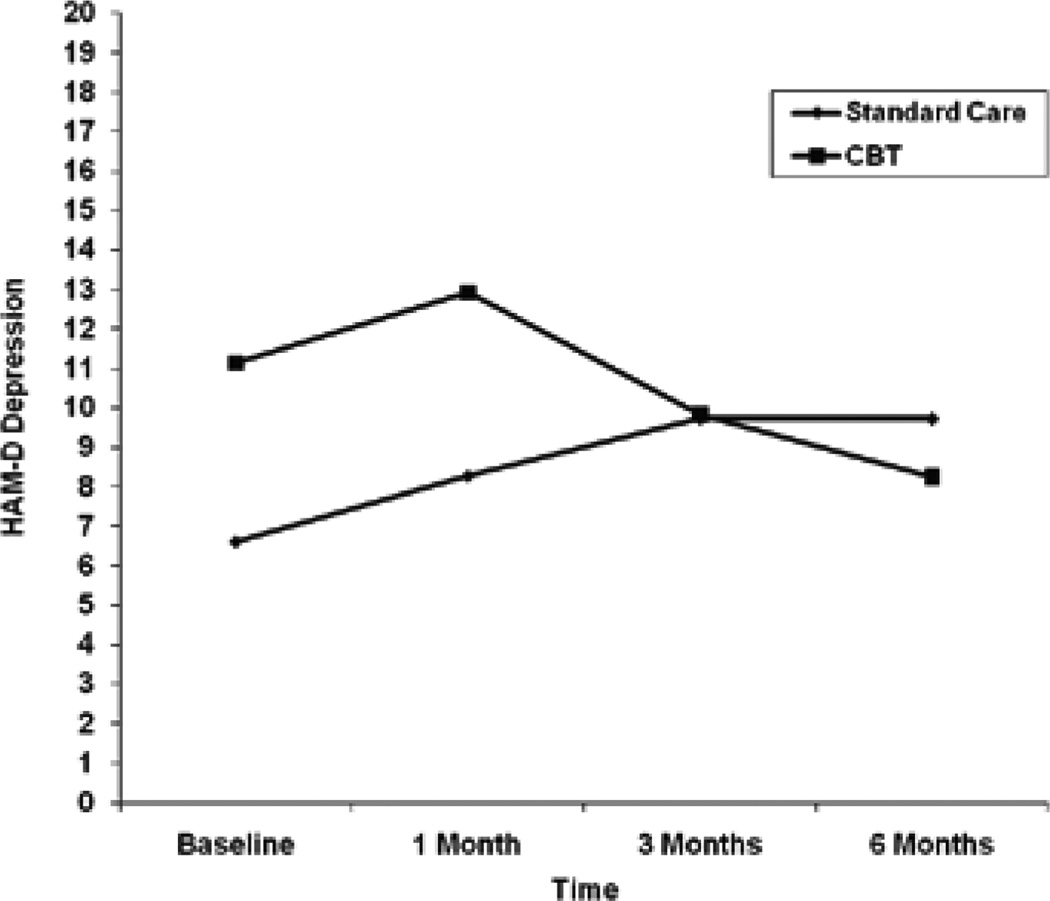
Table 4 shows observed means and mean differences in BDI-II, HAM-D, and proportion of illicit drug use days from baseline to each follow-up. Results for the random effects regression models for log transformed BDI-II and HAM-D scores indicated that there were no significant treatment group differences in change in depressive symptoms over time, and the parameter estimates did not change after controlling for antidepressant use (Table 3). Antidepressant use was significantly related to greater BDI-II (unstandardized parameter estimate=0.90; 95% CI=0.34, 1.47; p=0.002) and HAM-D (unstandardized parameter estimate=0.60; 95% CI=0.11, 1.08; p=0.02) scores over time. Despite a lack of significant treatment group differences, the observed mean trajectories suggested a greater and more consistent decline in both BDI-II and HAM-D scores over time for the CBT group (Figures 1 and 2). The mean estimated decrease in log BDI-II from baseline to the 6-month follow-up (derived from the unadjusted random effects regression model) was 0.07 for the SC group and 0.77 for the CBT group. The treatment effect was −0.70 (95% CI=−1.47, 0.07), which resulted in a relatively large effect size of d=0.85. The unadjusted mean estimated change in log HAM-D from baseline to the 6-month follow-up was −0.12 for the SC group, indicating a slight increase in HAM-D over time. The estimated mean change in log HAM-D was 0.41 for the CBT group, indicating a decline in HAM-D score over time. The treatment effect was −0.54 (95% CI=−1.15, 0.07), which resulted in a moderate effect size of d=0.72.
Table 4.
Observed Means and Mean Change for BDI, HAM-D and Proportion of Illicit Drug Use Days.
| Outcome | Baseline (sd) | 1 mos (sd) | 3 mos (sd) | 6 mos (sd) | Baseline-1 month (sd) |
Baseline-3 months (sd) |
Baseline-6 months (sd) |
|---|---|---|---|---|---|---|---|
| BDI-II | |||||||
| SC | 10.6 (7.85) | 11.9 (12.3) | 11.0 (10.6) | 11.1 (12.5) | 1.3 (7.3) | 0.4 (6.6) | 0.5 (7.5) |
| CBT | 16.9 (15.7) | 14.7 (13.19) | 12.9 (15.4) | 10.1 (13.6) | –3.2 (5.7) | –5.0 (6.7) | –7.8 (7.3) |
| HAM-D | |||||||
| SC | 6.6 (4.5) | 8.3 (6.8) | 9.7 (8.5) | 9.7 (11.6) | 1.7 (3.7) | 3.1 (6.4) | 3.1 (8.4) |
| CBT | 11.1 (7.5) | 12.9 (10.7) | 9.8 (9.0) | 8.3 (8.9) | 1.1 (7.8) | –1.5 (3.4) | –3.1 (4.3) |
| Drug Use | |||||||
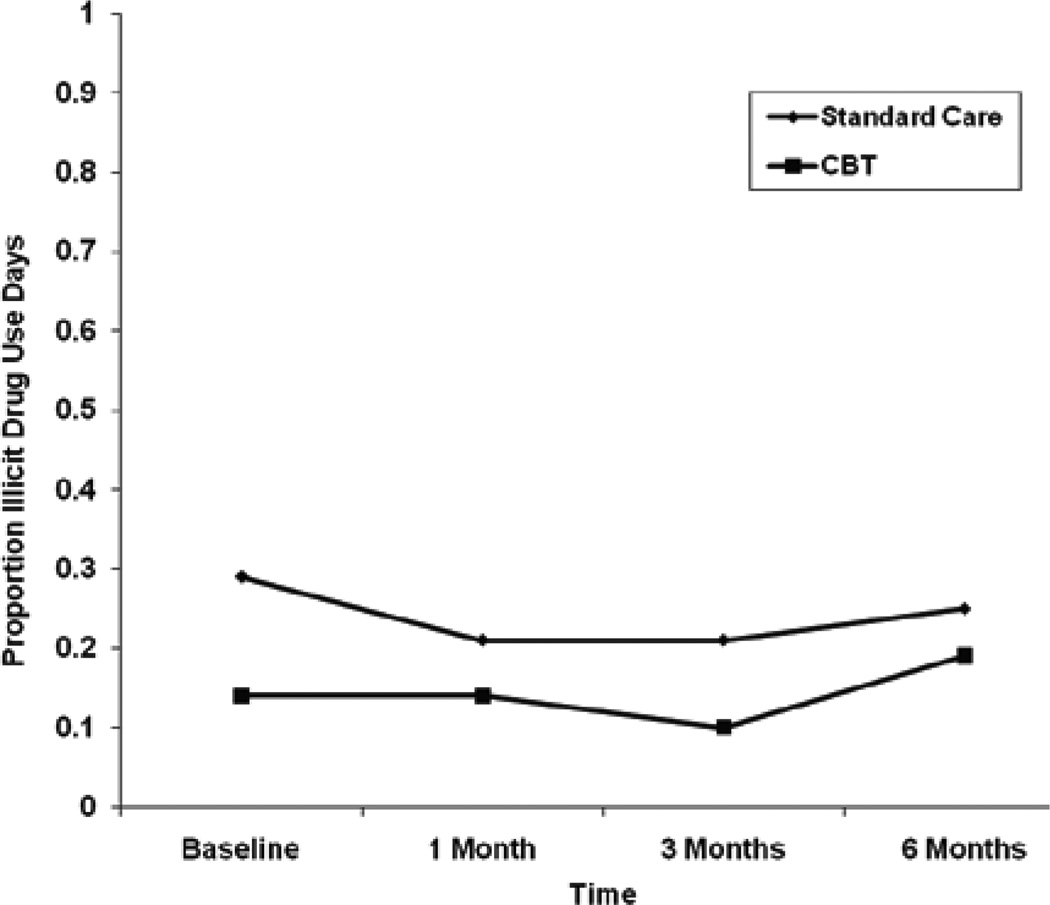
| SC | 0.29 (0.41) | 0.21 (0.41) | 0.21 (0.41) | 0.25 (0.43) | –0.09 (0.21) | –0.08 (0.21) | –0.04 (0.19) |
| CBT | 0.14 (0.20) | 0.14 (0.30) | 0.10 (0.29) | 0.18 (0.38) | 0.00 (0.26) | –0.03 (0.27) | .05 (0.39) |
Table 3.
Random Effects Regression Results for Repeated Primary Outcome Measures.
| Unstandardized Parameter Estimate (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Outcome Variables (Unadjusted) | Outcome Variables (Adjusted) | |||||
| Predictor Variables | BDI-II Depressiona | HAM-D Depressiona | Proportion Illicit Drug Use Days |
BDI-II Depressiona | HAM-D Depressiona | Proportion Illicit Drug Use Days |
| Intercept | 2.15 (1.82, 2.47) | 1.90 (1.63, 2.18) | 0.27 (0.16, 0.38) | 2.11 (1.79, 2.43) | 1.88 (1.60, 2.15) | 0.16 (–0.02, 0.34)b 0.03 (–0.15, 0.21)c |
| Linear Slope Baseline -6 months |
–0.01 (–0.15, 0.13) | 0.02 (–0.09, 0.14) | –0.004 (–0.05, 0.04) | –0.02 (–0.15, 0.10) | 0.01 (–0.09, 0.12) | –0.003 (–0.05, 0.04)b –0.01 (–0.05, 0.04)c |
| Baseline Treatment Difference |
0.27 (–0.20, 0.74) | 0.34* (–0.06, 0.75) | –0.13 (–0.29, 0.04) | 0.16 (–0.31, 0.63) | 0.27(–0.13, 0.67) | –0.14* (–0.30, 0.02)b –0.17* (–0.33, −0.02)c |
| Treatment Difference in Linear Slope Base- line–6 months |
–0.12 (–0.32, 0.09) | –0.09 (–0.26, 0.08) | 0.01 (–0.06, 0.08) | –0.12 (–0.31 0.07) | –0.09 (–0.25, 0.06) | 0.02 (–0.05, 0.09)b 0.02 (–0.05, 0.09)c |
Note: Adjusted parameter estimates control for time-varying antidepressant use for BDI-II and HAM-D outcomes, and for time-varying BDI-IIb and HAM-Dc for the illicit drug use outcome.
log transformed.
p<0.10
Figure 1.
Observed Mean Trajectory for BDI Depression.
Figure 2.
Observed Mean Trajectory for HAM-D Depression.
The random effects regression model for illicit drug use indicated that there were no significant treatment group differences in the proportion of illicit drug use days over time, and the parameter estimates did not change after controlling for BDI-II and HAM-D scores in separate models (Table 3). BDI-II scores were not significantly related to the proportion of illicit drug use days over time (unstandardized parameter estimate=0.05; 95% CI=−0.01, 0.12; p=0.103). However, greater HAM-D scores were significantly related to a greater proportion of illicit drug use days over time (unstandardized parameter estimate=0.13; 95% CI=0.05, 0.20; p=0.001). Both treatment groups showed little to no change in the proportion of illicit drug use days from baseline to 3 months and a slight increase from 3–6 months (Figure 3). The mean unadjusted change in the proportion of illicit drug use days from baseline to 6 months was 0.02 for the SC group and −0.05 for the CBT group. The effect size for the difference between the two groups in the change in the proportion of illicit drug use days was small (d=0.17).
Figure 3.
Mean Trajectory for Proportion of lllicit Drug Use Days.
Finally, logistic regression analyses indicated that the change in BDI-II and HAM-D from baseline to each of the three follow-up assessments was not significantly related to treatment adherence (Table 5).
Table 5.
Relation Between Change in BDI-II and HAM-D Scores and Treatment Adherence.
| Odds Ratio (95% CI) | |||
|---|---|---|---|
| Variable | Full Sample | CBT | Standard Care |
| Change in BDI-II Baseline to 1 Month |
1.00 (0.89, 1.12) | 0.83 (0.64, 1.07) | 1.04 (0.86, 1.24) |
| Change in BDI-II Baseline to 3 Months |
1.04 (0.92, 1.17) | 1.00 (0.84, 1.19) | 1.00 (0.82, 1.22) |
| Change in BDI-II Baseline to 6 Months |
1.00 (0.91, 1.10) | 0.92 (0.77, 1.10) | 0.96 (0.81, 1.14) |
| Change in HAM-D Baseline to 1 Month |
1.01 (0.89, 1.16) | 0.97 (0.83, 1.12) | 1.53 (0.82, 2.82) |
| Change in HAM-D Baseline to 3 Months |
1.30 (1.02, 1.65)* | 2.20 (0.96, 5.05) | 1.12 (0.90, 1.40) |
| Change in HAM-D Baseline to 6 Months |
1.08 (0.93, 1.25) | 1.05 (0.80, 1.39) | 1.02 (0.86, 1.22) |
p<0.05
Discussion
The 8-session CBT for depression intervention tested in the current study did not result in less depression-related antiviral treatment failure, better adherence to antiviral treatment, or better HCV RNA outcomes. Furthermore, there were no significant treatment group differences on depressive symptoms over time. However, the CBT intervention resulted in a relatively large change over time in BDI-II scores and a moderate change over time in HAM-D scores relative to the control group, and the estimated trajectories suggested a greater and more consistent decline in both BDI-II and HAM-D scores over time for the CBT group. While there were no significant treatment group differences on proportion of illicit drug use days, HAM-D scores were found to be significantly associated with a greater proportion of illicit drug use days over time.
One potential explanation for the lack of treatment effect on depression-related antiviral treatment failure, adherence to antiviral treatment, and HCV RNA outcomes is the relatively low rate of depression-related treatment failure, adherence to fewer than 24 injections, and detectable HCV RNA in this small sample. However, another explanation is that the type of psychosocial intervention employed in this study may not be the ideal choice to target the depressive symptoms that tend to occur during the course of HCV antiviral treatment. While CBT for depression has been found to be an efficacious means of addressing classical depressive symptoms (e.g., Brown et al., 1997 [47]) and resulted in moderate to large changes in depressive symptoms relative to the control group in this study, antiviral-induced depressive symptoms tend to consist of only mildly depressed mood and more prominent and problematic atypical depressive symptoms, such as agitation or neurovegetative symptoms [61]. It may be that the antiviral–induced symptoms that most strongly impact depression-related treatment failure, adherence to antiviral treatment, and HCV RNA levels are not effectively addressed with CBT for depression. However, we know of no studies that have attempted to ascertain the relative impact of various depressive symptoms on these antiviral treatment outcomes. As with our study, most studies in this area would not have a sufficient sample size to conduct these fine-grained analyses.
While new antiviral treatments for HCV are currently being investigated, these new agents will be used to enhance the treatment response rate and will maintain IFN and ribavirin as the core of the antiviral treatment regimen [62]. Therefore, in all likelihood, the neuropsychiatric symptoms that accompany HCV treatment currently will remain problematic with the emergence of the next generation of antiviral medications for the treatment of HCV. In light of the limited impact and potential problems associated with antidepressant medication use in this population, it is our belief that further work in the area of psychosocial intervention development could be worthwhile.
One potential avenue for psychosocial intervention development in this population is the use of an acceptance-based intervention, rooted in Acceptance and Commitment Therapy (ACT) [63]. These types of interventions represent a departure from traditional cognitive-behavioral therapies that focus on the goal of controlling thoughts and feelings; they are designed to produce acceptance behaviors aimed at private events that have interfered with accomplishing life goals, such as completing antiviral treatment for HCV. Accumulating evidence supports ACT as an efficacious treatment approach across a variety of clinical problem areas including affective disorders [64,65], anxiety disorders [66], substance use disorders [67,68], smoking [69,70], psychotic disorders [71], and chronic pain [72–74]. The accumulating empirical support for ACT-based interventions in these problem areas is particularly encouraging given the overlap that exists between the clinical presentation of these problem areas and the experiences of those with a history of drug use disorder undergoing treatment for HCV: depressed mood, anxious mood, negative cognitions, urges to use, and somatic discomfort. However, an acceptance-based intervention has not yet been tested in this population.
A key limitation of the current study is the small sample size. In addition, generalizability to other HCV-infected populations may be limited by our inclusion criteria, most notably the inclusion of only those patients engaged in MMT for six months or longer and those who did not currently meet criteria for a major depressive episode. We also did not equate for therapist contact time in the standard care condition and, therefore, cannot rule out the possibility that any treatment effects are due to more general social support received from the therapists rather than specific content contained in the CBT for depression intervention.
In summary, the results of this study are inconclusive regarding the use of CBT to address depressive symptoms among patients undergoing antiviral treatment for HCV. The trend findings of improved depressive symptom scores in the CBT group warrant further investigation. However, the intervention did not result in less depression-related antiviral treatment failure, better antiviral treatment adherence, or lower HCV RNA levels. Antidepressants have some demonstrated efficacy; however, their impact is limited, and they are not tolerated by everyone. Therefore, there is a need to explore other potential psychosocial interventions to be used alone or in conjunction with antidepressant medication.
Acknowledgements
This work was supported in part by Award Number R01DA016797 from the National Institute on Drug Abuse to Susan E. Ramsey. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes on Health. National Institutes on Health consensus development conference statement: Management of Hepatitis C: 2002--June 10–12, 2010. Hepatology. 2002;36:S3–S20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 3.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) and infection and HCV-related chronic disease. Centers for disease control and prevention. MMWR Recomm Rep. 1998;47:1–39. [PubMed] [Google Scholar]
- 5.Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 6.Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 7.Alter MJ, Margolis HS, Krawczynski K. The natural history of community-acquired hepatitis C in the United States. N Engl J Med. 1992;327:1899–1905. doi: 10.1056/NEJM199212313272702. [DOI] [PubMed] [Google Scholar]
- 8.Seeff LB, Buskell-Bales Z, Wright EC, Durako SJ, Alter HJ, et al. Long-term mortality after transfusion-associated non-A, non-B hepatitis. N Engl J Med. 1992;327:1906–1911. doi: 10.1056/NEJM199212313272703. [DOI] [PubMed] [Google Scholar]
- 9.Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 10.Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26 doi: 10.1002/hep.510260711. 62S–65S. [DOI] [PubMed] [Google Scholar]
- 11.Tremolada F, Casarin C, Tagger A, Robero ML, Realdi G, et al. Antibody to hepatitis C virus in post-transfusion hepatitis. Ann Intern Med. 1991;114:277–281. doi: 10.7326/0003-4819-114-4-277. [DOI] [PubMed] [Google Scholar]
- 12.Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36:S30–S34. doi: 10.1053/jhep.2002.36791. [DOI] [PubMed] [Google Scholar]
- 13.Deuffic-Burban S, Poynard T, Sulkowski MS, Wong JB. Estimating the future health burden of chronic hepatitis C and human immunodeficiency virus infections in the United States. J Viral Hepat. 2007;14:107–115. doi: 10.1111/j.1365-2893.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 14.Biggins SW, Terrault NA. Treatment of recurrent hepatitis C after liver transplantation. Clin Liver Dis. 2005;9:505–523. doi: 10.1016/j.cld.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: An update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garfein RS, Vlahov D, Galai N, Moherty MC, Nelson KE. Viral infections in short-term injection drug users: The prevalence of hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86:655–661. doi: 10.2105/ajph.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein MD, Maksad J, Clarke J. Hepatitis C disease among injection drug users: Knowledge, perceived risk and willingness to receive treatment. Drug Alcohol Depend. 2001;61:211–215. doi: 10.1016/s0376-8716(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 18.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 19.Hadziyannis SJ, Sette H, Jr, Morgan TR, Balan V, Diago M, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 20.Manns MP, McHutchinson JG, Gordon SC, Ristgi VK, Shiffman M. Peginterferon alfa-2b in combination with ribavirin compared to interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized controlled trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 21.Fried MW, Shiffman ML, Reddy RK, Smith C, Marino G, et al. Pegylated (40 KDa) interferon α-2a (PEGASYS (™)) in combination with ribavirin: Efficacy and safety results from a phase III, randomized, actively-controlled multicenter study. Gastroenterology. 2001;120:A55. [Google Scholar]
- 22.Dusheiko G. Side effects of alpha interferon in chronic hepatitis C. Hepatology. 1997;26:1125–1215. doi: 10.1002/hep.510260720. [DOI] [PubMed] [Google Scholar]
- 23.Janssen HL, Brouwer JT, van der Mast RC, Schalm SW. Suicide associated with alfa-interferon therapy for viral hepatitis. J Hepatol. 1994;21:241–243. doi: 10.1016/s0168-8278(05)80402-7. [DOI] [PubMed] [Google Scholar]
- 24.Renault PF, Hoofnagle JH, Park H, Mullen ED, Peters M, et al. Psychiatric complications of long-term interferon alpha therapy. Arch Intern Med. 1987;147:1577–1580. [PubMed] [Google Scholar]
- 25.Stritz D, Valentine AD, Meyers CA. Manic episodes in two patients treated with interferon alpha. J Neuropsychiatry Clin Neurosci. 1997;9:273–276. doi: 10.1176/jnp.9.2.273. [DOI] [PubMed] [Google Scholar]
- 26.Schaefer M, Mauss S. Hepatitis C treatment in patients with drug addiction: Clinical management of interferon-alpha-associated psychiatric side effects. Curr Drug Abuse Rev. 2008;1:177–187. doi: 10.2174/1874473710801020177. [DOI] [PubMed] [Google Scholar]
- 27.Ho SB, Nguyen H, Tetrick LL, Opitz GA, Basara ML, et al. Influence of psychiatric diagnoses on interferon-alpha treatment for chronic hepatitis C in a veteran population. Am J Gastroenterol. 2001;96:157–164. doi: 10.1111/j.1572-0241.2001.03468.x. [DOI] [PubMed] [Google Scholar]
- 28.Maddrey W. Safety of combination interferon alfa-2b/ribavirin therapy in chronic hepatitis C-relapsed and treatment naive patients. Semin Liver Dis. 1999;19:67–75. [PubMed] [Google Scholar]
- 29.Yokoyama A, Kimura Y, Shigemura J. Psychiatric side effects of interferon. Journal of Toxicological Sciences. 1996;21:93–96. doi: 10.2131/jts.21.93. [DOI] [PubMed] [Google Scholar]
- 30.McDonald E, Mann A, Thomas H. Interferons as mediators of psychiatric morbidity. An investigation in a trial of recombinant alpha-interferon in hepatitis-B carriers. Lancet. 1987;21:1175–1178. doi: 10.1016/s0140-6736(87)91319-5. [DOI] [PubMed] [Google Scholar]
- 31.Dieperink E, Willenbring ML, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: A review. Am J Psychiatry. 2000;157:867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- 32.Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-alpha-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44:104–112. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- 33.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: Recognition and management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rifai MA, Indest D, Loftis J, Hauser P. Psychiatric management of the hepatitis C patient. Curr Treat Options Gastroenterol. 2006;9:508–519. doi: 10.1007/s11938-006-0007-6. [DOI] [PubMed] [Google Scholar]
- 35.Zdilar D, Franco-Bronson K, Buchler N, Locala JA, Younossi ZM. Hepatitis C, interferon alfa, and depression. Hepatology. 2000;31:1207–1211. doi: 10.1053/jhep.2000.7880. [DOI] [PubMed] [Google Scholar]
- 36.Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, et al. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. J Affect Disord. 2002;72:237–241. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- 37.Horikawa N, Yamazaki T, Izumi N, Uchihara M. Incidence and clinical course of major depression in patients with chronic hepatitis type C undergoing interferon-alpha therapy: A prospective study. Gen Hosp Psychiatry. 2003;25:34–38. doi: 10.1016/s0163-8343(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 38.Miyaoka H, Otsubo T, Kamijima K, Ishii M, Onuki M, et al. Depression from interferon therapy in patients with hepatitis C. Am J Psychiatry. 1999;156:1120. doi: 10.1176/ajp.156.7.1120. [DOI] [PubMed] [Google Scholar]
- 39.Morasco BJ, Rifai MA, Loftis JM, Indest DW, Moles JK, et al. A randomized trial of paroxetine to prevent interferon-alpha-induced depression in patients wtih hepatitis C. J Affect Disord. 2007;103:83–90. doi: 10.1016/j.jad.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 40.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 41.Sockalingam S, Abbey SE. Managing depression during hepatitis C treatment. The Canadian Journal of Psychiatry. 2009;54:614–625. doi: 10.1177/070674370905400906. [DOI] [PubMed] [Google Scholar]
- 42.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, et al. Neurobehavioral effects of interferon-alfa in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 43.Robaeys G, Wichers MC, De Bie J, Koek GH, Buntinx F, et al. Does antidepressant medication in patients with hepatitis C undergoing interferon alfa treatment reduce therapeutic efficacy? Gut. 2009;58:145–146. doi: 10.1136/gut.2008.156919. [DOI] [PubMed] [Google Scholar]
- 44.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 45.Brown RA, Lewinsohn PM. A psychoeducational approach to the treatment of depression: Comparison of group, individual, and minimal contact procedures. J Consult Clin Psychol. 1984;52:774–783. doi: 10.1037//0022-006x.52.5.774. [DOI] [PubMed] [Google Scholar]
- 46.Brown RA, Lewinsohn PM. Coping with depression: Course workbook. Eugene, OR: Castalia Press; 1984. [Google Scholar]
- 47.Brown RA, Evans DM, Miller IW, Burgess ES, Mueller TI. Cognitive-behavioral treatment for depression in alcoholism. J Consult Clin Psychol. 1997;65:715–726. doi: 10.1037//0022-006x.65.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewinsohn PM, Graf M. Pleasant activities and depression. Journal of Consulting and Clinical Psychology. 1973;41:261–268. doi: 10.1037/h0035142. [DOI] [PubMed] [Google Scholar]
- 49.Ellis A, Harper RA. A guide to rational living. Hollywood, CA: Wilshire; 1961. [Google Scholar]
- 50.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. New York: Guilford Press; 1978. [Google Scholar]
- 51.Burns DD. Feeling good. New York: William Morrow; 1980. [Google Scholar]
- 52.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition. New York: New York State Psychiatric Institute; 1997. [Google Scholar]
- 53.Miller IW, Bishop S, Norman WH, Maddever H. The modified Hamilton Rating Scale for Depression: Reliability and validity. Psychiatry Res. 1984;14:131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- 54.Keller MB, Lavori PW, Friedman B, Nielson E, Endicott J, et al. The Longitudinal Interval Follow-up Evaluation: A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 55.Elkin I, Parloff MB, Hadley SW, Autry JH. NIMH Treatment of Depression Collaborative Research Program. Arch Gen Psychiatry. 1985;42:305–316. doi: 10.1001/archpsyc.1985.01790260103013. [DOI] [PubMed] [Google Scholar]
- 56.Sobell LC, Sobell MB. Timeline Followback user’s guide: A calendar method for assessing alcohol and drug use. Toronto, Ontario, Canada: Addiction Research Foundation; 1996. [Google Scholar]
- 57.Canner P. Covariate adjustment of treatment effects in clinical trials. Control Clin Trials. 1991;12:359–366. doi: 10.1016/0197-2456(91)90016-f. [DOI] [PubMed] [Google Scholar]
- 58.Senn S. Covariate imbalance and random allocation in clinical trials. Stat Med. 1989;8:467–475. doi: 10.1002/sim.4780080410. [DOI] [PubMed] [Google Scholar]
- 59.Steyerberg E, Bossyut P, Lee K. Clinical trials in acute myocardial infarction: Should we adjust for baseline characteristics? Am Heart J. 2000;139:745–751. doi: 10.1016/s0002-8703(00)90001-2. [DOI] [PubMed] [Google Scholar]
- 60.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 61.Patten SB, Barbui C. Drug-induced depression: A systematic review to inform clinical practice. Psychother Psychosom. 2004;73:207–215. doi: 10.1159/000077739. [DOI] [PubMed] [Google Scholar]
- 62.Ferenci P. Peginterferon and ribavirin in HCV: Improvement of sustained viral response. Best Pract Res Clin Gastroenterol. 2008;22:1109–1122. doi: 10.1016/j.bpg.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Hayes SC, Strosahl KD, Wilson KG. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change. New York: The Guilford Press; 1999. [Google Scholar]
- 64.Zettle RD, Hayes SC. Dysfunctional control by client verbal behavior: The context of reason-giving. Anal Verbal Behav. 1986;4:30–38. doi: 10.1007/BF03392813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zettle RD, Raines JC. Group cognitive and contextual therapies in the treatment of depression. J Clin Psychol. 1989;45:438–445. doi: 10.1002/1097-4679(198905)45:3<436::aid-jclp2270450314>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 66.Hayes SC. A contextual approach to therapeutic change. In: Jacobson N, editor. Psychotherapists in Clinical Practice. New York: Guilford Press; 1987. pp. 327–387. [Google Scholar]
- 67.Hayes SC, Wilson KG, Gifford EV, Bissett R, Batten S, et al. A preliminary trial of Twelve-Step Facilitation and Acceptance and Commitment Therapy with poly substance-abusing methadone-maintained opiate addicts. Behav Ther. 2004;35:667–688. [Google Scholar]
- 68.Heffner M, Eifert GH, Parker BT, Hernandez DH, Sperry JA. Valued directions: Acceptance and Commitment Therapy in the treatment of alcohol dependence. Cognitive and Behavioral Practice. 2003;10:378–383. [Google Scholar]
- 69.Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, et al. Distress Tolerance Treatment for early-lapse smokers: Rationale, program description, and preliminary findings. Behav Modif. 2008;32:302–332. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki MM, et al. Acceptance theory-based treatment for smoking cessation: An initial trial of acceptance and commitment therapy. Behavior Therapy. 2004;35:689–706. [Google Scholar]
- 71.Bach P, Hayes SC. The use of Acceptance and Commitment Therapy to prevent the rehospitalization of psychotic patients: A randomized controlled trial. J Consult Clin Psychol. 2002;70:1129–1139. doi: 10.1037//0022-006x.70.5.1129. [DOI] [PubMed] [Google Scholar]
- 72.Dahl JC, Wilson KG, Nilsson A. Acceptance and Commitment Therapy and the treatment of persons at risk for long-term disability resulting from stress and pain symptoms: A preliminary randomized trial. Behavior Therapy. 2004;35:785–801. [Google Scholar]
- 73.McCracken LM, Vowles KE, Eccleston C. Acceptance-based treatment for persons with complex, long standing chronic pain: A preliminary analysis of treatment outcome in comparison to a waiting phase. Behav Res Ther. 2005;43:1335–1346. doi: 10.1016/j.brat.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Vowles KE, McCracken LM, Eccleston C. Process of change in treatment for chronic pain: The contributions of pain, acceptance, and catastrophizing. Eur J Pain. 2007;11:779–787. doi: 10.1016/j.ejpain.2006.12.007. [DOI] [PubMed] [Google Scholar]