Abstract
Male bottlenose dolphins in Shark Bay, Western Australia form two levels of alliances; two to three males cooperate to herd individual females and teams of greater than three males compete with other groups for females. Previous observation suggested two alliance tactics: small four to six member teams of relatives that formed stable pairs or trios and unrelated males in a large 14-member second-order alliance that had labile trio formation. Here, we present evidence for a third level of alliance formation, a continuum of second-order alliance sizes and no relationship between first-order alliance stability and second-order alliance size. These findings challenge the ‘two alliance tactics’ hypothesis and add to the evidence that Shark Bay male bottlenose dolphins engage in alliance formation that likely places considerable demands on their social cognition.
Keywords: alliances, coalitions, social cognition
1. Introduction
Alliances and coalitions within social groups are considered to be a hallmark of social complexity [1,2]. Bottlenose dolphins (Tursiops sp.) in Shark Bay, Western Australia, were previously shown to exhibit two levels of male alliance formation within a large social network—a trait they share with humans but no other species [3,4]. Males in pairs and trios (= first-order alliances) cooperate to sequester individual females for ‘consortships’ and teams of greater than three males (= second-order alliances) cooperate to take and defend females from other groups [4].
Initial studies found stable male pairs and trios that associated in small second-order alliances (four to six males) often composed of relatives [4,5]. Later, we discovered a new alliance ‘tactic’: a large second-order ‘super-alliance’ whose 14 members were unrelated and engaged in a relatively labile trio formation [6,7]. Here, we present new results that challenge the ‘two alliance tactics’ hypothesis and reveal evidence of a third level of male alliance formation.
2. Material and methods
From July–November, 2001–2006, we studied alliances in a 600 km2 area along the east side of Peron Peninsula in Shark Bay. Data on male associations were collected on dolphin groups encountered in ‘surveys’ using a 10 m chain rule for group membership [8]. These data were used to generate half-weight association coefficients in socprog [9].
Data on second-order alliance stability were collected from instances of first-order alliance formation. A single consortship, lasting for any period of time, defined a single first-order alliance.
For each second-order alliance, we calculated an alliance stability index as 100 × [1 − (number of different alliances/number consortships)] for the group. This value will be low for a second-order alliance whose members combine in a large variety of pairs or trios to form consortships, and will be high for second-order alliances with stable first-order alliances. With a large number of consortships, the index for perfect stability will be similar for small and large groups, but if sample sizes are low this will not be the case (e.g. with 50 consortships perfect stability for a group of six with two trios will yield an index of 96 and a group of 12 with four trios 92, but with 10 consortships the numbers are 80 and 60). To compensate, we calculated the index as the ratio of the actual index to the index ‘expected’ if the alliances were completely stable.
To examine associations between second-order alliances and other male groups, we coded non-foraging survey groups for the presence of an alliance if it contained two or more members of that alliance. These data were used to calculate inter-alliance association coefficients and to test for inter-alliance partner preferences in socprog [9]. Further details on terms, methods and results are available in the electronic supplementary material.
3. Results
We focused our efforts on the most commonly found groups (table 1 summarizes data from 121 males that associated in 17 groups). The 121 males were observed in an average of 51 survey groups (range, 6–143; s.d. = 30.4). We documented 522 consortships, including 420 (80%) by male trios and 102 by male pairs. Individual males were observed in 1–35 consortships ( = 12.0; s.d. = 8.5).
= 12.0; s.d. = 8.5).
Table 1.
Characteristics of the 17 groups in the study. Significance was assessed at the 0.05 level with a two-tailed test using a Bonferroni correction (n = 14, we multiplied the socprog p-values by 78). Several other third-order alliance associations were recognized based on between group COA in the same range as ‘preferred partners’.
| alliance code | type | size | number of consortships | number different first-order alliances | alliance stability index | third-level associates (inter-group (COA)) | n for inter-group COA |
|---|---|---|---|---|---|---|---|
| RHP | first | 3 | 29 | n.a. | n.a. | PDa (16) | 82 |
| BB | first | 3 | 5 | n.a. | n.a. | SKa (15) | 30 |
| SK | first | 3 | 3 | n.a. | n.a. | BBa (15) | 11 |
| FCB | first | 3 | 4 | n.a. | n.a. | CBa (17) | 29 |
| PHG | first | 3 | 5 | n.a. | n.a. | CB (11) | 9 |
| HH | second | 6 | 26 | 6 | 83 | PB (16) | 39 |
| CB | second | 6 | 23 | 2 | 100 | FCBa (17); PHGa (11) | 66 |
| GG | second | 6 | 9 | 5 | 57 | 21 | |
| PD | second | 7 | 59 | 4 | 96 | KSa (10); RHPa (16) | 103 |
| RR | second | 7 | 26 | 21 | 21 | 48 | |
| HC | second | 7 | 23 | 7 | 80 | 68 | |
| BL | second | 8 | 28 | 22 | 25 | XF (11) | 53 |
| XF | second | 8 | 51 | 22 | 62 | BL (11) | 87 |
| WC | second | 10 | 52 | 18 | 69 | 38 | |
| SJ | second | 11 | 24 | 11 | 65 | 31 | |
| PB | second | 12 | 47 | 14 | 77 | HH (16) | 35 |
| KS | second | 14 | 108 | 36 | 69 | PDa (10) | 148 |
aIndicates preferred third-level associates based on permutation tests in socprog that excluded the SJ, PB and HH groups, whose winter range did not overlap the others.
Fifteen males associated in five trios that were not considered to be a part of any second-order alliance. There was a nearly continuous range in the size of the 12 second-order alliances, which had between six and 14 members (table 1). Of the 105 second-order alliance members, 102 males consorted females exclusively with members of their second-order alliance and three males engaged in one to four trio consortships with pairs of males from another second-order alliance. The alliance membership of one male was considered to be indeterminate. In total, of the 476 consortships by second-order alliance members, 15 (3%) were considered ‘anomalous’ (containing non-members).
Alliance size was not related to alliance stability (rs = −0.206, p = 0.52, n = 12; table 1). The two alliances that formed during the study were not large (seven and eight males) and had very low alliance stability values, but the removal of these two groups did not change the result (rs = −0.269, p = 0.45, n = 10).
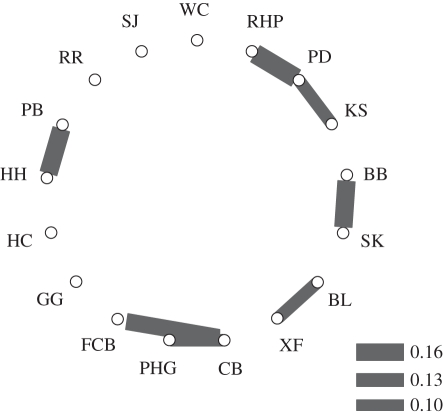
There were regular amicable low-level associations between particular second-order alliances and trios and a few second-order alliances. Permutation tests on 14 groups with extensive range overlap revealed significant association preferences between a pair of lone trios, two lone trios and a six-member second-order alliance, and a lone trio and a seven-member second-order alliance and an association that varied around significance between a seven-member and 14-member second-order alliance (table 1 and figure 1). Half-weight coefficients of association (COA) in the same range as those linking preferred third-order partners (10–17) were also found between two other second-order alliances (HH and PB = 16) and between XF and BL (11) during 2004–2006, the period of BL's inclusion in the analyses (table 1).
Figure 1.
Sociogram showing preferred third-order alliance associates based on permutation tests in socprog 2.4 (RHP and PD, PD and KS, CB and FCB) and other groups with between-group association coefficients in the same range (10–17) as those with significant associations (XF and BL, PB and HH, CB and PHG).
We observed seven conflicts during 2001–2006, involving members of three or more alliances, including four in which a female was taken, one probable failed theft attempt and two events that were joined in progress so we could not determine if a female was taken. Six events involved males from the KS, PD and RHP groups (figure 2), as follows:
2001: Three WC males attacked four KS males who had a female and who were with seven PD males who had two females. Thirty minutes after the attack seven more WC males arrived and the original three WC males took possession of the female. The KS males left after the fight and the PD and WC males remained together.
2001: We joined the conflict, involving nine KS, seven PD and seven WC males, in progress. The KS group left after the fight with a female, the seven PD group had two females throughout, but we did not see three WC males after they left so it was unclear if they had a female. The PD group stayed with some WC members after the fight and we observed petting between a PD and a WC male.
2002: All six GG males attacked four PD males and took their female. Within a minute, the four PD males were joined by six KS males and this group of 10 followed, but did not re-engage, the GG group for 30 min at 20–40 m. They then fell back to 150–200 m after 50 min, approached again to 20–30 m 65 min after theft, then fell back and were not seen again.
2002: Three KS males were approached by the seven PD members and the three RHP males. One PD trio had a female and the other took the female from the KS males.
2006: Four KS males attacked three PD males with a female and immediately the four other PD males and seven of the eight KS males in the area joined the group for totals of seven PD males and 11 KS males. One of the KS trios that joined had a female throughout the skirmish. Aggression (vocalizations and movement) escalated 20 min later when two RHP males (who had a female) entered the group. Four members of the RR alliance and a few immature unallied males were in the immediate vicinity and may have participated. Six minutes after the RHP males joined, the KS males split off but continued to follow the RHP and PD males, who remained together. The following day we encountered a resting group of three PD males and eight KS males. The three PD males still had the same female and the group included the four KS members that initiated the conflict the day before.
2006: The fight between 12 KS, three PD and eight WC males was joined in progress. After the fight, the WC males left with a female, and the KS and PD males remained together travelling. The PD males did not have a female and the KS group had two females. It is possible that WC took their female from three KS males who had her the day before.
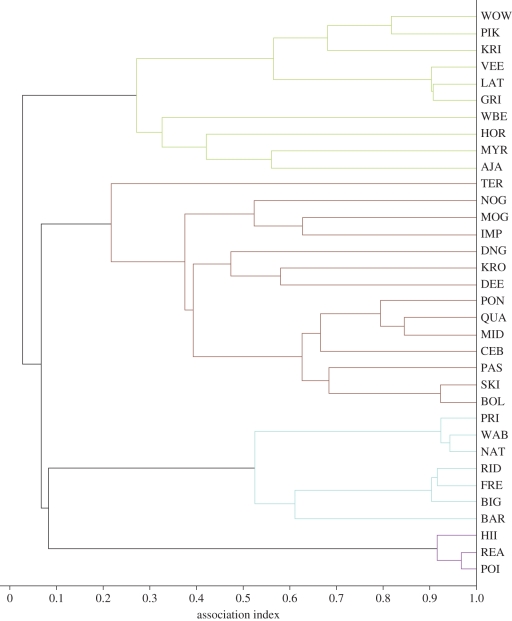
Figure 2.
Cluster diagram shows associations among 34 males in the WC (green), KS (brown), PD (blue) and RHP (purple) groups from 2001–2006. These groups were involved in six third-level alliance interactions (see text). The Average-linkage Cluster Diagram was generated in socprog 2.4 using half-weight association coefficients restricted to social, resting and travelling groups (WC n = 11–24; KS n = 24–77; PD n = 43–75; RHP n = 71–81).
4. Discussion
We reject the hypothesis of two distinct male alliance strategies in Shark Bay [5]. Instead, we found a continuum of second-order alliance sizes from 6–14 males. Both large and small second-order alliances may be stable for years [3].
We did not find a simple relationship between second-order alliance size and first-order alliance stability. The two groups that began consorting females during the study (BL and RR) had the lowest alliance stability values. Low alliance stability in younger groups is expected in systems with competition for social partners.
We found consistent low-level associations between particular male groups. Any association between males in different groups is surprising as the two levels of alliance formation we documented previously are clearly based on access to females. Our permutation analysis asked the question, ‘If a male is with at least one of his allies, do they exhibit preferences when associating with other alliances?’ We found that in some cases they do. These associations are not related to food resources as we restricted analyses to non-foraging groups. The fights involving greater than two groups suggest that these associations, like the second-order alliances, are employed in conflicts over females.
These fights were chaotic as they involved 13–23 males, so the only evidence of one group supporting another is based on who remained together when normal behaviour resumed after the fight.
Four of the five trios that did not have second-order alliance partners were older males that were adults consorting females in the 1980s as part of second-order alliances. The second-order alliance history of the other trio (PGH) is unknown. The need to have allies for female defence may explain the third-order associations that older male trios form with second-order alliances (e.g. CB with FCB) or each other (BB with SK) after their former allies have disappeared.
The dolphins' fission–fusion grouping pattern as well as variation in second-order alliance size may favour males having third-order alliance relationships. Second-order alliance partners may not always be present when rivals appear and even some third-order allies may not be enough (e.g. example 3).
Only humans and Shark Bay bottlenose dolphins are known to have multiple-level male alliances within a social network. It is unlikely a coincidence that humans and dolphins also have in common the largest brains, relative to body size, among mammals. Our evidence for a third level of alliance formation in the dolphins should refocus attention on the potential cognitive burdens for individuals embedded in such a system, where decisions at one level may have impacts at other levels (reviewed in [3]).
Acknowledgements
This study was supported by grants from the Australian Research Council (A19701144 and DP0346313), The Eppley Foundation for Research, The Seaworld Foundation, The W. V. Scott Foundation, The National Geographical Society's Committee for Research and Exploration and NSF (1316800). Accommodation was very generously provided by the Monkey Mia Dolphin Resort. Permits were obtained from the West Australian Department of Environment and Conservation. Many generous people helped out on this project. We thank Hal Whitehead for comments on the permutation analyses.
Footnotes
One contribution to a Special Feature on ‘Cognition in the wild’.
References
- 1.Cords M. 1997. Friendships, alliances, reciprocity and repair. In Machiavellian intelligence II (eds Whiten A., Byrne R.), pp. 24–49 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Harcourt A. H. 1992. Coalitions and alliances: are primates more complex than non-primates? In Coalitions and alliances in humans and other animals (eds Harcourt A. H., deWaal F. B. M.), pp. 445–471 Oxford, UK: Oxford University Press [Google Scholar]
- 3.Connor R. C. 2007. Complex alliance relationships in bottlenose dolphins and a consideration of selective environments for extreme brain size evolution in mammals. Phil. Trans. R. Soc. B 362, 587–602 10.1098/rstb.2006.1997 (doi:10.1098/rstb.2006.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connor R. C., Smolker R. A., Richards A. F. 1992. Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.). Proc. Natl Acad. Sci. USA 89, 987–990 10.1073/pnas.89.3.987 (doi:10.1073/pnas.89.3.987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krützen M., Sherwin W. B., Connor R. C., Barré L. M., Van de Casteele T., Mann J., Brooks R. 2003. Contrasting relatedness patterns in bottlenose dolphins (Tursiops sp.) with different alliance strategies. Proc. R. Soc. Lond. B 270, 497–502 10.1098/rspb.2002.2229 (doi:10.1098/rspb.2002.2229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connor R. C., Heithaus M. R., Barre L. M. 1999. Super-alliance of bottlenose dolphins. Nature 371, 571–572 10.1038/17501 (doi:10.1038/17501) [DOI] [Google Scholar]
- 7.Connor R. C., Heithaus M. R., Barre L. M. 2001. Complex social structure, alliance stability and mating access in a bottlenose dolphin ‘super-alliance’. Proc. R. Soc. Lond. B. 268, 263–267 10.1098/rspb.2000.1357 (doi:10.1098/rspb.2000.1357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smolker R. A., Richards A. F., Connor R. C., Pepper J. 1992. Association patterns among bottlenose dolphins in Shark Bay, Western Australia. Behaviour 123, 38–69 10.1163/156853992X00101 (doi:10.1163/156853992X00101) [DOI] [Google Scholar]
- 9.Whitehead H. 2006. SOCPROG: programs for the analysis of animal social structure. See http://myweb.dal.ca/hwhitehe/social.htm. [Google Scholar]