Abstract
Individual preference for the use of one limb over the other to explore the environment or manipulate objects is common trait among vertebrates. Here, we explore the hypothesis that limb preference is determined by the engagement of a particular cerebral hemisphere to analyse certain stimuli. We recorded the eye and foot preferences of 322 individuals from 16 species of Australian parrots while investigating potential food items. Across all species, eye preferences explained 99 per cent of the variation in foot use in Australian parrots. The vast majority of species showed significant relationships between eye and foot preferences at the population level.
Keywords: eye preference, handedness, laterality, cognition
1. Introduction
Despite the fact that the two hemispheres of the vertebrate brain look similar, they perform specialized cognitive functions. The right hemisphere generally executes rapid responses and attends to novelty, whereas the left hemisphere is involved in responses that require consideration of alternatives and is used to categorize stimuli [1]. This partitioning of information processing is referred to as cerebral lateralization. Lateralization is common place in vertebrates and is increasingly evident in invertebrates [2,3]. Cerebral lateralization is frequently overtly expressed as behavioural asymmetries. The most celebrated example is hand preferences in humans and other animals.
Parrots show foot preferences while perching and manipulating food items of a similar strength to those observed in humans. However, parrot species vary in their foot preference and the strength of laterality has previously been linked to discrimination and problem solving abilities [4]. Such variation between parrot species challenges the assumption that cerebral lateralization shows a conservative pattern across all vertebrates. While variation exists between species, there may also be variation within species. For example, approximately 10 per cent of humans are left handed but they do not necessarily show corresponding reversal of cognitive function [5]. Analysis of laterality in frequent situs inversus lines of zebrafish has shown that visceral, neuroanatomical and behavioural asymmetries are frequently coupled together but some behavioural responses defy the pattern and are always controlled by the same hemisphere [6]. Thus, the link between cerebral lateralization and laterality is far from clear.
Previous work on the chick model has shown that birds have a preferred hemisphere for discriminating food from non-food items [7]. Moreover, strongly lateralized pigeons and parrots are more efficient at forging discrimination tasks where grain must be picked out from a pebble matrix [4,8]. Many parrots use a preferred foot to manipulate food items and we suggest that foot preferences probably reflect the hemisphere that is controlling food discrimination processing. Here, we examined the foot and eye preferences of 16 species of Australian parrots to explicitly test the hypothesis that cerebral lateralization directly determines foot preferences.
2. Material and methods
All test subjects were made available through a number of zoos, wildlife parks and members of the Parrot Society of Australia. We were able to obtain suitable replicate numbers (n < 10) for a total of 16 species (table 1). Testing began by introducing a platform to the centre of the enclosure. After the birds had habituated to the platform, a small piece of fruit was placed on it. Using two stop watches, we recorded the dominant eye that subjects used to fixate on the food item as they approached it based on the orientation of the head. The foot used to grasp the food item was then recorded. In general, parrots grasp food items with the foot and rarely switch between feet once they start feeding [9]. Each individual was tested in this manner 10 times. Having tested nine species with fruit, we then tested seven species with brightly coloured, small wooden blocks. While the blocks represented a novel object, we reasoned their bright coloration would mimic fruit and we expected the parrots would investigate them in the same manner as they would potential food items.
Table 1.
Regression of left-foot preferences against left-eye preferences in 18 species of Australian parrots. All correlations were positive with the exception of the cockatiel.
| species | test stimuli | n | F | p | r2 |
|---|---|---|---|---|---|
| Bourke's parrot | block | 20 | 67.371 | <0.001 | 0.789 |
| budgie | block | 20 | 38.747 | <0.001 | 0.683 |
| cockatiel | block | 20 | 1.262 | 0.276 | 0.066 |
| crimson rosella | fruit | 12 | 31.91 | <0.001 | 0.761 |
| eclectus parrot | fruit | 20 | 20.782 | <0.001 | 0.536 |
| galah | fruit | 20 | 168.442 | <0.001 | 0.903 |
| king parrot | fruit | 20 | 1.742 | 0.203 | 0.088 |
| little corella | fruit | 20 | 15.907 | <0.001 | 0.469 |
| little lorikeet | block | 15 | 35.573 | <0.001 | 0.738 |
| Major Mitchell's cockatoo | fruit | 15 | 0.058 | 0.813 | 0.004 |
| rainbow lorikeet | block | 20 | 50.1 | <0.001 | 0.736 |
| red-rumped parrot | block | 20 | 23.74 | <0.001 | 0.569 |
| red-tailed black | fruit | 20 | 2.934 | 0.104 | 0.14 |
| red-winged parrot | fruit | 10 | 1.882 | 0.207 | 0.19 |
| sulphur-crested cockatoo | fruit | 20 | 25.549 | <0.001 | 0.587 |
| superb parrot | fruit | 20 | 14.01 | 0.002 | 0.406 |
| turquoise parrot | block | 10 | 44.307 | <0.001 | 0.847 |
| yellow-tailed black | fruit | 20 | 4.668 | 0.045 | 0.206 |
For each individual, we generated an eye and foot preference score ranging from 0 (completely right biased) to 100 (completely left biased). The mean and the standard error of each score were calculated at the species level. The relationship between foot preference and eye preference was analysed using linear regression. We performed two levels of analysis, one for each species and one using the species means. Analysis of the residuals showed that the data were normally distributed.
3. Results
Eleven of the 16 species examined showed significant correlations between eye preferences and hand preferences while viewing and manipulating food and potential food items (see the electronic supplementary material for species-specific regression figures). Of the five that showed non-significant correlations, four of them displayed limited variation in one or both traits (i.e. laterality was almost entirely fixed in the population). Only the cockatiel showed a large degree of variation in both traits but still showed a non-significant relationship between eye and foot preferences (table 1; figures in the electronic supplementary material).
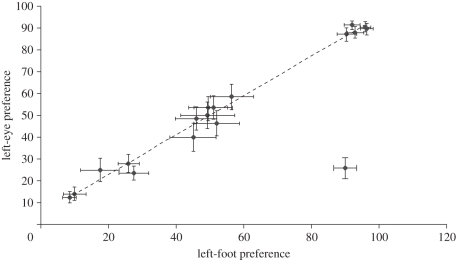
When the means of each of the species were analysed, a highly significant relationship between foot preference and eye preference was revealed (F1,16 = 46.884, p < 0.001; r2 = 0.73). Examination of figure 1 revealed a single outlier which was identified as the cockatiel. Removal of this species from the analysis greatly improved the explanatory power (F1,16 = 1075.415, p < 0.001; r2 = 0.99).
Figure 1.
Mean (±s.e.) hand and eye preferences for 16 species of Australian parrots. The regression line illustrated (r2 = 0.99) was calculated based on the exclusion of a single outlier.
4. Discussion
Australian parrot species vary tremendously in their foot preferences while manipulating food items [4]. Here, we show that this foot preference is strongly correlated with the eye that they use to scrutinize potential food items. Fixation on potential food items using a preferred eye explained 99 per cent of the variation in foot use when the parrots grasped the item. Only a single species defied the pattern. This result strongly suggests that cerebral lateralization is directly linked to behaviourally lateralized traits and provides a functional explanation for the evolution of handedness in vertebrates.
Our data suggest that functional partitioning of information processing in each hemisphere of the brain is highly correlated with the evolution and development of limb preferences while performing particular tasks. Rogers [10] has hypothesized that hand use in primates reflects the obligate use of one hemisphere and the extent to which handedness is expressed depends on the nature of the task rather than its complexity. However, laterality of behavioural traits existed long before the tetrapod divergence and we argue that cerebral lateralization drives the development of preferential limb use. Fishes, for example, show turn biases while exploring radial mazes [11] and even show preferences when using modified fins to explore novel objects that are associated with the preferred use of the ipsilateral eye [12]. Similarly, bees show enhanced recall of associations between odours and food rewards 6 h post-training when using the left antennae [13]. While this may be a case of convergent evolution, an alternative explanation suggests that the relationship between cerebral lateralization and limb preferences is an ancient and highly conserved evolutionary trait. We propose that limb preferences while performing certain tasks are a reflection of the dominant cerebral hemisphere that is involved in analysing the information related to the task at hand.
Significant relationships between eye preference and food preferences were revealed in 11 out of the 16 parrot species studied. In most of these 11 species, there was considerable individual variation across the laterality spectrum in both traits. The explanatory power of eye preference on foot preferences ranged from 20 to 90 per cent (mean 68%) although the lowest level was exhibited in a species comprised of highly lateralized individuals exhibiting little variation at the population level. In four species, the lateral bias was so strong at the population level that there was too little variation for the regression to work effectively. In these species, it seems that laterality has run to fixation. In only one species where considerable population variation was present, the cockatiel, did we find no relationship between eye and foot preference. It is unclear why the cockatiel should be exceptional in this regard, but we suggest that the variation in laterality in parrot species generally may be related to their feeding ecology. Cockatiels graze on small grass seeds that may require little coordination between the eyes and feet. This is unlikely to be the reason for the lack of correlation between foot and eye use in this species, however, given that many other small parrots also feed in this way.
A number of taxa have laterally placed eyes with little visual overlap in the optic field, therefore, laterality is likely to be strong in a wide range of animals. Most of the information that is received in each hemisphere from the contralateral eye is largely independent from the ipsilateral eye and is analysed separately. In the case of parrots, potential food items are processed with a specific hemisphere of the brain and the contralateral eye is then used to view the object. The hemisphere that is used to analyse certain stimuli can vary between species and even within species [4]. In order to make the most of their discriminatory capabilities, the potential food item is grasped with the corresponding foot and brought closer to the preferred eye for further scrutiny. Thus, one might expect that the link between visual asymmetry and the preferred limb used to explore the environment is a common feature in tetrapods and perhaps even some invertebrates.
Acknowledgements
This project was conducted with permission from the Macquarie University Ethics Board permit number 2007/034.
We thank the large number parrot breeders and staff from various zoos and animal parks for permitting us to conduct research on their birds. Also thanks to M. D. Magat for participating in data collection. This research was supported by the Australian Research Council and Macquarie University.
References
- 1.Rogers L. J., Zucca P., Vallortigara G. 2004. Advantages of having a lateralized brain. Proc. R. Soc. Lond. B 271, S420–S422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne R. A., Kuba M. J., Meisel D. V. 2004. Lateralized eye use in Octopus vulgaris shows antisymmetrical distribution. Anim. Behav. 68, 1107–1114 10.1016/j.anbehav.2003.11.027 (doi:10.1016/j.anbehav.2003.11.027) [DOI] [Google Scholar]
- 3.Vallortigara G. 2000. Comparative neuropsychology of the dual brain: a stroll through animals' left and right perceptual worlds. Brain Lang. 73, 189–219 10.1006/brln.2000.2303 (doi:10.1006/brln.2000.2303) [DOI] [PubMed] [Google Scholar]
- 4.Magat M., Brown C. 2009. Laterality enhances cognition in Australian parrots. Proc. R. Soc. B 276, 4155–4162 10.1098/rspb.2009.1397 (doi:10.1098/rspb.2009.1397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faurie C., Raymond M. 2004. Handedness frequency over more than ten thousand years. Proc. R. Soc. Lond. B 271, S43–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barth K. A., Miklosi A., Watkins J., Bianco I. H., Wilson S. W., Andrew R. J. 2005. fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr. Biol. 15, 844–850 10.1016/j.cub.2005.03.047 (doi:10.1016/j.cub.2005.03.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers L. J. 1990. Light input and the reversal of functional lateralization in the chicken brain. Behav. Brain Res. 38, 211–221 10.1016/0166-4328(90)90176-F (doi:10.1016/0166-4328(90)90176-F) [DOI] [PubMed] [Google Scholar]
- 8.Gunturkun O., Diekamp B., Manns M., Nottelmann F., Prior H., Schwarz A., Skiba M. 2000. Asymmetry pays: visual lateralization improves discrimination success in pigeons. Curr. Biol. 10, 1079–1081 10.1016/S0960-9822(00)00671-0 (doi:10.1016/S0960-9822(00)00671-0) [DOI] [PubMed] [Google Scholar]
- 9.Friedmann H., Davis M. 1938. ‘Left-handedness’ in parrots. Auk 55, 478–480 [Google Scholar]
- 10.Rogers L. J. 2009. Hand and paw preferences in relation to the lateralized brain. Phil. Trans. R. Soc. B 364, 943–954 10.1098/rstb.2008.0225 (doi:10.1098/rstb.2008.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown C., Braithwaite V. A. 2005. Effects of predation pressure on the cognitive ability of the poeciliid. Brachyraphis episcopi. Behav. Ecol. 16, 482–497 10.1093/beheco/ari016 (doi:10.1093/beheco/ari016) [DOI] [Google Scholar]
- 12.Bisazza A., Lippolis G., Vallortigara G. 2001. Lateralization of ventral fins use during object exploration in the blue gourami (Trichogaster trichopterus). Physiol. Behav. 72, 575–578 10.1016/S0031-9384(01)00417-6 (doi:10.1016/S0031-9384(01)00417-6) [DOI] [PubMed] [Google Scholar]
- 13.Rogers L. J., Vallortigara G. 2008. From antenna to antenna: lateral shift of olfactory memory recall by honey bees. PLoS ONE 3, e2340. 10.1371/journal.pone.0002340 (doi:10.1371/journal.pone.0002340) [DOI] [PMC free article] [PubMed] [Google Scholar]