Abstract
Begging by nestling birds has been used to test evolutionary models of signalling but theory has outstripped evidence. Eavesdropping predators potentially impose a cost on begging that ensures signal honesty, yet little experimental evidence exists for such a cost at active nests because the use of artificial nests, long playback bouts and absence of parents may have exaggerated costs. We broadcast short periods (1 h) of either nestling vocalizations or background noise at active white-browed scrubwren, Sericornis frontalis, nests. Nestlings called naturally during both treatments, allowing us to test whether elevated calling increases risk, a key but rarely tested assumption of evolutionary models. Predators visited nests exclusively during periods of elevated calling. Furthermore, playbacks affected neither adult visits nor nestling activity, suggesting that calling alone attracted predators. Adults gave alarm calls and nestlings usually called less when predators approached nests. Predation risk to broods is, therefore, likely to fluctuate substantially over short periods of time, depending on nestling hunger and whether adults or young have detected predators. This study confirms a present-day cost of nestling begging, demonstrates that this cost can be incurred over short periods and supports the importance of parent–offspring antipredator strategies in reducing predation risk.
Keywords: begging, predation, bioacoustics, nestling, white-browed scrubwren, communication
1. Introduction
Predation is the major cause of breeding failure in many bird species [1], and the risk posed by eavesdropping predators is an important component of evolutionarily stable models of nestling begging [2], but to date there is limited experimental evidence that nestling vocalizations at active nests attract predators [3]. Here, we report the predation risks of nestling vocalizations using realistic playbacks of nestlings at active songbird nests.
Previous studies have been valuable in showing that predators can be attracted to begging calls, but their ability to assess begging costs at active nests has been limited [3]. First, playback methods have probably exaggerated predation risk. Most studies have played back loops of nestling begging at high rates for long periods [4–6], and several have played back the begging calls of cavity or tree nesting species at ground nests [5–7], even though ground-nesting species tend to have more cryptic begging calls [8]. Additionally, most studies have compared the costs of begging at high rates to silence, even though nestlings are not silent for extended periods of time (but see [4,5,9]). Second, the use of artificial nests can be misleading. Most studies have used artificial nests, yet even when carefully placed they frequently have higher predation rates, and are depredated by different predators, than real nests at sites chosen by parents [10–12]. The use of dummy eggs can also bias our understanding of what predators visit nests, because damage to eggs may not reflect risk to nestlings, which can be vulnerable to different predators [10]. Finally, the use of artificial nests discounts adult and nestling behaviour, which could either increase or decrease predation risk (reviewed in [13,14]). Adult activity can attract predators [15], and nestling jostling could increase conspicuousness, regardless of vocal behaviour [16]. Alternatively, adults can mitigate risk by silencing nestlings with alarm calls, and nestlings can crouch or become silent when they detect predators nearby [14].
We examined the predation risk of nestling calling at active white-browed scrubwren, Sericornis frontalis, nests. Scrubwrens are an appropriate species for this study because nestlings beg when adults visit and also call between visits. Furthermore, scrubwrens use behavioural adaptations to manage nest predation risk: adults silence young with alarm calls, and nestlings go silent in response to the acoustic cues of a predator walking [17,18]. We assessed whether playback of nestling vocalizations at natural rates over a relatively short period (1 h) attracted predators to nests. We also examined whether adults and nestlings behaved in ways that could minimize risk when predators were present.
2. Material and methods
We conducted playback experiments in the Australian National Botanic Gardens in 2009. Scrubwrens are small, dome-nesting songbirds whose primary predators across much of southeastern Australia are pied currawongs, Strepera graculina [19]. Currawongs are territorial, omnivorous songbirds that hunt by sight and sound [18]. Scrubwren nests are extremely cryptic, and acoustic cues are probably a principal way that predators detect nests.
Nestlings call when parents visit the nest (begging calls: 200–800 ms, with sidebands and harmonics), as well as when parents are away (repeat calls: 50–150 ms, often without sidebands; [14]). Begging calls are usually louder than repeat calls (below) but only last several seconds, whereas bouts of repeat calls often go on for tens of minutes. Both call types can be audible from over 2 m away (T. Haff 2008–2009, personal observations). The rate and amplitude of begging and repeat calls increase as nestlings become hungrier [20].
We played back nestling vocalizations and control sounds at 21 nests containing 7–10 day old nestlings (electronic supplementary material). Playbacks of nestling vocalizations mimicked a brood of three, and consisted of 12 s of begging calls, played only during adult visits, and repeat calls, played continuously when adults were not at the nest, at a natural rate of three per second. Control sounds consisted of continuous amplified background noise, and tested whether nestling vocalizations or background noise affected the behaviour of predators, adults or nestlings. Nestlings were never completely silent, however, and so our playbacks contrasted hungry broods to average broods, instead of hungry broods to silent ones. Nestling calls and background sounds were recorded at each experimental nest when the young were 4–5 days old. Repeat and begging calls were played back at 65 and 80 dB at 20 cm, respectively, both at the upper natural range for 8 day old nestlings, and background noise was amplified to the same level as the background between amplified repeat calls (average 32 ± 2 dB among replicates). Vocalization playbacks were deliberately at the upper range of call rate and amplitude in order to mimic a hungry brood, as predation risk is predicted to increase with begging intensity [2].
Playbacks were 30 min long and were presented at nests twice on a single day, with at least 60 min between bouts, so that each nest was exposed to a total of 60 min of playback. We used the same playback at a nest on a single day in order to avoid carryover effects of brood noisiness on adult behaviour, and presented the alternate playback type the following day. Playback presentation order was alternated between nests, which were distributed across the 40 ha study site.
We measured predator attraction to nests as approach within 2 m. As controls, we measured: (i) adult activity, as the number of nest visits; and (ii) nestling vocal activity, as the total number of calls, mean duration (seconds) of measured calls and mean maximum power (dB) of calls. We tallied nestling calls in a subset of five randomly selected minutes of each playback trial in Raven 1.3, and measured call properties for two of those minutes, also randomly selected. In total, we tallied 27 687 and measured 11 275 calls. We compared whole broods, as we could not separate the calls of individuals. We also measured antipredator behaviour of nestlings and adults (mobbing calls by adults, silence by nestlings) by comparing calling during the 60 s after a predator arrived to calling during the same minute of the replicate playback at the same nest on the same day, when no predator was present. To minimize predation risk, we placed predator exclosures around nests, and ceased playback if a predator approached within 2 m. We analysed data using non-parametric statistics and a generalized linear model (GLM) with a binomial distribution and logit link function, conducted in SPSS 17.1 and R 2.1.
3. Results
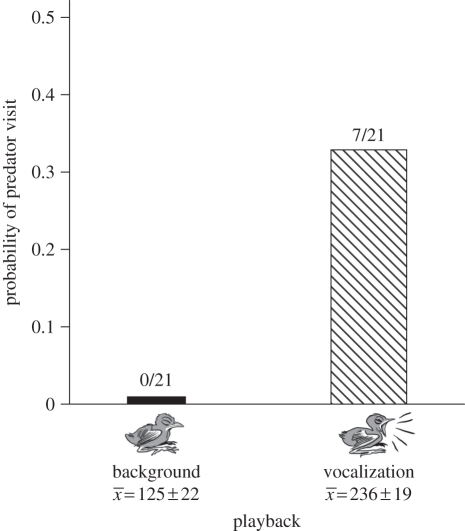
Predators, all currawongs, approached nests exclusively during nestling vocalization playbacks (seven visits during nestling vocalization, none during background playback; two-tailed binomial test p = 0.016; figure 1). No nest was approached more than once. Currawongs approached nests on foot, except for one individual that flew between perches. Combined playback and natural nestling calling during the vocalization playback approached the top of the range for natural broods (this study), and was greater than calling during the background playback (mean number of calls per minute ± s.e.: vocalization playback and natural calls combined 236.1 ± 18.6, background 125.2 ± 21.9, Wilcoxon Z = −3.88, p < 0.0001; mean duration combined calls: 0.12 ± 0.01 s, background 0.09 ± 0.05 s, Z = −2.0, p = 0.05; mean maximum power: combined calls 66.8 ± 0.9 dB, background 62.2 ± 1.3 dB, Z = −3.32, p = 0.001; figure 1).
Figure 1.
Probability of predator visits to nests, comparing mean number of calls per minute (±s.e.) during background and nestling vocalization playbacks.
Predator attraction to nestling calling was not confounded by changes in adult behaviour or nestling activity between treatments. There were no differences in scrubwren activity between treatments (mean adult visits: vocalization 10.9 ± 0.9, background 12.0 ± 1.0, paired t-test, t20 = −1.1, p = 0.3; mean number of nestling calls per minute: vocalization 104.0 ± 15.2, background 125.2 ± 21.9, Z = −1.04, p = 0.3; mean call duration: vocalization 0.10 ± 0.06 s, background 0.09 ± 0.05 s, Z = −8.52, p = 0.4; mean maximum power: vocalization 62.5 ± 1.2 dB, background 62.2 ± 1.3 dB, Z = −0.3, p = 0.8). Further, predator visits were not owing to between-nest differences in adult or nestling activity (binomial GLM including parental visits and nestling activity, 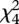 = 0.69, p = 0.95; mean parental visits, vocalization playback: predator visit 12.0 ± 1.4, no visit 12.0 ± 1.5; mean number of natural nestling calls per minute, vocalization playback: predator visit 89.3 ± 26.5, no visit 111.3 ± 18.9; mean duration: predator visit 0.11 ± 0.02 s, no visit 0.11 ± 0.02 s; mean maximum power: predator visit 61.1 ± 2.6 dB, no visit 62.86 ± 1.4 dB). Predators arrived an average of 16.3 ± 4.6 min after the start of playback, with two approaches on day 1 and five on day 2 (binomial test p = 0.45).
= 0.69, p = 0.95; mean parental visits, vocalization playback: predator visit 12.0 ± 1.4, no visit 12.0 ± 1.5; mean number of natural nestling calls per minute, vocalization playback: predator visit 89.3 ± 26.5, no visit 111.3 ± 18.9; mean duration: predator visit 0.11 ± 0.02 s, no visit 0.11 ± 0.02 s; mean maximum power: predator visit 61.1 ± 2.6 dB, no visit 62.86 ± 1.4 dB). Predators arrived an average of 16.3 ± 4.6 min after the start of playback, with two approaches on day 1 and five on day 2 (binomial test p = 0.45).
Adults and nestlings used defensive behaviours when a predator was near the nest. Adults gave alarm calls only when they saw a predator near the nest (mean number of alarms: currawong present 40.9 ± 27.6, not present 0.0, Wilcoxon Z = −2.02, p = 0.04), and nestlings tended to call less when a predator was nearby (mean number of calls: currawong present 5.7 ± 4.8, not present 28.7 ± 10.3, Z = −1.86, p = 0.06).
4. Discussion
Nestling vocalizations attracted avian predators to nests attended by parents. Neither adult nor natural nestling activity differed between playback types, indicating that predators were attracted by nestling calls alone. To our knowledge, this is the first experimental study demonstrating that nestling calling at active nests can attract predators. Further, we show that the cost of nestling calling can be incurred over short periods. This contrasts with previous studies, which have documented high predation rates after extended playback periods [4,5,7,9]. No nest was completely silent during ‘quiet’ playback treatments, yet predators only visited nests when calling was highest, confirming the begging model assumption that costs increase with signal exaggeration, so maintaining signal honesty [2]. Furthermore, because begging intensity varies in response to nestling hunger [20], risk will fluctuate temporally. Thus, although the daily predation rate on nests with young at our study site is 4 per cent [18], the risk to hungry broods is much higher (figure 1).
Adults gave mobbing calls, which silence young, when a predator was at the nest, and nestlings tended to call less during predator visits, consistent with results of previous playback experiments [17,18]. Nestling detection of danger was imperfect, however, and some broods continued to call when a predator was nearby. This occurred when adults were not present to alarm call and the predator flew to a nearby perch, instead of approaching on foot (T. Haff 2009, personal observations). We conclude that nestlings are most vulnerable when they are hungry, when parents are absent and when predators approach nests silently.
Our study suggests that begging can incur predation costs, and shows that past studies have not simply measured the ‘ghost of predation past’ [3]. Future studies at active nests incorporating incremental changes in nestling vocalizations, and separating out the relative influences of begging and repeat calling, will be valuable in helping to evaluate the true risks that nestlings take in crying out to be fed.
Acknowledgements
Permits were provided by the Australian National Botanic Gardens, Environment ACT, Australian Birds and Bat Banding Scheme and ANU Ethics Committee.
We thank Andrew Horn, Naomi Langmore and the Acoustic Ecology Laboratory for comments, Adam Searcy for field assistance and Ainsley Seago for artwork. The Australian Research Council funded the work.
References
- 1.Ricklefs R. E. 1969. An analysis of nesting mortality in birds. Smithson. Contrib. Zool. 9, 1–48 [Google Scholar]
- 2.Godfray H. C. J. 1995. Evolutionary theory of parent–offspring conflict. Nature 376, 133–138 10.1038/376133a0 (doi:10.1038/376133a0) [DOI] [PubMed] [Google Scholar]
- 3.Haskell D. G. 2002. Begging behaviour and nest predation. In Evolution of begging: competition, cooperation and communication (eds Wright J., Leonard M.), pp. 163–172 Dordrecht, The Netherlands: Kluwer Academic Publication [Google Scholar]
- 4.Dearborn D. C. 1999. Brown-headed cowbird nestling vocalizations and risk of nest predation. Auk 116, 448–457 [Google Scholar]
- 5.Haskell D. G. 1994. Experimental evidence that nestling begging behaviour incurs a cost due to nest predation. Proc. R. Soc. Lond. B 257, 161–164 10.1098/rspb.1994.0110 (doi:10.1098/rspb.1994.0110) [DOI] [Google Scholar]
- 6.Haskell D. G. 1999. The effect of predation on begging-call evolution in nestling wood warblers. Anim. Behav. 57, 893–901 10.1006/anbe.1998.1053 (doi:10.1006/anbe.1998.1053) [DOI] [PubMed] [Google Scholar]
- 7.Leech S. M., Leonard M. L. 1997. Begging and the risk of predation in nestling birds. Behav. Ecol. 8, 644–646 10.1093/beheco/8.6.644 (doi:10.1093/beheco/8.6.644) [DOI] [Google Scholar]
- 8.Briskie J. V., Martin P. R., Martin T. E. 1999. Nest predation and the evolution of nestling begging calls. Proc. R. Soc. Lond. B 266, 2153–2159 10.1098/rspb.1999.0902 (doi:10.1098/rspb.1999.0902) [DOI] [Google Scholar]
- 9.McDonald P. G., Wilson D. R., Evans C. 2009. Nestling begging increases predation risk, regardless of spectral characteristics or avian mobbing. Behav. Ecol. 20, 821–829 10.1093/beheco/arp066 (doi:10.1093/beheco/arp066) [DOI] [Google Scholar]
- 10.Thompson F. R., Burhans D. E. 2004. Differences in predators of artificial and real songbird nests: evidence of bias in artificial nest studies. Conserv. Biol. 18, 373–380 10.1111/j.1523-1739.2004.00167.x (doi:10.1111/j.1523-1739.2004.00167.x) [DOI] [Google Scholar]
- 11.Weidinger K. 2001. How well do predation rates on artificial nests estimate predation on natural passerine nests? Ibis 143, 632–641 10.1111/j.1474-919X.2001.tb04891.x (doi:10.1111/j.1474-919X.2001.tb04891.x) [DOI] [Google Scholar]
- 12.Fontaine J. J., Martel M., Markland H. A., Niklison A. A., Decker K. L., Martin T. E. 2007. Testing ecological and behavioral correlates of nest predation. Oikos 116, 1887–1894 10.1111/j.2007.0030-1299.16043.x (doi:10.1111/j.2007.0030-1299.16043.x) [DOI] [Google Scholar]
- 13.Lima S. L. 2009. Predators and the breeding bird: behavioral and reproductive flexibility under the risk of predation. Biol. Rev. 84, 485–513 10.1111/j.1469-185X.2009.00085.x (doi:10.1111/j.1469-185X.2009.00085.x) [DOI] [PubMed] [Google Scholar]
- 14.Magrath R. D., Haff T. M., Horn A. G., Leonard M. L. 2010. Calling in the face of danger: how predation risk affects acoustic communication by parent birds and their offspring. Adv. Study Behav. 41, 187–253 10.1016/S0065-3454(10)41005-0 (doi:10.1016/S0065-3454(10)41005-0) [DOI] [Google Scholar]
- 15.Martin T. E., Scott J., Menge C. 2000. Nest predation increases with parental activity: separating nest site and parental activity effects. Proc. R. Soc. Lond. B 267, 2287–2293 10.1098/rspb.2000.1281 (doi:10.1098/rspb.2000.1281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redondo T., Castro F. 1992. The increase in risk of predation with begging activity in broods of magpies Pica pica. Ibis. 134, 180–187 10.1111/j.1474-919X.1992.tb08395.x (doi:10.1111/j.1474-919X.1992.tb08395.x) [DOI] [Google Scholar]
- 17.Haff T. M., Magrath R. D. 2010. Vulnerable but not helpless: nestlings are fine-tuned to cues of approaching danger. Anim. Behav. 79, 487–496 10.1016/j.anbehav.2009.11.036 (doi:10.1016/j.anbehav.2009.11.036) [DOI] [Google Scholar]
- 18.Platzen D., Magrath R. D. 2004. Parental alarm calls suppress nestling vocalization. Proc. R. Soc. Lond. B 271, 1271–1276 10.1098/rspb.2004.2716 (doi:10.1098/rspb.2004.2716) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins P. J., Peter J. M. () 2002. Handbook of Australian, New Zealand and Antarctic birds, volume 6: pardalotes to shrike-thrushes. South Melbourne, Australia: Oxford University Press [Google Scholar]
- 20.Maurer G., Magrath R. D., Leonard M. L., Horn A. G., Donnelly C. 2003. Begging to differ: scrubwren nestlings beg to alarm calls and vocalize when parents are absent. Anim. Behav. 65, 1045–1055 10.1006/anbe.2003.2148 (doi:10.1006/anbe.2003.2148). [DOI] [Google Scholar]