Abstract
Although it has been shown that prosocial behaviour is heritable, it has not yet been established whether narrower aspects of prosociality are heritable, nor whether a common mechanism influences prosociality across its multiple domains. Here, we examine civic duty, work-place commitment and concern for the welfare of others with a study of prosocial obligations in 958 adult twin-pairs. Multivariate modelling indicated the existence of genetic factors underlying general prosocial obligations in females, with familial effects (genetic and shared-environment effects were indistinguishable) influencing this general mechanism in males. At the domain-specific level, modest genetic effects were observed in females for civic and work obligations, with shared-environment effects influencing welfare obligations. In males, genetic influences were observed for welfare obligation, with unique environments affecting work and civic duty.
Keywords: behaviour genetics, prosociality, obligations, civic duty, twins, welfare
1. Introduction
The ability to behave in a prosocial manner is a prerequisite for large-scale social living. The presence of empathy and helping behaviour even in infancy [1] is suggestive of an innate capacity to behave in a prosocial manner. Furthermore, twin studies capable of decomposing behaviour into additive genetic, shared- or family environmental, and unique-environmental influences have shown heritable components for both empathy [2] and prosociality [3–5] (but see [6]) with evidence for cross-culture generality in these effects [4].
While a biological basis for prosociality has been established, important domains of prosociality, such as civic obligations, obligations in work [7] and redistribution of wealth/welfare obligation [8], have not previously been addressed in a genetically informative study. In particular, it is unclear whether these specific prosocial obligations are, first, individually heritable, and, second, are underpinned by a single, genetically influenced, mental faculty [9].
To address these issues, we report a twin study examining the genetic and environmental structure of self-reported prosocial obligations across the three domains mentioned above. We hypothesized that a common mechanism underpinned each of the measured obligations, and that this common factor would be genetically influenced, in line with research demonstrating significant positive correlations between broad constellations of prosocial actions [10,11] and previous work demonstrating aspects of prosociality contain heritable variance [3–5].
2. Material and methods
(a). Participants
Phenotypic data were available for 958 pairs of twins contacted by the MacArthur Foundation Survey of Midlife Development in the United States [12]. Of the monozygotic (MZ) pairs, 167 were male (mean age = 44.64 ± 11.32) and 194 were female (mean age = 43.69 ± 12.20). Of the dizygotic (DZ) pairs, 136 were male (mean age = 44.64 ± 12.42), 210 were female (mean age = 45.78 ± 12.61), and 251 were opposite-sex pairs (mean age = 45.86 ± 11.73). The excess of females over males is comparable to previous twin research [13].
(b). Prosocial obligation measures
Participants completed three scales measuring different forms of prosocial obligations: civic obligation (four items), work obligation (three items) and welfare obligation (three items) [7], with a response scale from 0 to 10. An example item assessing civic obligation was: ‘how much obligation would you feel to testify in court about an accident you witnessed?’; for work obligation: ‘how much obligation would you feel to do more than most people would do on your kind of job?’; and for welfare obligation: ‘how much obligation would you feel to pay more for your healthcare so that everyone had access to healthcare?’ Cronbach's alpha [14] for the three prosocial domains was acceptable (civic, 0.78; work, 0.71; welfare, 0.81). For the purpose of examining an omnibus univariate model, the three prosocial obligations scales were summed to form a composite prosocial obligations measure. Prior to analysis, the effects of age and sex were regressed out, and standardized residuals were used in subsequent analyses [15].
(c). Analysis
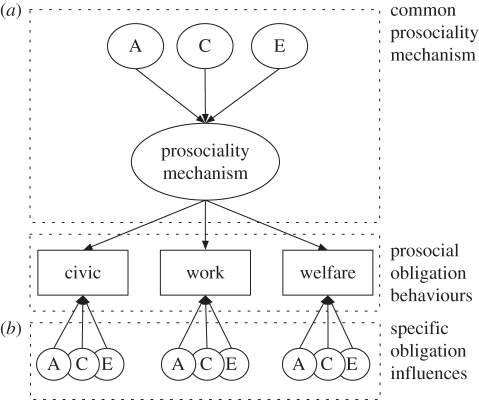
All analyses used full-information maximum-likelihood modelling, and structural equation modelling was conducted using OpenMx [16,17] and R [18]. The modelling used to test our predictions is outlined graphically in figure 1. The prediction of heritable and/or environment effects specific to each of the three prosociality measures is shown in the lower portion of the figure, where each obligation is accounted for by a combination of additive genetic (A), shared- (or familial) environment (C), and unique-environment (E) effects. The upper portion of figure 1 shows the predicted latent mechanism for domain-general prosociality, labelled here as ‘prosociality mechanism’. This functions as a common pathway affecting all obligation measures, and through which genetic and environmental influences must be mediated. If the fit of this common pathway model does not differ significantly from that of the saturated model, this would provide support for a model of prosocial obligations as involving a general psychological mechanism influencing each of the three measured prosocial domains.
Figure 1.
Common pathway model for prosocial obligations. The three measured prosocial obligations are shown in rectangles. Part (a) shows the putative common or general prosocial obligations mechanism. Part (b) represents putative genetic and environment influences for distinct prosocial obligations systems. A, additive genetic effects; C, common, or shared environment effects; E, unique-environment effects.
3. Results
For each of the obligation domains, same-sex DZ twins were more highly correlated than were opposite-sex DZ twin pairs, suggestive of sex-limitation. Equating male and female DZ pairs, and DZ same-sex twin pairs to DZ opposite-sex pairs, did not significantly worsen model fit for any of the variables; however, to avoid biases in our model estimates, we analysed males and females separately.
Intra-class twin correlations are detailed in table 1. The full results of the univariate modelling for each domain are presented in table 2. For males across each obligation domain, including the general prosocial obligation scale, additive genetic and shared-environment effects were individually non-significant, although removing both paths simultaneously significantly worsened the model fit indicating familial influences on each trait. For females, additive genetic and unique-environment effects were significant for both civic and work obligation, with the third domain, welfare obligation, showing significant support only for unique-environmental influences (although dropping shared-environment effects reduced fit substantially, p = 0.06). For the general prosocial obligation measure, familial effects were observed: additive genetic and shared-environment effects were individually non-significant, but could not be dropped simultaneously without worsening model fit.
Table 1.
Intra-class correlations for MZ, DZ same-sex, and DZ opposite-sex twin pairs for civic, work, welfare obligation and an omnibus summed measure of prosocial obligations.
| civic | work | welfare | prosociality | |
|---|---|---|---|---|
| MZ male twin pairs | 0.32 | 0.25 | 0.35 | 0.40 |
| MZ female twin pairs | 0.49 | 0.36 | 0.26 | 0.37 |
| DZ male twin pairs | 0.24 | 0.13 | 0.11 | 0.30 |
| DZ female twin pairs | 0.19 | 0.20 | 0.30 | 0.27 |
| DZ opposite-sex twin pairs | 0.06 | −0.01 | 0.05 | 0.06 |
Table 2.
Standardised univariate heritability estimates for civic, work, and welfare obligation, and an omnibus summed measure of prosocial obligations (95% CI in brackets).
| males |
females |
|||||
|---|---|---|---|---|---|---|
| A | C | E | A | C | E | |
| civic | 0.14 (0–0.44) | 0.17 (0–0.39) | 0.69 (0.56–0.83) | 0.50 (0.30–0.60) | 0 (0–0.15) | 0.50 (0.40–0.62) |
| work | 0.15 (0–0.37) | 0.08 (0–0.32) | 0.77 (0.63–0.91) | 0.38 (0–0.50) | 0 (0–0.29) | 0.62 (0.50–0.76) |
| welfare | 0.34 (0–0.46) | 0 (0–0.34) | 0.66 (0.54–0.81) | 0 (0–0.36) | 0.28 (0–0.37) | 0.72 (0.60–0.82) |
| general prosociality | 0.23 (0–0.48) | 0.13 (0–0.41) | 0.64 (0.52–0.79) | 0.32 (0–0.50) | 0.06 (0–0.33) | 0.62 (0.50–0.77) |
We next tested our full common-and-specific model of prosocial obligations. The phenotypic correlations between the three obligations variables were moderate-to-high (0.41–0.68). A confirmatory factor analysis indicated that neither a one-factor model nor a model with three uncorrelated obligation factors fitted the data well. By contrast, a hierarchical factor structure in which a super-ordinate ‘prosocial obligations’ factor loaded on the three obligations domains fitted well (see the electronic supplementary material). This indicated that both a common obligations factor and distinct obligations were present in the data.
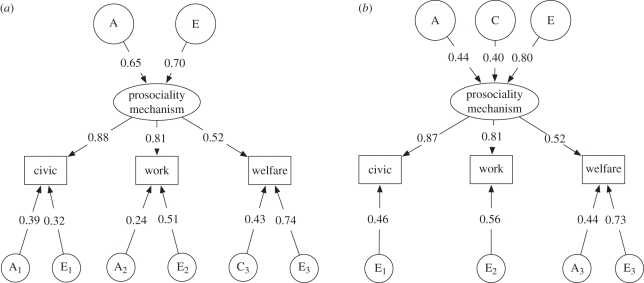
Having established the existence of a common prosocial obligations factor, we moved to biometric analyses. We compared the fit of the full common pathway model to the saturated model. The full common pathway model was a better fit to the data, when compared with the saturated model, for both males and females (Akaike information criterion: 765.49 versus 767.90, and 1295.93 versus 1296.68, respectively). We next moved to tests of goodness-of-fit for nested common pathway models using the χ2 test. For males, we could not drop all additive genetic and shared-environment effects simultaneously (Δχ28 = 34.78, p < 0.01); however, these effects were individually non-significant (Δχ24 = 2.89, p = 0.58; Δχ24 = 1.08, p = 0.90, respectively). As such, we retained both of these sources of influence for further analyses. Nested tests indicated that the specific genetic effects on civic and work obligation, and the shared-environment effects on civic, work, and welfare obligation were non-significant, and so were removed from the model (Δχ25 = 2.13, p = 0.83). All other paths were significant and so we retained this reduced common-pathway model as our preferred final model for males (figure 2).
Figure 2.
Final models of civic, work, and welfare prosocial obligations for (a) females and (b) males separately. Note: standardized path coefficients are shown, which when squared indicate the proportion of variance accounted for by that path. All paths are significant.
For females, the additive genetic effect on the common prosocial obligations factor was significant (Δχ21 = 8.55, p < 0.01); however, the shared-environment effect to the common factor could be dropped without significantly worsening fit (Δχ21 = 0, p = 1). At the domain-specific level, both additive genetic and shared-environment effects were non-significant (Δχ23 = 2.13, p = 0.55; Δχ23 = 1.84, p = 0.61, respectively); however, these influences could not be dropped simultaneously without significantly worsening fit (Δχ26 = 57.62, p < 0.01). Therefore, in line with the pattern of MZ–DZ intra-class correlations, we included genetic paths to civic and work obligation (reflecting the higher MZ to DZ correlations), and a shared-environment path to welfare obligation (reflecting the similar MZ to DZ correlations). The final model for females is presented in figure 2.
4. Discussion
The data confirmed the significant role of genetic influences on prosocial obligations, supporting both common and specific mechanisms, and suggesting a distinct pattern of effects between the sexes. For the common prosocial obligations mechanism, additive genetic factors accounted for 48 per cent of the variance for females. In males, however, while familial effects on this common mechanism were apparent, the available power did not allow us to distinguish between additive genetic and shared-environment effects. These findings suggest that further work, perhaps with an extended twin design [19], will be required to tease apart possible mechanisms for these effects. Potential influences include both assortative-mating for prosociality, non-additive genetic effects [20] and, importantly, gene–environment interaction (G × E) [20] between the sexes (see below).
At the domain-specific level, for males, welfare obligation showed a significant additive genetic component (with the other traits driven by unique-environment effects). For females, civic and work obligations showed significant additive genetic effects, with shared-environment influences underlying welfare obligations. The origin of these specific genetic influences on prosocial obligations is an open question. It may be that selection for stable division of work [21], civil conflicts [22] and welfare behaviours such as obligate food sharing [8] have been important in shaping specific adaptations linked to in-group cooperation.
Unique-environment effects were often large. While these effects partly reflect measurement error, they also potentially reflect gene–environment interaction (G × E) [20]. Given the likely differential family and social constraints placed on males and females enforcing compliance versus rewarding independence [23], it seems likely that genetic differences related to prosocial obligations may interact with these differentially experienced social environments. This view would be consistent with contingency models of prosocial obligations supported by research on the moderation of prosocial contributions by social exclusion [24] and knowledge of prosociality levels in the group [25].
In summary, the present data indicate that a common factor underlies prosocial obligations across three important social domains. The results also highlight important avenues for additional study in line with the contrasting observation of moderate-to-large additive genetic factors underlying this general prosocial obligations factor in females, with modest-to-moderate familial factors influencing general prosocial obligations in males. Further research is required to explore these possible gender differences and transmission modes, as well as the possible effects of contingency upon obligations.
Acknowledgements
This study was approved by the University of Edinburgh Department of Psychology research ethics committee.
References
- 1.Zahn-Waxler C., Radke-Yarrow M., Wagner E., Chapman M. 1992. Development of concern for others. Dev. Psych. 28, 126–136 10.1037/0012-1649.28.1.126 (doi:10.1037/0012-1649.28.1.126) [DOI] [Google Scholar]
- 2.Matthews K. A., Batson C. D., Horn J., Rosenman R. H. 1981. ‘Principles in his nature which interest him in the fortune of others…’: the heritability of empathic concern for others. J. Personal. 49, 237–247 10.1111/j.1467-6494.1981.tb00933.x (doi:10.1111/j.1467-6494.1981.tb00933.x) [DOI] [Google Scholar]
- 3.Rushton J. P. 2004. Genetic and environmental contributions to pro-social attitudes: a twin study of social responsibility. Proc. R. Soc. Lond. B 271, 2583–2585 10.1098/rspb.2004.2941 (doi:10.1098/rspb.2004.2941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hur Y.-M., Rushton J. P. 2007. Genetic and environmental contributions to prosocial behavior in 2- to 9-year-old South Korean twins. Biol. Lett. 3, 664–666 10.1098/rsbl.2007.0365 (doi:10.1098/rsbl.2007.0365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rushton J. P., Fulker D. W., Neale M. C., Nias D. K. B., Eysenck H. J. 1986. Altruism and aggression: the heritability of individual differences. J. Pers. Soc. Psychol. 50, 1192–1198 10.1037/0022-3514.50.6.1192 (doi:10.1037/0022-3514.50.6.1192) [DOI] [PubMed] [Google Scholar]
- 6.Krueger R. F., Hicks B. M., McGue M. 2001. Altruism and antisocial behaviour: independent tendencies, unique personality correlates, distinct etiologies. Psychol. Sci. 12, 397–402 10.1111/1467-9280.00373 (doi:10.1111/1467-9280.00373) [DOI] [PubMed] [Google Scholar]
- 7.Rossi A. S. 2004. Social responsibility to family and community In How healthy are we? A national study of wellbeing at midlife (eds Brim O. G., Ryff C. D., Kessler R. C.), pp. 550–585 Chicago, IL: University of Chicago Press [Google Scholar]
- 8.Gurven M. 2004. Reciprocal altruism and food sharing decisions among hunter–gatherers. Behav. Ecol. Sociobiol. 56, 366–380 10.1007/s00265-004-0793-6 (doi:10.1007/s00265-004-0793-6) [DOI] [Google Scholar]
- 9.Knafo A., Zahn-Waxler C., Van Hulle C., Robinson J. L., Rhee S. H. 2008. The developmental origins of a disposition towards empathy: genetic and environmental contributions. Emotion 8, 737–752 10.1037/a0014179 (doi:10.1037/a0014179) [DOI] [PubMed] [Google Scholar]
- 10.Carlo G., Randall B. A. 2002. The development of a measure of prosocial behaviours for late adolescents. J. Youth Adol. 31, 31–44 10.1023/A:1014033032440 (doi:10.1023/A:1014033032440) [DOI] [Google Scholar]
- 11.Eisenberg N., Guthrie I. K., Cumberland A., Murphy B. C., Shepard S. A., Zhou Q., Carlo G. 2002. Prosocial development in early adulthood: a longitudinal study. J. Pers. Soc. Psychol. 82, 993–1006 10.1037/0022-3514.82.6.993 (doi:10.1037/0022-3514.82.6.993) [DOI] [PubMed] [Google Scholar]
- 12.Kendler K. S., Thornton L. M., Gilman S. E., Kessler R. C. 2000. Sexual orientation in a U.S. national sample of twin and nontwin sibling pairs. Am. J. Psychiatry 157, 1843–1846 10.1176/appi.ajp.157.11.1843 (doi:10.1176/appi.ajp.157.11.1843) [DOI] [PubMed] [Google Scholar]
- 13.Lykken D. T., McGue M., Tellegen A. 1987. Recruitment bias in twin research: the rule of two-thirds reconsidered. Behav. Genet. 4, 343–362 10.1007/BF01068136 (doi:10.1007/BF01068136) [DOI] [PubMed] [Google Scholar]
- 14.Cronbach L. J. 1951. Coefficient alpha and the internal structure of tests. Psychometrika 16, 297–334 10.1007/BF02310555 (doi:10.1007/BF02310555) [DOI] [Google Scholar]
- 15.McGue M., Bouchard T. J. 1984. Adjustment of twin data for the effects of age and sex. Behav. Genet. 14, 325–343 10.1007/BF01080045 (doi:10.1007/BF01080045) [DOI] [PubMed] [Google Scholar]
- 16.Boker S., et al. In press OpenMx: an open source extended structural equation modeling framework. Psychometrika (doi:10.1007/s11336-010-9200-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boker S., et al. 2010. OpenMx: multipurpose software for statistical modelling. Version R package version 0.3.1-1246. See http://openmx.psyc.virginia.edu
- 18.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org [Google Scholar]
- 19.Eaves L. J., Last K. A., Young P. A., Martin N. G. 1978. Model-fitting approaches to the analysis of human behavior. Heredity 41, 249–320 10.1038/hdy.1978.101 (doi:10.1038/hdy.1978.101) [DOI] [PubMed] [Google Scholar]
- 20.Neale M., Cardon L. 1992. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 21.Wahl L. M. 2002. Evolving the division of labour: generalists, specialists and task allocation. J. Theor. Biol. 219, 371–388 10.1006/jtbi.2002.3133 (doi:10.1006/jtbi.2002.3133) [DOI] [PubMed] [Google Scholar]
- 22.Mitani J. C., Watts D. P., Amsler S. J. 2010. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 20, R507–R508 10.1016/j.cub.2010.04.021 (doi:10.1016/j.cub.2010.04.021) [DOI] [PubMed] [Google Scholar]
- 23.Mischel W., Shoda Y. 1995. A cognitive-affective system theory of personality: reconceptualizing situations, dispositions, dynamics, and invariance in personality structure. Psychol. Rev. 102, 246–268 10.1037/0033-295X.102.2.246 (doi:10.1037/0033-295X.102.2.246) [DOI] [PubMed] [Google Scholar]
- 24.Twenge J. M., Baumeister R. F., DeWall C. N., Ciarocco N. J., Bartels J. N. 2007. Social exclusion decreases prosocial behavior. J. Pers. Soc. Psychol. 96, 56–66 [DOI] [PubMed] [Google Scholar]
- 25.Gurven M. 2006. The evolution of contingent cooperation. Curr. Anthropol. 47, 185–192 10.1086/499552 (doi:10.1086/499552) [DOI] [Google Scholar]