Abstract
To avoid collisions when navigating through cluttered environments, flying insects must control their flight so that their sensory systems have time to detect obstacles and avoid them. To do this, day-active insects rely primarily on the pattern of apparent motion generated on the retina during flight (optic flow). However, many flying insects are active at night, when obtaining reliable visual information for flight control presents much more of a challenge. To assess whether nocturnal flying insects also rely on optic flow cues to control flight in dim light, we recorded flights of the nocturnal neotropical sweat bee, Megalopta genalis, flying along an experimental tunnel when: (i) the visual texture on each wall generated strong horizontal (front-to-back) optic flow cues, (ii) the texture on only one wall generated these cues, and (iii) horizontal optic flow cues were removed from both walls. We find that Megalopta increase their groundspeed when horizontal motion cues in the tunnel are reduced (conditions (ii) and (iii)). However, differences in the amount of horizontal optic flow on each wall of the tunnel (condition (ii)) do not affect the centred position of the bee within the flight tunnel. To better understand the behavioural response of Megalopta, we repeated the experiments on day-active bumble-bees (Bombus terrestris). Overall, our findings demonstrate that despite the limitations imposed by dim light, Megalopta—like their day-active relatives—rely heavily on vision to control flight, but that they use visual cues in a different manner from diurnal insects.
Keywords: flight, optic flow, insect vision, Megalopta, bumble-bee
1. Introduction
Nocturnal sweat bees, Megalopta genalis (Halictidae), live in hollowed-out sticks in the tangled understories of neotropical rainforests and are active in the dim light conditions that occur just before sunrise and after sunset [1,2]. To forage, these bees must negotiate the dark and cluttered environment around their nests, fly to a flowering tree to collect nectar and pollen and then find their way back home again [3]. Like all hymenopterans, Megalopta possess apposition compound eyes, which are adapted for vision in bright light. Although Megalopta have evolved optical specializations to capture more light than their day-active relatives, these enhancements are not sufficient to explain how Megalopta capture enough light to use vision in their dim habitat [4]. Despite this limitation, Megalopta use visual landmark information to locate their nests at very low light intensities [4].
Day-active insects such as honeybees [5,6], bumble-bees [7,8] and flies [9,10] use information extracted from the pattern of visual motion that occurs on the retina during flight (known as optic flow) to control groundspeed. A consequence of this strategy is that speed increases dramatically when horizontal optic flow cues are minimized [5,11]. Another behaviour known to be mediated by horizontal optic flow is the ‘centring’ response. When flying through narrow gaps, the day-active honeybee ‘centres’ between the nearby surfaces by balancing the rate of horizontal optic flow experienced in each eye [12]. This strategy ensures that the insect will maintain an equal distance between obstacles without the need for absolute distance measurements. When the rate of horizontal optic flow experienced on each eye becomes imbalanced, the insect attempts to restore the balance by flying nearer to the surface that provides the least horizontal optic flow.
As light intensity decreases, however, the perception of the pattern of visual motion is corrupted by noise and becomes decreasingly reliable. Do insects that are active in dim light also rely on optic flow to control flight, despite the reduced reliability of visual information? Here, we explore the limits of dim light vision by investigating whether Megalopta flying at low light intensities are also able to use optic flow cues for groundspeed control and centring. We also compare the visual flight-control strategies of Megalopta and a diurnal bee, Bombus terrestris (Apidae).
2. Material and methods
Nest sticks of M. genalis were collected on Barro Colorado Island in Panama and transferred to the experimental site (see [1] for site description). To explore the differences between the visual flight-control strategy of Megalopta and a day-active hymenopteran, we repeated the experiment using B. terrestris from a commercial hive (Koppert, UK) located outdoors near Lund, Sweden.
The experimental set-up consisted of a Perspex tunnel, 14 cm wide × 14.5 cm high × 50 cm long, mounted 65 cm above the ground. The nest/hive was placed at an opening in one end of the tunnel such that, to exit or enter their nest/hive, the bees had to fly along the tunnel's length. The walls of the tunnel were lined with either a pattern consisting of randomly placed black-and-white 3 × 3 cm squares (‘check’), or a pattern of alternating black-and-white 3 cm wide horizontal stripes (‘stripe’). For a bee flying along the tunnel, the check pattern provided strong horizontal optic flow cues, whereas the stripe pattern provided minimal horizontal visual cues. The effect of horizontal optic flow cues on flight control was tested under three conditions: (i) check/check—both walls displayed the check pattern, (ii) check/stripe—one wall displayed the check pattern and one displayed the stripe pattern, and (iii) stripe/stripe—both walls displayed the stripe pattern. The three conditions were presented to the bees in a randomized order, with each condition being presented four times. The numbers of recorded flights were 24 and 28 (check/check), 21 and 32 (check/stripe), 24 and 31 (stripe/stripe), for Megalopta and Bombus, respectively.
Flights of bees returning to their nest were recorded (at 25 and 50 frames s−1; Megalopta and bumble-bees, respectively) using a camera mounted underneath the tunnel. The top panel of the tunnel was sandblasted to make a light background against which the bees could be distinguished in the films. Recording sessions were performed during the normal foraging times (Megalopta: 45 min before sunrise and 45 min after sunset [1,2]; Bombus: two 30 min periods, at 10.30 and 13.30 h). Light intensity varied between 1–12 lux for Megalopta and 12 000–20 000 lux for Bombus.
Groundspeed was calculated by finding the longitudinal distance travelled between successive frames and dividing this value by the time step between the frames. This data was then averaged. Centring was calculated by finding the average lateral distance from the midline of the tunnel. For analysis, we calculated the groundspeed and centring over the first 25 cm of the tunnel to avoid including landing manoeuvres at the nest/hive.
The effect of experimental condition on groundspeed and centring was assessed using analysis of variances (ANOVAs) and Student's t-tests at the 5 per cent significance level. Linear mixed model analyses [13] using the lme function in R (release 1.26) with light intensity as a random effect were used to account for the level of variation, which is introduced by recording flights at different light intensities.
3. Results
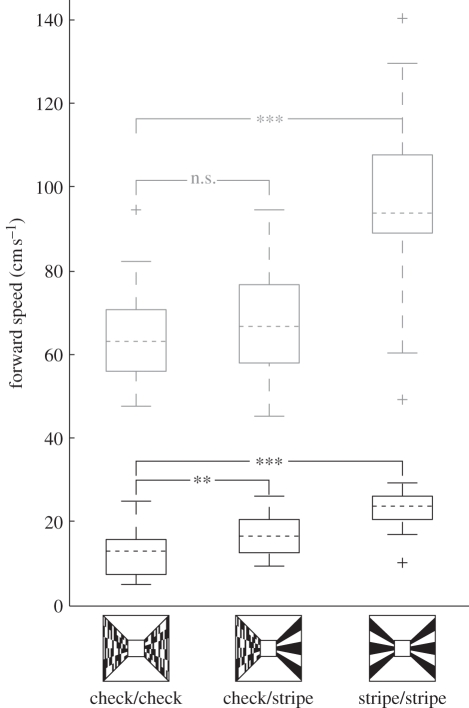
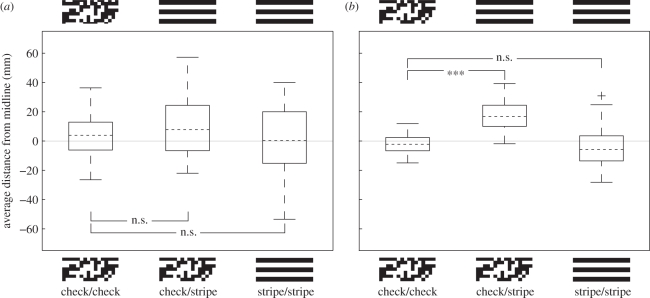
When the patterns on the walls of the tunnel provided decreasing amounts of horizontal optic flow cues—check/stripe, and stripe/stripe condition in comparison with the check/check condition—groundspeed in Megalopta increased (figure 1; check/stripe condition: t43 = 3.30, p = 0.002; stripe/stripe condition: t46 = 7.81, p < 0.0001). However, the amount of horizontal optic flow present in the tunnel had no effect upon the average centring (lateral position) of the bees as they flew along the tunnel (figure 2a; one-way ANOVA: F2/66 = 0.79, p = 0.46).
Figure 1.
The effect of changes in horizontal optic flow cues in the tunnel on the groundspeed of M. genalis (black boxes) and B. terrestris (grey boxes). Box limits represent the 25th and 75th percentiles of the data, dotted lines indicate the median, whiskers extend to the rest of the data, crosses indicate outliers. Both species increase their groundspeed when horizontal motion cues are minimized, but unlike Megalopta, bumble-bees do not fly faster when horizontal motion cues are removed from one wall. Significance codes: **p < 0.01; ***p < 0.001; n.s., not significant.
Figure 2.
The effect of changes in horizontal optic flow cues on centring in (a) M. genalis and in (b) B. terrestris. Thick black lines indicate the tunnel walls; light grey lines indicate the midline of the tunnel and the pattern (check or stripe) indicates the position of the patterns. Other details as in figure 1. In the check/stripe condition, bumble-bees fly closer to the stripe pattern; we see no such effect in Megalopta.
Interestingly, in all conditions, the diurnal bumble-bees fly considerably faster than Megalopta (figure 1). Like Megalopta, bumble-bees increase their groundspeed when horizontal motion cues are minimized (figure 1; stripe/stripe compared with check/check condition: t57 = 8.11, p < 0.0001). Unlike Megalopta, however, bumble-bees do not fly faster when horizontal motion cues are removed from only one wall (check/stripe compared with check/check condition: t58 = 0.78, p = 0.44). Another difference between Megalopta and bumble-bees is the effect of horizontal motion cues on centring. Whereas an asymmetry in horizontal optic flow did not affect centring in Megalopta, it caused the bumble-bees to fly closer to the wall that displayed the stripe pattern in the check/stripe condition (figure 2b; comparison with check/check condition: t58 = 6.68, p < 0.0001). There was no difference in the average distance from the midline between the stripe/stripe and the check/check conditions in bumble-bees (t57 = −0.75, p = 0.46).
4. Discussion
In this study, we compared the flight control of two different bee species adapted for flight at radically different light intensities. The most striking result of this comparison is the difference in the speed at which Megalopta and bumble-bees fly. When optic flow cues are strong, the mean groundspeed of bumble-bees in the tunnel is over five times faster than that of Megalopta. One way to improve visual reliability in dim light is to integrate the visual signal over time; a process called temporal summation. A consequence of temporal summation is the inability to detect high rates of optic flow, so the animal has to reduce its speed in order to perceive self-generated visual motion. The relatively low groundspeed of Megalopta lends support to the behavioural [14] and theoretical [15] indications that these bees use temporal summation to help them to perceive optic flow and to use it for flight control.
Further evidence for the importance of vision for flight control in Megalopta is provided by the finding that groundspeed increases when optic flow cues are minimized. This response is similar to the behaviour of honeybees [5,6] and bumble-bees [8,11], indicating that optic flow information is used in a similar manner for groundspeed control. However, other observed differences between the flight-control behaviours of Megalopta and bumble-bees suggest that Megalopta is using optic flow information in a different way to control flight. For example, in the check/stripe condition, bumble-bees fly closer to the stripe pattern in an apparent attempt to balance the optic flow experienced in each eye. We see no such effect in Megalopta. Groundspeed also increases in Megalopta in the check/stripe condition, even though they are maintaining the same distance from each wall. The differences in behaviour that we observe may be because Megalopta use optic flow cues from different parts of the visual field, such as the dorsal or ventral regions, to maintain a safe distance from nearby obstacles and to control groundspeed, or because they have reduced their reliance upon vision for flight control in favour of information that is not affected by light intensity, such as mechanosensory measurements of airspeed.
It is interesting to note that, when horizontal optic flow cues are strong, Megalopta show more variation in lateral position than bumble-bees. This may be owing to differences in the flight performance of the two species, but another possibility is that the sensory information which Megalopta uses to maintain a constant distance between the tunnel walls is noisier or less reliable than the information being used by the bumble-bees.
Overall, the results of this study demonstrate that the visual system of a nocturnal insect is capable of detecting optic flow information in dim light and using it for flight control. This is remarkable considering the sensory challenge of controlling flight in the complex environment of a dark rainforest.
Acknowledgements
This work was funded by the AFOSR/EOARD (grants FA8655-08-C-4004 and FA8655-07-C-4011), the Wenner Gren Foundation and the Swedish Research Council.
References
- 1.Wcislo W. T., Arneson L., Roesch K., Gonzalez V., Smith A., Fernandez H. 2004. The evolution of nocturnal behaviour in sweat bees, Megalopta genalis and M. ecuadoria (Hymenoptera: Halictidae): an escape from competitors and enemies? Biol. J. Linn. Soc. Lond. 83, 377–387 10.1111/j.1095-8312.2004.00399.x (doi:10.1111/j.1095-8312.2004.00399.x) [DOI] [Google Scholar]
- 2.Kelber A., Warrant E. J., Pfaff M., Wallen R., Theobald J. C., Wcislo W. T., Raguso R. A. 2006. Light intensity limits foraging activity in nocturnal and crepuscular bees. Behav. Ecol. 17, 63–72 10.1093/beheco/arj001 (doi:10.1093/beheco/arj001) [DOI] [Google Scholar]
- 3.Wcislo W. T., Tierney S. M. 2009. Behavioural environments and niche construction: the evolution of dim-light foraging in bees. Biol. Rev. Camb. Phil. Soc. 84, 19–37 10.1111/j.1469-185X.2008.00059.x (doi:10.1111/j.1469-185X.2008.00059.x) [DOI] [PubMed] [Google Scholar]
- 4.Warrant E. J., Kelber A., Gislen A., Greiner B., Ribi W., Wcislo W. T. 2004. Nocturnal vision and landmark orientation in a tropical halictid bee. Curr. Biol. 14, 1309–1318 10.1016/j.cub.2004.07.057 (doi:10.1016/j.cub.2004.07.057) [DOI] [PubMed] [Google Scholar]
- 5.Baird E., Srinivasan M. V., Zhang S., Cowling A. 2005. Visual control of flight speed in honeybees. J. Exp. Biol. 208, 3895–3905 10.1242/jeb.01818 (doi:10.1242/jeb.01818) [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan M., Zhang S., Lehrer M., Collett T. 1996. Honeybee navigation en route to the goal: visual flight control and odometry. J. Exp. Biol. 199, 237–244 [DOI] [PubMed] [Google Scholar]
- 7.Baird E., Kornfeldt T., Dacke M. 2010. Minimum viewing angle for visually guided ground speed control in bumblebees. J. Exp. Biol. 213, 1625–1632 10.1242/jeb.038802 (doi:10.1242/jeb.038802) [DOI] [PubMed] [Google Scholar]
- 8.Dyhr J. P., Higgins C. M. 2010. The spatial frequency tuning of optic-flow-dependent behaviors in the bumblebee Bombus impatiens. J. Exp. Biol. 213, 1643–1650 10.1242/jeb.041426 (doi:10.1242/jeb.041426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David C. T. 1982. Compensation for height in the control of groundspeed by Drosophila in a new ‘Barber's Pole’ wind tunnel. J. Comp. Physiol. A 147, 485–493 10.1007/BF00612014 (doi:10.1007/BF00612014) [DOI] [Google Scholar]
- 10.Fry S. N., Rohrseitz N., Straw A. D., Dickinson M. H. 2009. Visual control of flight speed in Drosophila melanogaster. J. Exp. Biol. 212, 1120–1130 10.1242/jeb.020768 (doi:10.1242/jeb.020768) [DOI] [PubMed] [Google Scholar]
- 11.Baird E., Kornfeldt T., Dacke D. 2010. Minimum viewing angle for visually guided ground speed control in bumble-bees. J. Exp. Biol. 13, 1625–1632 10.1242/jeb.038802 (doi:10.1242/jeb.038802) [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan M. V., Lehrer M., Kirchner W. H., Zhang S. W. 1991. Range perception through apparent image speed in freely flying honeybees. Vis. Neurosci. 6, 519–535 10.1017/S095252380000136X (doi:10.1017/S095252380000136X) [DOI] [PubMed] [Google Scholar]
- 13.McCulloch C. E., Searle S. R. 2001. Generalized, linear, and mixed models. New York, NY: John Wiley & Sons [Google Scholar]
- 14.Theobald J. C., Coates M. M., Wcislo W. T., Warrant E. J. 2007. Flight performance in night-flying sweat bees suffers at low light levels. J. Exp. Biol. 210, 4034–4042 10.1242/jeb.003756 (doi:10.1242/jeb.003756) [DOI] [PubMed] [Google Scholar]
- 15.Theobald J. C., Greiner B., Weislo W. T., Warrant E. J. 2006. Visual summation in night-flying sweat bees: a theoretical study. Vision Res. 46, 2298–2309 10.1016/j.visres.2006.01.002 (doi:10.1016/j.visres.2006.01.002) [DOI] [PubMed] [Google Scholar]