Abstract
During mating events, females of many primate species produce loud and distinct vocalizations known as ‘copulation calls’. The adaptive significance of these signals is considered to be in promoting the caller's direct reproductive success. Here, we investigated copulation calling in bonobos (Pan paniscus), a species in which females produce these vocalizations during sexual interactions with partners of both sexes. Females were more likely to call when mating with males than with females. We also observed a positive relationship between the likelihood of calling and partner rank, regardless of partner sex. Sexual activity generally increased with swelling size (an indicator of reproductive state) and, during their peak swelling, females called more with male than with female partners. Female bonobos are unusual among the non-human primates in terms of their heightened socio-sexuality. Our results suggest that in this species, copulation calls have undergone an evolutionary transition from a purely reproductive to a more general social function, reflecting the intrinsic evolutionary links between vocal behaviour and social cognition.
Keywords: primate vocalization, sexual selection, social intelligence
1. Introduction
Females of many primate species produce loud and distinct vocalizations during mating events. These signals, known as ‘copulation calls’ [1], are especially widespread among Old World species in which the females are promiscuous, live in multi-male multi-female groups and advertise their receptivity with sexual swellings [2]. While many functional hypotheses have been put forward, all essentially converge on the unifying notion that copulation calls are part of sexually selected strategies that promote the reproductive success of the caller [1,3]. For example, calls may attract mates and signal female receptivity, in turn inciting male–male competition directly [4] or via sperm competition [5]. Alternatively, calls may reduce risks of infanticide by promoting paternity uncertainty or enhancing mate guarding [6]. There is also some evidence of strategic call use, suggesting that copulation calls can provide a window into the cognitive processes underlying call production. In Barbary macaques (Macaca sylvanus), for example, females can elicit and time the onset of male ejaculation using copulation calls [7]. In chimpanzees (Pan troglodytes), females advertise matings with high-ranked males but inhibit calling in the presence of higher ranked female audiences, possibly as a means to reduce female competition [8].
Female bonobos (Pan paniscus) produce copulation calls during mating events [9]. For a number of reasons, this primate represents an intriguing species to study copulation calls. Females are the principally emigrating sex [10,11] but, despite the absence of kinship ties, they are highly gregarious and form strong bonds with one another [9,11]. Related to this is a heightened sexuality, frequently divorced from reproduction, with females commonly engaging in sexual interactions in all age and sex combinations [9,12,13]. Sex appears to also serve as a social tool, facilitating the integration of young females into their new community and their subsequently peaceful coexistence and affiliation with non-related group members (e.g. [9,11–13]).
Sexual interactions between bonobo females are known as ‘genital contacts’, whereby individuals embrace ventro-ventrally and swing their hips laterally, while keeping their vulvae in contact [12,13]. Intriguingly, females sometimes produce vocalizations during these events [9]. We term them copulation calls following recent acoustic analyses showing that these calls cannot be distinguished from those produced when mating with males [14]. The production of copulation calls during homosexual interactions is not well explained by existing theories of primate copulation calls, which all focus on reproductive significance.
The aim of the present study was to examine the function and usage of copulation calls in female bonobos by focusing especially on both homo- and heterosexual encounters and partner rank. We also examined whether a physical variable associated with reproductive state, sexual swelling size, influenced call production. Our general prediction was that, if copulation calls were used as a social rather than an exclusively reproductive signal, similar patterns of call production with both male and female partners should be found.
2. Material and methods
We conducted observations of three bonobo groups at Lola Ya Bonobo Sanctuary, Kinshasa, DR Congo, between September and November 2008 and between August and November 2009. The daily routine at the sanctuary remained the same throughout observation periods (electronic supplementary material). In 2008, we observed individuals in one of the two largest enclosures, henceforth ‘group 1a’. In 2009, we collected data from two groups housed in the same and the adjacent enclosure, henceforth ‘group 1b’ and ‘group 2’. Group composition in the first enclosure changed between the two study periods (group 1a: n = 9 females, n = 9 males, n = 4 infants; group 1b: n = 7 females, n = 9 males, n = 4 infants; group 2: n = 5 females, n = 11 males; n = 3 infants; electronic supplementary material, table S1). We pooled the data across the three groups and combined data for dyads that met again in the second year (n = 9 female–female dyads; n = 19 male–female dyads).
In bonobos, sexual behaviour can take a variety of forms, such as heterosexual copulations (with pelvic thrusts and intromission), mountings, homosexual genital contacts and genital stimulation using an object or body part. Here, we recorded behaviours and vocalizations of females engaging in copulations with males or genital contacts with females. Copulation calls were acoustically distinct and never observed in contexts other than sexual interactions. We conducted observations using all-day focal and ad libitum sampling methods (approx. n = 1093 h), balanced across individuals. We recorded female vocalizations at distances of 3–20 m using a Sennheiser MKH816T directional microphone and Marantz PMD660 solid-state recorder (sampling rate of 44.1 kHz, 16 bits accuracy). Bonobo copulation calls typically consist of a single or succession of high-frequency squeaks and screams that usually begin during the copulation [9] (electronic supplementary material, figure S1). We also recorded female reproductive states following veterinary assessments and collected daily records of swelling sizes, using Furuichi's [15] 4-point scale based on degree of wrinkling.
To assess the influence of dominance, we used hierarchies calculated for another study on the same study groups and period [14], based on the outcome of dyadic agonistic interactions. We used ‘fleeing upon aggression’ as a behavioural marker for subordinance [16]. Dominance relationships and linearity were calculated with the Matman matrix analysis program (Noldus v. 1.1). We calculated and tested the adjusted linearity index h′, corrected for unknown relationships [16–18], as well as the directional consistency index (electronic supplementary material, table S2). For significantly linear hierarchies, we calculated individual cardinal ranks, using normalized David's scores corrected for chance (electronic supplementary material; tables S1 and S2) [16,18]. Using regression plots of the rank scores, we divided the females into either high or low ranks, based on their position in the hierarchies (high: n = 6; low: n = 8). For the males, the absence of significant linearity prevented calculations of cardinal ranks. We thus assigned high-rank status to any male who dominated (i.e. elicited fleeing behaviour) at least 50 per cent of the other males in the group (high: n = 7; low: n = 17, electronic supplementary material, tables S1–S2).
3. Results
(a). Effect of context and partner rank
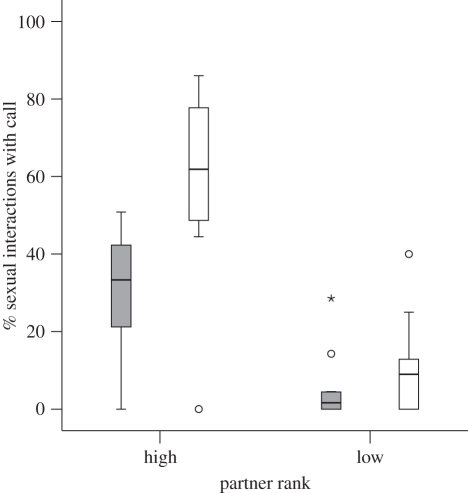
Overall, we observed n = 1100 female–male copulations and n = 674 female–female genital contacts. When taking into account the number of available male and female partners, females engaged in significantly more sexual interactions with other females than with males (mean n interactions (female partners) = 9.93, mean n interactions (male partners) = 5.63; paired t-test = 3.443, d.f. = 13, p = 0.004; electronic supplementary material, table S3). We investigated whether the type of sexual interaction (homo- versus heterosexual) and the dominance rank of the partner (high versus low) influenced call production. Results from a two-way repeated measures ANOVA on the factors of sexual interaction type and partner rank revealed that sexual interaction type had a significant effect on call production, with females being significantly more likely to call with male than with female partners (mean calling with males = 32.88%; versus with females = 16.43%; F1,10 = 14.621, p = 0.003). Each female contributed one data point for each level of both factors (n = 11; n = 3 females had to be excluded owing to insufficient data).
There was a general main effect of partner rank, with females being more likely to call with high-ranked than with low-ranked partners, regardless of partner sex (mean calling high-ranked males = 59.86%; high-ranked females = 28.22%; low-ranked males = 9.17%; low-ranked females = 2.96%; F1,10 = 54.734, p < 0.001). Finally, although we found similar rank effects for the likelihood of calling in both homo- and heterosexual interactions, there was a significant interaction between partner sex and partner rank, revealing a steeper decline in call production with low-ranked male than with female partners (F1,10 = 7.512, p = 0.021; figure 1).
Figure 1.
Box plot indicating the percentage of homo- and heterosexual interactions accompanied by copulation calls as a function of partner rank. Outliers are marked with circles and extreme cases with asterisks. Shaded bars, female–female interaction; white bars, female–male interaction.
(b). Effect of sexual swelling size
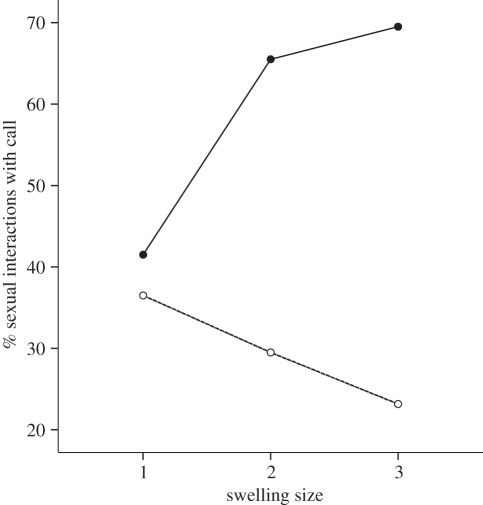
We investigated whether swelling size influenced sexual activity and call production, controlling for the number of observation days per swelling size. Results from a two-way repeated-measures ANOVA on the factors of swelling size (zero to three) and partner sex (homo- versus heterosexual) revealed that swelling size had a significant effect on rates of sexual activity (F3,24 = 21.362, p < 0.001), with females engaging in more sexual interactions as their swelling sizes increased (pairwise comparisons of swelling sizes with Bonferroni corrections: zero versus one, p = 0.075; zero versus two, p = 0.005; zero versus three, p = 0.002; one versus three, p = 0.013; one versus two and two versus three, p > 0.05). We found no significant effect of partner sex on rates of sexual activity (F1,8 = 1.170, p = 0.311), although there was a trend in sexual interactions with females increasing more steeply during the period of maximum tumescence compared with sexual interactions with males.
Finally, we examined whether changes in swelling size influenced copulation call production. Results from a two-way ANOVA with the factors swelling size (size zero excluded owing to insufficient contributions) and partner sex (male or female) on the proportion of sexual interactions accompanied by calls (n = 6 cycling females) revealed that female swelling size did not significantly increase the likelihood of call production overall (F2,10 = 0.345, p > 0.05), although females called more with male partners. While the interaction was not statistically significant, call production increased with increasing swelling size for heterosexual copulations but decreased with increasing swelling size for homosexual interactions (figure 2). Pairwise comparisons revealed that, during their peak swelling, females were significantly more likely to call with male than with female partners (Wilcoxon matched-pairs test: Z6 = −2.366, p = 0.018).
Figure 2.
Line graph indicating the percentage of homosexual and heterosexual interactions accompanied by copulation calls as a function of female swelling size. Solid line, male–female interaction; dashed line, female–female interaction.
4. Discussion
We investigated the function and use of bonobo copulation calling by focusing on both homo- and heterosexual interactions. Results highlighted the social significance of copulation calls in this species and suggested that, similar to other sexual behaviour, calling has become partly detached from its original reproductive function. Females engaged in frequent sexual interactions with both males and females and produced copulation calls in both contexts. In another study, we showed that copulation calls given with male and female partners were largely acoustically indistinguishable [14]. Females called more during sexual interactions with males than with females, but partner rank was a key variable in both contexts. Swelling size was associated with increased sexual activity, but corresponding increases in call production only occurred during heterosexual matings, while decreased calling during homosexual interactions was observed as trends. In summary, our results suggest that, while bonobo copulation calls have maintained some features relating to their putative original reproductive function, as indicated for example by enhanced production during peak swelling phases for heterosexual compared with homosexual interactions, they have acquired an additional communicative function to be used in social contexts.
In previous studies with other primates, rank effects in copulation calling have been explained as reproductive strategies, such as to promote mate guarding [1]. However, we also found a comparably strong rank effect with female partners. This highlights the social aspect of copulation calling in bonobos, something that has not received much attention so far. The social use of a reproductive signal is, however, consistent with a broader functional transition of sexuality in this species. Sex serves as a social tool, for example, by facilitating the formation of female aggregations and the development of intra-sexual bonds, allowing females to coexist peacefully, to form coalitions and to exert social power [9,11–13]. Although further work is required to test the communicative function of these calls and their effects on receivers, copulation calls appear to help females to advertise their sexual interactions, especially with high-ranked partners, a behaviour that appears to be part of a broader strategy to form associations with socially important group members. This is consistent with evidence from the wild, which has shown that newly immigrated females frequently engage in sexual interactions and especially target established, high-ranking females with whom they seek to form affiliations [19]. Our findings are compatible with this pattern and suggest that copulation calls in bonobos may function in social advertisement. Alternatively, given that females called more with high-ranked partners, these calls may also function to signal appeasement or subordinate status. Whether these hypotheses have any functional relevance for other primates will need to be addressed by further research.
Acknowledgements
We received full ethical approval for all aspects of this study from the Lola Ya Bonobo Scientific Coordinator and Scientific Committee of ‘Les Amis des Bonobos du Congo’ (ABC).
We are very grateful to all at Lola Ya Bonobo Sanctuary, in particular Claudine Andre, Fanny Mehl, Dominique Morel, Valery Dhanani, Stany Mokando, Jean-Claude Nzumbi and Philippe Kunaka. Our special gratitude goes to Brian Hare for his advice and support. We thank Mike Oram and Christie Marr for statistical advice and Simon Townsend and Jeroen Stevens for their comments. This study was funded by the Leverhulme Trust (UK) and the Lucie Burgers Stichting.
References
- 1.Pradhan G. R., Engelhardt A., Van Schaik C. P., Maestripieri D. 2006. The evolution of female copulation calls in primates: a review and a new model. Behav. Ecol. Sociobiol. 59, 333–343 10.1007/s00265-005-0075-y (doi:10.1007/s00265-005-0075-y) [DOI] [Google Scholar]
- 2.Dixson A. 1998. Primate sexuality. Oxford, UK: Oxford University Press [Google Scholar]
- 3.Maestripieri D., Roney J. 2005. Primate copulation calls and postcopulatory female choice. Behav. Ecol. 16, 106–113 10.1093/beheco/arh120 (doi:10.1093/beheco/arh120) [DOI] [Google Scholar]
- 4.Cox C. R., LeBoeuf B. J. 1977. Female incitation of male competition: a mechanism in sexual selection. Am. Nat. 111, 317–335 10.1086/283163 (doi:10.1086/283163) [DOI] [Google Scholar]
- 5.Semple S. 1998. The function of Barbary macaque copulation calls. Proc. R. Soc. Lond. B 265, 287–291 10.1098/rspb.1998.0294 (doi:10.1098/rspb.1998.0294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Connell S. M., Cowlishaw G. 1994. Infanticide avoidance, sperm competition and mate choice: the function of copulation calls in female baboons. Anim. Behav. 48, 687–694 10.1006/anbe.1994.1288 (doi:10.1006/anbe.1994.1288) [DOI] [Google Scholar]
- 7.Pfefferle D., Brauch K., Heistermann M., Hodges J., Fischer J. 2008. Female Barbary macaque (Macaca sylvanus) copulation calls do not reveal the fertile phase but influence mating outcome. Proc. R. Soc. B 275, 571–578 10.1098/rspb.2007.1499 (doi:10.1098/rspb.2007.1499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsend S., Deschner T., Zuberbühler K. 2008. Female chimpanzees use copulation calls flexibly to prevent social competition. PLoS ONE 3, 2431. 10.1371/journal.pone.0002431 (doi:10.1371/journal.pone.0002431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kano T. 1992. The last ape. Pygmy chimpanzee behavior and ecology. Stanford, CA: Stanford University Press [Google Scholar]
- 10.Gerloff U., Hartung B., Fruth B., Hohmann G., Tautz D. 1999. Intracommunity relationships, dispersal pattern and paternity success in a wild living community of bonobos (Pan paniscus) determined from DNA analysis of faecal samples. Proc. R. Soc. Lond. B 266, 1189–1195 10.1098/rspb.1999.0762 (doi:10.1098/rspb.1999.0762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuichi T. 1989. Social interactions and the life history of female Pan paniscus in Wamba, Zaire. Int. J. Primatol. 188, 855–875 [Google Scholar]
- 12.de Waal F. B. M. 1987. Tension regulation and nonreproductive functions of sex in captive bonobos. Natl Geogr. Res. 3, 318–335 [Google Scholar]
- 13.Hohmann G., Fruth B. 2000. Use and function of genital contacts among female bonobos. Anim. Behav. 60, 107–120 10.1006/anbe.2000.1451 (doi:10.1006/anbe.2000.1451) [DOI] [PubMed] [Google Scholar]
- 14.Clay Z. 2010. Vocal communication in bonobos (Pan paniscus): studies in the contexts of food discovery and sex. PhD thesis, University of St Andrews, St Andrews, UK [Google Scholar]
- 15.Furuichi T. 1987. Sexual swelling, receptivity, and grooming of wild pygmy chimpanzee females at Wamba, Zaire. Primates 28, 309–318 10.1007/BF02381014 (doi:10.1007/BF02381014) [DOI] [Google Scholar]
- 16.Stevens J. M. G., Vervaecke H., de Vries H., van Elsacker L. 2007. Sex differences in the steepness of dominance hierarchies in captive bonobo groups. Int. J. Primatol. 28, 1417–1430 10.1007/s10764-007-9186-9 (doi:10.1007/s10764-007-9186-9) [DOI] [Google Scholar]
- 17.de Vries H. 1998. Finding a dominance order most consistent with a linear hierarchy: a new procedure and review. Anim. Behav. 55, 827–843 10.1006/anbe.1997.0708 (doi:10.1006/anbe.1997.0708) [DOI] [PubMed] [Google Scholar]
- 18.de Vries H., Stevens J., Vervaecke H. 2006. Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 71, 585–592 10.1016/j.anbehav.2005.05.015 (doi:10.1016/j.anbehav.2005.05.015) [DOI] [Google Scholar]
- 19.Idani G. 1991. Social relationships between immigrant and resident bonobo (Pan paniscus) females at Wamba. Folia Primatol. 57, 83–95 10.1159/000156568 (doi:10.1159/000156568) [DOI] [PubMed] [Google Scholar]