Abstract
Preferences for mates within and between species are often harmonious, as traits that females prefer are usually more developed in conspecifics than heterospecifics. This need not be the case, however. When it is not, conflict between these arenas of mate choice can be resolved if females attend to different cues for each task. But this raises the potential for correlations among preferences to limit the opportunity for these two processes to operate independently. Here, we show that, within individual female pygmy swordtails (Xiphophorus pygmaeus), directional preferences for conspicuous ornamentation are inversely associated with discrimination against a sympatric heterospecific, Xiphophorus cortezi. Thus, mate choice among and within species need not be separate, independent processes; instead, they can be mechanistically intertwined. As a consequence, different arenas of mate choice can constrain one another, even when females assess multiple cues.
Keywords: mate choice, reproductive character displacement, Xiphophorus, Poeciliidae
1. Introduction
Mate choice depends on the evaluation and integration of multiple perceptual cues produced by potential partners. These cues, and their concomitant preferences, are often redundant, acting in concert to signal both within-species attractiveness as well as species identity [1]. Under some circumstances, however, sexual selection and species recognition can be in direct conflict. There are numerous examples of reproductive character displacement of both male traits and female preferences among sympatric species (reviewed in [2]). Several studies have shown that directional preferences for elaborate male traits can be lost or reduced in populations where these traits are shared by sympatric heterospecifics [3–5].
When such a trade-off arises in a single trait, females can avoid a conflict between intra and interspecific mate choice by attending to multiple cues [6,7]. For example, females in a population of pygmy swordtails, Xiphophorus pygmaeus, prefer males of larger size [8]. Indeed, females show a preference for the sympatric Xiphophorus cortezi when species-typical vertical bar patterns and pheromone cues are experimentally removed; however, females show strong preferences for conspecifics when presented with these additional traits. By attending to multiple cues, therefore, females avoid a compromise between sexual selection and species recognition.
Female responses to multiple cues, however, may be constrained by phenotypic correlations among multiple preferences [9]. Correlations among female responses across contexts could be owing to any number of factors, including pleiotropic effects of the same genetic locus, linkage disequilibrium among underlying loci, sensory trade-offs or cognitive limits on attention. The magnitude and the direction of these correlations are critical to the evolutionary relationship between species recognition and sexual selection [10].
In this study, we addressed these correlations by examining individual variation in female preferences in the pygmy swordtail X. pygmaeus. In this species, males are usually small, inconspicuous and lack courtship, in contrast with the large, ornamented, courting males of other swordtail species. Nevertheless, females show preferences for sexually dimorphic visual traits that have been secondarily lost in their own species, choosing large, courting Xiphophorus nigrensis males over conspecifics [11]. It is important to note here that X. pygmaeus and X. nigrensis are allopatric.
Xiphophorus pygmaeus is sympatric with another large, courting and ornamented swordtail, X. cortezi [12]. Females avoid X. cortezi males on the basis of multiple cues [7,13]. We sought to ask if ‘hidden’ preferences for traits of large size and courtship exhibited towards allopatric X. nigrensis could confound discrimination against large, courting sympatric X. cortezi. Specifically, we determined if hidden preferences for allopatric heterospecific males are negatively correlated with discrimination against sympatric heterospecific males. To do so, we quantified, within individual females, the strength of preference for X. nigrensis versus discrimination against X. cortezi.
2. Material and methods
All fishes used were from stocks maintained in large outdoor tanks at the Brackenridge Field Laboratory, University of Texas, Austin, TX. Females had no prior experience with heterospecifics, either sympatric or allopatric ones. Xiphophorus pygmaeus were wild-caught or first-generation offspring of individuals collected at three localities (figure 1; about 30 adult individuals per locality per year) on the Río Huichihuayán, San Luis Potosi (SLP), Mexico; X. nigrensis males were from individuals collected at sites on the Río Choy, SLP and X. cortezi were from populations sympatric with X. pygmaeus. Fish were maintained in large, semi-natural populations at the Brackenridge Field Laboratory, University of Texas at Austin. To acclimatize fishes to the laboratory and to standardize sexual motivation [8], 30 days prior to testing, fishes were housed in groups, segregated by sex and species, in 40 l aquaria.
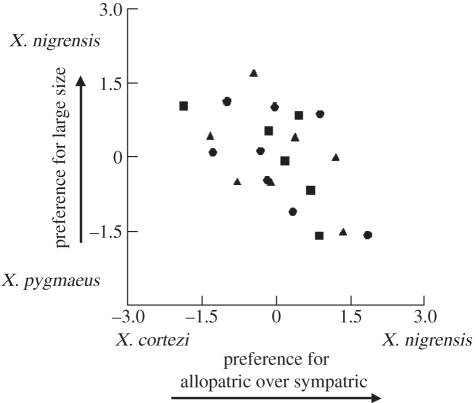
Figure 1.
Individual female preferences for X. nigrensis versus X. cortezi and versus X. pygmaeus. Filled circles, Nacimiento; filled squares, Huichihuayán; filled triangles, Y-griega.
We used standard dichotomous choice tests [14] to evaluate whether responses to sympatric heterospecifics were correlated with preferences for large-size within-individual females. We used a 220 l aquarium, 120 × 32 cm and filled to a depth of 40 cm. The aquarium was divided along its length by Plexiglas dividers into two flanking sections 21 cm wide and a central section. This central section was further subdivided into left and right ‘preference’ areas 27 cm wide and a neutral area 23 cm wide. We placed males in opposite flanking sections and placed the female in a 12 cm diameter cylinder in the centre of the aquarium. We allowed animals to acclimatize for 10 min prior to testing. The cylinder was then lifted, allowing the female to move freely about the central section. A small clump of Java moss (Vesicularia dubyana) was provided for cover in the centre of the aquarium. The female's position (left, centre or right) was recorded for 10 min using a computer event-recording programme. A female was operationally defined as present in a section if her eye appeared in that section. The males were then switched to control for side biases, after which we repeated the acclimatization and testing periods.
We presented females with a choice between live X. cortezi and X. nigrensis males courting matched for size (standard length). Immediately before or afterwards, we presented the same X. nigrensis versus a smaller X. pygmaeus male. To correct for interpopulation variation in preference, we standardized each female's net preference as the z-score with respect to her population. We computed the Spearman rank correlation coefficient between net preferences in the two trials.
3. Results
There was a significant negative correlation between preference for X. nigrensis over X. cortezi and preference for the same X. nigrensis over X. pygmaeus (n = 21, Spearman rank correlation coefficient = −0.522, p < 0.01; figure 1). Untransformed net preferences are shown in the electronic supplementary material, figure S1. We did not detect a difference in net preference among the populations for X. nigrensis over X. pygmaeus (n = 21, Kruskal–Wallis test statistic = 0.303, p = 0.86) or for X. nigrensis over X. cortezi (n= 21, Kruskal–Wallis test statistic = 3.89, p = 0.14). There was no effect of order on preference (paired t-test on preference for X. nigrensis: n = 21, t = 0.10, p = 0.92).
4. Discussion
Species preferences and body-size preferences were correlated within females. Individuals that avoided X. cortezi relative to other heterospecifics showed a weakened preference for body size; that is, they were less likely to prefer the larger X. nigrensis over the smaller X. pygmaeus. At both population and individual levels, females that discriminated more against X. cortezi versus X. nigrensis were thus less attracted to the larger male X. nigrensis versus X. cortezi.
The genetics [9] and psychological mechanisms [6,15] underlying female response to multiple cues are key determinants of how between- and within-species preferences can interact. In X. pygmaeus, female assessment of multiple cues allows for rejection of sympatric heterospecifics despite directional biases for more-ornamented males [7]. Nevertheless, directional preferences covary with responses to heterospecifics. The ancestral bias for large size in Xiphophorus [16,17] compromises rejection of sympatric congeners with respect to allopatric ones, even when body size itself is uninformative; perhaps females with a strong-size bias simply fail to attend to other traits.
Secondary loss of directional preferences [16,17] may provide an opportunity for females to attend to more subtle and more complex cues in mate choice. In our study, a broadly permissive [16] preference for body size appears to be supplanted by a more refined assessment probably based on a combination of olfactory cues and higher order visual cues such as motor patterns, body shape and vertical bar morphology. Counterintuitively, the loss of a strong preference along one axis can lead to enhanced discrimination among complex signals (see [18]).
Within-individual covariation among behaviours, or ‘behavioural syndromes’, has garnered much recent attention [19]. Comparatively little attention has been paid, however, to how preferences for multiple traits covary among females [20]. Indeed, it is often assumed that preferences for different traits, especially in different modalities, are free to evolve independently. In evolutionary psychology, selection is often posited to favour domain-specific modules, specifically adapted to a particular task [21,22]; e.g. mate evaluation versus species recognition. Our results, however, suggest that choice behaviour is not independent across tasks, and that domain-general constraints may be important to behavioural evolution. Further studies of how individual females respond to suites of stimuli should prove fundamental to our understanding of how the psychological architecture of mate choice influences sexual selection and reproductive isolation.
Acknowledgements
The Institutional Animal Care and Use Committee of the University of Texas at Austin approved the experimental procedures.
We are grateful to the federal government of Mexico (permiso de pesca de fomento #020398-213-03) for permission to collect. The Brackenridge Field Laboratory provided fish-rearing facilities. A. McCormick and P. Shah assisted with choice tests. F. García de León provided essential logistical support. Z. Culumber, N. Ratterman, J. B. Johnson, S. Scobell, C. Carlson, V. Smith, R. Cui, M. Jennions and four anonymous reviewers provided helpful comments. Funding was provided by an NSF grant to M.J.R. and an NSF DDIG to G.G.R.
References
- 1.Ryan M. J., Rand A. S. 1993. Species recognition and sexual selection as a unitary problem in animal communication. Evolution 47, 647–657 10.2307/2410076 (doi:10.2307/2410076) [DOI] [PubMed] [Google Scholar]
- 2.Pfennig K. S., Pfennig D. W. 2009. Character displacement: ecological and reproductive responses to a common evolutionary problem. Q. Rev. Biol. 84, 253–276 10.1086/605079 (doi:10.1086/605079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pryke S. R., Andersson S. 2008. Female preferences for long tails constrained by species recognition in short-tailed red bishops. Behav. Ecol. 19, 1116–1121 10.1093/beheco/arn100 (doi:10.1093/beheco/arn100) [DOI] [Google Scholar]
- 4.Collins S. A., Luddem S. T. 2002. Degree of male ornamentation affects female preference for conspecific versus heterospecific males. Proc. R. Soc. Lond. B 269, 111–117 10.1098/rspb.2001.1864 (doi:10.1098/rspb.2001.1864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfennig K. S. 2000. Female spadefoot toads compromise on mate quality to ensure conspecific matings. Behav. Ecol. 11, 220–227 10.1093/beheco/11.2.220 (doi:10.1093/beheco/11.2.220) [DOI] [Google Scholar]
- 6.Castellano S., Cermelli P. 2006. Reconciling sexual selection to species recognition: a process-based model of mating decision. J. Theor. Biol. 242, 529–538 10.1016/j.jtbi.2006.04.001 (doi:10.1016/j.jtbi.2006.04.001) [DOI] [PubMed] [Google Scholar]
- 7.Hankison S. J., Morris M. R. 2003. Avoiding a compromise between sexual selection and species recognition: female swordtail fish assess multiple species-specific cues. Behav. Ecol. 14, 282–287 10.1093/beheco/14.2.282 (doi:10.1093/beheco/14.2.282) [DOI] [Google Scholar]
- 8.Morris M. R., Wagner W. E., Jr, Ryan M. J. 1996. A negative correlation between trait and mate preference in Xiphophorus pygmaeus. Anim. Behav. 52, 1193–1203 10.1006/anbe.1996.0267 (doi:10.1006/anbe.1996.0267) [DOI] [Google Scholar]
- 9.Van Homrigh A., Higgie M., McGuigan K., Blows M. W. 2007. The depletion of genetic variance by sexual selection. Curr. Biol. 17, 528–532 10.1016/j.cub.2007.01.055 (doi:10.1016/j.cub.2007.01.055) [DOI] [PubMed] [Google Scholar]
- 10.Higgie M., Blows M. W. 2008. The evolution of reproductive character displacement conflicts with how sexual selection operates within a species. Evolution 62, 1192–1203 10.1111/j.1558-5646.2008.00357.x (doi:10.1111/j.1558-5646.2008.00357.x) [DOI] [PubMed] [Google Scholar]
- 11.Ryan M. J., Wagner W. E. J. 1987. Asymmetries in mating preferences between species: female swordtails prefer heterospecific males. Science 236, 595–597 10.1126/science.236.4801.595 (doi:10.1126/science.236.4801.595) [DOI] [PubMed] [Google Scholar]
- 12.Rauchenberger M., Kallman K. D., Morizot D. C. 1990. Monophyly and geography of the Río Panuco basin swordtails (genus Xiphophorus) with descriptions of four new species. Am. Mus. Novitates 2975, 1–41 [Google Scholar]
- 13.Hankison S. J., Morris M. R. 2001. Sexual selection and species recognition in the pygmy swordtail, Xiphophorus pygmaeus: conflicting preferences. Behav. Ecol. Sociobiol. 51, 140–145 10.1007/S00265-001-0425-3 (doi:10.1007/S00265-001-0425-3) [DOI] [Google Scholar]
- 14.Kingston J. J., Rosenthal G. G., Ryan M. J. 2003. The role of sexual selection in maintaining a colour polymorphism in the pygmy swordtail, Xiphophorus pygmaeus. Anim. Behav. 65, 735–743 10.1006/anbe.2003.2110 (doi:10.1006/anbe.2003.2110) [DOI] [Google Scholar]
- 15.Rowe C. 1999. Receiver psychology and the evolution of multicomponent signals. Anim. Behav. 58, 921–931 10.1006/anbe.1999.1242 (doi:10.1006/anbe.1999.1242) [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal G. G., Evans C. S. 1998. Female preference for swords in Xiphophorus helleri reflects a bias for large apparent size. Proc. Natl Acad. Sci. USA 95, 4431–4436 10.1073/pnas.95.8.4431 (doi:10.1073/pnas.95.8.4431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong B. B. M., Rosenthal G. G. 2006. Female disdain for swords in a swordtail fish. Am. Nat. 167, 136–140 10.1086/498278 (doi:10.1086/498278) [DOI] [PubMed] [Google Scholar]
- 18.Slovic P., Fischhoff B., Lichtenstein S. 1977. Behavioral decision theory. Annu. Rev. Psychol. 28, 1–39 10.1146/annurev.ps.28.020177.000245 (doi:10.1146/annurev.ps.28.020177.000245) [DOI] [Google Scholar]
- 19.Sih A., Bell A. M., Johnson J. C., Ziemba R. E. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277 10.1086/422893 (doi:10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 20.Brooks R., Endler J. A. 2001. Female guppies agree to differ: phenotypic and genetic variation in mate-choice behavior and the consequences for sexual selection. Evolution 55, 1644–1655 [DOI] [PubMed] [Google Scholar]
- 21.Ermer E., Cosmides L., Tooby J. 2007. Functional specialization and the adaptationist program. In The evolution of mind: fundamental questions and controversies (eds Gangstead S., Simpson J.), pp. 153–160 New York, NY: The Guilford Press [Google Scholar]
- 22.Tooby J., Cosmides L. 1992. The psychological foundations of culture. In The adapted mind: evolutionary psychology and the generation of culture (eds Barkow J., Cosmides L., Tooby J.), pp. 19–136 New York, NY: Oxford University Press [Google Scholar]