Abstract
We report the spontaneous modification and use of sticks to fish for termites, above the ground, in wild blonde capuchins (Cebus flavius). These critically endangered Neotropical primates inhabit remnants of the Atlantic Forest. They used two previously undescribed techniques to enhance their termite capture success: nest tapping and stick rotation. The current ecologically based explanation for tool use in wild capuchins (i.e. terrestrial habits and bipedalism) must be viewed cautiously. Instead, remarkable manual skills linked to a varied diet seem important in promoting tool use in different contexts. The repertoire of tool-using techniques employed by wild capuchins has been expanded, highlighting the behavioural versatility in this genus.
Keywords: tool use, manual skills, cognition, blonde capuchins, primates
1. Introduction
Because tools play a central role in human culture and evolution, their manufacture and use by non-human animals are a fascinating subject of investigation [1]. While it is known that wild animals of different taxonomic groups are capable of using tools [2], a flexible tool repertoire has been commonly ascribed only to chimpanzees and orang-utans (e.g. [3,4]). However, growing evidence, obtained in dry regions of Brazil (savannah-like vegetation), suggests that capuchins are also versatile users of tools (see Ottoni & Izar [5] for a review).
The arid environment where tool use has been observed in capuchins has influenced current explanations for the disjunct distribution of tool use across dry and humid habitats. Moura & Lee [6], for example, suggested that food scarcity (motivational factor) and terrestrial habits (through which monkeys had access to tubers, roots and some insects by digging with stones) were the main factors for the occurrence of tool use in those primates. More recently, Ottoni & Izar [5] concluded that food scarcity is of peripheral importance, after noting that tool use can occur in groups of capuchins with provisioning and is absent in others during periods of food scarcity. The latter proposed that terrestrial habits, which free the monkey's hands to transport tools as they travel bipedally and also provide stable and relatively flat surfaces to practise tool-using behaviour, are the most important behavioural factors promoting tool use in wild capuchins [4]. Given the limited data on tool use in wild capuchins, the propositions supporting the appearance of tool use remain unclear.
Termites are abundant in all tropical biomes, and are a nutritious food source for many animals, including humans [7]. The use of sticks to extract termites by wild chimpanzees was originally observed in the forests of Gombe [8], and this phenomenon rapidly became one of the best known and most influential discoveries involving animal tool use.
We report here the spontaneous modification and use of sticks to fish for termites observed in one group of wild blonde capuchins (Cebus flavius) living in a fragment of Atlantic Forest (figure 1a). This species was recently rediscovered, after presumed extinction [9]. We also report the use of two techniques employed sequentially by the capuchins during termite fishing that have never been described in other non-human primates, including chimpanzees. Experiments with humans playing the role of termite fishers in the monkeys' habitat were also conducted to test the effectiveness of the fishing techniques employed by the animals.
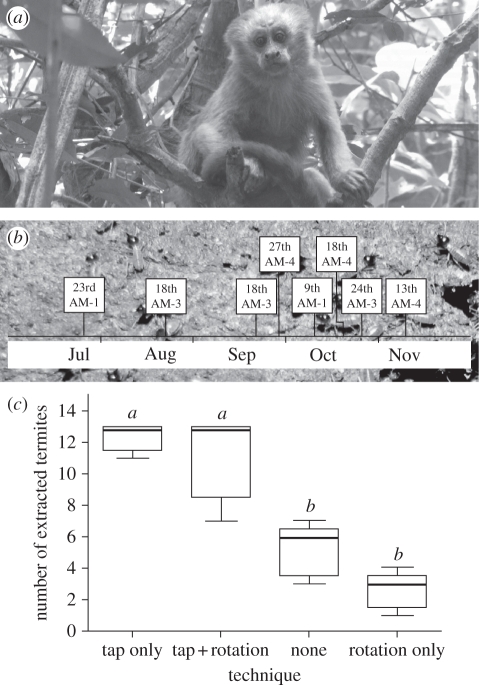
Figure 1.
(a) A blonde capuchin (Cebus flavius) in the study site. (b) Distribution of termite fishing activity across the observational period (days and months are in italic; AM refers to ‘adult male’ and the number to its identity). Background image: Nasutitermes sp. (c) Tapping increased the rate of collection; rotation did not. The term ‘none’ means that the tapping and rotating techniques were not employed. Illustrated are medians and interquartile ranges. Statistics: Mann–Whitney U-test, adjusted via a sequential Bonferroni correction (tap only versus tap + rotation: U = 10.5, p = 0.7484a,a; tap only versus none: U = 0, p = 0.0079a,b; tap only versus rotation only: U = 0, p = 0.0079a,b; tap + rotation versus none: U = 0.5, p = 0.0162a,b; tap + rotation versus rotation only: U = 0, p = 0.0079a,b; none versus rotation only: U = 2.5, p = 0.0463b,b); n1 = n2 = 5 in all cases; after correction: different letters = p ≤ 0.05; similar letters = n.s.).
2. Material and methods
(a). Study site
The study site (6°33′32.1″S, 35°07′56.5″W) has approximately 300 ha of Atlantic Forest that belongs to three different owners. We obtained permission to work in ASPLAN/Mamanguape/PB segment (94 ha of forest). General characteristics of the vegetation: canopy, about 20 m high; emergent trees, up to 25 m high; understorey, juvenile trees and smaller tree species. The sugar cane monoculture dominates the landscape around the fragment.
Aspects of the climate in the Mamanguape district: rainy season, April–October; average annual temperature, 24–26°C; average annual rainfall, 1750–2000 mm; average annual humidity, 80 per cent [10].
(b). Subjects
We studied a group of six tufted blonde capuchins (four adult males, one adult female and one juvenile male). It is unclear whether this group composition is normal for this species. Each individual was reliably identified through their physical characteristics (body size, scars and variation in fur colour). They were habituated to the observers C.B.C.B. and M.B.
The diet of these monkeys included fruits, insects, spiders and small vertebrates. On three occasions individuals were seen eating sugar cane. Foraging, excluding termite fishing, was a daily activity displayed by all individuals of the study group. Termite fishing was the only form of tool use observed.
(c). Observations
Fieldwork was carried out from July to November 2009 (140 h of direct observation across 72 days), at distances ranging from 5 to 30 m from the animals. The observational time was unevenly distributed across the 72 days (117 min d−1, ±11.95 s.e.m.), because the animals were not always located in the part of the fragment for which we received permission to work. All data, including the dietary observations, were collected using focal animal and ad libitum techniques [11]. Observations started when the monkeys were located in the forest. Behaviours were recorded using a notepad.
(d). Evaluating the efficiency of tapping and rotating techniques
We chose, in situ, 20 termite nests, similar in size (mean circumference: 48.5 cm, ±2.16 s.e.m.; mean height: 28.5 cm, ±1.09 s.e.m.) to those visited by C. flavius, to evaluate how tapping and rotating affected capture of termites. We used sticks of the plant Ouratea sp., the same used by the monkeys, to perforate the nests. The experimental design involved four different situations (five nests used in each situation): (i) before introducing the stick, we tapped the walls of the nest, but we did not rotate the stick while introducing it into the nest; (ii) we tapped the nest and perforated it by applying a rotating movement on the stick; (iii) no tapping or rotation were used; and (iv) we did not tap the nest, but rotation was employed. Used sticks were immediately placed into individual plastic bags, each of them sealed. We subsequently counted the termites collected on each stick.
(e). Statistical analyses
We compared the number of termites collected per stick across all pairwise combinations of the four experimental fishing techniques using the Mann–Whitney U-test; we adjusted p-values via a sequential Bonferroni correction [12]. Significance after correction was set at p ≤ 0.05, two-tailed.
3. Results
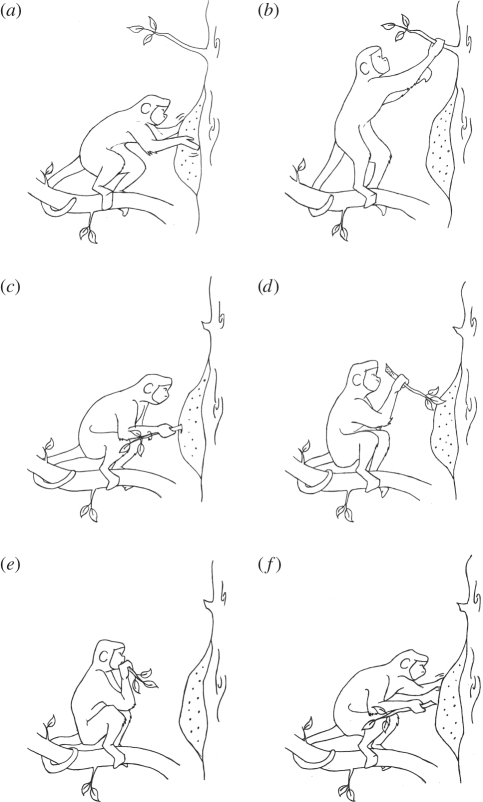
Three out of six study animals (all adult males) were observed eight times (in 8 of 72 days) (figure 1b) collecting termites (Nasutitermes sp.) from different nests in the canopy (5–10 m above the ground). The behaviour consisted of three main steps: (i) the monkey approached the nest, reaching for and then tapping (firmly and quickly, as when capuchins tap hard surfaces) the nest exterior immediately in front of him using both palms (when in front of the nest, the body in a squatting position; semi-prehensile tail used to anchor the body on a stable horizontal branch (10–15 cm in diameter); figure 2a,c–f); (ii) the monkey tore off a branchlet (hereafter, stick) approximately 20–30 cm long from the tree where the nest was located (to tear off the stick, the capuchin may temporarily adopt another body position; figure 2b), perforated the nest with it (approximately in the middle) and then inserted it into the nest (5–10 cm deep). The monkey, always with the right hand, inserted the stick by rotating while pressing the basal portion of the stick against the nest at the opening where it was inserted. In all events, the animal continuously rotated the stick using the right-hand while perforating the nest. On two occasions, two individuals reduced the length of the stick by breaking part of its basal portion (which is free of leaves and thicker than the apices); and (iii) the monkey pulled the stick out of the nest, inspected the stick, and ate the termites attached to it (figure 2c–e). In six visits, the procedure of inserting the stick was repeated three or four times re-using the first perforation. In these subsequent repetitions, the monkeys rotated the stick in the same way. Between insertions, the monkey tapped the nest close to the perforation opening always with the palm of the left hand (the right hand holding the stick). In all cases, after finishing, sticks were discarded. Each episode of fishing for termites, from the arrival at the nest to the act of discarding the stick, lasted between 40 and 60 s (including repetitions).
Figure 2.
The sequence of behaviours displayed by the blonde capuchins when fishing for termites. (a) Tapping the nest. (b) Tearing off a branchlet. (c) Rotating and inserting the stick into the nest. (d) Inspecting the stick. (e) Eating the termites. (f) Tapping the nest with the left hand.
The influence of tapping the nest and rotating the stick on the number of termites extracted per insertion was assessed by human subjects. Tapping had a strong positive influence, while rotation had no clear effect (figure 1c). However, rotation affected a different aspect of efficiency. Without rotation, the stick broke and had to be replaced in four occasions (out of 10). No stick broke when rotation was employed (i.e. extending the useful life of the tool).
4. Discussion
Fishing for termites is clearly present in wild blonde capuchins. Worthy of note, tool-using was performed above the ground, whereas tool-using in capuchins has been commonly associated with terrestrial habits and bipedal postures [4]. Although we agree that the expression of certain tool-using behaviours, like employing stones and anvils to crack nuts [13], can be promoted by terrestrial habits, terrestriality cannot be invoked to explain our findings. However, bipedal postures free the hands to manipulate objects [1]. This is a common point observed among studies in arid regions and our study in the Atlantic Forest. With their bodies in a squatting position, the blonde capuchins relied upon free hands to fish for termites. They could adopt these postures in part because their semi-prehensile tail helped anchor them against gravity [14]. This allowed efficient bimanual action involving tapping the walls of the nest, and inserting and rotating the stick. Tapping the walls of the nest and rotating the stick have not been reported previously for chimpanzees or any other non-human primates. They indicate effective problem solving and effective deployment of sensitive manual actions.
Data gathered on wild chimpanzees' tool use have shown a greater than expected variability within a population, and across populations inhabiting similar environments, a confounding factor for ecologically based explanations [3]. Our results similarly suggest that linking tool use to ecological conditions in capuchins must be done cautiously. Without denying the influence of social phenomena, the importance of manual skills (viewed as resultants of mental processes promoting complex goal-directed actions) has been highlighted for understanding problem-solving in apes [15]. When fishing for termites, blonde capuchins expressed bimanual role differentiation, manual laterality, object modification and sequences of manual actions, all features supported by having two hands free. Such features are also found in other capuchins and, together with their varied diet [16], seem to be important factors enabling tool use to emerge in different contexts. Further specific studies on blonde capuchins inhabiting isolated fragments will help to address questions about the behavioural distribution and flexibility of fishing for termites across populations. Critically endangered [17] and vastly unknown, all efforts should be directed to study and protect this fascinating species.
Acknowledgements
The study was non-invasive and complied with Brazilian law.
We thank two anonymous referees for valuable comments, the ASPLAN/PB and Mônica Montenegro for logistical assistance, Adelmar Bandeira and Auristela Albuquerque for identifying the termites, Lucilene dos Santos for identifying the plant used as a tool and Fernanda Oliveira for the illustrations in this study. Supported by the UFRPE (UR09/2009-04) and the CNPq (479483/2009-4).
References
- 1.Visalberghi E., Fragaszy D. 2006. What is challenging about tool use? The capuchin's perspective. In Comparative cognition: experimental explorations of animal intelligence (eds Wasserman E. A., Zentall T. R.), pp. 529–552 New York, NY: Oxford University Press [Google Scholar]
- 2.Bentley-Condit V. C., Smith E. O. 2010. Animal tool use: current definitions and an updated comprehensive catalog. Behaviour 147, 185–32A(–152) 10.1163/000579509X12512865686555 (doi:10.1163/000579509X12512865686555) [DOI] [Google Scholar]
- 3.Sanz C. M., Morgan D. B. 2007. Chimpanzee tool technology in the Goualougo Triangle, Republic of Congo. J. Hum. Evol. 52, 420–433 10.1016/j.jhevol.2006.11.001 (doi:10.1016/j.jhevol.2006.11.001) [DOI] [PubMed] [Google Scholar]
- 4.Van Schaik C. P., Deaner R. O., Merrill M. Y. 1999. The conditions for tool use in primates: implications for the evolution of material culture. J. Hum. Evol. 36, 719–741 10.1006/jhev.1999.0304 (doi:10.1006/jhev.1999.0304) [DOI] [PubMed] [Google Scholar]
- 5.Ottoni E. B., Izar P. 2008. Capuchin monkey tool use: overview and implications. Evol. Anthropol. 17, 171–178 10.1002/evan.20185 (doi:10.1002/evan.20185) [DOI] [Google Scholar]
- 6.Moura A. C., de A., Lee P. C. 2004. Capuchin stone tool use in Caatinga dry forest. Science 306, 1909. 10.1126/science.1102558 (doi:10.1126/science.1102558) [DOI] [PubMed] [Google Scholar]
- 7.Solavan A., Paulmurugan R., Wilsanand V. 2006. Effect of the subterranean termite used in the South Indian folk. Ind. J. Tradit. Knowl. 5, 376–379 [Google Scholar]
- 8.Goodall J. 1963. My life among wild chimpanzees. Natl Geogr. 124, 272–308 [Google Scholar]
- 9.Oliveira M. M., Langguth A. 2006. Rediscovery of Marcgrave's capuchin monkey and designation of a neotype for Simia flavia Schreber, 1774 (Primates, Cebidae). Bol. Mus. Nac. 523, 1–16 [Google Scholar]
- 10.Nimer E. 1979. Climatologia do Brasil. Rio de Janeiro, Brasil: IBGE [Google Scholar]
- 11.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–267 10.1163/156853974X00534 (doi:10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 12.Rice W. R. 1989. Analyzing tables of statistical tests. Evolution 43, 223–225 10.2307/2409177 (doi:10.2307/2409177) [DOI] [PubMed] [Google Scholar]
- 13.Visalberghi E., Addessi E., Truppa V., Spagnoletti N., Ottoni E., Izar P., Fragaszy D. 2009. Selection of effective stone tools by bearded capuchin monkeys. Curr. Biol. 19, 213–217 10.1016/j.cub.2008.11.064 (doi:10.1016/j.cub.2008.11.064) [DOI] [PubMed] [Google Scholar]
- 14.Garber P. A., Rehg J. A. 1999. The ecological role of the prehensile tail in white-faced capuchins (Cebus capucinus). Am. J. Phys. Anthropol. 110, 325–339 (doi:10.1002/(SICI)1096-8644(199911)110:3<325::AID-AJPA5>3.0.CO;2-D) [DOI] [PubMed] [Google Scholar]
- 15.Byrne R. 2004. The manual skills and cognition that lie behind hominid tool use. In The evolution of thought: evolutionary origins of great ape intelligence (eds Russon A. E., Begun D. R.), pp. 31–44 Cambridge, UK: Cambridge University Press; (doi: 10.2277/0521783356) [Google Scholar]
- 16.Fragaszy D. M., Visalberghi E., Fedigan L. M. 2004. The complete capuchin: the biology of the genus Cebus. Cambridge, UK: Cambridge University Press [Google Scholar]
- 17.IUCN 2010. Red list of threatened species (Cebus flavius). See www.iucnredlist.org (accessed on 20 December 2010).