Abstract
Insect societies integrate many information sources to organize collective activities such as foraging. Many ants use trail pheromones to guide foragers to food sources, but foragers can also use memories to find familiar locations of stable food sources. Route memories are often more accurate than trail pheromones in guiding ants, and are often followed in preference to trail pheromones when the two conflict. Why then does the system expend effort in producing and acquiring seemingly redundant and low-quality information, such as trail pheromones, when route memory is available? Here we show that, in the ant Lasius niger, trail pheromones and route memory act synergistically during foraging; increasing walking speed and straightness by 25 and 30 per cent, respectively, and maintaining trail pheromone deposition, but only when used together. Our results demonstrate a previously undescribed major role of trail pheromones: to complement memory by allowing higher confidence in route memory. This highlights the importance of multiple interacting information sources in the efficient running of complex adaptive systems.
Keywords: synergy, trail pheromone, route memory, multiple information sources
1. Introduction
The integration of multiple information sources is necessary for the functioning of adaptive biological systems at cell, organ, organism and society levels [1–4] and also in technological systems [5]. The successful coordination of the many individuals in a colony of social insects typically involves the gathering and transfer of information from several sources [6–8]. Workers may gain information either by interacting with their environment (private information) or by interacting with their nest-mates (social information) [9,10].
During foraging, workers commonly transfer information in order to enhance the ability of the colony to forage efficiently in a constantly changing environment. Honeybees, for example, use the waggle dance to inform nest-mates about the location of food patches in the environment [11], whereas many ants use trail pheromones. Pheromone trails, however, do not provide perfect information and naive foragers frequently make mistakes when using trail pheromones at trail bifurcations [12–15]. Grüter et al. [12] found that in Lasius niger, only 70 per cent of foragers chose a trail with high levels of trail pheromone at a bifurcation. Route memories, on the other hand, may be acquired rapidly, leading to 95 per cent correct choices by L. niger workers at a bifurcation after only three previous visits to a food source on one branch [12]. In situations where these information types conflict, many species follow route memories in preference to trail pheromones [12,13,16–18], as do honeybees when social and private information conflicts [19]. Aphid-tending ants may return to stable food sources for days, weeks or months [16,20,21]. Why then do ants continue laying pheromones on a trail past the initial recruitment phase when they have more reliable route memories? One possibility is that pheromones are only used by naive ants. However, this does not explain why pheromone deposition continues past the initial recruitment. Could pheromones interact with route memory to play a yet unknown function for the vast majority of foragers that are travelling along familiar routes? We propose an alternative hypothesis that trail pheromones may complement route memory. Foraging ants may be using the trail pheromone as a reassurance marker, like a white line on a road, allowing ants to reduce the speed/accuracy trade-off by reducing the foragers' need to spend time checking their location or avoiding straying from trails. Foraging efficiency could thus be increased on familiar trails. We therefore tested whether the presence of trail pheromone causes an increase in walking speed and path straightness, and a decrease in the rate of U-turning in both experienced and naive ants.
2. Material and methods
We videoed foragers of the common garden ant L. niger which had or had not visited the food source before, walking over a straight walkway divided into three sections. The middle section was unmarked by trail pheromone and home-range markings (markings deposited passively by walking ants that may inform them of the local frequency of nest-mates or other ants), while the nest side and feeder end sections were marked either with trail pheromone and home-range markings or just home-range markings (figure 1). Walking speed, sinuosity, U-turn rates and trail pheromone deposition rates were determined from the video. Details of the experimental procedure are provided as electronic supplementary material, S2.
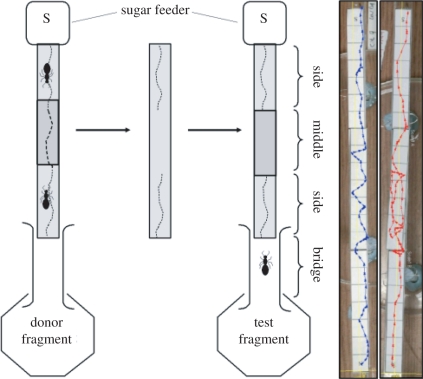
Figure 1.
Apparatus used to create a walkway marked by trail pheromone. An ant colony is split into two equal fragments. The donor fragment is allowed access to a 21 cm long walkway covered in printer paper with a 1 M sucrose feeder at one end. The middle 7 cm section has an additional paper overlay. Once either 1 or 20 ants had deposited pheromone at least once on the middle section, the 7 cm overlay is discarded and the 21 cm walkway cleared of ants. This was then used to replace the walkway in the test fragment colony. Simultaneously, the test ants are also visiting a sucrose feeder at the end of a 21 cm walkway. Ants are marked individually at the feeder and allowed to make one or three return visits. The trail pheromone-marked walkway from the donor colony is then used to replace the original walkway. A fresh 7 cm paper overlay is placed over the middle section to mask any possible marks, and discarded and replaced every time an ant walks over it. On the right are two representative paths of an experienced ant returning to the feeder. The blue path (left) is of an ant with one previous visit to the feeder. The red path (right) is an ant with three previous visits to the feeder. Both paths are outbound walks on a heavily pheromone-marked walkway.
3. Results
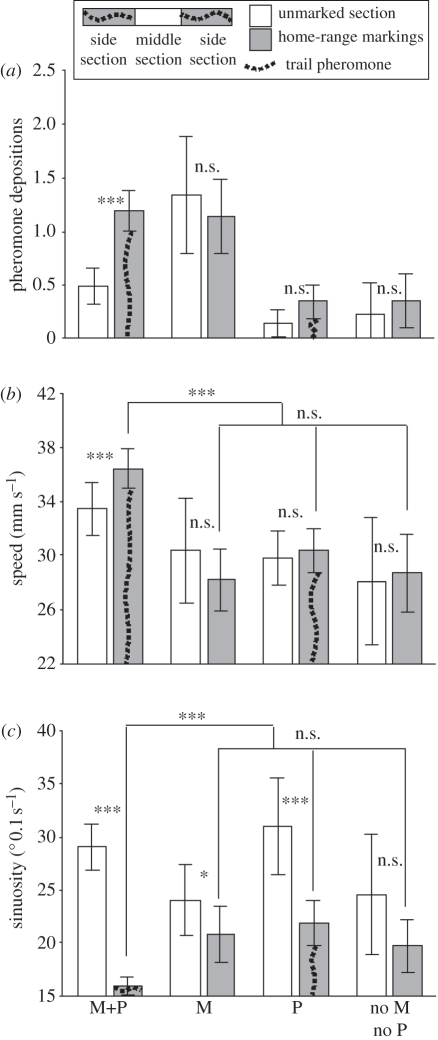
We found that experienced ants walked 30 per cent faster and 30 per cent straighter on walkways marked by trail pheromone versus unmarked walkways, 20 per cent faster and 37 per cent straighter than naive ants walking on pheromone-marked walkways, and 29 per cent faster and 24 per cent straighter than naive ants walking on unmarked walkways [generalized linear mixed model (GLMM), speed; all comparison t > 4, p < 0.001, sinuosity; all comparisons t > 2.88, p < 0.003] (electronic supplementary material, table S1 and figure 2b,c). This shows that there is a synergistic interaction between the two sources of information, as no increase in walking speed or straightness occurred when only one of the information sources was present. Ants with route memory that step off a pheromone-marked path also reduce their trail pheromone deposition rate significantly, by 59 per cent (electronic supplementary material, table S1 and figure 2a). This explains why previous authors [22] reported no effect of trail pheromone on path sinuosity and speed in L. niger: only in combination with route memory do these effects occur. Stepping off a path marked with trail pheromones causes an increase in U-turning rates regardless of the presence of route memory (GLMM, Z > 2.78, p < 0.01, electronic supplementary material, table S1).
Figure 2.
Comparing behaviours on middle and side trail sections. Side trail sections always have home-range markings plus trail pheromones where indicated. Middle sections are always unmarked. (a) Pheromone laying reduced when experienced ants step off a pheromone-marked section. (b,c) Walking speed increased and sinuosity decreased when experienced ants walk on substrate marked with pheromone. M, route memory; P, side sections marked with trail pheromone. Annotations refer to significance level (GLMM, ***p < 0.001, **p < 0.01, *p ≤ 0.05, n.s. = p > 0.05). Thick connecting lines present statistical comparisons. Whiskers on bars represent 2 s.d.
The strength of the trail pheromone (one ant passage or 20 ant passages, see the electronic supplementary material, S2) had no effect on trail pheromone deposition rates (GLMM, t = 0.035, p > 0.5), U-turning rates (GLMM, t = 1.24, p > 0.5), walking speed (GLMM, t = 1.39, p > 0.05) or path sinuosity (GLMM, t = 0.101, p > 0.5). This suggests that the presence of trail pheromone releases an all-or-nothing effect in foraging ants and agrees with previous research on L. niger in which more heavily marked trails did not lead to greater accuracy in trail choice at a T-junction [12].
Experience level, home-range markings, and direction of travel also affected ant behaviour—see the electronic supplementary material, S1 and table S1 for details.
4. Discussion
The presence of trail pheromones seems to ‘reassure’ experienced foragers that they have not strayed from the trail. This allows a reduced investment in error checking, leading to increased speed until a lack of trail pheromone indicates that they have strayed. In real terms, ants can walk faster and straighter, relying on the lack of pheromones to inform them that they have strayed from the trail. The trade-off between speed and accuracy is a common problem for many animals [23]. Here, with the presence of trail pheromone information reassuring the foragers, the need to make this trade-off can be lessened by allowing ants to increase foraging speed without sacrificing accuracy. The reduction in pheromone deposition shown by experienced foragers when they step off a marked path will also have the effect of maintaining path integrity, avoiding erroneous informational cascades [24] by ensuring that ants which do make an error will not compound this error by marking false paths with trail pheromone. But why require a route memory for the cessation of pheromone deposition when suddenly leaving a pheromone-marked path? We suggest that this cessation does not occur on the first return trip from the feeder so as to allow the formation of new continuous trails by ants on their first return journey while maintaining trail cohesiveness of established trails.
It is often assumed that social insect foragers have to decide between social information and memory [7,10]. Our results show that the combination of these seemingly mutually exclusive information sources leads to the emergence of adaptive properties in the colony's foraging system and leads to further questions concerning interactions between information sources in insect societies and other complex adaptive systems.
Acknowledgements
We thank Thomas Collett for comments on a previous version of this manuscript. T.C. was supported by a PhD studentship from BBSRC. G.C. was funded by a postdoctoral fellowship from the Swiss National Science Foundation (SNSF grant no: PA00P3_129134). S.J. was funded by a Sussex University GTP studentship.
References
- 1.Bro-Jørgensen J. 2010. Dynamics of multiple signalling systems: animal communication in a world in flux. Trends Ecol. Evol. 25, 292–300 10.1016/j.tree.2009.11.003 (doi:10.1016/j.tree.2009.11.003) [DOI] [PubMed] [Google Scholar]
- 2.King C. 2009. New insights into the differentiation and function of T follicular helper cells. Nat. Rev. Immunol. 9, 757–766 10.1038/nri2644 (doi:10.1038/nri2644) [DOI] [PubMed] [Google Scholar]
- 3.Cahill J. F., McNickle G. G., Haag J. J., Lamb E. G., Nyanumba S. M., St. Clair C. C. 2010. Plants integrate information about nutrients and neighbors. Science 328, 1657. 10.1126/science.1189736 (doi:10.1126/science.1189736) [DOI] [PubMed] [Google Scholar]
- 4.Camazine S., Deneubourg J., Franks N. R., Sneyd J., Theraula G., Bonabeau E. 2003. Self-organization in biological systems. Princeton, NJ: Princeton University Press [Google Scholar]
- 5.Tanenbaum A. S. 2003. Computer networks. Englewood Cliffs, NJ: Prentice Hall & PTR [Google Scholar]
- 6.Robinson E. J. H., Jackson D. E., Holcombe M., Ratnieks F. L. W. 2005. Insect communication: ‘no entry’ signal in ant foraging. Nature 438, 442. 10.1038/438442a (doi:10.1038/438442a) [DOI] [PubMed] [Google Scholar]
- 7.Leadbeater E., Chittka L. 2007. Social learning in insects: from miniature brains to consensus building. Curr. Biol. 17, 703–713 10.1016/j.cub.2007.06.012 (doi:10.1016/j.cub.2007.06.012) [DOI] [PubMed] [Google Scholar]
- 8.Ratnieks F. L. W. 2008. Biomimicry: further insights from ant colonies? Bio-Inspired Comput. Commun. 5151, 58–66 10.1007/978-3-540-92191-2_6 (doi:10.1007/978-3-540-92191-2_6) [DOI] [Google Scholar]
- 9.Dall S. R., Giraldeau L., Olsson O., McNamara J. M., Stephens D. W. 2005. Information and its use by animals in evolutionary ecology. Trends Ecol. Evol. 20, 187–193 10.1016/j.tree.2005.01.010 (doi:10.1016/j.tree.2005.01.010) [DOI] [PubMed] [Google Scholar]
- 10.Kendal R. L., Coolen I., Van Bergen Y., Laland K. N. 2005. Trade-offs in the adaptive use of social and asocial learning. Adv. Stud. Behav. 35, 333–379 10.1016/S0065-3454(05)35008-X (doi:10.1016/S0065-3454(05)35008-X) [DOI] [Google Scholar]
- 11.von Frisch K. 1967. The dance language and orientation of bees. Cambridge, MA: Harvard University Press [Google Scholar]
- 12.Grüter C., Czaczkes T. J., Ratnieks F. L. W. 2010. Decision making in ant foragers (Lasius niger) facing conflicting private and social information. Behav. Ecol. Sociobiol. 65, 141–148 10.1007/s00265-010-1020-2 (doi:10.1007/s00265-010-1020-2) [DOI] [Google Scholar]
- 13.Harrison J. F., Fewell J. H., Stiller T. M., Breed M. D. 1989. Effects of experience on use of orientation cues in the giant tropical ant. Anim. Behav. 37, 869–871 10.1016/0003-3472(89)90076-6 (doi:10.1016/0003-3472(89)90076-6) [DOI] [Google Scholar]
- 14.Deneubourg J., Pasteels J., Verhaeghe J. 1983. Probabilistic behaviour in ants: a strategy of errors? J. Theor. Biol. 105, 259–271 10.1016/S0022-5193(83)80007-1 (doi:10.1016/S0022-5193(83)80007-1) [DOI] [Google Scholar]
- 15.Jeanson R., Ratnieks F. L. W., Deneubourg J. 2003. Pheromone trail decay rates on different substrates in the Pharaoh's ant, Monomorium pharaonis. Physiol. Entomol. 28, 192–198 10.1046/j.1365-3032.2003.00332.x (doi:10.1046/j.1365-3032.2003.00332.x) [DOI] [Google Scholar]
- 16.Rosengren R., Fortelius W. 1986. Ortstreue in foraging ants of the Formica rufa group: hierarchy of orienting cues and long-term memory. Insect Soc. 33, 306–337 10.1007/BF02224248 (doi:10.1007/BF02224248) [DOI] [Google Scholar]
- 17.Avebury 1906. Ants, bees and wasps, vol. Xl The International Scientific Series London, UK: Kegan Paul [Google Scholar]
- 18.Beugnon G., Fourcassie V. 1988. How do red wood ants orient during diurnal and nocturnal foraging in a three dimensional system? II. Field experiments. Insect. Soc. 35, 106–124 10.1007/BF02224142 (doi:10.1007/BF02224142) [DOI] [Google Scholar]
- 19.Grüter C., Balbuena M. S., Farina W. M. 2008. Informational conflicts created by the waggle dance. Proc. R. Soc. B 275, 1321–1327 10.1098/rspb.2008.0186 (doi:10.1098/rspb.2008.0186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinet Y., Pasteels J. M. 1996. Spatial specialization of the foragers and foraging strategy in Lasius fuliginosus (Latreille) (Hymenoptera, Formicidae). Insect Soc. 43, 333–346 10.1007/BF01258407 (doi:10.1007/BF01258407) [DOI] [Google Scholar]
- 21.Salo O., Rosengren R. 2001. Memory of location and site recognition in the ant Formica uralensis (Hymenoptera: Formicidae). Ethology 107, 737–752 10.1046/j.1439-0310.2001.00702.x (doi:10.1046/j.1439-0310.2001.00702.x) [DOI] [Google Scholar]
- 22.Breton J., Fourcassie V. 2004. Information transfer during recruitment in the ant Lasius niger L. (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 55, 242–250 10.1007/s00265-003-0704-2 (doi:10.1007/s00265-003-0704-2) [DOI] [Google Scholar]
- 23.Chittka L., Skorupski P., Raine N. E. 2009. Speed-accuracy tradeoffs in animal decision making. Trends Ecol. Evol. 24, 400–407 10.1016/j.tree.2009.02.010 (doi:10.1016/j.tree.2009.02.010) [DOI] [PubMed] [Google Scholar]
- 24.Bikhchandani S., Hirshleifer D., Welch I. 1992. A theory of fads, fashion, custom, and cultural change as informational cascades. J. Politic. Econ. 100, 992–1026 10.1086/261849 (doi:10.1086/261849) [DOI] [Google Scholar]