Abstract
Large-brained diurnal mammals with complex social systems are known to plan where and how to reach a resource, as shown by a systematic movement pattern analysis. We examined for the first time large-scale movement patterns of a solitary-ranging and small-brained mammal, the mouse lemur (Microcebus murinus), by using the change-point test and a heuristic random travel model to get insight into foraging strategies and potential route-planning abilities. Mouse lemurs are small nocturnal primates inhabiting the seasonal dry deciduous forest in Madagascar. During the lean season with limited food availability, these lemurs rely on few stationary food resources. We radio-tracked seven lemurs and analysed their foraging patterns. First change-points coincided with out-of-sight keystone food resources. Travel paths were more efficient in detecting these resources than a heuristic random travel model within limits of estimated detection distance. Findings suggest that even nocturnal, solitary-ranging mammals with small brains plan their route to an out-of-sight target. Thus, similar ecological pressures may lead to comparable spatial cognitive skills irrespective of the degree of sociality or relative brain size.
Keywords: route planning, spatial cognition, mouse lemurs, solitary forager, change-point test, non-human primates
1. Introduction
In order to unravel how ecological pressure shapes animal spatial cognitive abilities, large-scale movement patterns during foraging in nature must be quantified. Foraging strategies and spatial navigation have been studied for diurnal central-place foraging insects, mice and rats, migrating or homing birds and group-living non-human primates [1,2].
Cognitive processes allowing animals to get around can be explored through a new analytic tool, the change-point test (CPT; [3]), allowing investigators to statistically determine whether a travel is directed to a certain location. In anthropoid primates with large brains and complex social systems [1,2], route-planning abilities have been demonstrated by linking change-points (points at which travel direction is changed) to biologically relevant behavioural contexts, showing that they were out-of-sight of the route starting point and that other available resources were passed and observed routes were not explained by a random travel search (i.e. a straight-line search strategy in a random direction).
In this study, we examined for the first time movement patterns of a solitary-ranging and small-brained mammal, the mouse lemur (Microcebus murinus), to assess individual decision-making and potential route-planning abilities by using the CPT and a heuristic random travel model. Most mammals are small and nocturnal, but few of them can be studied as individuals ranging in large-scale space. Mouse lemurs are a valuable exception. They are arboreal prosimians, which forage rapidly in all strata of dense Malagasy forests. They inhabit seasonal dry deciduous forests and use few stationary keystone food resources (KFRs) such as gum and honeydew of insect larvae during the lean season [4,5]. Mouse lemurs revisit the same feeding sites regularly and show the preferred direction when leaving their nest [4]. Feeding platform experiments showed that they selectively revisit baited platforms and seem to use a Euclidean map for relocation [6,7]. Based on this knowledge, we inferred that observed foraging routes are planned to a first KFR if mouse lemurs (i) orient their travel to an out-of sight KFR that coincides with the first change-point, as determined by CPT, (ii) pass other available resources reaching a first KFR, and (iii) travel to a first KFR more efficiently than a heuristic travel model based on a straight travel in a random direction.
2. Material and methods
(a). Study site and use of global positioning system
The study was performed in northwestern Madagascar during the dry seasons 2005 and 2006. We worked in a 30.6 ha patch of a dry deciduous forest [4]. For mapping purpose, we used a global positioning system (GPS) (Magellan-Explorist 100). The mean measure precision of the device was 10.1 m (±1.6; 720 point measures).
(b). Focal observations and analysis
Seven females of M. murinus were captured and radiocollared (see [4] for methods). Three females were radiotracked in 2005, two in 2006 and additional two in both years. We followed each animal during consecutive nights between 6.00 and 12.00 h. Animals were in visual contact for 58 per cent (±18%) of the time.
The animal's position, called the waypoint, was noted using GPS coordinates, whenever the animal showed a change in behaviour or moved more than 10 m from its actual position. Tiny mouse lemurs were located visually by using head lamp-induced eye reflection. If visual contact was lost, we noted waypoints whenever we got a strong radio signal. Time data of each waypoint and the behaviour of an animal were recorded continuously [4]. Each waypoint was linked to one of three behavioural contexts: travelling, feeding or resting. The sleeping site of each individual was localized radiotelemetrically during the daytime. All feeding and sleeping sites were flagged and their spatial positions determined by GPS.
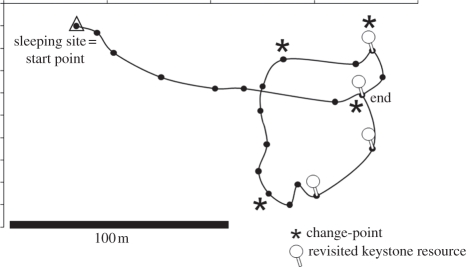
A total of 22 nocturnal foraging routes with at least 15 different waypoints were examined. We used the CPT [3] to assess when an individual changed its travel direction first. We performed the statistical analysis on all foraging routes using the program R [3]. Each route started at the sleeping site and ended at the last important KFR (the feeding site where the animal was seen for more than 6 min, capturing the majority of feeding events and more than 90% of the total time spent feeding). The CPT was applied to a route from the last keystone resource until a change-point was discovered. This point became the starting point for applying the CPT again and so on until identifying the first change-point of the route. We tested the value 1 to 6 to determine the most appropriate value q [3]. A total of 214 iterations of the CPT were run (see figure 1 for a route example). The greatest number of change-points was obtained with q = 5, p < 0.05 (29 change-points identified; median = seven change-points per animal; range: 3–7). Fourteen out of 22 routes contained one or more change-points (five animals; two to three routes per animal) for which we further explored the behavioural context.
Figure 1.
Route of one focal individual analysed using the CPT. Asterisks indicate significant directional changes detected with q = 5 and α = 0.05.
For each foraging route, we determined three distance parameters: (i) the distance travelled by a focal individual = total distance between all more-than-10-m-apart waypoints (= mean measure precision of our GPS device, see above), (ii) the distance between the starting point and each waypoint, to estimate the mean and maximum distance from the sleeping site, and (iii) the beeline distance to the first change-point. For each parameter, we calculated a mean for each individual and year and calculated the median over the seven individuals.
We determined whether available KFRs (i.e. all KFRs visited in a night) were passed before reaching the first change-point. An individual was passing a resource when it was not visited, but in the field of detection. We tested three possible detection distances: 5, 10 and 20 m.
We simulated a random search to determine the relative efficiency of finding keystone resources using a heuristic travel model similar to that of Asensio et al. [8] (see the electronic supplementary material for details). Briefly, the search model started at the sleeping site and simulated an animal travelling in a random direction and in a straight line until it encountered a KFR or the border of its home range. The encounter with a resource was considered to have occurred as soon as the line touched a resource and its buffer defined by the sensory detection distance of the animal. Since the detection field of the KFR is unavailable for mouse lemurs, we performed calculations using three distances: 5, 10 and 20 m. We used the same efficiency index as defined by Asensio et al. [8] to evaluate the search efficiency of our model ((number of KFR visited by the lemur − number of KFR found by the random model)/total number of KFR into the home range, ranges from −1 to +1, with negative efficiency values implying that the random search model is performing better than mouse lemurs, whereas positive values imply that the mouse lemurs are more efficient). This index was calculated for each of the 14 routes analysed using the CPT and containing one or more change-points (five animals; two to three routes per animal). Efficiency index was compared between the three detection distances using a Friedman test.
We assessed route overlaps between the different routes of each animal by creating a buffer of 5 m around each route and calculating the percentage of the overlapping area between all routes and a median over all individuals.
For route visualization and calculation, geographical coordinates of all waypoints were loaded in Arcview GIS 3.3 (ESRI Inc.). We analysed the data using the program SPSS v. 17.0 (SPSS Inc.) and by applying non-parametric exact two-tailed tests. The statistical significance level was p = 0.05 for all tests.
3. Results
The distance travelled by a focal animal per half night was 462 m (range: 349–517 m). Lemurs roamed in a radius of 94 m (range: 28–127 m) around the sleeping site and were seen at a maximum distance of 143 m (range: 52–177 m) from their sleeping site.
First change-points were situated at 145 m (range: 70–170 m) from the sleeping site. Six out of nine first change-points were associated with feeding, one with resting and two with the travelling context. Five out of the six feeding sites were revisited several times over different observation nights. The animals spent 14 min (range: 1–76) on the first change-points. This is significantly longer than the time spent at a randomly chosen waypoint of the same route segment (0.1 min; range: 0.1–1.3; Wilcoxon matched-pair test n = 9; T = 1; p = 0.031).
All animals were observed passing one to two available KFRs before reaching the first change-point. Findings were similar for all three detection distances.
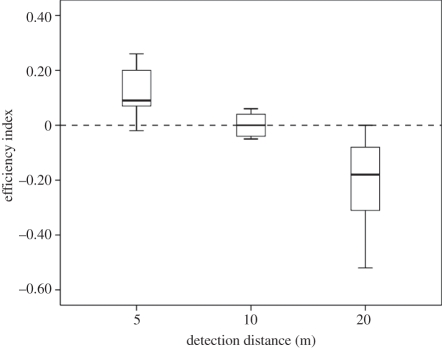
The efficiency indices calculated after simulation of the random search model differed significantly between the tested detection distances (Friedman test, χ2 = 9.6, d.f. = 2, p = 0.002, figure 2). Mouse lemurs travelled more efficiently than the random model for the distance 5 m. The heuristic random model performed better than mouse lemurs only for the distance 20 m. Mouse lemurs and the random model performed similarly for a 10 m distance.
Figure 2.
Search efficiency index for detection distances of 5, 10 and 20 m. Line = median, box = 25–75% and whisker = non-outlier range.
Overlap between different routes for an animal was 21 per cent (range 9–30%), indicating a similar range of values as found for groups of apes [8].
4. Discussion
Our study revealed that in a small-brained, solitary foraging mammal, the mouse lemur, first travel direction changes using CPT coincided with KFRs that were more than 100 m from the sleeping site. Mouse lemurs were better at detecting resources than a random travel model when the detection distance was less than 10 m. Visibility distance for diurnal and arboreal apes foraging on fruits in the canopy is estimated at 21 m [8]. Even if mouse lemur's vision is adapted to nocturnal conditions [9], it is highly unlikely that such tiny animals in a dense forest detect food at 20 m as diurnal anthropoids do. If using sensory cues to find food, mouse lemurs may actually rely more on olfactory signals [10]. Information on their olfactory detection distances is not available yet and remains limited for mammals in general. We did not observe odour plume monitoring and travel speed trade-offs as shown for other wild macrosmatic mammals, e.g. coatis [11]. The average distance at which coatis detect fruit plots is 9 m [11]. Since both gum and homopteran secretions are less smelly than ripe fruits, it is unlikely that mouse lemurs detect these resources at a distance of 9 m. We therefore conclude that 10 m or more is an unrealistic detection distance but 5 m a realistic estimation for mouse lemurs. Altogether findings support that mouse lemurs are not travelling randomly but plan their first visit to a KFR when leaving their sleeping site.
According to our study, mouse lemurs show high partial between-day overlapping of routes comparable to gibbons [8]. However, our dataset did not allow determining which mental spatial representation mouse lemurs use to navigate. Ongoing experiments will show whether they use a Euclidian representation in a small-scale range and a topological representation in a large-scale range as suggested by the model of Poucet [12].
All in all, a small-brained solitary mammal shows similar cognitive abilities as large-brained and highly social mammals such as monkeys and apes. Thus, our findings suggest that spatial skills evolved independently of large brains and sociality.
Acknowledgements
We thank R. W. Byrne, L. A. Bates, N. Asensio as well as four anonymous reviewers for their help and comments and all Malagasy authorities as well as C. Schopf, B. Randrianambinina and S. Rasoloharijaona for logistic support. The study complies with the current laws of Madagascar and was financially supported by the DAAD and the committee for gender equality from the University of Veterinary Medicine Hannover.
Footnotes
One contribution to a Special Feature on ‘Cognition in the Wild’.
References
- 1.Janson C. H., Byrne R. 2007. What wild primates know about resources: opening up the black box. Anim. Cogn. 10, 357–367 10.1007/s10071-007-0080-9 (doi:10.1007/s10071-007-0080-9) [DOI] [PubMed] [Google Scholar]
- 2.Shettleworth S. J. 1998. Cognition, evolution and behaviour, p. 688 New York, NY: Oxford University Press [Google Scholar]
- 3.Byrne R. W., Noser R., Bates L. A., Jupp P. E. 2009. How did they get here from there? Detecting changes of direction in terrestrial ranging. Anim. Behav. 77, 619–631 10.1016/j.anbehav.2008.11.014 (doi:10.1016/j.anbehav.2008.11.014) [DOI] [Google Scholar]
- 4.Joly M., Zimmermann E. 2007. First evidence for relocation of stationary food resources during foraging in a strepsirhine primate (Microcebus murinus). Am. J. Primatol. 69, 1045–1052 10.1002/ajp.20418 (doi:10.1002/ajp.20418) [DOI] [PubMed] [Google Scholar]
- 5.Radespiel U., Reimann W., Rahelinirina M., Zimmermann E. 2006. Feeding ecology of sympatric mouse lemur species in northwestern Madagascar. Int. J. Primatol. 27, 311–321 10.1007/s10764-005-9005-0 (doi:10.1007/s10764-005-9005-0) [DOI] [Google Scholar]
- 6.Joly M., Scheumann M., Zimmermann E. 2008. Wild mouse lemurs revisit artificial feeding platforms: implications for field experiments on sensory and cognitive abilities in small primates. Am. J. Primatol. 70, 892–896 10.1002/ajp.20560 (doi:10.1002/ajp.20560) [DOI] [PubMed] [Google Scholar]
- 7.Luhrs M. L., Dammhahn M., Kappeler P. M., Fichtel C. 2009. Spatial memory in the grey mouse lemur (Microcebus murinus). Anim. Cogn. 12, 599–609 10.1007/s10071-009-0219-y (doi:10.1007/s10071-009-0219-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asensio N., Brockelman W. Y., Malaivijitnond S., Reichard U. H. 2011. Gibbon travel paths are goal oriented. Anim. Cogn. (doi:10.1007/s10071-010-0374-1) [DOI] [PubMed] [Google Scholar]
- 9.Dkhissi-Benyahya O., Szel A., Degrip W. J., Cooper H. M. 2001. Short and mid-wavelength cone distribution in a nocturnal Strepsirrhine primate (Microcebus murinus). J. Comp. Neurol. 438, 490–504 10.1002/cne.1330 (doi:10.1002/cne.1330) [DOI] [PubMed] [Google Scholar]
- 10.Siemers B. M., Goerlitz H. R., Robsomanitrandrasana E., Piep M., Ramanamanjato J. B., Rakotondravony D., Ramilijaona O., Ganzhorn J. U. 2007. Sensory basis of food detection in wild Microcebus murinus. Int. J. Primatol. 28, 291–304 10.1007/s10764-007-9135-7 (doi:10.1007/s10764-007-9135-7) [DOI] [Google Scholar]
- 11.Hirsch B. T. 2010. Tradeoff between travel speed and olfactory food detection in ring-tailed coatis (Nasua nasua). Ethology 116, 671–679 [Google Scholar]
- 12.Poucet B. 1993. Spatial cognitive maps in animals—new hypotheses on their structure and neural mechanisms. Psychol. Rev. 100, 163–182 10.1037/0033-295X.100.2.163 (doi:10.1037/0033-295X.100.2.163) [DOI] [PubMed] [Google Scholar]