Abstract
Objective
High-sensitivity C-reactive protein (hsCRP) levels are closely associated with abdominal obesity, metabolic syndrome, and atherosclerotic cardiovascular disease. The JUPITER trial has encouraged using hsCRP ≥2 mg/L to guide statin therapy; however the association of hsCRP to atherosclerosis, independent of obesity, remains unknown.
Methods and Results
We studied 6,760 participants from the Multi-Ethnic Study of Atherosclerosis (MESA). Participants were stratified into 4 groups: non-obese/low hsCRP, non-obese/high hsCRP, obese/low hsCRP, and obese/high hsCRP. Using multivariable logistic and robust linear regression, we described the association with subclinical atherosclerosis, using coronary artery calcium (CAC) and carotid intima-media thickness (cIMT). Mean BMI was 28.3 ± 5.5 kg/m2, and median hsCRP was 1.9 mg/L (0.84 – 4.26). High hsCRP, in the absence of obesity, was not associated with CAC and was mildly associated with cIMT. Obesity was strongly associated with CAC and cIMT independent of hsCRP. When obesity and high hsCRP were both present, there was no evidence of multiplicative interaction. Similar associations were seen among 2,083 JUPITER-eligible individuals.
Conclusions
High hsCRP, as defined by JUPITER, was not associated with CAC and was mildly associated with cIMT in the absence of obesity. In contrast, obesity was associated with both measures of subclinical atherosclerosis independent of hsCRP status.
Keywords: obesity, hsCRP, high sensitivity C-reactive protein, subclinical atherosclerosis, coronary artery calcium, carotid intima-media thickness
INTRODUCTION
High-sensitivity C-reactive protein (hsCRP) is an inflammatory marker that is closely associated with abdominal obesity1, metabolic syndrome2, and atherosclerotic cardiovascular disease3. The recent Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial has encouraged the use of hsCRP ≥2 mg/L mg/L as a screening tool for statin therapy4. This approach is useful for identifying increased absolute risk5–6, although the mechanism of increased cardiovascular risk associated with hsCRP, and its relationship to obesity, remain unclear.
Indeed, while hsCRP has previously been described as having an independent mechanistic role in atherosclerosis7, several lines of evidence call into question a causal role for CRP8. For example, a large population-based study9 and a Mendelian randomization study10 have not supported a causal association of hsCRP with atherosclerosis. In prior studies of subclinical atherosclerosis, variable or negative associations have been seen with inflammatory markers11–14, while measures of overweight and obesity have been strongly associated with atherosclerosis15–18.
In the wake of the JUPITER trial, a more thorough understanding of hsCRP is required. To further explore the implications of hsCRP as an indicator of increased risk, we conducted a stratified analysis describing the relationship between obesity, high hsCRP, and subclinical atherosclerosis using data from the Multi-Ethnic Study of Atherosclerosis (MESA).
METHODS
The Multi-Ethnic Study of Atherosclerosis (MESA)
We used baseline data from the NIH/NHLBI-funded Multi-Ethnic Study of Atherosclerosis (MESA, 2000–2002). The MESA study design and participant recruitment have been previously published19. In summary, MESA enrolled 6,814 men and women from 4 different ethnic groups (Whites, Chinese, African-American, and Hispanic), aged 45 to 84, into a population-based prospective cohort study aimed at describing the prevalence, progression, and significance of subclinical atherosclerosis. Patients were enrolled from 6 geographically distinct centers in the United States. All participants were free of known cardiovascular disease at enrollment. Baseline weight of over 300 pounds is an exclusion criteria for MESA.
All participants gave informed consent, and the study was approved by the institutional review boards at all 6 MESA field centers.
Patient Population
All MESA participants had anthropomorphic measurements of obesity taken at baseline. A total of 6,762 (99%) individuals had baseline measurement of hsCRP. At baseline (2000–2002), all participants received 2 baseline cardiac CT scans for the evaluation of coronary artery calcification (CAC), and 6,726 (99%) had a baseline carotid ultrasound for measurement of carotid intima-media thickness (cIMT). Patients without baseline hsCRP or cIMT measurements were more likely to be female and African-American.
For subsequent analyses, we identified a subset of our study who fit JUPITER entry criteria (retaining the low hsCRP group for this analysis): Men age 50 and above, women age 60 and above, LDL-C <130 mg/dL, not on lipid-lowering therapy, free of diabetes, triglycerides ≤500 mg/dL, and creatinine ≤2 mg/dL.
At baseline, 417 men were younger than 50 years old, 1,546 women were younger than 60 years old, 2,167 participants had LDL-C ≥130 mg/dL, 1,101 were on lipid-lowering therapy, 971 had diabetes, 34 had triglycerides ≥500 mg/dL, and 34 had a creatinine >2 mg/dL. Excluding these individuals resulted in a “MESA JUPITER” subpopulation of 2,083 participants (see study flow diagram, Supplemental Figure).
Definition of Obesity and Metabolic Syndrome
Obesity was defined as a BMI ≥30 kg/m2, or a waist circumference >102 cm for males and >88 cm for females when BMI ≥25 kg/m2. Overweight was defined as BMI 25 – 29.9 kg/m2.
The metabolic syndrome was identified according to the modified National Cholesterol Education Program Adult Treatment Panel III (NCEP ATPIII) definition. In summary, at least 3 of the following 5 criteria must be met: waist circumference >102 cm in males and >88 cm in females, fasting glucose ≥100 mg/dL or on hypoglycemic therapy, HDL-C <40 mg/dL in men and <50 mg/dL in women, triglycerides ≥150 mg/dL, and systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or on anti-hypertensive medication. Metabolic syndrome score is the total number of NCEP ATPIII criteria present.
hsCRP and Study Covariates
hsCRP was determined by BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc., Deerfield, IL). The lower limit of detection was 0.17 mg/L. As established by the JUPITER trial, hsCRP ≥2 mg/L was considered elevated.
Family historywas obtained by asking the participantswhether any immediate family member (parents, siblings,and children) had a prior myocardial infarction. Participants were classified as current cigarette smokers, formersmokers, or never smokers. Medication use was defined as present use of prescription medications for the treatment of hypertension or hypercholesterolemia.
Using a Dinamap Pro 1000 automatedoscillometric sphygmomanometer (Critikon), restingblood pressure was measured three times with the participant in the seatedposition. The average of the last two measures was used in the analyses. A central laboratory (University of Vermont, Burlington)measured levels of total and HDL cholesterol, triglycerides, plasma glucose, and high-sensitivity C-reactive protein in bloodsamples obtained after a 12-hour fast. Diabetes was definedaccording to American Diabetes Association (ADA) guidelines as a fasting plasma glucose level >126 mg/dL or a history of medical treatment for diabetes. Replacement of smoking and diabetes with pack-years and fasting glucose in the study models resulted in minimal change with no overall impact on study conclusions.
Cardiac CT Protocol
Cardiac CT was performed at 3 sites using a cardiac-gated electron-beam CT scanner (Imatron C-150XL, GE-Imatron, San Francisco, CA), and at 3 sites using 4-slice multidetector CT. All participants were scanned over phantoms of known physical calcium concentration. Patients were scanned twice, with scores averaged. Images were read at the MESA CT reading center (Harbor-UCLA).
The MESA scanning protocol has been described previously20. Images slices were obtained with the participant supine, with no couch angulation, during a single breath hold. A minimum of 35 contiguous images were obtained, beginning above the left main coronary artery and proceeding below both ventricles. Section thickness of 3 mm, field of view 35 cm, and matrix 512 × 512 were used to reconstruct the raw data. Nominal section thickness was 3.0 mm for electron beam CT and 2.5 mm for 4-detector row CT. Spatial resolution, expressed as the smallest voxel able to be discriminated, was 1.38 mm3 (0.68 × 0.68 × 3.00 mm) for electron beam CT and 1.15 mm3 (0.68 × 0.68 × 2.50 mm) for 4-detector row CT. The kappa statistic for agreement on presence of CAC was 0.92, and the mean rescan percentage absolute difference in CAC>0 was 20.1%.
For this study, CAC was considered both as a binary measure (present vs. not present) and a continuous measure (Agatston score).
Carotid Ultrasound
The right and left common carotid arteries were imaged by trained technicians according to a common scanning protocol using high resolution B-mode ultrasonography with a Logiq 700 machine (General Electric Medical Systems, Waukesha, Wisconsin). The MESA ultrasound reading center (Tufts Medical Center) measured maximal IMT of the common carotid artery as the mean of the maximum IMT of the near and far walls onthe right and left sides.
For this study, cIMT was considered both as a binary measure (>75th percentile amongst MESA participants, as suggested in some guidelines21) and as continuous measure.
Statistical Analysis
We divided our total and MESA JUPITER populations into 4 study groups:
-
Group 1
Non-obese, hsCRP <2 mg/L (reference group)
-
Group 2
Non-obese, hsCRP ≥2 mg/L
-
Group 3
Obese, hsCRP <2 mg/L
-
Group 4
Obese, hsCRP ≥2 mg/L
For secondary analyses, similar study groups were constructed stratifying by NCEP ATPIII metabolic syndrome in place of obesity.
We analyzed baseline characteristics of the study participants according to the 4 main study groups. Frequencies and proportions were reported for categorical variables, and either means with standard deviations or medians with interquartile ranges were reported for continuous variables based on normality of distribution. Chi-square tests, Fisher’s exact tests, one-way ANOVA, or Kruskal-Wallis tests were used for comparison of variables between groups. To examine correlations, a Spearman correlation matrix between BMI, waist circumference, and hsCRP was constructed.
Multivariable regression models were used to determine the independent associations between our study groups and subclinical atherosclerosis. When considering measures of subclinical atherosclerosis as categorical variables, we conducted prevalence regression using a generalized estimating equation with logit link and binomial distribution. The measure of association from this model was interpreted as the prevalence ratio. When considering measures of subclinical atherosclerosis as continuous variables, we used robust linear regression.
Multivariable analyses were conducted in three groups: total study population (age, gender, race adjusted), MESA JUPITER population (age, gender, race adjusted), MESA JUPITER population (fully adjusted model). The fully-adjusted model included the following covariates: age, gender, race, systolic blood pressure, diastolic blood pressure, smoking (never/former/current), LDL-C, HDL-C, triglycerides, and anti-hypertensive medications. Additional models were conducted including BMI and hsCRP as continuous measures to adjust for the possibility of residual confounding. Interaction terms for age, gender, and race were tested, and discarded due to non-significance. Education, a measure of socioeconomic status, was tested in the models but discarded due to lack of significance.
To confirm the prognostic significance of our findings, we also constructed age, gender, and race adjusted Cox proportional hazards models for our 4 study groups for the prediction of coronary heart disease (CHD) and cardiovascular disease (CVD) events. CHD events consisted of myocardial infarction, death from coronary heart disease, definite angina, probable angina followed by coronary revascularization, or resuscitated cardiac arrest. CVD events consisted of myocardial infarction, angina, resuscitated cardiac arrest, stroke (not TIA), CHD death, stroke death, other atherosclerotic death, or other CVD death. A complete descriptionof the MESA follow-up methods is available at www.mesa-nhlbi.org.
All analyses used a 5% two-sided significance level. Calculations were performed using STATA software, version 8.2.
RESULTS
Baseline Characteristics – Entire MESA Population
The mean age of the 6,760 study participants was 62 ± 10 years. Approximately 53% were female, with mean calculated 10-year Framingham risk for the entire cohort of 8.2 ± 7%. The mean BMI was 28.3 ± 5.5 kg/m2, and median hsCRP was 1.9 mg/L (0.84 – 4.26). Approximately 52% were obese, and 48% had high hsCRP.
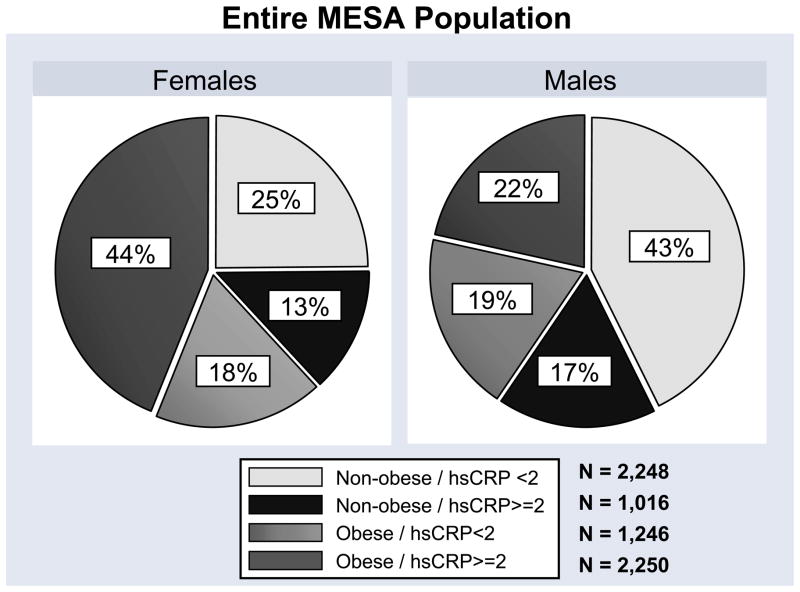
In general, females were more likely to be obese and have high hsCRP. For females, 25% were non-obese with low hsCRP, while 43% of males fit into this group. Approximately 44% of females were both obese and had high hsCRP, while just 22% of males fit this group (Figure 1).
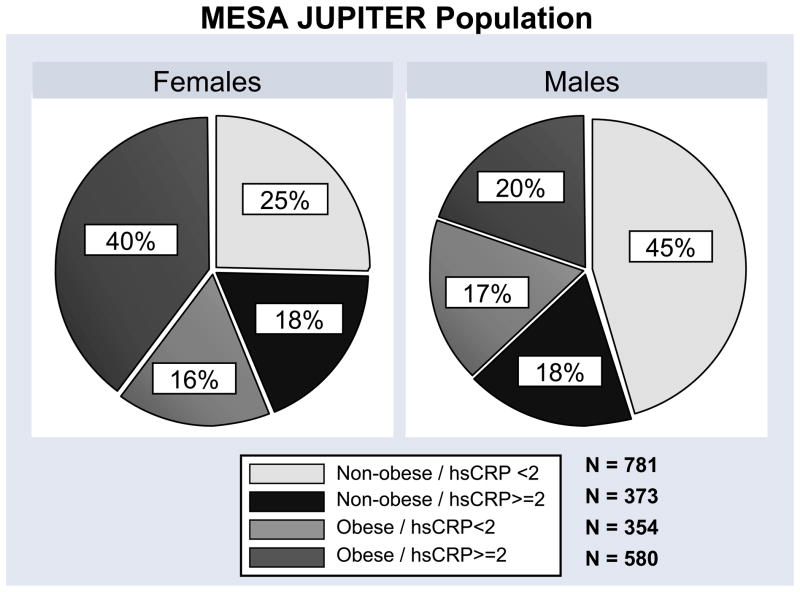
Figure 1.
Distribution of the study groups by gender for the entire MESA population and the MESA JUPITER subpopulation.
The number of participants in the 4 study groups and their characteristics are shown in Table 1. Patients in the obese, high hsCRP group were more likely to be African-American and have hypertension, diabetes, and a family history of myocardial infarction. In this group, levels of HDL-C were lower and triglyceride levels were higher, which is characteristic of increasing metabolic syndrome prevalence. However the traditional 10-year Framingham risk score for CHD, which does not include obesity, hsCRP, or metabolic syndrome, was slightly lower in this group. The number of JUPITER-eligible participants in the 4 study groups is shown in Figure 1.
Table 1.
Baseline characteristics of the study group – MESA (2000–2002).
| Characteristic | Total (N = 6,760) | Non-obese hsCRP <2 (N = 2,248) | Non-obese hsCRP ≥2 (N = 1,016) | Obese hsCRP <2 (N = 1,246) | Obese hsCRP ≥2 (N = 2,250) | P |
|---|---|---|---|---|---|---|
| Age, years | 62.2 ± 10 | 61.7 ± 11 | 63.0 ± 10 | 62.5 ± 10 | 62.0 ± 10 | <0.001 |
| Gender, women | 53% | 39% | 47% | 52% | 69% | <0.001 |
| Race | ||||||
| ▪Whites | 39% | 40% | 40% | 41% | 35% | <0.001 |
| ▪Chinese | 12% | 24% | 12% | 7% | 2% | |
| ▪African American | 27% | 20% | 25% | 28% | 36% | |
| ▪Hispanic | 22% | 16% | 23% | 24% | 26% | |
| BMI, kg/m2 | 28.3 ± 5 | 24.2 ± 3 | 24.8 ± 3 | 30.6 ± 4 | 32.9 ± 5 | <0.001 |
| Waist circumference (cm) | 98.2 ± 14 | 87.3 ± 9 | 88.9 ± 8 | 106 ± 10 | 109 ± 12 | <0.001 |
| hsCRP, mg/L | 1.92 (0.84 – 4.26) | 0.74 (0.43 – 1.20) | 3.81 (2.70 – 6.18) | 1.08 (0.68 – 1.49) | 4.71 (3.16 – 8.54) | <0.001 |
| Systolic blood pressure, mmHg | 127 ± 22 | 122 ± 21 | 126 ± 22 | 128 ± 21 | 130 ± 21 | 0.04 |
| Diastolic blood pressure, mmHg | 72 ± 10 | 72 ± 10 | 72 ± 11 | 73 ± 10 | 72 ± 10 | 0.07 |
| Hypertension | 45% | 33% | 43% | 49% | 55% | <0.001 |
| Fasting glucose, mg/dL | 105 ± 31 | 100 ± 24 | 102 ± 35 | 105 ± 28 | 110 ± 35 | <0.001 |
| Diabetes | 14% | 10% | 9% | 15% | 20% | <0.001 |
| Creatinine, mg/dL | 0.96 ± 0.3 | 0.96 ± 0.2 | 0.96 ± 0.2 | 0.96 ± 0.3 | 0.94 ± 0.4 | <0.001 |
| Smoking | <0.001 | |||||
| ▪Former | 37% | 36% | 36% | 37% | 38% | |
| ▪Current | 13% | 11% | 19% | 11% | 14% | |
| LDL, mg/dL | 117 ± 31 | 116 ± 31 | 117 ± 32 | 118 ± 31 | 118 ± 32 | 0.07 |
| HDL, mg/dL | 51 ± 15 | 53 ± 16 | 52 ± 17 | 49 ± 13 | 49 ± 13 | <0.001 |
| Triglycerides, mg/dL | 111 (78 – 161) | 95 (69 – 138) | 108 (78 – 156) | 120 (85 – 165) | 125 (88 – 181) | <0.001 |
| Family history of heart attack | 43% | 37% | 40% | 46% | 48% | <0.001 |
| Metabolic Syndrome Score | 2.2 ± 1.4 | 1.3 ± 1.1 | 1.6 ± 1.2 | 2.8 ± 1.1 | 3.0 ± 1.1 | <0.001 |
| Metabolic Syndrome | 41% | 15% | 22% | 57% | 67% | <0.001 |
| Medications for hypertension | 37% | 26% | 32% | 42% | 48% | <0.001 |
| Medications for cholesterol | 16% | 14% | 12% | 22% | 17% | <0.001 |
| 10-yr FRS (%) | 8.2 ± 7% | 8.1 ± 7% | 9.0 ± 8% | 8.7 ± 7% | 7.6 ± 7% | <0.001 |
| CAC (%) | 50% | 48% | 51% | 54% | 48% | 0.003 |
| cIMT (mm) | 0.870 ± 0.19 | 0.837 ± 0.19 | 0.876 ± 0.20 | 0.884 ± 0.19 | 0.893 ± 0.19 | 0.05 |
Both obesity and subclinical inflammation are considered central features of the metabolic syndrome. Therefore, we conducted a focused comparison of metabolic variables between the non-obese, high hsCRP group (Group 2) and the obese, low hsCRP group (Group 3). Participants in the obese, low hsCRP group were more likely to have features of the metabolic syndrome than the non-obese, low hsCRP group. Fasting glucose, blood pressure, and triglycerides were higher, and HDL-C lower in this group corresponding to more features of the metabolic syndrome.
Correlation between BMI, Waist Circumference, and hsCRP – Entire MESA Population
BMI and hsCRP were found to be correlated (Spearman rank correlation coefficient 0.42 p<0.0001). There was a greater correlation between anthropomorphic measures of obesity and hsCRP among women compared to men (Table 2). In women, the correlation coefficient between BMI and hsCRP was ρ=0.48 (p<0.0001), and between waist circumference and hsCRP it was ρ=0.44 (p<0.0001). For men, these correlation coefficients were ρ=0.35 and ρ=0.37, respectively (p<0.0001, gender/BMI interaction term p<0.0001).
Table 2.
Correlation between BMI, waist circumference, and hsCRP - MESA (2000–2002).
| Correlation coefficient between: | Males | Females |
|---|---|---|
| ρ BMI, hsCRP | 0.348 | 0.482 |
| ρ Waist Circumference, hsCRP | 0.365 | 0.438 |
| ρ BMI, Waist Circumference | 0.881 | 0.859 |
| Scenario | ||
| If obese, likelihood hsCRP ≥2 mg/dL | 53% | 71% |
| If hsCRP ≥2 mg/dL, likelihood obese | 56% | 77% |
All individual correlation coefficients were statistically significant at p<0.001.
Stated in clinically applicable terms, there was a 71% probability that obese females had high hsCRP. When hsCRP was high, approximately 77% of females were found to be obese. For males, the probabilities were 53% and 56%, respectively.
Association between Obesity, hsCRP, and Subclinical Atherosclerosis
Table 3 shows the results of the primary multivariable analyses. The reference group for these analyses was the non-obese, low hsCRP group (Group 1).
Table 3.
Association between obesity, hsCRP, and subclinical atherosclerosis -MESA (2000–2002).
| Odds Ratio | β-coefficient | |||
|---|---|---|---|---|
| CAC>0 | CIMT (>75th percentile) | CAC score (log CAC + 1) | Maximum CIMT (mm) | |
| Total Population Age, gender, race adjusted | ||||
| Non-obese/hsCRP<2 | 1 (ref) | 1 (ref) | 0 (ref) | 0 (ref) |
| Non-obese/hsCRP≥2 | 1.18 (1.00 – 1.40) | 1.34 (1.11 – 1.62) | 0.12 (−0.05 – 0.29) | 0.27 (0.15 – 0.38) |
| Obese/hsCRP<2 | 1.51 (1.29 – 1.76) | 1.63 (1.36 – 1.95) | 0.48 (0.33 – 0.64) | 0.48 (0.37 – 0.58) |
| Obese/hsCRP≥2 | 1.51 (1.32 – 1.74) | 2.17 (1.86 – 2.54) | 0.45 (0.31 – 0.59) | 0.66 (0.57 – 0.76) |
| MESA JUPITER population* Age, gender, race adjusted | ||||
| Non-obese/hsCRP<2 | 1 (ref) | 1 (ref) | 0 (ref) | 0 (ref) |
| Non-obese/hsCRP≥2 | 1.11 (0.85 – 1.47) | 1.35 (0.99 – 1.83) | 0.10 (−0.20 – 0.40) | 0.37 (0.18 – 0.55) |
| Obese/hsCRP<2 | 1.68 (1.26 – 2.22) | 1.43 (1.05 – 1.95) | 0.63 (0.32 – 0.93) | 0.50 (0.31 – 0.69) |
| Obese/hsCRP≥2 | 1.28 (1.00 – 1.64) | 2.33 (1.78 – 3.05) | 0.47 (0.20 – 0.74) | 0.68 (0.51 – 0.85) |
| MESA JUPITER population* Fully adjusted** | ||||
| Non-obese/hsCRP<2 | 1 (ref) | 1 (ref) | 0 (ref) | 0 (ref) |
| Non-obese/hsCRP≥2 | 1.04 (0.79 – 1.38) | 1.31 (0.96 – 1.80) | 0.01 (−0.28 – 0.31) | 0.34 (0.15 – 0.52) |
| Obese/hsCRP<2 | 1.44 (1.07 – 1.94) | 1.28 (0.93 – 1.77) | 0.44 (0.13 – 0.75) | 0.40 (0.21 – 0.59) |
| Obese/hsCRP≥2 | 1.01 (0.78 – 1.31) | 2.10 (1.58 – 2.80) | 0.20 (−0.08 – 0.48) | 0.54 (0.37 – 0.72) |
MESA JUPITER population (N=2,083): Men age ≥50 and women ≥60 with LDL-C <130 mg/dL, not on lipid-lowering therapy, without diabetes, triglycerides ≤500 mg/dL, and creatinine ≤2 mg/dL
Fully adjusted model: Adjusted for age, gender, race, systolic blood pressure, diastolic blood pressure, smoking, LDL-C, HDL-C, triglycerides, anti-hypertensive medications
β-coefficient should be interpreted as the absolute difference between each category and the reference category.
After adjustment for age, race, and gender, individuals in Group 2 (non-obese, high hsCRP) had a minimal association with CAC>0, which was not apparent in the MESA JUPITER subpopulation (prevalence ratio [PR] 1.11, 95% confidence interval [CI] 0.85 –1.47). In contrast, individuals in Group 3 (obese, low hsCRP) had a strong association with CAC>0, which remained similar in the MESA JUPITER population (PR 1.68, 95% CI 1.26–2.22). Among MESA JUPITER individuals in Group 4 (obese, high hsCRP), the prevalence ratio was 1.28 (95% CI 1.00–1.64), consistent with no multiplicative interaction between obesity and high hsCRP.
Adjusted for age, race, and gender, individuals in Group 2 (non-obese, high hsCRP) had a modestly increased prevalence ratio for cIMT>75th percentile that was similar, but no longer significant, in the MESA JUPITER subpopulation (PR 1.35, 95% CI 0.99–1.83). In contrast, in Group 3 (obese, low hsCRP) there was a persistent statistically significant increase in cIMT (PR 1.43, 95% CI 1.05–1.95) among the MESA JUPITER population. The prevalence ratio for the MESA JUPITER population in Group 4 (obese, hsCRP) was 2.33 (95% CI 1.78–3.05). Formal testing revealed no evidence of multiplicative interaction (p=0.35) between obesity and high hsCRP.
Similar trends were seen when CAC was considered as a continuous variable (Table 3). Group 2 (non-obese, high hsCRP) was not associated with CAC. Within Group 3 (obese, low hsCRP), there was a strong association with CAC (β=0.63, 95% CI 0.32–0.93). In Group 4, when both obesity and high hsCRP were present, the β=0.47 (95% CI 0.20–0.74), indicating no multiplicative interaction.
Similar trends were also seen when cIMT was considered as a continuous variable (Table 3). Group 2 (non-obese, high hsCRP) was mildly associated with increased cIMT (β=0.37, 95% CI 0.18–0.55). Within Group 3 (obese, low hsCRP), there was a moderate association with cIMT (β=0.50, 95% CI 0.31–0.69). The strongest absolute difference was seen when both obesity and high hsCRP were present (Group 4: β=0.68, 95% CI 0.51–0.85). Formal testing revealed no evidence of multiplicative interaction (p=0.56) between obesity and high hsCRP.
The results for the fully adjusted model in the total population are shown in Supplemental Table I. We also conducted similar analyses after substituting overweight for obesity (Supplemental Table II). Overall results and conclusions are similar to the analyses using obesity described above.
Association between Metabolic Syndrome, hsCRP, and Subclinical Atherosclerosis
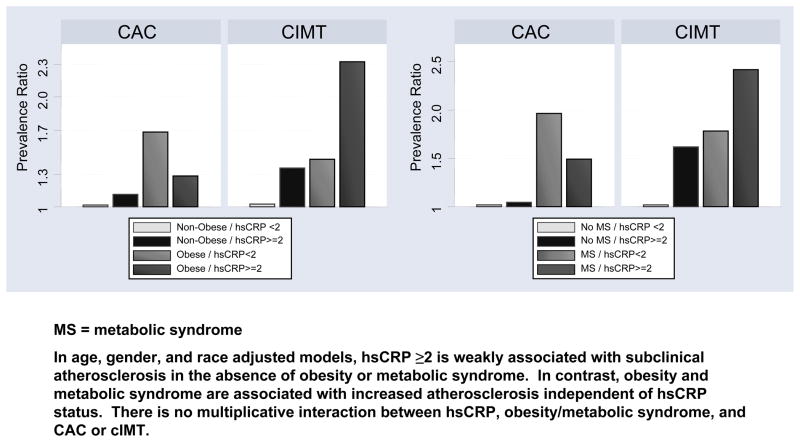
Table 4 shows the results of the multivariable analysis stratifying by hsCRP status and presence of metabolic syndrome. In general, results were similar to the above stratifying by hsCRP and obesity. High hsCRP was associated with cIMT, but not CAC, in the absence of metabolic syndrome. Metabolic syndrome was strongly correlated with both CAC and cIMT. When both metabolic syndrome and cIMT were present, there was no evidence of multiplicative interaction.
Table 4.
Association between metabolic syndrome, hsCRP, and subclinical atherosclerosis – MESA (2000–2002)
| Odds Ratio | β-coefficient | |||
|---|---|---|---|---|
| CAC>0 | CIMT (>75th percentile) | CAC score (log CAC + 1) | Maximum CIMT (mm) | |
| Total Population Age, gender, race adjusted | ||||
| No Met Syn/hsCRP<2 | 1 (ref) | 1 (ref) | 0 (ref) | 0 (ref) |
| No Met Syn/hsCRP≥2 | 1.08 (0.93 – 1.25) | 1.43 (1.21 – 1.70) | 0.08 (−0.06 – 0.23) | 0.32 (0.22 – 0.42) |
| Met Syn/hsCRP<2 | 1.59 (1.34 – 1.88) | 1.79 (1.49 – 2.15) | 0.63 (0.46 – 0.79) | 0.47 (0.36 – 0.59) |
| Met Syn/hsCRP≥2 | 1.72 (1.49 – 1.99) | 2.35 (2.00 – 2.76) | 0.59 (0.45 – 0.74) | 0.67 (0.57 – 0.76) |
| MESA JUPITER population* Age, gender, race adjusted | ||||
| No Met Syn/hsCRP<2 | 1 (ref) | 1 (ref) | 0 (ref) | 0 (ref) |
| No Met Syn/hsCRP≥2 | 1.01 (0.80 – 1.29) | 1.62 (1.23 – 2.12) | 0.06 (−0.20 – 0.33) | 0.37 (0.21 – 0.54) |
| Met Syn/hsCRP<2 | 1.96 (1.43 – 2.70) | 1.78 (1.29 – 2.45) | 0.79 (0.46 – 1.11) | 0.44 (0.24 – 0.65) |
| Met Syn/hsCRP≥2 | 1.49 (1.15 – 1.95) | 2.42 (1.83 – 3.21) | 0.62 (0.34 – 0.91) | 0.65 (0.46 – 0.83) |
| MESA JUPITER population* Fully adjusted** | ||||
| No Met Syn/hsCRP<2 | 1 (ref) | 1 (ref) | 0 (ref) | 0 (ref) |
| No Met Syn/hsCRP≥2 | 0.97 (0.76 – 1.24) | 1.60 (1.21 – 2.12) | 0.01 (−0.26 – 0.27) | 0.35 (0.19 – 0.52) |
| Met Syn/hsCRP<2 | 1.69 (1.21 – 2.35) | 1.52 (1.10 – 2.12) | 0.58 (0.25 – 0.91) | 0.30 (0.09 – 0.51) |
| Met Syn/hsCRP≥2 | 1.24 (0.94 – 1.63) | 2.13 (1.58 – 2.87) | 0.38 (0.08 – 0.67) | 0.50 (0.31 – 0.68) |
MESA JUPITER population (N=2,083): Men age ≥50 and women ≥60 with LDL-C <130 mg/dL, not on lipid-lowering therapy, without diabetes, triglycerides ≤500 mg/dL, and creatinine ≤2 mg/dL
Fully adjusted model: Adjusted for age, gender, race, systolic blood pressure, diastolic blood pressure, smoking, LDL-C, anti-hypertensive medications
β-coefficient should be interpreted as the absolute difference between each category and the reference category.
Figure 2 summarizes the age and gender-adjusted prevalence ratios for the 4 study groups in graphical form (MESA JUPITER subpopulation).
Figure 2.
Association between obesity, metabolic syndrome (MS), hsCRP ≥2, and subclinical atherosclerosis in the MESA JUPITER population - MESA (2000–2002).
No Residual Confounding With Exposure Dichotomization or Gender Interaction
When anthropomorphic variables and hsCRP were included into the models as continuous variables, hsCRP was not significantly associated with either CAC or cIMT, while BMI and waist circumference were significantly associated with both CAC and cIMT. There was no statistically significant interaction between the 4 main study groups and age (study group x age, p=0.14), gender (study group x gender, p=0.41), or race (study group x race, p=0.56) in the prediction of subclinical atherosclerosis among MESA JUPITER participants.
No Effect Modification by JUPITER Inclusion/Exclusion Criteria
LDL-C greater than or less 130 mg/dL, use of cholesterol-lowering therapy, and diabetes status were not statistically-significant effect modifiers of the association between hsCRP and subclinical atherosclerosis.
Correlation with CHD and CVD Events
Median follow-up of the participants was 5.8 years. Within the MESA JUPITER population, there were a total of 82 CHD events and 117 CVD events. Trends were similar to those seen for subclinical atherosclerosis, although results did not achieve statistical significance due to limited power. The multivariable adjusted hazard ratio for CHD and CVD events for Group 2 (non-obese, high hsCRP) was 0.88 (95% CI 0.46 – 1.73) and 1.05 (95% CI 0.59 – 1.90), respectively. For Group 3 (obese, low hsCRP), the hazard ratios were 1.25 (95% CI 0.69 – 2.29) and 1.11 (95% CI 0.66 – 1.90). For Group 4 (obese, high hsCRP), the hazard ratios were 1.33 (95% CI 0.73 – 2.32) and 1.39 (95% CI 0.87 – 2.21), respectively.
DISCUSSION
In this large multi-ethnic cohort of participants free of baseline clinical cardiovascular disease, high hsCRP in the absence of obesity was not associated with CAC and was mildly associated with cIMT. Obesity was strongly associated with CAC and cIMT independent of hsCRP. When obesity and high hsCRP were both present, there was no evidence of multiplicative interaction. Similar associations were seen in when considering only the MESA JUPITER subpopulation.
Obesity and hsCRP
One proposed mechanistic link between obesity and the development of atherosclerosis is subclinical inflammation, resulting from innate and acquired immune responses22, 23. CRP is an acute phase plasma protein that is synthesized in the liver in response to inflammatory cytokines, and therefore is used as a non-specific marker of inflammation. Our study (ρ=0.42) confirms the close correlation between measures of obesity and hsCRP seen in other more homogenous cohorts1. Indeed, amongst over 19,000 participants in the Reasons for Geographic And Racial Differences in Stroke (REGARDS) study, obesity was more strongly correlated with elevated hsCRP than any other demographic or clinical variable24.
Results from our study indicate that circulating levels of hsCRP do not fully account for the association between obesity and atherosclerosis. As illustrated by the “metabolic syndrome” clinical phenotype, the vascular biology of obesity is complex with links to several other emerging atherosclerotic risk factors including prediabetes, atherogenic dyslipidemia, decreased adiponectin, leptin resistance, decreased plasminogen activator inhibitor-1 (PAI-1), and endothelial dysfunction including microalbuminuria25, 26. In our study, the addition of hsCRP as the sole marker of inflammation did not add to the association between the metabolic syndrome and subclinical atherosclerosis.
Inflammation, hsCRP, and Atherosclerosis
While there is a clear role for hsCRP in cardiovascular risk prediction independent of obesity and physical fitness27, 28, there is currently controversy over whether CRP plays a causal role in atherosclerotic cardiovascular disease (CVD)8, 29.
Studies supporting a causal role point to evidence that CRP binds to LDL and is present in atherosclerotic plaques30. However, recent basic science research has questioned a direct atherogenic mechanism. For example, direct injection of CRP into mice and rats illicits relatively little cellular level activity and little vascular inflammation31. Transgenic rabbits that express high amounts of human CRP have no additional aortic or coronary atherosclerosis, despite evidence of human CRP within the vessel wall32.
Several recent large clinical studies have added to the basic science evidence suggesting that CRP is not causal. For example, Zacho et al. studied a large population-based sample, showing that polymorphisms in the CRP gene are associated with marked increases in CRP levels yet do not predict the incidence of adverse ischemic cardiovascular events9. Elliot et al. carried out a Mendelian randomization study of the most closely associated single-nucleotide polymorphism (SNP) in the CRP locus, as well as other well-established CRP genetic variants, amongst over 100,000 patients10. The SNP and other variants were associated with CRP levels, but not with incidence of coronary heart disease. This suggests that hsCRP may reflect a secondary inflammatory response, and not the cause, of atherosclerosis.
Our study adds to prior studies demonstrating a weak association between hsCRP and subclinical atherosclerosis by studying a large multiethnic sample, stratifying by obesity, and including both CAC and cIMT11–14. Since atherosclerosis underlies the development of most clinical cardiovascular events, our findings suggest that if CRP does indeed have a causal role in CVD, the mechanism for its effect likely relies on pathways other than increased subclinical atherosclerosis. Additional research is needed to define the role of CRP in non-atherosclerotic pathways such as thrombosis, reduced fibrinolysis, and plaque instability8.
In our study, the association of high hsCRP with cIMT>75% percentile was stronger than that seen with CAC>0. Prior studies using different cutpoints have suggested a strong association between CRP and cIMT33, as compared to CAC11–14. The implications of atherosclerosis in these vascular beds may be different. The stronger association of hsCRP with cIMT may explain why some studies have found hsCRP to be more predictive of stroke than coronary heart disease34. Within MESA, CAC predicts all cardiovascular events (coronary heart disease, stroke, and fatal cardiovascular disease) better than cIMT, although cIMT is more strongly predictive of stroke35. cIMT also predicts stroke better than CAC amongst individuals >70 years old in the Cardiovascular Health Study36.
The MESA JUPITER Subpopulation
In our study, there were similar mild associations between high hsCRP and subclinical atherosclerosis in the entire MESA population and in the MESA JUPITER subpopulation. Neither LDL<130, exclusion of diabetes, or the exclusion of patients on prior lipid-lowering therapy modified the relationship between hsCRP and subclinical atherosclerosis. As such, the biology of hsCRP does not appear to differ for the specific MESA JUPITER population. Since prior research indicates that high hsCRP (at least in single biomarker approaches37) appears to be associated with increased absolute risk, our results suggest a continuing need to define the mechanism of hsCRP-related risk in all populations.
Limitations
This study is limited by its cross-sectional nature. While we are able to describe the statistical associations of obesity, hsCRP, and subclinical atherosclerosis, we are unable to establish the temporal relationships or causality. While our events analysis shows the same trends as those seen for subclinical atherosclerosis, these results must be considered exploratory given the small number of events when defining small subgroups within MESA.
While the multi-ethnic makeup of this study increases generalizability, it may limit precise characterization of obesity. Waist circumference, which was an integral part of our definition of obesity, varies for different ethnicities and geographic locations38. The impact of different waist circumference thresholds according to ethnicity has not yet been thoroughly described within MESA. Another limitation is that hsCRP was measured just once at baseline in MESA.
Conclusions and Future Directions
In this study, we show that high hsCRP as defined by JUPITER (≥2 mg/L) was weakly associated with subclinical atherosclerosis in the absence of obesity. In contrast, obesity is associated with increased atherosclerosis independent of hsCRP status. Further research, of a longitudinal nature, is needed to understand the potential independent mechanisms of risk imparted by obesity, subclinical inflammation as measured by hsCRP, and the possibility of their synergistic combination. Based on our study, it does not appear that isolated identification of hsCRP ≥2 mg/L is an optimal tool for identifying individuals expected to have an increased burden of atherosclerosis. Identifying obesity may be more valuable for this purpose.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
This research was supported by contracts N01-HC-95159 through N01-HC-95167 and N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Dr. Budoff is on the Speaker’s Bureau for GE Healthcare
Abbreviations List
- JUPITER
Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin
- MESA
Multi-Ethnic Study of Atherosclerosis
- hsCRP
high sensitivity C-reactive protein
- CAC
coronary artery calcium
- cIMT
carotid intima-media thickness
- BMI
body mass index
REFERNCES
- 1.Brooks GC, Blaha MJ, Blumenthal RS. Relation of C-Reactive Protein to Abdominal Adiposity. Am J Cardiol. 2010;106:56 – 61. doi: 10.1016/j.amjcard.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Festa A, D’Agostino R, Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–7. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 3.Musunuru K, Kral BG, Blumenthal RS, Fuster V, Campbell CY, Gluckman TJ, Lange RA, Topol EJ, Willerson JT, Desai MY, Davidson MH, Mora S. The use of high-sensitivity assays for C-reactive protein in clinical practice. Nat Clin Pract Cardiovasc Med. 2008;5:621–35. doi: 10.1038/ncpcardio1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 5.Yang EY, Nambi V, Tang Z, Virani SS, Boerwinkle E, Hoogeveen RC, Astor BC, Mosley TH, Coresh J, Chambless L, Ballantyne CM. Clinical Implications of JUPITER (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) in a U.S. Population – Insights From the ARIC (Atherosclerosis Risk in Communities) Study. J Am Coll Cardiol. 2009;54:2388–2395. doi: 10.1016/j.jacc.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cushman M, McClure LA, Lakoski SG, Jenny NS. Eligibility for statin therapy by the JUPITER trial criteria and subsequent mortality. Am J Cardiol. 2010;105:77–81. doi: 10.1016/j.amjcard.2009.08.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mora S, Ridker PM. Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)--can C-reactive protein be used to target statin therapy in primary prevention? Am J Cardiol. 2006;97:33A–41A. doi: 10.1016/j.amjcard.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Danesh J, Pepys MB. C-reactive protein and coronary disease: is there a causal link? Circulation. 2009;120:2036–9. doi: 10.1161/CIRCULATIONAHA.109.907212. [DOI] [PubMed] [Google Scholar]
- 9.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 10.Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC, Peden JF, Erdmann J, Braund P, Engert JC, Bennett D, Coin L, Ashby D, Tzoulaki I, Brown IJ, Mt-Isa S, McCarthy MI, Peltonen L, Freimer NB, Farrall M, Ruokonen A, Hamsten A, Lim N, Froguel P, Waterworth DM, Vollenweider P, Waeber G, Jarvelin MR, Mooser V, Scott J, Hall AS, Schunkert H, Anand SS, Collins R, Samani NJ, Watkins H, Kooner JS. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reilly MP, Wolfe ML, Localio AR, Rader DJ. Study of Inherited Risk of Coronary Atherosclerosis. C-reactive protein and coronary artery calcification: The Study of Inherited Risk of Coronary Atherosclerosis (SIRCA) Arterioscler Thromb Vasc Biol. 2003;23:1851–6. doi: 10.1161/01.ATV.0000092327.60858.4A. [DOI] [PubMed] [Google Scholar]
- 12.Khera A, de Lemos JA, Peshock RM, Lo HS, Stanek HG, Murphy SA, Wians FH, Jr, Grundy SM, McGuire DK. Relationship between C-reactive protein and subclinical atherosclerosis: the Dallas Heart Study. Circulation. 2006;113:38–43. doi: 10.1161/CIRCULATIONAHA.105.575241. [DOI] [PubMed] [Google Scholar]
- 13.Jenny NS, Brown ER, Detrano R, Folsom AR, Saad MF, Shea S, Szklo M, Herrington DM, Jacobs DR., Jr Associations of inflammatory markers with coronary artery calcification: results from the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2010;209:226–9. doi: 10.1016/j.atherosclerosis.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamirani YS, Pandey S, Rivera JJ, Ndumele C, Budoff MJ, Blumenthal RS, Nasir K. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis. 2008;201:1–7. doi: 10.1016/j.atherosclerosis.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 15.See R, Abdullah SM, McGuire DK, Khera A, Patel MJ, Lindsey JB, Grundy SM, de Lemos JA. The Association of Differing Measures of Overweight and Obesity With Prevalent Atherosclerosis: The Dallas Heart Study. J Am Coll Cardiol. 2007;50:752–759. doi: 10.1016/j.jacc.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 16.Burke GL, Bertoni AG, Shea S, Tracy R, Watson KE, Blumenthal RS, Chung H, Carnethon MR. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:928–35. doi: 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nasir K, Campbell CY, Santos RD, Roguin A, Braunstein JB, Carvalho JA, Blumenthal RS. The association of subclinical coronary atherosclerosis with abdominal and total obesity in asymptomatic men. Prev Cardiol. 2005;8(3):143–8. doi: 10.1111/j.1520-037x.2005.4362.x. [DOI] [PubMed] [Google Scholar]
- 18.Cassidy AE, Bielak LF, Zhou Y, Sheedy PF, 2nd, Turner ST, Breen JF, Araoz PA, Kullo IJ, Lin X, Peyser PA. Progression of subclinical coronary atherosclerosis: does obesity make a difference? Circulation. 2005;111:1877–82. doi: 10.1161/01.CIR.0000161820.40494.5D. [DOI] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 20.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac ct in population-based studies: Standardized protocol of multi-ethnic study of atherosclerosis (MESA) and coronary artery risk development in young adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 21.Naghavi M, Falk E, Hecht HS, Jamieson MJ, Kaul S, Berman D, Fayad Z, Budoff MJ, Rumberger J, Naqvi TZ, Shaw LJ, Faergeman O, Cohn J, Bahr R, Koenig W, Demirovic J, Arking D, Herrera VL, Badimon J, Goldstein JA, Rudy Y, Airaksinen J, Schwartz RS, Riley WA, Mendes RA, Douglas P, Shah PK. SHAPE Task Force. From vulnerable plaque to vulnerable patient--Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol. 2006;98:2H–15H. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Rocha VZ, Lippy P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 23.Libby P, Ridker PM, Hansson GK. Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cushman M, McClure LA, Howard VJ, Jenny NS, Lakoski SG, Howard G. Implications of increased C-reactive protein for cardiovascular risk stratification in black and white men and women in the US. Clin Chem. 2009;55:1627–36. doi: 10.1373/clinchem.2008.122093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaha MJ, Bansal S, Rouf R, Golden SH, Blumenthal RS, Defilippis AP. A practical “ABCDE” approach to the metabolic syndrome. Mayo Clin Proc. 2008;83:932–41. doi: 10.4065/83.8.932. [DOI] [PubMed] [Google Scholar]
- 26.Qasim A, Mehta NN, Tadesse MG, Wolfe ML, Rhodes T, Girman C, Reilly MP. Adipokines, Insulin Resistance, and Coronary Artery Calcification. J Am Coll Cardiol. 2008;52:231–6. doi: 10.1016/j.jacc.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rana JS, Arsenault BJ, Després JP, Côté M, Talmud PJ, Ninio E, Jukema JW, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. Inflammatory biomarkers, physical activity, waist circumference, and risk of future coronary heart disease in healthy men and women. Eur Heart J. 2009 Feb 18; doi: 10.1093/eurheartj/ehp010. [DOI] [PubMed] [Google Scholar]
- 28.Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boekholdt SM, Kastelein JJ. C-reactive protein and cardiovascular risk: more fuel to the fire. Lancet. 2010;375:95–6. doi: 10.1016/S0140-6736(09)62098-5. [DOI] [PubMed] [Google Scholar]
- 30.Zhang YX, Cliff WJ, Schoefl GI, Higgins G. Coronary C-reactive protein distribution: its relation to development of atherosclerosis. Atherosclerosis. 1999;145:375–9. doi: 10.1016/s0021-9150(99)00105-7. [DOI] [PubMed] [Google Scholar]
- 31.Clapp BR, Hirschfield GM, Storry C, Gallimore JR, Stidwill RP, Singer M, Deanfield JE, MacAllister RJ, Pepys MB, Vallance P, Hingorani AD. Inflammation and endothelial function: direct vascular effects of human C-reactive protein on nitric oxide bioavailability. Circulation. 2005;111:1530–6. doi: 10.1161/01.CIR.0000159336.31613.31. [DOI] [PubMed] [Google Scholar]
- 32.Koike T, Kitajima S, Yu Y, Nishijima K, Zhang J, Ozaki Y, Morimoto M, Watanabe T, Bhakdi S, Asada Y, Chen YE, Fan J. Human C-reactive protein does not promote atherosclerosis in transgenic rabbits. Circulation. 2009;120:2088–2094. doi: 10.1161/CIRCULATIONAHA.109.872796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao JJ, Arnold AM, Manolio TA, Polak JF, Psaty BM, Hirsch CH, Kuller LH, Cushman M. Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: the Cardiovascular Health Study. Circulation. 2007;116:32–8. doi: 10.1161/CIRCULATIONAHA.106.645606. [DOI] [PubMed] [Google Scholar]
- 34.Everett BM, Kurth T, Buring JE, Ridker PM. The relative strength of C-reactive protein and lipid levels as determinants of ischemic stroke compared with coronary heart disease in women. J Am Coll Cardiol. 2006;48:2235–42. doi: 10.1016/j.jacc.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–9. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman AB, Naydeck BL, Ives DG, Boudreau RM, Sutton-Tyrrell K, O’Leary DH, Kuller LH. Coronary artery calcium, carotid artery wall thickness, and cardiovascular disease outcomes in adults 70 to 99 years old. Am J Cardiol. 2008;101:186–92. doi: 10.1016/j.amjcard.2007.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HC, Greenland P, Rossouw JE, Manson JE, Cochrane BB, Lasser NL, Limacher MC, Lloyd-Jones DM, Margolis KL, Robinson JG. Multimarker prediction of coronary heart disease risk. J Am Coll Cardiol. 2010;55:2080–2091. doi: 10.1016/j.jacc.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 38.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.